Abstract
Many species use chemicals to communicate. In humans, there is increasing evidence that chemicals conveyed by the body are extremely important in interpersonal relationships. However, many aspects of chemical communication remain to be explored to fully understand this function in humans. The aim of this article is to identify relevant challenges in this field, with a focus on human attractiveness in the context of reproduction, and to put forward roadmaps for future studies that will hopefully extend to a wider range of social interactions. The first challenge consists in not being limited to body (mal)odours from the axilla. Preliminary data on how the odour of the face and head is perceived are presented. Second, there is a crucial need to increase our knowledge of the chemical bases of human chemical communication. Third, cross-cultural approaches must not be overlooked, because they have a major input in understanding the universal and culture-specific aspects of chemical communication. Fourth, the influence of specific cultural practices such as contraceptive and fragrance use is likely to be prominent and, therefore, needs to be well described. The fifth and last challenge for research projects in this field is the integration of different disciplines such as behavioural sciences, social sciences, neurosciences and microbiology.
This article is part of the Theo Murphy meeting issue ‘Olfactory communication in humans’.
Keywords: olfaction, human attractiveness, sex differences, culture
1. Introduction
The human body conveys a complex mixture of hundreds of chemical compounds (alcohols, carboxylic acids, ketones, aldehydes, esters, hydrocarbons, etc.), which are transformed from precursors secreted by excretory skin glands [1,2] through the action of skin bacteria (Corynebacteria, Proprionibacteria, Staphylococcus) (see [3]) and that constitute body odour. This chemical blend has a fixed fraction, which is genetically determined [4,5] and used as a marker of individuality [6,7]. It also possesses a variable fraction, which depends, for example, on diet [8,9], age [10] or physiological state [11,12]. There is empirical and societal evidence that odours produced by human bodies are not just some unwelcome emissions that a large proportion of individuals from many societies try to eliminate with the help of hygienic and cosmetic practices: they are sought, and they clearly affect daily interactions with others, especially those close to us. Scientific studies back up these observations by providing solid reasons to believe that chemical communication occurs in humans, i.e. communication through chemicals emitted by an emitter's body and perceived by a receiver through the olfactory and/or trigeminal systems.
Mother–infant communication, for example, is likely modulated by chemicals produced by the mother and perceived through the olfactory system of her baby. Indeed, mothers' areolar glands produce secretions that are attractive to newborns [13] and that are positively associated with breastfeeding success [14] (see [15]). But human chemical communication has been most extensively studied in the context of mate choice, mostly by investigating perceived attractiveness and preferences for a potential reproductive partner. Studies using self-reports show that body odour is rated as a key factor in a person's attractiveness when compared with several sensory and behavioural characteristics of a potential partner [16], although mainly in female raters [16,17]. Studies in patients with olfactory disorders show that olfactory loss has serious adverse effects on the quality of social interactions (see [18]), such as partnership insecurity or decreased sexual activity [19]. It must be pointed out here that a significant part of the research in this area has been driven by the fascination of the scientific community as well as the public by pheromones. In particular, many studies have focused on the behavioural effects of male steroid compounds, such as androstadienone. But the scientific justification for such a focus––to the detriment of all the other components of human biological odour––is highly debatable [20] and, although androstadienone is likely to have psychological effects [21,22], it does not seem to meet the concept of a sex pheromone [23,24] (see also [25]). In sum, while pheromones have been defined [26] and identified in several animal species (insects [27] and mammals [28,29]), their existence in humans has only been speculated and not rigorously demonstrated. In addition, their functional processing has been a subject of debate. Namely, the widespread belief that they would be processed through the accessory (vomeronasal organ, VNO) rather than the main olfactory system has been challenged by the fact that (i) the occlusion or absence of VNO in humans has no effect on putative pheromone perception [30] and that (ii) in other mammals (e.g. rabbit) pheromones are processed through the main (not the accessory) olfactory system [28,31,32].
Another section of research in this area provided significant advances in understanding human chemical communication by postulating that human body odour carries specific and relevant information about the individuals, which is transferred from one individual to another (chemosignalling; see [33]). Based on previous literature in non-human animals (e.g. [34]), perceptual studies in humans have revealed preferences for biological odours that provide cues to biological quality of a potential sexual partner with regard to genetics (including the major histocompatibility complex, MHC), reproductive status and health. People have been shown to prefer and choose MHC-dissimilar mates based on their body odour [35], at least in some populations [36], a mechanism believed to favour the production of MHC-heterozygous offspring who are more resistant to pathogens (see also [37,38]). This outcome is also served by a preference for MHC-diverse (heterozygous) mates, which has been shown to be a more robust phenomenon in a recent meta-analysis (while mixed evidence was found––notably as a function of samples' ethnical heterogeneity––for MHC-dissimilarity) [39]. Preferences for other indicators of mate fitness, which have been evolved because of their beneficial effects on the offspring (biological quality, contribution to child care), seem to be mediated by body odours. For example, women within the fertile phase of the menstrual cycle prefer the body odours of men with greater body bilateral symmetry (indicating a better developmental stability, which is a marker of good physical condition) [40], and for socially dominant men [41]. Men's preference for women's cues of fertility is another adaptive phenomenon that seems to be (in part) mediated by body odour, as shown by men's preference for odour samples collected during women's fertile phase of the menstrual cycle [12,42]. Additionally, human body odour communicates information about health status (see [43]), which is one aspect of mate fitness. Compared with healthy donors, the odour of sick persons is more aversive [11] and more intensely activates brain networks involved in odour processing [44]. Information about body condition related to nutritional state is also conveyed by body odour [45], as well as about gender [46,47] and age [10]. Finally, body odours are involved in the communication of emotions (see also [48,49]; meta-analysis in [50]) and of personality traits (such as dominance and neuroticism) [51], both of which are involved in the initiation and maintenance of romantic relationships [52].
In spite of these numerous pieces of evidence regarding the existence of a chemical communication in humans, especially in the context of mate choice, the mechanisms of such a communication are poorly documented to date. The aim of this article is to identify in the following sections five relevant challenges in the field and to put forward potential avenues for future studies to better understand chemical communication. The context of mate choice is used as a background here because it is the most largely documented, probably owing to the fact that reproduction is a fundamental function in living organisms and to the rich evolutionary theoretical framework available in this domain. However, it must be kept in mind that the identified challenges also apply (and should be extended) to other social contexts. Current knowledge is almost exclusively based on one odour source, the axilla, and on several unpleasant odorous compounds (malodours): Challenge 1 therefore consists in going beyond malodours from the axilla. Especially, odour sources that have a behavioural relevance in interactions with the actual or a potential mate (such as the hands or the face) should clearly be within the scope of investigation. We started facing this challenge and provide some new data on the perception of head/face odour from men and women. Challenge 2 consists in increasing our knowledge of the chemistry of human body odour. Most of the studies mentioned in the previous paragraph use perceptual approaches, and little is known about the chemical bases of human chemosignalling. Exploring how this question was studied until today reveals several limitations, as well as heterogeneity and relative paucity of investigations. Challenge 3 is a warning against confining investigations to the usually studied WEIRD populations (Western, Educated, Industrialized, Rich, and Democratic countries) [53] and an explicit incentive to extend them to different cultures, which to date has only been done occasionally. This allows testing to what extent hypothesized mechanisms of odour-based attractiveness may be common to the human species and to identify cultural specificities. About cultural specificities, two major aspects constitute Challenge 4: contraception and fragrance use. These very widespread practices seem to interfere with or complement natural chemical communication. Finally, significant advances in the field will be possible only with combined efforts from different disciplines: this constitutes Challenge 5. This last challenge presents how the conjunction of behavioural sciences, social sciences, neurosciences and microbiology will help to increase our understanding of human chemical communication.
2. Challenge 1: beyond the axilla
Although many areas of the body produce a smell, most studies on human chemical communication have investigated odours from the axilla. This can be explained in part by the belief that axilla has taken over the communication role from the anogenital region owing to the upright human posture [54], but also by cosmetic motives (necessity to understand the formation of malodours to better neutralize them), and by the fact that in adults, it is one of the most odorous body regions (richest in apocrine sweat glands) and one of the easiest to collect (discreet sampling, limited contamination by exogenous odours). Other odour sources, largely ignored so far, may be relevant though.
For example, the perception of vaginal and breath odours has been occasionally investigated as a function of hormonal status and gender. Vaginal odours were found to have lower intensity and unpleasantness during the late follicular phase (near ovulation) of female donors, compared to the other phases of the menstrual cycle [55]. The authors, however, raise some doubts regarding a possible function of vaginal odours in attraction because they remained in the unpleasant pole of hedonic valence even around ovulation. Breath odours of men and women were categorized as male or female at a better than chance frequency [46], which seems to be driven by the propensity to rate male odours as more intense and less pleasant than female odours (see also [56]). Another study found that individuals can be discriminated on the basis of olfactory cues from their hands; female raters' performance in this task was improved when the two donors were genetically unrelated, and differed on their diet [57]. More recently, a comprehensive approach consisted in comparing the responses of men exposed to women's tears versus a control saline solution [58]. Without conscious perception of an odour, tears caused men's sexual arousal to decrease, which was associated with a decrease in physiological arousal and in the activation of the cerebral substrates of sexual arousal (but see [59] and the following debate [60,61]). Another study from the same group (see also [62]) focused on a widespread social practice: greeting by hand-shaking [63]. This behaviour may be used to sample conspecifics' chemosignals since participants increased the sniffing of their own hands after a handshake. How hand sniffing behaviour after greeting a person of the opposite sex could be related with the evaluation processes of a potential reproductive partner is an aspect that should be investigated further. Finally, other body odour sources have been investigated for other purposes than studying human attractiveness to conspecifics. These studies often favour analytical views of the emitted odour over behavioural approaches. Their aims range from (i) managing malodorous emanations (e.g. from feet [64], urine [65], flatus [66], but also related to ageing [67]), (ii) diagnosing diseases (e.g. cancer, from breath [68]), (iii) forensic applications (e.g. odours from hands [69], internal organs [70]), to (iv) understanding the spreading of diseases (owing to blood-sucking insects attracted by human hand odour [71] or feet odour [72]).
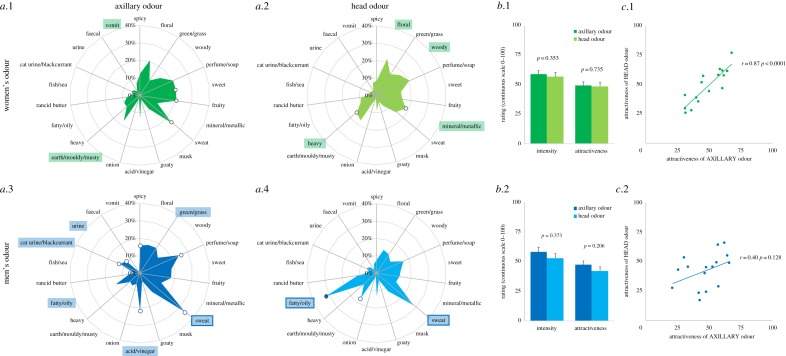
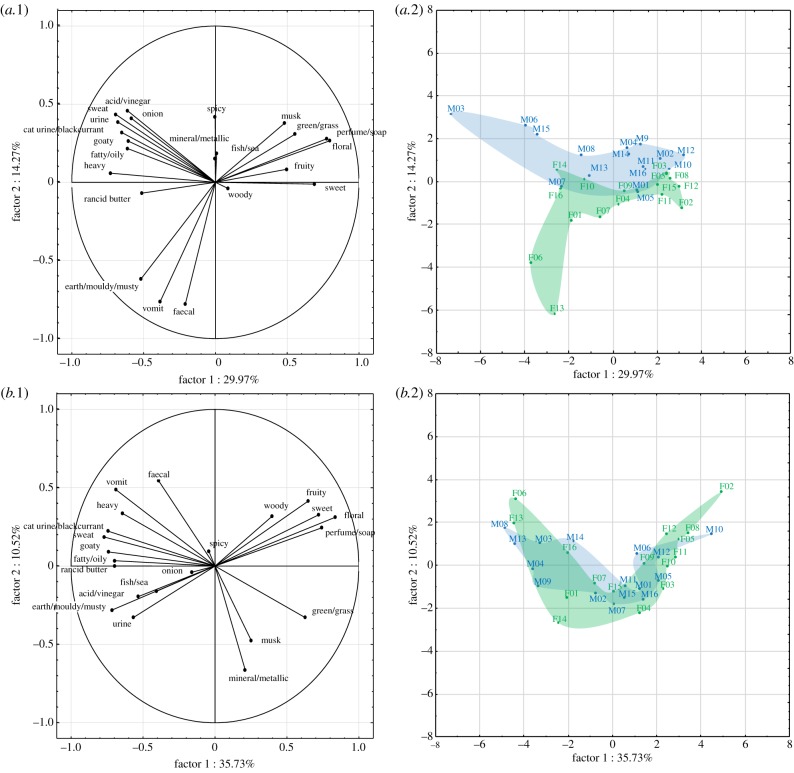
Returning to body odour attractiveness, it is crucial to consider odours that may be meaningful during interactions with the partner, namely odours emitted by body parts that are sampled voluntarily or––more likely––in a non-conscious manner. This includes more particularly the head (face, hair, neck and mouth), the hands, the axilla and the genital areas. Here, we present the results of a perceptual study comparing the odour of the head and of the axilla (for details of our methods, see electronic supplementary material). Young heterosexual adults, of European descent and non-smokers, took part either as donors (N = 16 men and 16 women, including 8 contraceptive pill users and 8 non‐pill users) or as raters (N = 16 men and 17 women, including 9 non-pill users and 8 pill users). Donors followed a strict food/hygiene protocol and sampled the odour of their axilla and their head (face, neck and scalp) over 6 days. Raters evaluated the attractiveness and intensity of opposite-sex odour samples on continuous scales, and described them by choosing the most appropriate terms among a list of both positive and negative descriptors from a list of 22 items (electronic supplementary material, table S1). Qualitative variations can be described as a function of gender (figure 1a, descriptors highlighted by colours). Men's odours were perceived as sweatier than female samples (axilla and head), but also more fatty/oily (head). Women's axillary odours were more earthy/mouldy/musty and smelled more like vomit than male samples, and their head odour was more floral, woody, heavy and mineral/metallic. Odour sources also differed (figure 1a, descriptors highlighted by dots). Compared to axillary odour, head odour was heavier and more mineral/metallic in women, and more fatty/oily and earthy/mouldy/musty in men. On average, head and axillary odours did not differ in intensity or attractiveness, which were moderate (figure 1b). A principal component analysis (PCA) conducted on the individual's qualitative profiles showed that the highest part of the variance (30% and 36% for axilla and head odours, respectively, first factor) was explained by valence as shown by the contribution of descriptors such as ‘floral’, ‘perfume/soap’ versus ‘sweat’, ‘heavy’, ‘cat urine’ (figure 2a.1 and 2b.1). The second factor (14% and 11% of the total variance, respectively) could be interpreted as pathogenic threat, since for both odour sources terms related to organic decay (‘vomit' and ‘faecal’) contribute importantly to this dimension (figure 2a.1 and 2b.1; in opposition to ‘mineral/metallic’ for head odour, figure 2b.1). Examination of how odour donors are represented in these two-dimensional spaces (figure 2a.2 and 2b.2) suggests that there is a higher variability between individuals, i.e. spatial distance between them, with regard to the negatively connoted descriptors (left side of the graphs) than for the positive descriptors (right side of the graphs). This was true both for head odours (bottom graph, figure 2b.2) and even more for axillary odours (top graph, figure 2a.2) for which men and women seem to present different patterns of unpleasant features. The diversity in body odour quality between individuals may thus rest upon chemical compounds that are rather unpleasant, especially in the axilla. A relevant question for research in the coming years is whether olfactory attractiveness is based on some (to be defined) pleasant attributes, or on the absence of some (to be defined, too) unpleasant attributes that are cues of poor health/of other unwanted features in a reproductive partner, or on both (the second option being supported by the fact that diversity is higher within the unpleasant pole of descriptors).
Figure 1.
Perception of human body odour from the axilla and head of women (top, green, N = 16) and men (bottom, blue, N = 16). (a) Descriptive profiles (in percentage of times each descriptor was chosen): terms differing by more than 5% between men and women are highlighted (in green when higher in women, in blue when higher in men) and frames indicate a difference greater than 10%; terms differing by more than 5% and 10% between head and axilla are indicated by empty and filled dots, respectively. (b) Average intensity and attractiveness ratings (mean + s.e.m.) for axillary and head odours, compared by paired-sample t-tests. (c) Pearson correlations between axillary and head odours' attractiveness.
Figure 2.
PCA on the descriptive data of (a) the axilla and (b) the head odour of women (green, N = 16) and men (blue, N = 16). The figures represent the projections of the variables (descriptive terms, a.1 & b.1) and of the cases (odour donors, a.2 & b.2) on the first bi-dimensional spaces that explained nearly half of the variance.
Interestingly, head and axillary odour attractiveness were highly correlated in women (r = 0.87, p < 0.0001) and significantly less in men (r = 0.40, p = 0.128; figure 1c; comparison between rs: p = 0.028). It cannot be excluded that a bias could have accounted for these results: a potential greater use of perfumed cosmetics in women may have homogenized the olfactory signature of different body parts (through residual compounds diffusing all over the body, for example, despite the hygienic restriction protocol we applied) rendering axillary and head odour more similar than in participants using less fragrant products. But another possibility that would be worth exploring in the future is that the odour of the head could convey different cues from the axilla in male donors. Some of the most interesting questions to address in the future are: which information is conveyed by each odour source? by which mechanisms? and which odour source has more weight in determining the attractiveness of a potential reproductive partner?
Associated with another set of studies showing that odours of the breast—a body area rich in sebaceous glands as is the face—may serve mother–infant communication [13,14], the unique data presented above support the idea that more effort should be made to investigate odours produced in the facial area owing to their potentially relevant informative content. While axillary odours mostly originate in the bacterial transformation of compounds conveyed by apocrine sweat, the face and scalp possess a high concentration of sebaceous glands (and some apocrine glands) and probably host a different microflora, which is likely to result in odours differing in quality compared to the axilla. Whether these odours differently impact potential partners' attractiveness remains to be tested, with comprehensive approaches including the testing of behavioural effects. Qualitative measures using descriptive terms is a useful approach to investigate the sensory profiles of body odours (see also [73]), but also to describe their intra- and inter-individual variations (gender differences, variations according to hormonal status), and eventually to link the odours' perceptual and chemical profiles.
3. Challenge 2: body odour chemistry
In humans and other mammals, the study of chemical communication is much more recent than in other organisms such as plants or insects [74]. In the past two decades, the chemical composition of human scents has received increasing interest in areas such as medical diagnosis [68] and disease prevention [75,76], forensics [69,70], emergency search [77] and more occasionally in the field of cognitive psychology and affective neuroscience (e.g. communication of emotions [78,79]). The chemical underpinnings of human chemical communication remain mostly unknown, likely because explorations of the characteristics of human scent using analytical chemistry are very heterogeneous and generally confined to very specific issues such as malodour management [67,80,81].
The chemicals emitted by the human body are volatile organic compounds (VOCs, which are targeted when investigating communication through the olfactory system), but also semi- and non-volatile compounds that emanate from the skin, urine, sperm, vaginal secretions, sweat and breath. Various sampling methods have been used to analyse human body odour (see [76] for a review), including (i) solvent extraction, where compounds are collected on a medium (e.g. cotton, gauze and glass) and then extracted with a solvent (e.g. hexane, dichloromethane and ether); (ii) solid-phase micro-extraction (SPME) and stir bar sorptive extraction (SBSE), where volatiles are trapped on an adsorbing polymer coated on a small glass fibre or a stir bar, and then liberated by thermal desorption for analysis; and (iii) other types of VOC-traps for the dynamic headspace adsorption on various porous polymers (e.g. Tenax, Porapak Q). Analysis is then often performed with gas chromatography–mass spectrometry (GC-MS), which combines the excellent resolutive capacity of GC with the power of MS for structure identification (usually assisted by the use of mass spectra databases). GC-MS is particularly adapted to highly volatile compounds of low molecular weight and polarity (as are most animal scent compounds). Alternatives include liquid chromatography LC-MS [82], which is more adapted for detection of non-volatile compounds, proton-transfer-reaction PTR-(Q or QTOF)-MS [78], allowing for real-time quantification of VOCs (see [83]), selected ion flow tube SIFT-MS [84] or multi-capillary column-ion mobility spectrometry MCC-IMS [85]. Each method has its advantages and drawbacks, such as isolation of irrelevant compounds with solvent extraction (compounds that are not volatile at natural body temperature) or SPME (artefacts originating from the trapping polymer) [86]. Choosing the most suited technique must be carefully weighted [87] depending on the studied substrate (human scents are complex and diverse) and the pursued goal (e.g. understanding an individual's full chemical signature for forensic applications versus investigating olfactory detection of airborne attractive compounds). Indeed, two different methods may provide very different outcomes, as shown for example by a comparison of SPME and solvent extraction, which revealed only 15 molecules in common (against 92 and 58 compounds found with each technique, respectively [88]). To comprehensively and exhaustively understand human body odour, using complementary techniques and comparing them as it has been done in plant science [89,90] can prove to be useful [86,88,91,92], although inevitably costly and time-consuming.
To date, our knowledge about the chemical composition of human body odour remains heterogeneous and incomplete. Diverse methods are used for sampling (collection time from a few minutes to several hours; during daytime or night-time; contact or non-contact; diverse media such as sterile gauze, cotton, wool, viscose, glass beads and t-shirts [93–95]), and for analysing (see above). Outcomes are therefore hardly comparable, which is worsened by the small number of donors involved and by the large number of VOCs found [77]. These VOCs are sometimes listed without discussing their origin: it has been estimated that nearly one-third of the molecules reported in mammals are either misnamed or not likely to have been produced by the studied organisms [96], therefore emphasizing the need for a critical attitude towards the origins and roles of the compounds found. The complexity and fluctuations of human body odour (owing to diet, physiological state, inter-individual genetic variability and exogenous compounds introduced using drugs and cosmetics) constitute a serious challenge. Exogenous compounds related to fragranced cosmetics are difficult to eliminate even with a specific hygiene protocol before sampling [88,97] and others are owing to contaminations of the samples at every stage of the processing, indicating that special precautions should be taken to diminish this risk [87]. In sum, more research is needed in this area, always with extreme care at all stages of processing, especially regarding undesirable contamination, and standardization of methodologies would be welcome. To do so, recruiting volunteers who have a natural/perfume-free lifestyle could help in obtaining more relevant chemical profiles. Also, repeated measures over time for a given individual would be helpful. Replications of previous studies are needed too, as in other fields of science, which could be facilitated by open-access practices [87] that allow for cross-analyses and reduction in redundant experiments. Finally, significant progress could be achieved on chemical profile characterization with the help of new generations of ‘artificial noses’, namely electronic devices mimicking the nose and detecting odorants through sensors. The field of artificial olfaction has recently achieved a significant expansion and offers devices with increasingly improving performance based on bioinspired sensors [98]. Historically used for quality control of the environment or of food and beverages, artificial noses are increasingly employed in disease diagnosis and could very well extend their applications to detection of body odour compounds.
4. Challenge 3: cross-cultural studies
The study of how body odours may be involved in human reproductive function should not be limited to the usually studied WEIRD populations [53]. Including different populations allows us to test to what extent hypothesized mechanisms of odour-based attractiveness may be common to the human species and to describe cultural specificities. For example, it has been proposed that if human body odour contains compounds that are relevant in human attractiveness, then olfactory receptor genes responsible for their detection would be highly conserved in the human species [20]. In the light of this, the extensively studied putative pheromones androstenone and androstadienone seem to be poorly credible candidates. Indeed, at least one in five people cannot detect them [23,99] and strong genetically based inter-individual variability has been described for their perceived quality [100]. In line with this, a significant part of the population (80–95% of Asians, versus 0–3% of Europeans and Africans) possesses a non-functional variant of the ABCC11 gene, which has a key function in the formation of body odour, resulting in the absence of androgen (and carboxylic acid) compounds and a much weaker body odour [101]. These molecules may thus not be as relevant in human mate choice as scientists once thought.
Considering the more complex body odour in its entirety, cross-cultural studies remain scarce. Anthropologists have pointed out ethnicity-related differences in body odour judgements, such as the fact that white and black people perceive the other group as ‘foul-smelling’ [102]. A proposed explanation was that such an olfactory classification of people demarcates social groups and contributes to maintaining boundaries between them. However, whether this has biological and perceptual grounds remains under-studied to date, probably because this is an ethically sensitive topic. A few studies compared Asian, African and Caucasian people on several aspects, though. African participants were found to have the largest apocrine glands and highest levels of sweat secretion, while Asian participants have fewer apocrine glands and a lower level of axillary odour [103]. The emission of some carboxylic acids also differs between ethnic groups [104], and differences related to donors' ethnicity were reported in terms of pleasantness and intensity ratings [105], which are likely genetic, although diet- and hygiene-related influences cannot be excluded.
Cultural norms and practices may indeed influence body odour emission and perception. For example, the rules of interpersonal distances during social interactions (theory of proxemics [106]) vary according to culture [107] and may have a role in chemical communication, as hypothesized in some cultural comparisons of body odour perception [108,109]. Cultural variations do also exist in emotional responses to odours [110,111] and in the language used to evoke odours (see [112]). Cultural variations in cosmetic and drug practices may also be influential, as detailed hereafter.
5. Challenge 4: pill and fragrance use
Cultural practices are known to interact with body odour emission and perception: hormonal contraception and cosmetic practices are among the most significant ones. Using hormonal contraceptives seems to cause counter-adaptive effects, such as inverting preferences related to the immune system (preference for the smell of individuals with a similar MHC [113], while the reverse could be expected in naturally cycling women on the basis of some studies [35]; but see [39]). Studies using various smells [114,115] and body odours [116] show that olfactory sensitivity of pill users is equivalent to that of non-pill users in the luteal (non-fertile) phase of their menstrual cycle, both groups having lower sensitivity than non-pill users in the follicular (fertile) phase of their cycle. The same pattern occurs for body odour emission, with body odour attractiveness being highest around ovulation [12] and lowest in the luteal phase and in women using the pill [42]. Hormonal contraception use is very widespread in Western societies (41% of French women aged 15–49 years [117]) and its influence on mate preferences [118] and more largely on the brain and behaviour [119] is being increasingly studied. Therefore, how it interferes with olfactory attractiveness of a partner and how initiation/cessation of it impacts the relationship deserves more attention in the future.
Another practice that might influence chemical communication is the ancient and widespread use of fragranced daily hygiene products. Masking or embellishing people's body odour is one of the major functions of this practice, but the nature of perfume–body odour interactions is complex and to date not fully understood, all the more so as most studies on human chemical communication require perfume removal in donors to focus on the biological odour and to limit exogenous variations in body odour samples. There are chemical interactions with perfume depending on the person's natural odour type [120], potentially leading to the formation of new aromatic compounds [121]. There is some evidence that fragrance has a disruptive effect on judgements of masculinity [122], personality traits [123] and gender [124], and that it modifies other social judgements such as perceived likeability and friendliness [125]. Some experiments support the idea, however, that fragrances may interact with body odour in a complementary manner. An Individual's natural olfactory signature is conserved even when they use perfume, as shown by a discrimination task [126], and the use of fragrance creates individually specific odour mixtures leading to the maintenance of the inter-individual variability [127]. Comparing people's own perfume versus a fragrance imposed by the experimenter, several studies show that people choose perfumes in an individual fashion to complement their own natural scent [126,127]. This is very consistent with the fact that individual preferences for perfume ingredients (for use on self) correlate with a person's MHC genotype [128,129]. Perfumes selected for oneself may thus enhance the individual genetic/olfactory signature (see also [130] for a discussion of perfume–body odour interaction being an example of culture–gene coevolution), and the selection of perfume by a biologically related individual (sister versus girlfriend or oneself) enhances the attractiveness of the perfume/men's body odour blend [131]. Human body odour is very complex in itself, and understanding the hardly predictable perceptual quality that emerges when it is mixed with a perfume represents an additional significant challenge for the field of human chemical communication. In the future, particular attention should be paid to the fact that not only conscious processes (as measured by raters' verbal responses in the studies cited above), but also non-conscious processes, are likely to result in fragrance-related changes in social behaviours. To finally widen the scope of investigation, other hygienic practices may be worth considering to understand the impact of human behaviours aimed at odour control, such as how, and how often people wash their body.
6. Challenge 5: interdisciplinarity
Inputs from different disciplines are obviously required to understand chemical communication. Beyond the necessity to multiply analytical chemistry approaches (discussed in Challenge 2), other approaches using measures of overt behaviour, theoretical frameworks of the social sciences, neuroscientific methods and analysis of the skin microbiome will undoubtedly be beneficial to the field.
Human chemical communication has been studied using mostly verbal descriptions of how body odour samples are perceived, either with rating scales (pleasantness, intensity, attractiveness, etc.) or with preference tasks (see §1). Indirect tasks have also been used, requiring social stimuli (e.g. faces [23], real persons [132]) to be rated after being primed by or in the presence of a body odour or a body odour compound. However, how chemosignals influence our biology and cognition, and ultimately our behaviours, is likely to be a covert process, which is not necessarily measurable with conscious verbal methods. Future research would certainly gain in developing paradigms to measure overt behaviours. For example, motor behaviours are sometimes (but rarely) measured, especially when language is lacking: e.g. head orientation of infants towards spatially distributed odours [133], or reach-to-grasp action towards an object in children with autism [134]. Other motor behaviours appear to be relevant, such as odour sampling behaviours, measured indirectly, e.g. by recording the frequency with which someone brings his/her own hand near the face after hand-shaking [63], or directly, e.g. by recording airflow variations in the nostrils (sniffing behaviour [135]). The latter, however, has never been used to our knowledge in the context of social communication in humans (but see [136] for non-human hominids). Ideally, behaviours displayed in real social interactions (e.g. speed dating experiment, see [132]) and indicating attraction or repulsion towards someone would be very informative. However, although being highly ecologically valid, such approaches face numerous experimental biases, such as uncontrolled sensory and behavioural factors inherent to the interacting person. Therefore, crafty alternatives could be developed, such as associating the diffusion of body odours with artificial standardized individuals, using virtual avatars [137] or physically present manikins [138].
Besides these methodological perspectives in terms of outcome variables, taking advantage of the theoretical frameworks provided by social sciences would help to give a more integrated view. Some models have been proposed to conceptualize social interactions, such as the perceptual model of intergroup relations (PMIR) [139]. The PMIR can be fed with several existing research on human body odour from various disciplines [140], to explain how olfaction mediates the interactions between group belonging (gender, culture/ethnicity, religion and social status) and intergroup perception/relationships. Although this model can be recruited for many types of social interactions (colleagues, friends, strangers, etc.), in the case of reproduction and mate choice, gender-related aspects are of major interest. Indeed, this framework can help in modelling how one's particular biological odour influences his/her gender identity (e.g. level of gender conformity/nonconformity [141]), as well as the reverse, i.e. how one can modulate his/her gender identity by manipulating the odour blend conveyed by his/her body (through hygienic and cosmetic practices). This in turn may have implications for the relationships with other individuals, depending on their gender (e.g. implications for sexual orientation). Tightly related to this, the concept of stereotype is also worth considering as it may have a considerable influence on body odour perception. Gender stereotypes have seldom been investigated in the field of social olfaction (see [142] for perfumes, and [105,143] for body odour), despite being a potentially significant source of cognitive modulation of body odour perception.
To understand the mechanisms by which chemicals emitted by a potential mate influence receivers' responses, we need to have a better knowledge of the central processing of these chemosignals. The neural networks involved in the perception of social chemosignals have been explored mostly for androstadienone, which was found to activate––even in the absence of conscious perception––a brain network that is not exclusively associated with olfaction and that is related to social cognition and attention [144,145]. Differences were found in the hypothalamus as a function of perceiver's sex ([146], but see [147]) and sexual orientation [148,149], and interpreted as being the underpinnings of sex-differentiated behavioural responses. In more naturalistic approaches, it was found that body odours induced increased neural activity in a large network including cingulate cortex, occipital and angular gyri, but not the primary olfactory areas [150]. Different networks are recruited depending on the origin of the body odour: network for processing fearful stimuli (stranger versus friend odour) [150], kin recognition (sister versus friend odour) [151] and rewarding stimuli (infant's odour) [152]. Some findings are more directly related with body odour attractiveness: exposure to sadness tears [58] and to body odour of sexually aroused donors [153] modulates activations in the brain substrates of sexual arousal (namely fusiform gyrus and hypothalamus). Further research is needed to identify the neural networks involved in body odour attractiveness processing, considering, for example, other odour sources than the axilla (see Challenge 1).
While neuroscientific approaches provide an invaluable methodological asset to understand how receivers process potentially relevant chemosignals, there is a need to better understand how these chemosignals are produced and the mechanisms by which they convey information that is relevant to mate choice (and more generally to other social interactions). In this perspective, analytical chemistry has been identified as key discipline in Challenge 1, but biochemistry and microbiology are at least as important. Indeed, microorganisms are key contributors to the formation of human body odour. Precursor molecules that are present in the (odourless) secretions of skin glands (apocrine, eccrine, apoeccrine and sebaceous glands) are transformed through enzymatic activity of the resident bacteria into compounds that are more or less volatile. This process has been mostly explored for one production site: the axilla [1,154–158]. The main families of bacteria identified on the skin are Corynebacteria, Propionibacteria and Staphylococci [1,159], with more men having a coryneform-dominant microflora (two-thirds, versus one-third of the women) [160]. Different types of bacteria have been found to produce different compounds, resulting in various odour types: Corynebacteria are responsible for the production of carboxylic acids (such as 3-methyl-2-hexenoic acid and 3-hydroxy-3-methylhexanoic acid) and androgen steroids (such as 5α-androst-16-en-3-one, 5α-androst-16-en-3α-ol and 4,16-androstadien-3-one) that contribute to a typical pungent axillary odour, while a more acid and sulfur-like odour is given by Staphylococci through the production of short-chain fatty acids and thio-alcohols (such as 3-methyl-3-sulfanylhexan-1-ol) [1,80,158]. The axilla is an ideal field of exploration in this regard because it contains a high concentration of all types of skin glands, hosts a dense population of microorganisms and produces numerous volatile compounds. Other odour-producing areas of the body, however, are likely less prolific and thus more difficult to investigate, but they probably constitute an inescapable challenge for future research in human chemical communication. Deeper explorations would also be welcome regarding individual differences but also the factors of variations in time and their potential influence on interpersonal behaviour.
7. Conclusion
Chemical communication in humans is a relatively new field of research, with most of the research effort being concentrated in the past two decades. Major advances have been made, but many aspects remain poorly understood for various reasons, including the fact that this is a recent topic, and focus has been made on some odours and on some social contexts to the detriment of others (e.g. mate choice versus other types of social interactions such as parent–child and friends) and that researchers are facing numerous technical, methodological and disciplinary limitations. Although this is a ‘young’ area of research, investigators are making constant progress. The challenges developed in this article are destined to provide guiding principles for future studies to contribute to elucidating the social function of odours in various contexts of social interactions.
Supplementary Material
Acknowledgments
We would like to thank Mariam Khidasheli, Ines Mehu-Blantar, Alexia Lamblin, as well as Myriam Troccaz, Christian Starkenman and Isabelle Cayeux (Firmenich), for their respective inputs in terms of bibliography, data collection and methodology. We are also very grateful to two anonymous reviewers for their valuable and constructive comments on an earlier version of the manuscript.
Ethics
Protocol approved by the Committee on Research Ethics of the Faculty of Psychology and Education Sciences at the University of Geneva.
Data accessibility
Ferdenzi C, Delplanque S. 2020 Dataset related to: 'Interdisciplinary challenges for elucidating human olfactory attractiveness' (Version 1) [Dataset]. Zenodo. (doi:10.5281/zenodo.3676952)
Authors' contributions
C.F. designed the study, carried out the data collection, carried out the statistical analyses and drafted the manuscript; S.R.O. co-drafted and critically revised the manuscript; S.D. participated in the design of the study and critically revised the manuscript; N.B. critically revised the manuscript; M.B. co-drafted and critically revised the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This research was financed by grants from the Fondation Fyssen Paris (grant no. 173867, to C.F.), the French National Research Agency (grant no. ANR-12-PDOC-0016, to C.F.), the Swiss National Science Foundation (grant no. 100014_130036) and from the National Centre of Competence in Research ‘Affective Sciences’ (grant no. 51NF40-104897).
References
- 1.Leyden JJ, McGinley KJ, Holzle E, Labows JN, Kligman AM. 1981. The microbiology of the human axilla and its relationship to axillary odor. J. Invest. Dermatol. 77, 413–416. ( 10.1111/1523-1747.ep12494624) [DOI] [PubMed] [Google Scholar]
- 2.Wilke K, Martin A, Terstegen L, Biel SS. 2007. A short history of sweat gland biology. Int. J. Cosmet. Sci. 29, 169–179. ( 10.1111/j.1467-2494.2007.00387.x) [DOI] [PubMed] [Google Scholar]
- 3.Natsch A, Emter R. 2020. The specific biochemistry of human axilla odour formation viewed in an evolutionary context. Phil. Trans. R. Soc. B 375, 20190269 ( 10.1098/rstb.2019.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts SC, Gosling LM, Spector TD, Miller P, Penn DJ, Petrie M. 2005. Body odor similarity in noncohabiting twins. Chem. Senses 30, 651–656. ( 10.1093/chemse/bji058) [DOI] [PubMed] [Google Scholar]
- 5.Kuhn F, Natsch A. 2009. Body odour of monozygotic human twins: a common pattern of odorant carboxylic acids released by a bacterial aminoacylase from axilla secretions contributing to an inherited body odour type. J. R. Soc. Interface 6, 377–392. ( 10.1098/rsif.2008.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penn DJ, et al. 2007. Individual and gender fingerprints in human body odour. J. R. Soc. Interface 4, 331–340. ( 10.1098/rsif.2006.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchal S, Bregeras O, Puaux D, Gervais R, Ferry B. 2016. Rigorous training of dogs leads to high accuracy in human scent matching-to-sample performance. PLoS ONE 11, e0146963 ( 10.1371/journal.pone.0146963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havlicek J, Lenochova P. 2006. The effect of meat consumption on body odor attractiveness. Chem. Senses 31, 747–752. ( 10.1093/chemse/bjl017) [DOI] [PubMed] [Google Scholar]
- 9.Fialova J, Roberts SC, Havlicek J. 2016. Consumption of garlic positively affects hedonic perception of axillary body odour. Appetite 97, 8–15. ( 10.1016/j.appet.2015.11.001) [DOI] [PubMed] [Google Scholar]
- 10.Mitro S, Gordon AR, Olsson MJ, Lundström JN. 2012. The smell of age: perception and discrimination of body odors of different ages. PLoS ONE 7, e38110 ( 10.1371/journal.pone.0038110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson MJ, et al. 2014. The scent of disease: human body odor contains an early chemosensory cue of sickness. Psychol. Sci. 25, 817–823. ( 10.1177/0956797613515681) [DOI] [PubMed] [Google Scholar]
- 12.Singh D, Bronstad PM. 2001. Female body odour is a potential cue to ovulation. Proc. R. Soc. Lond. B 268, 797–801. ( 10.1098/rspb.2001.1589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doucet S, Soussignan R, Sagot P, Schaal B. 2009. The secretion of areolar (Montgomery's) glands from lactating women elicits selective, unconditional responses in neonates. PLoS ONE 4, e7579 ( 10.1371/journal.pone.0007579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doucet S, Soussignan R, Sagot P, Schaal B. 2012. An overlooked aspect of the human breast: areolar glands in relation with breastfeeding pattern, neonatal weight gain, and the dynamics of lactation. Early Hum. Dev. 88, 119–128. ( 10.1016/j.earlhumdev.2011.07.020) [DOI] [PubMed] [Google Scholar]
- 15.Schaal B, Saxton T, Loos H, Soussignan R, Durand K. 2020. Olfaction scaffolds the developing human from neonate to adolescent and beyond. Phil. Trans. R. Soc. B 375, 20190261 ( 10.1098/rstb.2019.0261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herz RS, Inzlicht M. 2002. Sex differences in response to physical and social factors involved in human mate selection. The importance of smell for women. Evol. Hum. Behav. 23, 359–364. ( 10.1016/s1090-5138(02)00095-8) [DOI] [Google Scholar]
- 17.Havlicek J, Saxton TK, Roberts SC, Jozifkova E, Lhota S, Valentova J, Flegr J. 2008. He sees, she smells? Male and female reports of sensory reliance in mate choice and non-mate choice contexts. Pers. Individ. Dif. 45, 565–570. ( 10.1016/j.paid.2008.06.019) [DOI] [Google Scholar]
- 18.Oleszkiewicz A, Kunkel F, Larsson M, Hummel T. 2020. Consequences of undetected olfactory loss for human chemosensory communication and well-being. Phil. Trans. R. Soc. B 375, 20190265 ( 10.1098/rstb.2019.0265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croy I, Nordin S, Hummel T. 2014. Olfactory disorders and quality of life—an updated review. Chem. Senses 39, 185–194. ( 10.1093/chemse/bjt072) [DOI] [PubMed] [Google Scholar]
- 20.Wyatt TD. 2015. The search for human pheromones: the lost decades and the necessity of returning to first principles. Proc. R. Soc. B 282, 20142994 ( 10.1098/rspb.2014.2994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob S, McClintock MK. 2000. Psychological state and mood effects of steroidal chemosignals in women and men. Horm. Behav. 37, 57–78. ( 10.1006/hbeh.1999.1559) [DOI] [PubMed] [Google Scholar]
- 22.Bensafi M, Tsutsui T, Khan R, Levenson RW, Sobel N. 2004. Sniffing a human sex-steroid derived compound affects mood and autonomic arousal in a dose-dependent manner. Psychoneuroendocrinology 29, 1290–1299. ( 10.1016/s0306-4530(04)00046-0) [DOI] [PubMed] [Google Scholar]
- 23.Ferdenzi C, Delplanque S, Atanassova R, Sander D. 2016. Androstadienone's influence on the perception of facial and vocal attractiveness is not sex specific. Psychoneuroendocrinology 66, 166–175. ( 10.1016/j.psyneuen.2016.01.016) [DOI] [PubMed] [Google Scholar]
- 24.Hummer TA, McClintock MK. 2009. Putative human pheromone androstadienone attunes the mind specifically to emotional information. Horm. Behav. 19, 548–559. ( 10.1016/j.yhbeh.2009.01.002) [DOI] [PubMed] [Google Scholar]
- 25.Wyatt TD. 2020. Reproducible research into human chemical communication by cues and pheromones: learning from psychology's renaissance. Phil. Trans. R. Soc. B 375, 20190262 ( 10.1098/rstb.2019.0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlson P, Lüscher M. 1959. Pheromones: a new term for a class of biologically active substances. Nature 183, 55–56. ( 10.1038/183055a0) [DOI] [PubMed] [Google Scholar]
- 27.Butenandt A, Beckmann R, Stamm D, Hecker Z. 1959. Über den Sexual-Lockstoff des Seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z. Naturforsch. B 14, 283–284. [Google Scholar]
- 28.Schaal B, Coureaud G, Langlois D, Ginies C, Semon E, Perrier G. 2003. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature 424, 68–72. ( 10.1038/nature01739) [DOI] [PubMed] [Google Scholar]
- 29.Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. 2010. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male's odour. BMC Biol. 8, 75 ( 10.1186/1741-7007-8-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frasnelli J, Lundström JN, Boyle JA, Katsarkas A, Jones-Gotman M. 2011. The vomeronasal organ is not involved in the perception of endogenous odors. Hum. Brain Mapp. 32, 450–460. ( 10.1002/hbm.21035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson R, Distel H. 1986. Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol. Behav. 37, 123–128. ( 10.1016/0031-9384(86)90394-x) [DOI] [PubMed] [Google Scholar]
- 32.Baxi KN, Dorries KM, Eisthen HL. 2006. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 29, 1–7. ( 10.1016/j.tins.2005.10.002) [DOI] [PubMed] [Google Scholar]
- 33.Lübke KT, Pause BM. 2015. Always follow your nose: the functional significance of social chemosignals in human reproduction and survival. Horm. Behav. 68, 134–144. ( 10.1016/j.yhbeh.2014.10.001) [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki K, Boyse EA, Miké V, Thaler HT, Mathieson BJ, Abbott J, Boyse J, Zayas ZA, Thomas L. 1976. Control of mating preferences in mice by genes in the major histocompatibility complex. J. Exp. Med. 144, 1324–1335. ( 10.1084/jem.144.5.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wedekind C, Seebeck T, Bettens F, Paepke AJ. 1995. MHC-dependent mate preferences in humans. Proc. R. Soc. Lond. B 260, 245–249. ( 10.1098/rspb.1995.0087) [DOI] [PubMed] [Google Scholar]
- 36.Chaix R, Cao C, Donnelly P. 2008. Is mate choice in humans MHC-dependent? PLoS Genet. 4, e1000184 ( 10.1371/journal.pgen.1000184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Havlíček J, Winternitz J, Roberts SC. 2020. Major histocompatibility complex-associated odour preferences and human mate choice: near and far horizons. Phil. Trans. R. Soc. B 375, 20190260 ( 10.1098/rstb.2019.0260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schäfer L, Sorokowska A, Sauter J, Schmidt AH, Croy I. 2020. Body odours as a chemosignal in the mother–child relationship: new insights based on an human leucocyte antigen-genotyped family cohort. Phil. Trans. R. Soc. B 375, 20190266 ( 10.1098/rstb.2019.0266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winternitz J, Abbate JL, Huchard E, Havlíček J, Garamszegi LZ. 2017. Patterns of MHC-dependent mate selection in humans and nonhuman primates: a meta-analysis. Mol. Ecol. 26, 668–688. ( 10.1111/mec.13920) [DOI] [PubMed] [Google Scholar]
- 40.Thornhill R, Gangestad SW. 1999. The scent of symmetry: a human sex pheromone that signals fitness? Evol. Hum. Behav. 20, 175–201. ( 10.1016/S1090-5138(99)00005-7) [DOI] [Google Scholar]
- 41.Havlicek J, Roberts SC, Flegr J. 2005. Women's preference for dominant male odour: effects of menstrual cycle and relationship status. Biol. Lett. 1, 256–259. ( 10.1098/rsbl.2005.0332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuukasjarvi S, Eriksson CJP, Koskela E, Mappes T, Nissinen K, Rantala MJ. 2004. Attractiveness of women's body odors over the menstrual cycle: the role of oral contraceptives and receiver sex. Behav. Ecol. 15, 579–584. ( 10.1093/beheco/arh050) [DOI] [Google Scholar]
- 43.Sarolidou G, Axelsson J, Kimball BA, Sundelin T, Regenbogen C, Lundström JN, Lekander M, Olsson MJ. 2020. People expressing olfactory and visual cues of disease are less liked. Phil. Trans. R. Soc. B 375, 20190272 ( 10.1098/rstb.2019.0272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regenbogen C, Axelsson J, Lasselin J, Porada DK, Sundelin T, Peter MG, Lekander M, Lundström JN, Olsson MJ. 2017. Behavioral and neural correlates to multisensory detection of sick humans. Proc. Natl Acad. Sci. USA 114, 6400–6405. ( 10.1073/pnas.1617357114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fialová J, Hoffmann R, Roberts SC, Havlíček J. 2019. The effect of complete caloric intake restriction on human body odour quality. Physiol. Behav. 210, 112554 ( 10.1016/j.physbeh.2019.05.015) [DOI] [PubMed] [Google Scholar]
- 46.Doty RL, Green PA, Ram C, Yankell SL. 1982. Communication of gender from human breath odors: relationship to perceived intensity and pleasantness. Horm. Behav. 16, 13–22. ( 10.1016/0018-506X(82)90002-2) [DOI] [PubMed] [Google Scholar]
- 47.Chen D, Haviland-Jones J. 1999. Rapid mood change and human odors. Physiol. Behav. 68, 241–250. ( 10.1016/S0031-9384(99)00147-X) [DOI] [PubMed] [Google Scholar]
- 48.de Groot JHB, Kirk PA, Gottfried JA. 2020. Encoding fear intensity in human sweat. Phil. Trans. R. Soc. B 375, 20190271 ( 10.1098/rstb.2019.0271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pause BM, Storch D, Lübke KT. 2020. Chemosensory communication of aggression: women's fine-tuned neural processing of male aggression signals. Phil. Trans. R. Soc. B 375, 20190270 ( 10.1098/rstb.2019.0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Groot JHB, Smeets MAM. 2017. Human fear chemosignaling: evidence from a meta-analysis. Chem. Senses 42, 663–673. ( 10.1093/chemse/bjx049) [DOI] [PubMed] [Google Scholar]
- 51.Sorokowska A, Sorokowski P, Szmajke A. 2012. Does personality smell? Accuracy of personality assessments based on body odour. Eur. J. Personal. 26, 496–503. ( 10.1002/per.848) [DOI] [Google Scholar]
- 52.Mahmut MK, Croy I. 2019. The role of body odors and olfactory ability in the initiation, maintenance and breakdown of romantic relationships – A review. Physiol. Behav. 207, 179–184. ( 10.1016/j.physbeh.2019.05.003) [DOI] [PubMed] [Google Scholar]
- 53.Henrich J, Heine SJ, Norenzayan A. 2010. The weirdest people in the world? Behav. Brain Sci. 33, 61–83. ( 10.1017/S0140525X0999152X) [DOI] [PubMed] [Google Scholar]
- 54.Pawłowski B. 1999. Loss of oestrus and concealed ovulation in human evolution: the case against the sexual-selection hypothesis. Curr. Anthropol. 40, 257–275. ( 10.1086/200017) [DOI] [Google Scholar]
- 55.Doty RL, Ford M, Preti G, Huggins GR. 1975. Changes in the intensity and pleasantness of human vaginal odors during the menstrual cycle. Science 190, 1316–1318. ( 10.1126/science.1239080) [DOI] [PubMed] [Google Scholar]
- 56.Hold B, Schleidt M. 1977. The importance of human odour in non-verbal communication. Z. Tierpsychol. 43, 225–238. ( 10.1111/j.1439-0310.1977.tb00072.x) [DOI] [PubMed] [Google Scholar]
- 57.Wallace P. 1977. Individual discrimination of humans by odor. Physiol. Behav. 19, 577–579. ( 10.1016/0031-9384(77)90238-4) [DOI] [PubMed] [Google Scholar]
- 58.Gelstein S, Yeshurun Y, Rozenkrantz L, Shushan S, Frumin I, Roth Y, Sobel N. 2011. Human tears contain a chemosignal. Science 331, 226–230. ( 10.1126/science.1198331) [DOI] [PubMed] [Google Scholar]
- 59.Gračanin A, van Assen MA, Omrčen V, Koraj I, Vingerhoets AJ. 2017. Chemosignalling effects of human tears revisited: does exposure to female tears decrease males' perception of female sexual attractiveness? Cogn. Emot. 31, 139–150. ( 10.1080/02699931.2016.1151402) [DOI] [PubMed] [Google Scholar]
- 60.Gračanin A, Vingerhoets AJ, van Assen MA. 2017. Response to comment on ‘Chemosignalling effects of human tears revisited: does exposure to female tears decrease males’ perception of female sexual attractiveness?' Cogn. Emot. 31, 158–159. ( 10.1080/02699931.2016.1182471) [DOI] [PubMed] [Google Scholar]
- 61.Sobel N. 2017. Revisiting the revisit: added evidence for a social chemosignal in human emotional tears. Cogn. Emot. 31, 151–157. ( 10.1080/02699931.2016.1177488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perl O, Mishor E, Ravia A, Ravreby I, Sobel N. 2020. Are humans constantly but subconsciously smelling themselves? Phil. Trans. R. Soc. B 375, 20190372 ( 10.1098/rstb.2019.0372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frumin I, et al. 2015. A social chemosignaling function for human handshaking. Elife 4, e05154 ( 10.7554/eLife.05154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caroprese A, Gabbanini S, Beltramini C, Lucchi E, Valgimigli L. 2009. HS-SPME-GC-MS analysis of body odor to test the efficacy of foot deodorant formulations. Skin Res. Technol. 15, 503–510. ( 10.1111/j.1600-0846.2009.00399.x) [DOI] [PubMed] [Google Scholar]
- 65.Troccaz M, Niclass Y, Anziani P, Starkenmann C. 2013. The influence of thermal reaction and microbial transformation on the odour of human urine. Flavour Fragr. J. 28, 200–211. ( 10.1002/ffj.3143) [DOI] [Google Scholar]
- 66.Ohge H, Furne JK, Springfield J, Ringwala S, Levitt MD. 2005. Effectiveness of devices purported to reduce flatus odor. Am. J. Gastroenterol. 100, 397–400. ( 10.1111/j.1572-0241.2005.40631.x) [DOI] [PubMed] [Google Scholar]
- 67.Haze S, Gozu Y, Nakamura S, Kohno Y, Sawano K, Ohta H, Yamazaki K. 2001. 2-Nonenal newly found in human body odor tends to increase with aging. J. Invest. Dermatol. 116, 520–524. ( 10.1046/j.0022-202x.2001.01287.x) [DOI] [PubMed] [Google Scholar]
- 68.Bajtarevic A, et al. 2009. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 9, 348 ( 10.1186/1471-2407-9-348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Curran AM, Prada PA, Furton KG. 2010. The differentiation of the volatile organic signatures of individuals through SPME-GC/MS of characteristic human scent compounds. J. Forensic Sci. 55, 50–57. ( 10.1111/j.1556-4029.2009.01236.x) [DOI] [PubMed] [Google Scholar]
- 70.Dubois L, Stefanuto P-H, Perrault K, Delporte G, Delvenne P, Focant J-F. 2019. Comprehensive approach for monitoring human tissue degradation. Chromatographia 82, 857–871. ( 10.1007/s10337-019-03710-3) [DOI] [Google Scholar]
- 71.Bernier UR, Kline DL, Barnard DR, Schreck CE, Yost RA. 2000. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal. Chem. 72, 747–756. ( 10.1021/ac990963k) [DOI] [PubMed] [Google Scholar]
- 72.Verhulst NO, et al. 2013. Relation between HLA genes, human skin volatiles and attractiveness of humans to malaria mosquitoes. Infect. Genet. Evol. 18, 87–93. ( 10.1016/j.meegid.2013.05.009) [DOI] [PubMed] [Google Scholar]
- 73.Allen C, Havlíček J, Williams K, Roberts SC. 2018. Perfume experts' perceptions of body odors: toward a new lexicon for body odor description. J. Sens. Stud. 33, e12314 ( 10.1111/joss.12314) [DOI] [Google Scholar]
- 74.Dudareva N. 2004. Biochemistry of plant volatiles. Plant Physiol. 135, 1893–1902. ( 10.1104/pp.104.049981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verhulst NO, Umanets A, Weldegergis BT, Maas JPA, Visser TM, Dicke M, Smidt H, Takken W. 2018. Do apes smell like humans? The role of skin bacteria and volatiles of primates in mosquito host selection. J. Exp. Biol. 221, jeb185959 ( 10.1242/jeb.185959) [DOI] [PubMed] [Google Scholar]
- 76.Dormont L, Bessière J-M, Cohuet A. 2013. Human skin volatiles: a review. J. Chem. Ecol. 39, 569–578. ( 10.1007/s10886-013-0286-z) [DOI] [PubMed] [Google Scholar]
- 77.Agapiou A, Amann A, Mochalski P, Statheropoulos M, Thomas CLP. 2015. Trace detection of endogenous human volatile organic compounds for search, rescue and emergency applications. TrAC, Trends Anal. Chem. 66, 158–175. ( 10.1016/j.trac.2014.11.018) [DOI] [Google Scholar]
- 78.Williams J, Stönner C, Wicker J, Krauter N, Derstroff B, Bourtsoukidis E, Klüpfel T, Kramer S. 2016. Cinema audiences reproducibly vary the chemical composition of air during films, by broadcasting scene specific emissions on breath. Sci. Rep. 6, 25464 ( 10.1038/srep25464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smeets MAM, et al. 2020. Chemical fingerprints of emotional body odor. Metabolites 10, 84 ( 10.3390/metabo10030084). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Starkenmann C, Niclass Y, Troccaz M, Clark AJ. 2005. Identification of the precursor of (S)-3-methyl-3-sulfanylhexan-1-ol, the sulfury malodour of human axilla sweat. Chem. Biodivers. 2, 705–716. ( 10.1002/cbdv.200590048) [DOI] [PubMed] [Google Scholar]
- 81.Natsch A, Gfeller H, Gygax P, Schmid J. 2005. Isolation of a bacterial enzyme releasing axillary malodor and its use as a screening target for novel deodorant formulations. Int. J. Cosmet. Sci. 27, 115–122. ( 10.1111/j.1467-2494.2004.00255.x) [DOI] [PubMed] [Google Scholar]
- 82.Hooton K, Han W, Li L. 2016. Comprehensive and quantitative profiling of the human sweat submetabolome using high-performance chemical isotope labeling LC–MS. Anal. Chem. 88, 7378–7386. ( 10.1021/acs.analchem.6b01930) [DOI] [PubMed] [Google Scholar]
- 83.Williams J, Ringsdorf A. 2020. Human odour thresholds are tuned to atmospheric chemical lifetimes. Phil. Trans. R. Soc. B 375, 20190274 ( 10.1098/rstb.2019.0274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turner C, Parekh B, Walton C, Spanel P, Smith D, Evans M. 2008. An exploratory comparative study of volatile compounds in exhaled breath and emitted by skin using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 22, 526–532. ( 10.1002/rcm.3402) [DOI] [PubMed] [Google Scholar]
- 85.Ruzsanyi V, Mochalski P, Schmid A, Wiesenhofer H, Klieber M, Hinterhuber H, Amann A. 2012. Ion mobility spectrometry for detection of skin volatiles. J. Chromatogr. B 911, 84–92. ( 10.1016/j.jchromb.2012.10.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prada PA, Curran AM, Furton KG. 2011. The evaluation of human hand odor volatiles on various textiles: a comparison between contact and noncontact sampling methods. J. Forensic Sci. 56, 866–881. ( 10.1111/j.1556-4029.2011.01762.x) [DOI] [PubMed] [Google Scholar]
- 87.Drea CM, Boulet M, Delbarco-Trillo J, Greene LK, Sacha CR, Goodwin TE, Dubay GR. 2013. The ‘secret’ in secretions: methodological considerations in deciphering primate olfactory communication. Am. J. Primatol. 75, 621–642. ( 10.1002/ajp.22143) [DOI] [PubMed] [Google Scholar]
- 88.Gallagher M, Wysocki CJ, Leyden JJ, Spielman AI, Sun X, Preti G. 2008. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 159, 780–791. ( 10.1111/j.1365-2133.2008.08748.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agelopoulos NG, Pickett JA. 1998. Headspace analysis in chemical ecology: effects of different sampling methods on ratios of volatile compounds present in headspace samples. J. Chem. Ecol. 24, 1161–1172. ( 10.1023/A:1022442818196) [DOI] [Google Scholar]
- 90.Tholl D, Boland W, Hansel A, Loreto F, Röse USR, Schnitzler J-P. 2006. Practical approaches to plant volatile analysis. Plant J. 45, 540–560. ( 10.1111/j.1365-313X.2005.02612.x) [DOI] [PubMed] [Google Scholar]
- 91.Dormont L, Bessiere J-M, McKey D, Cohuet A. 2013. New methods for field collection of human skin volatiles and perspectives for their application in the chemical ecology of human–pathogen–vector interactions. J. Exp. Biol. 216, 2783–2788. ( 10.1242/jeb.085936) [DOI] [PubMed] [Google Scholar]
- 92.Kücklich M, Möller M, Marcillo A, Einspanier A, Weiß BM, Birkemeyer C, Widdig A. 2017. Different methods for volatile sampling in mammals. PLoS ONE 12, e0183440 ( 10.1371/journal.pone.0183440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Curran AM, Rabin SI, Prada PA, Furton KG. 2006. On the definition and measurement of human scent: response by Curran et al. J. Chem. Ecol. 32, 1617–1623. ( 10.1007/s10886-006-9096-x) [DOI] [PubMed] [Google Scholar]
- 94.Preti G, Willse A, Labows JN, Leyden JJ, Wahl J, Kwak J. 2006. On the definition and measurement of human scent: comments on Curran et al. J. Chem. Ecol. 32, 1613–1616. ( 10.1007/s10886-006-9095-y) [DOI] [PubMed] [Google Scholar]
- 95.Birkemeyer CS, Thomsen R, Jänig S, Kücklich M, Slama A, Weiß BM, Widdig A. 2016. Sampling the body odor of primates: cotton swabs sample semivolatiles rather than volatiles. Chem. Senses 41, 525–535. ( 10.1093/chemse/bjw056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charpentier MJE, Barthes N, Proffit M, Bessiere J-M, Grison C. 2012. Critical thinking in the chemical ecology of mammalian communication: roadmap for future studies. Funct. Ecol. 26, 769–774. ( 10.1111/j.1365-2435.2012.01998.x) [DOI] [Google Scholar]
- 97.Labows J, Preti G, Hoelzle E, Leyden J, Kligman A. 1979. Analysis of human axillary volatiles: compounds of exogenous origin. J. Chromatogr. B 163, 294–299. ( 10.1016/S0378-4347(00)81417-6) [DOI] [PubMed] [Google Scholar]
- 98.Barbosa AJM, Oliveira AR, Roque ACA. 2018. Protein- and peptide-based biosensors in artificial olfaction. Trends Biotechnol. 36, 1244–1258. ( 10.1016/j.tibtech.2018.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amoore JE. 1991. Specific anosmias. In Smell and taste in health and disease (eds Getchell TV, Doty RL, Bartoshuk LM, Snow JB), pp. 655–664. New York, NY: Raven Press. [Google Scholar]
- 100.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. 2007. Genetic variation in a human odorant receptor alters odour perception. Nature 449, 468–472. ( 10.1038/nature06162) [DOI] [PubMed] [Google Scholar]
- 101.Martin A, Saathoff M, Kuhn F, Max H, Terstegen L, Natsch A. 2010. A functional ABCC11 allele is essential in the biochemical formation of human axillary odor. J. Investig. Dermatol. 130, 529–540. ( 10.1038/jid.2009.254) [DOI] [PubMed] [Google Scholar]
- 102.Classen C. 1992. The odor of the other: olfactory symbolism and cultural categories. Ethos 20, 133–166. ( 10.1525/eth.1992.20.2.02a00010) [DOI] [Google Scholar]
- 103.Hart R. 1980. Human body odor. Nexus 1. [Google Scholar]
- 104.Prokop-Prigge KA, Greene K, Varallo L, Wysocki CJ, Preti G. 2016. The effect of ethnicity on human axillary odorant production. J. Chem. Ecol. 42, 33–39. ( 10.1007/s10886-015-0657-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parma V, Redolfi N, Alho L, Rocha M, Ferreira J, Silva CF, Soares SC. 2019. Ethnic influences on the perceptual properties of human chemosignals. Physiol. Behav. 210, 112544 ( 10.1016/j.physbeh.2019.05.005) [DOI] [PubMed] [Google Scholar]
- 106.Hall ET. 1966. The hidden dimension. New York, NY: Doubleday. [Google Scholar]
- 107.Sorokowska A, et al. 2017. Preferred interpersonal distances: a global comparison. J. Cross Cult. Psychol. 48, 577–592. ( 10.1177/0022022117698039) [DOI] [Google Scholar]
- 108.Schleidt M, Hold B, Attili G. 1981. A cross-cultural study on the attitude towards personal odors. J. Chem. Ecol. 7, 19–31. ( 10.1007/BF00988632) [DOI] [PubMed] [Google Scholar]
- 109.Ferdenzi C, Mustonen S, Tuorila H, Schaal B. 2008. Children's awareness and uses of odor cues in everyday life: a Finland–France comparison. Chemosens. Percept. 1, 190–198. ( 10.1007/s12078-008-9020-6) [DOI] [Google Scholar]
- 110.Ferdenzi C, et al. 2013. Affective semantic space of scents. Towards a universal scale to measure self-reported odor-related feelings. Food Qual. Prefer. 30, 128–138. ( 10.1016/j.foodqual.2013.04.010) [DOI] [Google Scholar]
- 111.Sorokowska A, et al. 2018. Global study of social odor awareness. Chem. Senses 43, 503–513. ( 10.1093/chemse/bjy038) [DOI] [PubMed] [Google Scholar]
- 112.Arshamian A, Manko P, Majid A. 2020. Limitations in odour simulation may originate from differential sensory embodiment. Phil. Trans. R. Soc. B 375, 20190273 ( 10.1098/rstb.2019.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roberts SC, Gosling LM, Carter V, Petrie M. 2008. MHC-correlated odour preferences in humans and the use of oral contraceptives. Proc. R. Soc. B 275, 2715–2722. ( 10.1098/rspb.2008.0825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doty RL, Cameron EL. 2009. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 97, 213–228. ( 10.1016/j.physbeh.2009.02.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Caruso S, Grillo C, Agnello C, Maiolino L, Intelisano G, Serra A. 2001. A prospective study evidencing rhinomanometric and olfactometric outcomes in women taking oral contraceptives. Hum. Reprod. 16, 2288–2294. ( 10.1093/humrep/16.11.2288) [DOI] [PubMed] [Google Scholar]
- 116.Lundström JN, McClintock MK, Olsson MJ. 2006. Effects of reproductive state on olfactory sensitivity suggest odor specificity. Biol. Psychol. 71, 244–247. ( 10.1016/j.biopsycho.2005.07.001) [DOI] [PubMed] [Google Scholar]
- 117.Bajos N, Rouzaud-Cornabas M, Panjo H, Bohet A, Moreau C. 2014. La crise de la pilule en France: vers un nouveau modèle contraceptif? Population et Sociétés (INED) 511. [Google Scholar]
- 118.Alvergne A, Lummaa V. 2010. Does the contraceptive pill alter mate choice in humans? Trends Ecol. Evol. 25, 171–179. ( 10.1016/j.tree.2009.08.003) [DOI] [PubMed] [Google Scholar]
- 119.Pletzer B. 2015. Editorial: From sex differences in neuroscience to a neuroscience of sex differences: new directions and perspectives. Front. Neurosci. 9, 330 ( 10.3389/fnins.2015.00330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ostrovskaya A, Landa PA, Sokolinsky M, Rosalia AD, Maes D. 2002. Study and identification of volatile compounds from human skin. J. Cosmet. Sci. 53, 147–148. [Google Scholar]
- 121.Behan JM, Macmaster AP, Perring KD, Tuck KM. 1996. Insight into how skin changes perfume. Int. J. Cosmet. Sci. 18, 237–246. ( 10.1111/j.1467-2494.1996.tb00154.x) [DOI] [PubMed] [Google Scholar]
- 122.Allen C, Cobey KD, Havlíček J, Roberts SC. 2016. The impact of artificial fragrances on the assessment of mate quality cues in body odor. Evol. Hum. Behav. 37, 481–489. ( 10.1016/j.evolhumbehav.2016.05.001) [DOI] [Google Scholar]
- 123.Sorokowska A, Sorokowski P, Havlicek J. 2016. Body odor based personality judgments: the effect of fragranced cosmetics. Front. Psychol. 7, 530 ( 10.3389/fpsyg.2016.00530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lindqvist A. 2012. Preference and gender associations of perfumes applied on human skin. J. Sens. Stud. 27, 490–497. ( 10.1111/joss.12014) [DOI] [Google Scholar]
- 125.Gaby JM, Zayas V. 2017. Smelling is telling: human olfactory cues influence social judgments in semi-realistic interactions. Chem. Senses 42, 405–418. ( 10.1093/chemse/bjx012) [DOI] [PubMed] [Google Scholar]
- 126.Allen C, Havlicek J, Roberts SC. 2015. Effect of fragrance use on discrimination of individual body odor. Front. Psychol. 6, 1115 ( 10.3389/fpsyg.2015.01115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lenochova P, Vohnoutova P, Roberts SC, Oberzaucher E, Grammer K, Havlicek J. 2012. Psychology of fragrance use: perception of individual odor and perfume blends reveals a mechanism for idiosyncratic effects on fragrance choice. PLoS ONE 7, e33810 ( 10.1371/journal.pone.0033810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Milinski M, Wedekind C. 2001. Evidence for MHC-correlated perfume preferences in humans. Behav. Ecol. 12, 140–149. ( 10.1093/beheco/12.2.140) [DOI] [Google Scholar]
- 129.Hämmerli A, Schweisgut C, Kaegi M. 2012. Population genetic segmentation of MHC-correlated perfume preferences. Int. J. Cosmet. Sci. 34, 161–168. ( 10.1111/j.1468-2494.2011.00696.x) [DOI] [PubMed] [Google Scholar]
- 130.Havlicek J, Roberts SC. 2013. The perfume–body odour complex: an insightful model for culture–gene coevolution? In Chemical signals in vertebrates 12 (eds East ML, Dehnhard M), pp. 185–195. New York, NY: Springer. [Google Scholar]
- 131.Sobotkova M, Fialova J, Roberts SC, Havlicek J. 2017. Effect of biological relatedness on perfume selection for others: preliminary evidence. Perception 46, 498–515. ( 10.1177/0301006616682514) [DOI] [PubMed] [Google Scholar]
- 132.Saxton TK, Lyndon A, Little AC, Roberts SC. 2008. Evidence that androstadienone, a putative human chemosignal, modulates women's attributions of men's attractiveness. Horm. Behav. 54, 597–601. ( 10.1016/j.yhbeh.2008.06.001) [DOI] [PubMed] [Google Scholar]
- 133.Marlier L, Schaal B. 2005. Human newborns prefer human milk: conspecific milk odor is attractive without postnatal exposure. Child Dev. 76, 155–168. ( 10.1111/j.1467-8624.2005.00836.x) [DOI] [PubMed] [Google Scholar]
- 134.Parma V, Bulgheroni M, Tirindelli R, Castiello U. 2013. Body odors promote automatic imitation in autism. Biol. Psychiatry 74, 220–226. ( 10.1016/j.biopsych.2013.01.010) [DOI] [PubMed] [Google Scholar]
- 135.Mainland J, Sobel N. 2006. The sniff is part of the olfactory percept. Chem. Senses 31, 181–196. ( 10.1093/chemse/bjj012) [DOI] [PubMed] [Google Scholar]
- 136.Jaenig S, Weiss BM, Widdig A. 2018. Comparing the sniffing behavior of great apes. Am. J. Primatol. 80, e22872 ( 10.1002/ajp.22872) [DOI] [PubMed] [Google Scholar]
- 137.Kim K, Rosenthal MZ, Gwaltney M, Jarrold W, Hatt N, McIntyre N, Swain L, Solomon M, Mundy P. 2015. A virtual joy-stick study of emotional responses and social motivation in children with autism spectrum disorder. J. Autism Dev. Disord. 45, 3891–3899. ( 10.1007/s10803-014-2036-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Endevelt-Shapira Y, et al. 2018. Altered responses to social chemosignals in autism spectrum disorder. Nat. Neurosci. 21, 111–119. ( 10.1038/s41593-017-0024-x) [DOI] [PubMed] [Google Scholar]
- 139.Xiao YJ, Coppin G, Bavel JJV. 2016. Perceiving the world through group-colored glasses: a perceptual model of intergroup relations. Psychol. Inquiry 27, 255–274. ( 10.1080/1047840X.2016.1199221) [DOI] [Google Scholar]
- 140.Ferdenzi C, Rouby C, Bensafi M. 2016. The social nose: importance of olfactory perception in group dynamics and relationships. Psychol. Inquiry 27, 299–305. ( 10.1080/1047840X.2016.1215207) [DOI] [Google Scholar]
- 141.Novakova L, Valentova JV, Havlicek J. 2013. Olfactory performance is predicted by individual sex-atypicality, but not sexual orientation. PLoS ONE 8, e80234 ( 10.1371/journal.pone.0080234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sczesny S, Stahlberg D. 2002. The influence of gender-stereotyped perfumes on leadership attribution. Eur. J. Soc. Psychol. 32, 815–828. ( 10.1002/ejsp.123) [DOI] [Google Scholar]
- 143.Mutic S, Moellers EM, Wiesmann M, Freiherr J. 2015. Chemosensory communication of gender information: masculinity bias in body odor perception and femininity bias introduced by chemosignals during social perception. Front. Psychol. 6, 1980 ( 10.3389/fpsyg.2015.01980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jacob S, Kinnunen LH, Metz J, Cooper M, McClintock MK. 2001. Sustained human chemosignal unconsciously alters brain function. Neuroreport 12, 2391–2394. ( 10.1097/00001756-200108080-00021) [DOI] [PubMed] [Google Scholar]
- 145.Gulyás B, Kéri S, O'Sullivan BT, Decety J, Roland PE. 2004. The putative pheromone androstadienone activates cortical fields in the human brain related to social cognition. Neurochem. Int. 44, 595–600. ( 10.1016/j.neuint.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 146.Savic I, Berglund H, Gulyas B, Roland P. 2001. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron 31, 661–668. ( 10.1016/S0896-6273(01)00390-7) [DOI] [PubMed] [Google Scholar]
- 147.Burke SM, Veltman DJ, Gerber J, Hummel T, Bakker J. 2012. Heterosexual men and women both show a hypothalamic response to the chemo-signal androstadienone. PLoS ONE 7, e40993 ( 10.1371/journal.pone.0040993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Savic I, Berglund H, Lindstrom P. 2005. Brain response to putative pheromones in homosexual men. Proc. Natl Acad. Sci. USA 102, 7356–7361. ( 10.1073/pnas.0407998102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berglund H, Lindstrom P, Savic I. 2006. Brain response to putative pheromones in lesbian women. Proc. Natl. Acad. Sci. USA 103, 8269–8274. ( 10.1073/pnas.0600331103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lundström JN, Boyle JA, Zatorre RJ, Jones-Gotman M. 2008. Functional neuronal processing of body odors differs from that of similar common odors. Cereb. Cortex 18, 1466–1474. ( 10.1093/cercor/bhm178) [DOI] [PubMed] [Google Scholar]
- 151.Lundström JN, Boyle JA, Zatorre RJ, Jones-Gotman M. 2009. The neuronal substrates of human olfactory based kin recognition. Hum. Brain Mapp. 30, 2571–2580. ( 10.1002/hbm.20686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lundström JN, Mathe A, Schaal B, Frasnelli J, Nitzsche K, Gerber J, Hummel T. 2013. Maternal status regulates cortical responses to the body odor of newborns. Front. Psychol. 4, 597 ( 10.3389/fpsyg.2013.00597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou W, Chen D. 2008. Encoding human sexual chemosensory cues in the orbitofrontal and fusiform cortices. J. Neurosci. 28, 14 416–14 421. ( 10.1523/JNEUROSCI.3148-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Labows J, Mcginley K, Kligman A. 1982. Perspectives on axillary odor. J. Soc. Cosmet. Chem. 33, 193–202. [Google Scholar]
- 155.Zeng X, Leyden JJ, Lawley HJ, Sawano K, Nohara I, Preti G. 1991. Analysis of characteristic odors from human male axillae. J. Chem. Ecol. 17, 1469–1492. ( 10.1007/BF00983777) [DOI] [PubMed] [Google Scholar]
- 156.Natsch A. 2013. Bacteria and human (mal)-odours. Flavour Fragr. J. 28, 199 ( 10.1002/ffj.3162) [DOI] [Google Scholar]
- 157.Troccaz M, Gaïa N, Beccucci S, Schrenzel J, Cayeux I, Starkenmann C, Lazarevic V. 2015. Mapping axillary microbiota responsible for body odours using a culture-independent approach. Microbiome 3, 3 ( 10.1186/s40168-014-0064-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gower DB, Holland KT, Mallet AI, Rennie PJ, Watkins WJ. 1994. Comparison of 16-androstene steroid concentrations in sterile apocrine sweat and axillary secretions: interconversions of 16-androstenes by the axillary microflora—a mechanism for axillary odour production in man? J. Steroid. Biochem. Mol. Biol. 48, 409–418. ( 10.1016/0960-0760(94)90082-5) [DOI] [PubMed] [Google Scholar]
- 159.Natsch A. 2017. Biochemistry and genetics of human axilla odor. In Springer handbook of odor (ed. Buettner A.), pp. 123–124. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 160.Jackman P, Noble W. 1983. Normal axillary skin microflora in various populations. Clin. Exp. Dermatol. 8, 259–268. ( 10.1111/j.1365-2230.1983.tb01778.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Ferdenzi C, Delplanque S. 2020 Dataset related to: 'Interdisciplinary challenges for elucidating human olfactory attractiveness' (Version 1) [Dataset]. Zenodo. (doi:10.5281/zenodo.3676952)