Abstract
For humans, like other social animals, behaviour acts as a first line of defence against pathogens. A key component is the ability to detect subtle perceptual cues of sick conspecifics. The present study assessed the effects of endotoxin-induced olfactory and visual sickness cues on liking, as well as potential involved mechanisms. Seventy-seven participants were exposed to sick and healthy facial pictures and body odours from the same individual in a 2 × 2 factorial design while disgust-related facial electromyography (EMG) was recorded. Following exposure, participants rated their liking of the person presented. In another session, participants also answered questionnaires on perceived vulnerability to disease, disgust sensitivity and health anxiety. Lower ratings of liking were linked to both facial and body odour disease cues as main effects. Disgust, as measured by EMG, did not seem to be the mediating mechanism, but participants who perceived themselves as more prone to disgust, and as more vulnerable to disease, liked presented persons less irrespectively of their health status. Concluding, olfactory and visual sickness cues that appear already a few hours after the experimental induction of systemic inflammation have implications for human sociality and may as such be a part of a behavioural defence against disease.
This article is part of the Theo Murphy meeting issue ‘Olfactory communication in humans’.
Keywords: sickness cues, body odour, face, disease avoidance, disgust
1. Introduction
Humans are among the most social species on Earth [1]. Although social interactions are rewarding, they also carry costs, such as those of contagious diseases [2,3]. Owing to their long-term coexistence with pathogens, humans and other animals have developed an immune system that acts as a defence against pathogens. The costs of an immune response itself are also high, both in terms of metabolic costs and of inhibition of normal functioning, thereby promoting starvation and predation and reducing reproductive opportunities [4]. This inhibition is a result of behavioural changes often referred to as sickness behaviour, which generally helps with recovery [5] and possibly decreases the spread of disease to kin [4]. Examples of sickness behaviour are fatigue, social withdrawal, loss of appetite and physical inactivity [6].
It has been suggested that the behavioural avoidance of sick individuals is the first, and probably the most cost-effective line of defence against infection [7]. Moreover, statistical modelling indicates substantial disease-containing effects from small alterations in patterns of inter-individual contact [8]. These two points vouch for the importance of a behavioural defence against disease. This defence includes the capability of detecting sickness-relevant cues, followed by adaptive behaviours such as avoidance [9,10], and is often referred to as part of a behavioural immune system [11].
An association between disease and specific human body odour cues has been found for certain types of diseases, both contagious and non-contagious [12]. The role of body odour cues in disease detection was recently addressed for the first time by two experimental studies which indicated that individuals, following an induction of systemic and transient inflammation by way of an endotoxin lipopolysaccharide (LPS), had a more aversive body odour just a few hours after induction [13,14]. Similar findings have also indicated that visual cues, such as the skin coloration [15], gait pattern [16] and perceived (emotional expressions) as well as physical facial features (eye openness, skin colour, angle of corner of mouth [17,18]) of LPS-treated individuals might serve as cues for disease detection.
Starting with the assumption that a behavioural defence would benefit from being able to integrate cues between senses in order to improve accuracy and speed of detection [19,20], a recent study investigated whether integration of visual and olfactory stimuli could facilitate disease detection using the LPS bacterial model [21]. Visual (facial pictures) and olfactory (body odour) sickness cues were presented to participants, both separately and in combination, while imaging the brain (functional magnetic resonance imaging). A negative main effect of face sickness on liking ratings of the presented stimuli was found and a statistical tendency for further dislike was found as a function of body odour sickness. Besides widespread neural activation of modality-specific cortical odour and visual processing networks as a function of disease cues, activations of areas relating to multisensory integration, such as the intraparietal sulcus, superior temporal sulcus and orbitofrontal cortex, were also observed.
When sickness cues are detected, adaptive psychological responses, such as disgust, will cause avoidance behaviour [7]. In fact, many cues that convey the presence of pathogens are also disgust elicitors [22]. While it seems likely that disgust responses are central for the initiation of avoidance behaviour to pathogenic threats, it remains unknown whether disgust is instrumental when pathogenic threats are more subtle.
It is reasonable that different personality traits may be predisposing individuals to the detection of disease cues. For instance, individuals who perceive themselves as more vulnerable to disease are more alert towards people or inanimate objects that might pose an infection risk, and when they detect them, they might express greater avoidance [7,23]. In parallel, health anxiety is also connected to disease detection. Health anxiety is defined as a constant fear of somatic sickness, resulting in misinterpretations of non-threatening cues and avoidance of stimuli connected to health anxiety [24]. Specifically, it has been found that individuals with severe health anxiety tend to experience more disgust towards others, perceive them as less healthy and also rate the risk of contagion as greater compared to individuals with low health anxiety [25]. In line with perceived vulnerability to disease (PVD) and health anxiety, disgust sensitivity is also associated with behavioural avoidance [26].
In the present study, following the same assumption as in Regenbogen et al. [21] that visual and olfactory stimuli could facilitate disease detection, a combination of both visual and olfactory disease cues was presented to observers to assess potential main or interaction effects of visual and olfactory disease cues on social liking. We assessed ‘liking’ of individuals in response to disease cues because liking predicts approach/avoidance behaviour [27]. As a possible mediator of dislike, we added electromyography (EMG) measures of muscles associated with the emotion of disgust. As a secondary aim, we also investigated if individuals reporting to be more vulnerable to disease, more sensitive to disgust, or are higher in health anxiety will perceive the disease cues differently from those with low scores for these personality traits. One possibility is that they may be overly sensitive to possible disease cues (true or not) in an effort to protect themselves from infection. Another is that their ability to dissociate between sick and healthy cues will be different (either better or worse) depending on the trait scores.
2. Methods
(a). Participants
A total of 77 healthy participants took part in the present study (mean age 30 years; of which, 49 women). There was no significant difference in age between female and male participants (t75 = −0.227, p = 0.821). Participants were recruited via posters, Karolinska Institutet's online recruitment system (SONA system) and posts on social media (Facebook). Inclusion criteria were greater than or equal to 18 years of age, self-reported normal or corrected to normal vision, self-reported normal sense of smell, non-smokers, not pregnant and finally, an ability to speak and understand Swedish. All participants provided written informed consent and were remunerated for their participation with two movie tickets. The study was approved by the regional ethical board (2017/55-31/4).
(b). Procedure
The study consisted of two sessions. Before the sessions started, all participants did an odour identification task using a short version of the Sniffin’ Sticks odour identification test to assess their olfactory function. During the first session, participants completed several questionnaires collecting socio-demographic data and measuring their sensitivity to disgust, health anxiety and PVD. After the first session was completed, the experimenter prepared the participants for the recording of facial EMG. During the second session, they gave liking ratings following the simultaneous presentation of face and body odour stimuli while EMG was recorded.
(c). Sniffin’ sticks test
Prior to the experimental procedure, every participant was tested with a four-item version of the Sniffin’ Sticks odour identification test. The odours used were orange, leather, cinnamon and peppermint. The Sniffin’ Sticks test assesses nasal chemosensory performance using pen-like odour-dispensing devices [28]. All met the criterion for inclusion, three out of four correct cued identifications.
(d). Session 1: questionnaires
During session 1 of the study, the participants were asked to complete questionnaires. First, they provided information about age, sex, education, smoking habits, pregnancy, use of contraceptives, date of last day of menstruation and perceived sense of olfactory acuity. Directly after the administration of the demographic questionnaires and before the second session of the study took place, the below three self-reported instruments were administered. In an explorative manner, we wanted to see whether traits relating to vulnerability to disease, disgust sensitivity and health anxiety would, somehow, be associated with how well participants could differ between sick and healthy or whether they had a general tendency to dislike others. The participants after they completed the questionnaires moved to another room for the experimental part.
(i). Perceived vulnerability to disease
PVD is a 15-item self-report instrument that assesses chronic beliefs about disease transmission [29]. The questionnaire consists of two subscales. The germ subscale assesses discomfort in situations with potential transmission of pathogens and has been found to correlate with measures of disgust sensitivity. The infectability subscale measures perceived susceptibility to infectious diseases and is associated with measures of health anxiety [29].
(ii). Disgust scale-revised
The disgust scale revised (DS-R), a 27-item self-report instrument, was used to assess participants' disgust sensitivity. A validation study of the DS-R revealed a three-factor structure with acceptable internal consistency and split-half reliability [30]. The three factors are: core disgust, assessing aversive reactions to the threat of disease; animal-reminder disgust, elicited by reminders of one's mortality and animalistic nature; and contamination disgust, measuring aversive reactions to threat of contamination [30,31]. The DS-R also contains two ‘catch’ questions which help with identification and removal of inattentive participants.
(iii). Health anxiety inventory
Health Anxiety Inventory (HAI) is a screening measure of clinical health anxiety, based on the validated HAI [24]. HAI consists of 27 items, combined to one total score of health anxiety as well as three separate subscales. The subscales measure different aspects of illness-related ideas of health anxiety: negative consequences of illness, reassurance seeking and avoidance behaviours [24].
(e). Stimuli
The present study used the same facial pictures and body odours as used in Regenbogen et al. [21]. That study includes all the details regarding the procedure for collecting the facial pictures and body odours [21]. In short, healthy individuals (nine women, 13 men; mean age 23 years), hereafter called donors, participated in a within-subject, double-blind and placebo-controlled study. The donors were injected with either the endotoxin LPS injection (Escherichia coli endotoxin, Lot HOK354, CAT number 1235503, United States Pharmacopeia, Rockville, MD, USA) at 2.0 ng kg−1 body weight diluted in 0.9% NaCl or a placebo injection (0.9% NaCl). Each donor received both treatments in sessions separated by a month and in balanced order. Whereas in Regenbogen et al., only a small subset (four healthy and four sick body odours, but different subsets for each participant) of the available body odours was used, we here presented all the 44 available sick and healthy body odour samples to all of the participants. It needs to be mentioned that the body odours were only sparsely used in the Regenbogen study which better allowed us to re-use them in the present study. The body odours were stored in the freezer in between the two studies to keep their integrity as well as possible [32].
The visual stimuli used here were facial photos of these donors. The facial photos were taken 2 h after the LPS injection, when sick, and 2 h after the placebo injection, when healthy. The donors were seated on a stool against a white background a metre away from the camera. For all the pictures, a Nikon D90 (Nikon Corp., Tokyo, Japan) was used. The focal length was 50 mm (a 50 mm lens was used), aperture speed at 1/125, and 200 ISO. For the lighting, a studio flash set-up with two ElinChrome softboxes was used. The same flash lighting was standardized across all photos. All donors were asked to keep a neutral facial expression and were restricted from wearing any make-up. Post-processing of the facial photos included cropping of pictures to 1.989 × 2.188 pixels, so all faces were of similar size. An oval shape (354 × 483 pixels) was used to crop the faces to remove hair from the image for the subsequent behavioural experiment.
For the collection of the olfactory stimuli, donors avoided strenuous exercise and alcohol during 48 h before collection of the body odour samples. During 24 h before collection of body odour samples, donors avoided strong spices (e.g. Mexican, Indian, Thai food), garlic and asparagus to limit residual odours in their body odour. The morning of odour samples collection, donors had a light breakfast (e.g. one glass of orange juice and two slices of bread with a little jam) and drank a glass of water after breakfast. On this day, donors used an unscented liquid soap (Lactacyd, ACO Hud Nordic AB, Upplands Väsby, Sweden) and shampoo (Apoliva, Apoteket AB, Stockholm, Sweden) provided by us to shower and wash their armpits, and they were instructed to not use any odourized products (e.g. perfume, deodorant, body lotion or other scented cosmetics). For the sampling of the olfactory stimuli, all donors wore tight T-shirts (type BB301, 50% cotton/50% polyester; American Apparel, London, UK) for 5 h, with nursing pads sewn into the armpit regions following treatment both for LPS and placebo. After the completion of 5 h, all pads were removed from the T-shirts and were stored in a freezer at −35°C in 1 l freezer bags (Toppits, Minden, Germany). More information regarding the protocol can be found in [33].
(f). Session 2: ratings
During the second block, participants were exposed to the 22 donors' facial pictures and body odours in both a sick and a healthy version in a factorial design. For each donor, olfactory and visual stimuli combinations (sick/sick; sick/healthy; healthy/sick; healthy/healthy) were presented in a randomized order, amounting to 88 presentations for each participant (figure 1). The stimuli (face and odour pairs coming from the same donor) were presented in a randomized order for each participant. Before each stimulus presentation, a fixation cross was presented for 5 s which indicated that a stimulus combination would be presented. When the experimental session (presented in detail below) started, the experimenter removed the lid from the jar and presented the odour to the participant by placing the jar a few centimetres below the nostril. Each stimulus combination (facial picture and odour) was presented for 5 s. After each presentation, a visual analogue scale (VAS) was presented on the screen for 5 s, so that the participants could rate how much they liked the person being presented. The VAS ranged from 0 to 100 where ‘I don't like this person at all’ represented the lower end, and ‘I like this person very much’ the higher end. Hence, 50 represents neither like nor dislike of the person.
Figure 1.
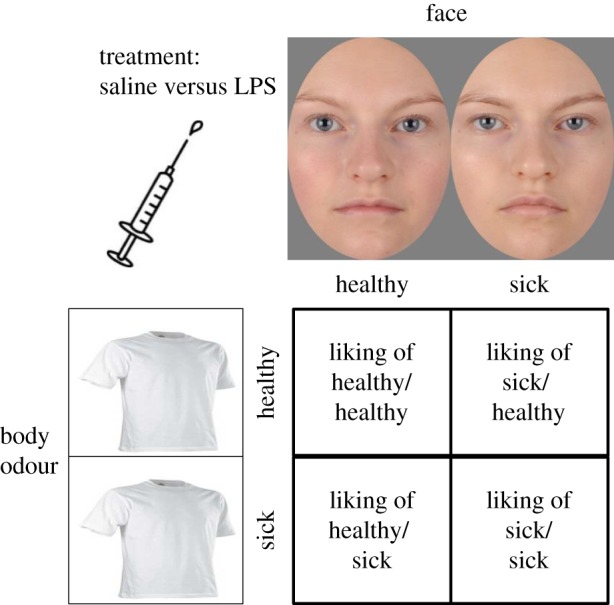
Body odours and facial pictures of 22 individuals collected when sick (LPS injection) and when healthy (saline injection) were presented in a factorial design resulting in 88 unique combinations of face and odour. Each participant rated the person presented on a liking scale. Pictures represent an example of how a face can vary between the healthy and the sick condition. (Online version in colour.)
(g). Facial electromyography recording
Facial EMG activity associated with disgust was recorded from the corrugator supercilii region located at the forehead above the left eyebrow (frowning), and from the levator labii superiores al nasi region located on the cheek close to the left nose wing (lifting upper lip). A ground electrode was positioned at the forehead border of the hair line, all electrode placement following the recommendation of van Boxtel [34]. After cleaning relevant skin areas with 50% ethanol solution, surface electrode pairs (recording area 0.4 cm diameter) were filled with electrode gel (Signa gel, Parker, Cortech Solutions) and mounted to target areas. EMG signal was recorded using an AD Instruments differential BioAmp amplifier controlled by a PC using LabChart7 pro software (AD Instruments, Boulder, CO, USA) with the following settings: input impedance of 200 MΩ, amplification range ±5 µV to ±100 mV, gain accuracy ±1.5%, common mode rejection ratio 85 dB at 60 Hz, 1000 Hz sampling rate, mains filter, first-order 10 Hz high-pass filter and fourth-order Bessel 500 Hz low-pass filter at −3 dB [35]. Offline data processing for statistical analysis comprised 20 Hz high-pass filtering, down sampling to 100 Hz, rectifying and smoothing with a window size of 200 ms. Rectifying and smoothing took place in a research environment using Jupyter Notebook running Python 3.7. The data were preprocessed by the usage of Pandas and NumPy as main libraries. Target/baseline ratios were calculated for baseline correction [34].
(h). Statistical analysis
(i). Perceptual ratings
The effects of face and odour on liking ratings were analysed using linear mixed model analyses conducted in R.3.4.1, using the package lme4 and lmertest. A conservative mixed-effect model was used where intercepts and slopes were allowed to vary between participants and donors. The model we used was: lmer (ratings ∼ face + odour + (1 + face + odour|participant) + (1 + face + odour|donor). To test for interaction, the following model was used: lmer (ratings ∼ face × odour + (1 + face × odour|participant) + (1 + face × odour|donor). Random intercepts for ‘participant’ and ‘donor’ were used to account for variance between participants and donors, respectively.
(iii). Facial electromyography recording
For the facial EMG recording, a linear mixed-model analysis in R.3.4.1 using the package lme4 and lmertest was used as well. For the mixed-effect model used here, only intercepts were allowed to vary between participants and donors owing to convergence problem when the random slope was included in the model. As an example, the model for the effect of face and odour on levator labii muscle would be the following: lmer (levator ∼ face + odour + (1|participant) + (1|donor)).
(iv). Questionnaires
Questionnaire responses were analysed using the Pearson correlation (two-tailed) with a threshold value for statistical significance set to p < 0.05. This statistical analysis was performed using Microsoft Office Excel and IBM SPSS Statistic (v. 23). Regarding the liking ratings, each participant received a mean liking score. In addition, differences between ratings of healthy and sick faces and healthy and sick odours were calculated and combined into one mean difference. Twelve participants were excluded from the DS-R analysis owing to incomplete answers and getting caught on ‘catch’-questions. In addition, correlations between PVD, HAI and DS-R were calculated as well (see the electronic supplementary material).
3. Results
(a). Effect of sickness on liking ratings
The ratings of liking of the presented persons as a function of face and body odour sickness were analysed (table 1; electronic supplementary material, figure S1).
Table 1.
Linear mixed model investigating the effect of face and odour sickness on liking ratings. (For each variable, the estimate, the standard error of the mean (s.e.m.), the confidence interval (CI), the t-value, the degrees of freedom (d.f.) and the p-values are given.)
| ratings | predictors | B | s.e.m. | 95% CI | t | d.f. | p-value |
|---|---|---|---|---|---|---|---|
| liking | intercept | 40.83 | 2.43 | [35.9, 45.7] | |||
| face | −2.04 | 0.57 | [−3.2, −0.9] | −3.58 | 22.41 | 0.001 | |
| odour | −3.32 | 1.51 | [−6.3, −0.3] | −2.20 | 22.94 | 0.038 |
As hypothesized, there was a main effect on liking of face sickness (t22.4 = −3.586, p = 0.002, d = −0.40), as well as for body odour sickness (t22.9 = −2.202, p = 0038, d = −0.25) compared to the healthy ones (figure 2). There was no significant interaction effect between face and odour sickness on liking ratings (t70.3 = 1.204, p = 0.232, d = 0.15). In summary, the results indicate that both face and odour sickness decrease liking of persons.
Figure 2.
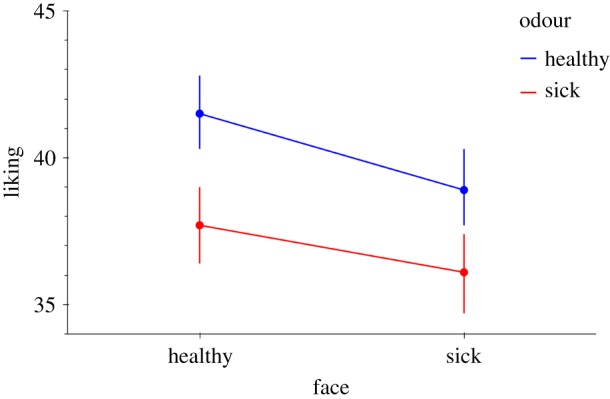
Interaction plot depicting the effects of face and odour sickness on liking of the bimodally presented person. There is no statistically significant interaction, but both factors ‘face’ sickness and ‘odour’ sickness are significant (see text). The liking scale ranges from 0 (I don't like this person at all) to 100 (I like this person very much), and where 50 indicates neither like, nor dislike. Error bars represent standard errors. (Online version in colour.)
(b). Facial electromyography recording
Analyses showed no significant activation of the levator labii region in response to sickness in faces (t6591) = −0.484, p = 0.6, d = −0.05) or odours (t6591 = 0.381, p = 0.7, d = 0.04). The corrugator supercilii region also showed no significant activation in response to sickness in faces (t6591 = 0.195, p = 0.8, d = 0.01) or odours (t6591 = −0.648, p = 0.5, d = −0.06) (see the electronic supplementary material, figures S2 and S3).
(c). Questionnaires
The extent to which the different questionnaires and their subscales shared variance was assessed through correlation analyses. The analyses showed weak to moderately significant positive correlations between PVD, DS-R and HAI (see the electronic supplementary material, table S1). There were no significant correlations between individuals' difference between liking ratings of sick and healthy odours and faces (see Methods), and their scores on the questionnaires. For overall mean liking of presented persons, we found no significant correlations between the HAI scores and overall mean liking of presented persons but significant negative correlations between mean liking and PVD germ subscale (r = −0.293, n = 77, p = 0.01). Negative correlations were also found between mean liking and disgust scale total score (r = −0.250, n = 65, p = 0.04), as well as between mean liking and DS-R core disgust subscale score (r = −0.289, n = 65, p = 0.02). Furthermore, four linear multiple regression models were performed, one for each questionnaire's subscales and one for the three total score of each questionnaire. The mean liking ratings were the dependent variable in all models. In the regression analysis, a significant regression equation was found for the PVD germ subscale (F2,62 = 2.452, p < 0.03) with an R2 of 0.073 (table 2).
Table 2.
Multiple regression analysis summary for total scores and subscales of PVD, DS-R and HAI predicting liking ratings.
| variable | B | s.e.m. | 95% CI | t | p-value |
|---|---|---|---|---|---|
| total scores | |||||
| intercept | 48.12 | 4.63 | [38.8, 57.4] | 10.37 | 0.000 |
| PVD total | −0.02 | 0.12 | [−0.26, 0.21] | −0.22 | 0.823 |
| DS-R total | −0.14 | 0.09 | [−0.32, 0.04] | −1.55 | 0.126 |
| HAI total | −0.62 | 0.29 | [−0.65, 0.53] | −0.21 | 0.834 |
| PVD subscales | |||||
| intercept | 45.24 | 3.82 | [37.6, 52.8] | 11.84 | 0.000 |
| germ | −0.36 | 0.16 | [−0.68, −0.03] | −2.21 | 0.031 |
| infectability | 0.1 | 0.15 | [−0.20, 0.42] | 0.68 | 0.497 |
| DS-R subscales | |||||
| intercept | 48.52 | 4.22 | [40.1, 56.9] | 11.49 | 0.000 |
| core | −0.40 | 0.22 | [−0.86, 0.04] | −1.77 | 0.079 |
| animal | 0.06 | 0.22 | [−0.39, 0.51] | 0.26 | 0.792 |
| contamination | 0.05 | 0.41 | [−0.76, 0.87] | 0.13 | 0.894 |
| HAI subscales | |||||
| intercept | 39.50 | 3.19 | [33.1, 45.8] | 12.37 | 0.000 |
| negative consequences | −0.16 | 0.66 | [−1.49, 1.17] | −0.24 | 0.809 |
| avoidance | 0.11 | 0.12 | [−0.13, 0.36] | 0.93 | 0.352 |
| reassurance | −0.05 | 0.14 | [−0.35, 0.23] | −0.38 | 0.703 |
4. Discussion
In a factorial design, we showed a negative effect of both face and body odour sickness on the liking of presented persons. This indicates that the cue types add to each other and testifies to the benefit of multimodal perception of sickness. The results further indicated that humans who perceive themselves as more vulnerable to disease and more prone to disgust dislike presented persons more, irrespective of health status, possibly by being oversensitive to cues even remotely suggesting a risk of contagion [36].
The observed dislike as a function of sick faces is in line with several previous studies which showed that visual sickness detection was possible using the same experimental sickness model as here [16–18,21]. Although several findings so far are based on subjective ratings of perceived facial traits and emotions, some studies, as noted above, also identify objective physical traits that change as a function of sickness, such as angle of corner of the mouth, eye openness and skin coloration [15,18].
As with face sickness, body odour sickness in the present study had a negative effect on the liking ratings of presented persons. Thus, both seeing and smelling a person with a recently activated inflammatory response reduces liking of the person. Previous studies have shown that sick body odours are rated as more unpleasant compared to healthy body odours [13,14], which probably drives the dislike associated with the sick body odours in the present study. This study corroborates our previous study [21] in which we also found that sick body odours drove dislike of individuals, only now with statistical significance. This could be owing to that in the present study, using the same sample pads, the odours were presented from jars. This technique lets participants sample the odorant from a saturated head-space which is expected to yield stronger and clearer odours than from olfactometers that flow air over the surface of the odorous material.
As noted, it is known that congruent cues from different sensory systems, in particular if they are weak, result in faster and more accurate detection when combined [19,20,37]. Considering that the sickness cues in the present study are relatively subtle, one could expect that sick faces combined with sick body odours would result in a synergistic integration beyond additivity, possibly coming out as a significant interaction (cf. figure 2). However, although additive, the present study could not show such an interaction between visual and olfactory sickness cues, despite that sick body odours and faces are both perceived as more ‘sick’ [13,17] and therefore, in theory, could add synergistically, especially, if the cues alone are weak. This could simply indicate that these cues are not synergistic enough to drive a statistically significant interaction. It could also mean that the dislike is to some extent mediated by different mechanisms for sick faces and body odours. Indeed, it has been shown that sick body odours are perceived as more unpleasant [13,14], whereas sick faces are perceived as expressing more negative emotions [18].
Overall, our results support the notion of a behavioural defence that enables us to detect and avoid the source of sickness cues. As noted in the Introduction, there is also support for the influence of personality traits on how we relate to aspects of sickness. In line with that, the present results showed a negative relation between the PVD questionnaire germ subscale and liking ratings, suggesting that individuals' PVD can affect their social behaviour, and that this is mediated by a dislike of others. This is consistent with previous results showing that individuals high in PVD are more likely to avoid objects, individuals or actions that might carry an infection [7,10], but notably also individuals with non-infectious traits, such as disabilities or obesity [38]. Furthermore, it has been shown that exposure to sickness cues in humans with high PVD scores leads to less agreeableness and low levels of openness, suggesting that self-perception biases facilitate avoidance behaviour [39]. Interestingly, another study has shown that people who score high in PVD tend to make more false-positive errors when they are exposed to stimuli only heuristically associated with sickness [40]. In summary, the present results support previous findings indicating that PVD and less social behaviour are associated [29].
The present results did not show significant correlations between HAI and liking ratings. These results are not in line with previous research where it has been shown that health anxiety is related to perceiving healthy faces as less healthy and less attractive [25]. This may be owing to that HAI is validated and used to measure health anxiety in a clinical context differentiating between clinical and non-clinical cases, and in the present study, we did not have participants with severe health anxiety.
It has been suggested that disgust in several ways is associated with disease avoidance [7,41]. In addition to the raters’ PVD, their own disgust sensitivity was negatively correlated with the liking ratings of others. As higher disgust sensitivity is associated with fewer recent infections [42], this suggests that disgust sensitivity acts as a protective mechanism against infection by way of liking and approaching others less. Furthermore, stimuli that elicit disgust can increase immune markers, such as immunoglobulin A and tumour necrosis factor α that are involved in systemic inflammation. This has been interpreted as if the immune system is triggered by disease cues in anticipation of pathogen exposure [43]. Other studies have argued that disgust could be a mediator between sickness stimuli and avoidance [22]. Indeed, some authors argue that pathogen disgust and disease avoidance are strongly interconnected, thus, they are either used interchangeably or considered functionally the same [44,45]. These observations are in accordance with the notion that stimulus-induced disgust has evolved to protect us from infection by fulfilling a sickness avoidance function [22].
Several studies in the past have indeed shown increased facial muscle activity in response to disgust after exposure to visual [46–48] and olfactory stimuli [49,50]. However, in the current study, we found no significant increase in disgust-related muscle activity and therefore no evidence of that stimulus-induced physiological disgust mediated the sickness-related dislike of others.
Although there are observations that vouch for the involvement of disgust in a behavioural defence, disgust is not necessarily the cause of the avoidance [51]. In the current study, the body odours were weak and generally not very disgusting (or unpleasant, as indicated in Regenbogen et al. [21]). The sick faces that we used as visual stimuli did not either have any profound sickness symptom, such as red spots, nasal discharge or open ulcers that could be clear disgust elicitors. Therefore, it is possible that the present disease cues were either not strong enough disgust elicitors to activate measurable physiological (EMG) responses or that the decreased liking we have observed as a function of sickness cues was not primarily driven by disgust. Arguably, sickness cues should also be able to operate on the positive end of an approach–avoidance dimension. It makes sense that healthy individuals with looks and body odour that merit approach rather than avoidance could when sick be perceived less worthy of approach in order to decrease the probability of infection. In this way, a lowered approach value can contribute to disease avoidance without disgust being involved.
A limitation of the current study is that it only assessed perceived liking in an effort to address behavioural avoidance towards sick individuals. Although, as noted, liking predicts approach of others [27], further studies should test overt avoidance behaviour as a function of sickness cues. Moreover, the above results are based on an experimental sickness model. Future studies would benefit from using a natural sickness model, such as an upper respiratory infection, to test the effects of sickness cues on components of a behavioural defence in humans. Another possible limitation of the present study is the high number of stimuli the participants had to rate. That could have potentially led to odour adaptation and consequently affected the ratings the participants gave. However, we inserted an extra 5 s in the stimulus presentation protocol where no stimuli but a fixation cross was presented to counteract adaptation.
5. Conclusion
The present findings show that olfactory and visual sickness cues appear within hours after the induction of systemic inflammation in otherwise healthy individuals. As a result, they are liked less which is a possible precursor of an avoidance behaviour. We also demonstrate that participants who perceive themselves as more vulnerable to disease and have high sensitivity to disgust tend to dislike individuals in general. Altogether these results offer support for the notion of a behavioural defence that protects us from contagion by altering social behaviour.
Supplementary Material
Acknowledgements
We thank the two anonymous referees whose comments helped improve this manuscript. We thank Tasos Bouzikas for help with figures and the preprocessing of EMG data. We thank Anastasija Aviri and Katarina Holmgren for help with data collection. We thank Alessandro Davoli, Malin Burman and Marina Atho for help with the scoring of questionnaires. We thank Julie Lasselin for help with the experimental sickness protocol.
Ethics
Photos of sick and healthy individuals were obtained in the Center for Clinical Research at Danderyd Hospital, Stockholm, Sweden, and all participants and research staff were blind to the conditions, except one physician for safety reasons. All photographed subjects gave written informed consent after the study protocol had been fully explained and received a compensation of 3500 SEK (approx. 370 euros). For the rating procedure, all participants signed an informed consent form and they received two cinema tickets in compensation for their participation. The study was approved by the regional ethical review board in Stockholm, Sweden (Registration number 2017/55-31/4).
Data accessibility
Data are available at: https://doi.org/10.6084/m9.figshare.c.4547381.
Authors' contributions
M.J.O. developed the study concept. M.J.O. and G.S. designed the study with contributions from all the authors. G.S. collected and analysed the data. M.J.O. and G.S. drafted the manuscript, and J.A., B.A.K., T.S., C.R., J.N.L. and M.L. provided critical revisions. All authors approved the final version of the manuscript for submission.
Competing interests
We declare we have no competing interests.
Funding
Swedish Research Council grant nos 2012-1125 and 2016-02742 (to M.J.O.), Swedish Foundation for Humanities and Social Sciences grant no. P12-1017 (to M.J.O.), the Knut and Alice Wallenberg Foundation grant no. KAW 2012.0141 (to J.N.L.) and Swedish Research Council grant no. 2014-1346 (to J.N.L.).
References
- 1.Young SN. 2008. The neurobiology of human social behaviour: an important but neglected topic. J. Psychiatry Neurosci. 33, 391–392. [PMC free article] [PubMed] [Google Scholar]
- 2.Hamrick N, Cohen S, Rodriguez MS. 2002. Being popular can be healthy or unhealthy: stress, social network diversity, and incidence of upper respiratory infection. Health Psychol. 21, 294–298. ( 10.1037/0278-6133.21.3.294) [DOI] [PubMed] [Google Scholar]
- 3.Nettle D. 2005. An evolutionary approach to the extraversion continuum. Evol. Hum. Behav. 26, 363–373. ( 10.1016/j.evolhumbehav.2004.12.004) [DOI] [Google Scholar]
- 4.Shakhar K, Shakhar G. 2015. Why do we feel sick when infected: can altruism play a role? PLoS Biol. 13, e1002276 ( 10.1371/journal.pbio.1002276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. ( 10.1038/nrn2297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dantzer R. 2009. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 29, 247–264. ( 10.1016/j.iac.2009.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaller M. 2011. The behavioural immune system and the psychology of human sociality. Phil. Trans. R. Soc. B 366, 3418–3426. ( 10.1098/rstb.2011.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole SW. 2006. The complexity of dynamic host networks. In Complex systems science in biomedicine (eds Deisboeck TS, Kresh JY), pp. 605–629. Boston, MA: Springer US. [Google Scholar]
- 9.Schaller M, Park J, Faulkner J. 2003. Prehistoric dangers and contemporary prejudices. Eur. Rev. Soc. Psychol. 14, 105–137. ( 10.1080/10463280340000036) [DOI] [Google Scholar]
- 10.Schaller M, Murray DR, Bangerter A. 2015. Implications of the behavioural immune system for social behaviour and human health in the modern world. Phil. Trans. R. Soc. B 370, 20140105 ( 10.1098/rstb.2014.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaller M, Park JH. 2011. The behavioral immune system (and why it matters). Curr. Dir. Psychol. Sci. 20, 99–103. ( 10.1177/0963721411402596) [DOI] [Google Scholar]
- 12.Shirasu M, Touhara K. 2011. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J. Biochem. 150, 257–266. ( 10.1093/jb/mvr090) [DOI] [PubMed] [Google Scholar]
- 13.Olsson MJ, et al. 2014. The scent of disease: human body odor contains an early chemosensory cue of sickness. Psychol. Sci. 25, 817–823. ( 10.1177/0956797613515681) [DOI] [PubMed] [Google Scholar]
- 14.Gordon AR, Kimball BA, Sorjonen K, Karshikoff B, Axelsson J, Lekander M, Lundström JN, Olsson MJ. 2018. Detection of inflammation via volatile cues in human urine. Chem. Senses 43, 711–719. ( 10.1093/chemse/bjy059) [DOI] [PubMed] [Google Scholar]
- 15.Henderson AJ, Lasselin J, Lekander M, Olsson MJ, Powis SJ, Axelsson J, Perrett DI. 2017. Skin colour changes during experimentally-induced sickness. Brain Behav. Immun. 60, 312–318. ( 10.1016/j.bbi.2016.11.008) [DOI] [PubMed] [Google Scholar]
- 16.Sundelin T, Karshikoff B, Axelsson E, Höglund CO, Lekander M, Axelsson J. 2015. Sick man walking: perception of health status from body motion. Brain Behav. Immun. 48, 53–56. ( 10.1016/j.bbi.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 17.Axelsson J, Sundelin T, Olsson MJ, Sorjonen K, Axelsson C, Lasselin J, Lekander M. 2018. Identification of acutely sick people and facial cues of sickness. Proc. R. Soc. B 285, 20172430 ( 10.1098/rspb.2017.2430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarolidou G, Axelsson J, Sundelin T, Lasselin J, Regenbogen C, Sorjonen K, Lundström JN, Lekander M, Olsson MJ. 2019. Emotional expressions of the sick face. Brain Behav. Immun. 80, 286–291. ( 10.1016/j.bbi.2019.04.003) [DOI] [PubMed] [Google Scholar]
- 19.Stein BE, Stanford TR. 2008. Multisensory integration: current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 9, 255–266. ( 10.1038/nrn2331) [DOI] [PubMed] [Google Scholar]
- 20.Stevenson RA, et al. 2014. Identifying and quantifying multisensory integration: a tutorial review. Brain Topogr. 27, 707–730. ( 10.1007/s10548-014-0365-7) [DOI] [PubMed] [Google Scholar]
- 21.Regenbogen C, Axelsson J, Lasselin J, Porada DK, Sundelin T, Peter MG, Lekander M, Lundström JN, Olsson MJ. 2017. Behavioral and neural correlates to multisensory detection of sick humans. Proc. Natl Acad. Sci. USA. 114, 6400–6405. ( 10.1073/pnas.1617357114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis V, Biran A. 2001. Dirt, disgust, and disease. Is hygiene in our genes? Perspect. Biol. Med. 44, 17–31. ( 10.1353/pbm.2001.0001) [DOI] [PubMed] [Google Scholar]
- 23.Ackerman JM, Becker DV, Mortensen CR, Sasaki T, Neuberg SL, Kenrick DT. 2009. A pox on the mind: disjunction of attention and memory in the processing of physical disfigurement. J. Exp. Soc. Psychol. 45, 478–485. ( 10.1016/j.jesp.2008.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salkovskis PM, Rimes KA, Warwick HMC, Clark DM. 2002. The Health Anxiety Inventory: development and validation of scales for the measurement of health anxiety and hypochondriasis. Psychol. Med. 32, 843–853. ( 10.1017/S0033291702005822) [DOI] [PubMed] [Google Scholar]
- 25.Hedman E, Lekander M, Karshikoff B, Ljótsson B, Axelsson E, Axelsson J. 2016. Health anxiety in a disease-avoidance framework: investigation of anxiety, disgust and disease perception in response to sickness cues. J. Abnorm. Psychol. 125, 868–878. ( 10.1037/abn0000195) [DOI] [PubMed] [Google Scholar]
- 26.Fan Q, Olatunji BO. 2013. Individual differences in disgust sensitivity and health-related avoidance: examination of specific associations. Pers. Individ. Dif. 55, 454–458. ( 10.1016/j.paid.2013.04.007) [DOI] [Google Scholar]
- 27.Cialdini RB, Goldstein NJ. 2004. Social influence: compliance and conformity. Annu. Rev. Psychol. 55, 591–621. ( 10.1146/annurev.psych.55.090902.142015) [DOI] [PubMed] [Google Scholar]
- 28.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. Sniffin' sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 22, 39–52. ( 10.1093/chemse/22.1.39) [DOI] [PubMed] [Google Scholar]
- 29.Duncan LA, Schaller M, Park JH. 2009. Perceived vulnerability to disease: development and validation of a 15-item self-report instrument. Pers. Individ. Dif. 47, 541–546. ( 10.1016/j.paid.2009.05.001) [DOI] [Google Scholar]
- 30.Olatunji BO, Williams NL, Tolin DF, Abramowitz JS, Sawchuk CN, Lohr JM, Elwood LS. 2007. The disgust scale: item analysis, factor structure, and suggestions for refinement. Psychol. Assess. 19, 281–297. ( 10.1037/1040-3590.19.3.281) [DOI] [PubMed] [Google Scholar]
- 31.Olatunji BO, Haidt J, McKay D, David B. 2008. Core, animal reminder, and contamination disgust: three kinds of disgust with distinct personality, behavioral, physiological, and clinical correlates. J. Res. Pers. 42, 1243–1259. ( 10.1016/j.jrp.2008.03.009) [DOI] [Google Scholar]
- 32.Lenochova P, Roberts SC, Havlicek J. 2009. Methods of human body odor sampling: the effect of freezing. Chem. Senses 34, 127–138. ( 10.1093/chemse/bjn067) [DOI] [PubMed] [Google Scholar]
- 33.Lasselin J, et al. 2017. Lipopolysaccharide alters motivated behavior in a monetary reward task: a randomized trial. Neuropsychopharmacology 42, 801–810. ( 10.1038/npp.2016.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Boxtel A. 2010. Facial EMG as a tool for inferring affective states. In Proc. of Measuring Behaviour 2010 (eds AJ Spink, F Grieco, O Krips, L Loijens, L Noldus, P Zimmerman), pp. 104–108. Wageningen, The Netherlands: Noldus Information Technology.
- 35.Merletti R. 1999. Standard for reporting EMG data. J. Electromyogr. Kinesiol. 9, 3–4. [Google Scholar]
- 36.Thornhill R, Fincher CL. 2014. The parasite-stress theory of values and sociality: infectious disease, history and human values worldwide. Dordrecht, The Netherlands: Springer.
- 37.Stein BE, Stanford TR, Rowland BA. 2014. Development of multisensory integration from the perspective of the individual neuron. Nat. Rev. Neurosci. 15, 520–535. ( 10.1038/nrn3742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JH, Schaller M, Crandall CS. 2007. Pathogen-avoidance mechanisms and the stigmatization of obese people. Evol. Hum. Behav. 28, 410–414. ( 10.1016/j.evolhumbehav.2007.05.008) [DOI] [Google Scholar]
- 39.Mortensen CR, Becker DV, Ackerman JM, Neuberg SL, Kenrick DT. 2010. Infection breeds reticence: the effects of disease salience on self-perceptions of personality and behavioral avoidance tendencies. Psychol. Sci. 21, 440–447. ( 10.1177/0956797610361706) [DOI] [PubMed] [Google Scholar]
- 40.Miller SL, Maner JK. 2012. Overperceiving disease cues: the basic cognition of the behavioral immune system. J. Pers. Soc. Psychol. 102, 1198–1213. ( 10.1037/a0027198) [DOI] [PubMed] [Google Scholar]
- 41.Oaten M, Stevenson RJ, Case TI. 2009. Disgust as a disease-avoidance mechanism. Psychol. Bull. 135, 303–321. ( 10.1037/a0014823) [DOI] [PubMed] [Google Scholar]
- 42.Stevenson RJ, Case TI, Oaten MJ. 2009. Frequency and recency of infection and their relationship with disgust and contamination sensitivity. Evol. Hum. Behav. 30, 363–368. ( 10.1016/j.evolhumbehav.2009.02.005) [DOI] [Google Scholar]
- 43.Stevenson RJ, Hodgson D, Oaten MJ, Moussavi M, Langberg R, Case TI, Barouei J. 2012. Disgust elevates core body temperature and up-regulates certain oral immune markers. Brain Behav. Immun. 26, 1160–1168. ( 10.1016/j.bbi.2012.07.010) [DOI] [PubMed] [Google Scholar]
- 44.Aunger R, Curtis V. 2013. The anatomy of motivation: an evolutionary-ecological approach. Biol. Theory 8, 49–63. ( 10.1007/s13752-013-0101-7) [DOI] [Google Scholar]
- 45.Lieberman D, Patrick C. 2014. Are the behavioral immune system and pathogen disgust identical? Evol. Behav. Sci. 8, 244–250. ( 10.1037/ebs0000018) [DOI] [Google Scholar]
- 46.Künecke J, Hildebrandt A, Recio G, Sommer W, Wilhelm O. 2014. Facial EMG responses to emotional expressions are related to emotion perception ability. PLoS ONE 9, e84053 ( 10.1371/journal.pone.0084053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitton AE, Henry JD, Rendell PG, Grisham JR. 2014. Disgust, but not anger provocation, enhances levator labii superioris activity during exposure to moral transgressions. Biol. Psychol. 96, 48–56. ( 10.1016/j.biopsycho.2013.11.012) [DOI] [PubMed] [Google Scholar]
- 48.Wolf K, Mass R, Ingenbleek T, Kiefer F, Naber D, Wiedemann K. 2005. The facial pattern of disgust, appetence, excited joy and relaxed joy: an improved facial EMG study. Scand. J. Psychol. 46, 403–409. ( 10.1111/j.1467-9450.2005.00471.x) [DOI] [PubMed] [Google Scholar]
- 49.Bensafi M, Rouby C, Farget V, Bertrand B, Vigouroux M, Holley A. 2002. Psychophysiological correlates of affects in human olfaction. Neurophysiol. Clin. 32, 326–332. ( 10.1016/S0987-7053(02)00339-8) [DOI] [PubMed] [Google Scholar]
- 50.He W, Boesveldt S, de Graaf C, de Wijk RA. 2014. Dynamics of autonomic nervous system responses and facial expressions to odors. Front. Psychol. 5, 110 ( 10.3389/fpsyg.2014.00110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaller M. 2014. When and how disgust is and is not implicated in the behavioral immune system. Evol. Behav. Sci. 8, 251–256. ( 10.1037/ebs0000019) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at: https://doi.org/10.6084/m9.figshare.c.4547381.


