Abstract
The study of human chemical communication benefits from comparative perspectives that relate humans, conceptually and empirically, to other primates. All major primate groups rely on intraspecific chemosignals, but strepsirrhines present the greatest diversity and specialization, providing a rich framework for examining design, delivery and perception. Strepsirrhines actively scent mark, possess a functional vomeronasal organ, investigate scents via olfactory and gustatory means, and are exquisitely sensitive to chemically encoded messages. Variation in delivery, scent mixing and multimodality alters signal detection, longevity and intended audience. Based on an integrative, 19-species review, the main scent source used (excretory versus glandular) differentiates nocturnal from diurnal or cathemeral species, reflecting differing socioecological demands and evolutionary trajectories. Condition-dependent signals reflect immutable (species, sex, identity, genetic diversity, immunity and kinship) and transient (health, social status, reproductive state and breeding history) traits, consistent with socio-reproductive functions. Sex reversals in glandular elaboration, marking rates or chemical richness in female-dominant species implicate sexual selection of olfactory ornaments in both sexes. Whereas some compounds may be endogenously produced and modified (e.g. via hormones), microbial analyses of different odorants support the fermentation hypothesis of bacterial contribution. The intimate contexts of information transfer and varied functions provide important parallels applicable to olfactory communication in humans.
This article is part of the Theo Murphy meeting issue ‘Olfactory communication in humans’.
Keywords: condition-dependent chemical signal, glandular microbiome, human, olfactory communication, sensory ecology, strepsirrhine primate
1. Introduction
Olfactory communication is ubiquitous and critical among mammals, including primates, but, relative to visual and vocal communication, remains neglected as a field of study. Indeed, Westernized cultures even lack a common nomenclature to identify odours [1]. This research bias reflects enduring, albeit waning, views about the diminished olfactory sensitivity of primates. In early evolutionary models, researchers assumed an ancestral olfactory state and, based on anatomical structures (e.g. olfactory bulb volume relative to total brain volume), inferred two reductions in primate olfactory sensitivity, one for all primates in general (relative to other mammals) and another for haplorhines (or ‘dry-nosed’ primates) more specifically (reviewed in [2]). Haplorhines comprise platyrrhines (New World monkeys) and catarrhines, which include Old World monkeys and hominoids (apes and humans). Based on the loss of an accessory or vomeronasal organ (VNO) in catarrhines [3] or on olfactory receptor (OR) pseudogenization [4], some researchers attributed these reductions to a trade-off between primate olfactory sensitivity and trichromatic colour vision. Based on the retention of functional OR genes, however, others suggested a gradual loss in olfactory sensitivity in every lineage, but one that could not be explained by trichromacy [5]. Such inconsistencies suggest that the alleged sensory trade-off has been overstated (reviewed in [6]).
Some researchers have additionally questioned the universal loss in catarrhines of the VNO [7,8], have placed the human OR gene repertoire on par with that of certain platyrrhines [5] and have documented acute olfactory sensitivity to certain, socially relevant odorants across anthropoids [9], including humans [10]. A more recent phylogenetic model of continuous trait evolution, using parent–daughter comparisons, also challenges the characterization of dual reductions by suggesting oppositional diversification trajectories for haplorrhines and strepsirrhines (or ‘wet-nosed’ primates) [2]. Accordingly, (i) the purported loss of sensitivity in catarrhines, particularly hominoids, appears to have been exaggerated and (ii) olfaction in strepsirrhines is inconsistent with gradual reduction; rather, it has been further selected and elaborated (reviewed in [6]). The first point has been supported by a growing literature on functional scent glands [11,12] and intraspecific olfactory communication [13–15] in catarrhines, including humans [16–19]. The second point is consistent with the retention of a functional VNO, a large repertoire of OR genes, a well-developed rhinarium, numerous turbinates, a diversity of specialized scent glands and the sophisticated use of and reliance on scent by strepsirrhines [20–22].
The present review is thus focused on the latter group (figure 1), but highlights empirical and methodological aspects of strepsirrhine chemical communication that are relevant to humans (and other haplorhines). It begins by surveying the historical perspectives, breadth of evidence and various methodologies related to the study of signal design, delivery and perception (§2). Unlike the emergent patterns of chemical cues, the perception of which exclusively benefits the receiver, the reliable information encoded in chemical signals is evolutionarily selected, because it changes the behaviour or perception of the receiver in a manner that generally benefits both sender and receiver [24,25]. The next section presents comparative and integrative studies, primarily out of one laboratory, to reveal the expression and transfer of condition-dependent information, typically in the service of mutually profitable, socio-reproductive functions (§3). These topics are then summarized (§4) and punctuated with suggestions for future research.
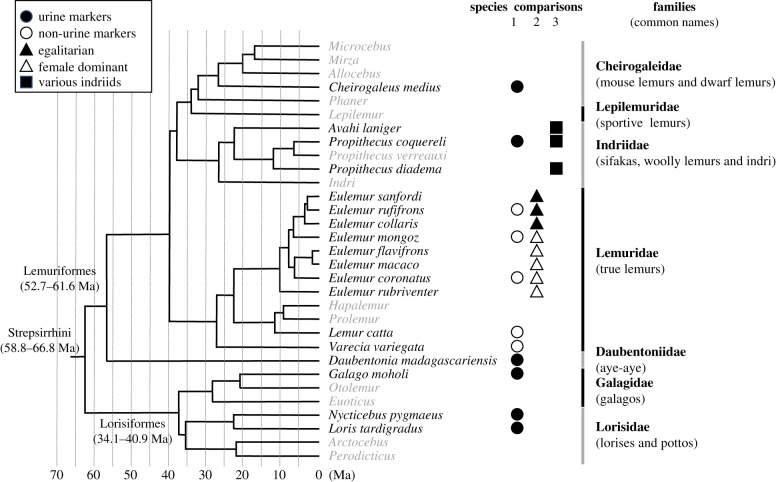
Figure 1.
The strepsirrhine lineage of primates, modified with permission from dos Reis et al. [23], showing the main species reported upon (in black) and the remaining genera (in grey). Symbols represent the species included in three comparative studies (§3), for which shadings represent different categories of animals.
2. Strepsirrhine chemical signals: design, delivery, perception
Strepsirrhines represent nearly one-third of extant, primate species (figure 1), with all members relying heavily on olfaction. These species have a restricted geographical range; lorisiforms occur in parts of Africa and Asia and lemuriforms are limited (i.e. 100% endemism) to the island of Madagascar [26]. Owing to infrequent, ancient colonization events, Malagasy animals [27], including lemurids [28], have a long history of isolation and underwent unparalleled adaptive radiations. Lemurs thus represent an ‘experiment of nature’, both at macro- [29] and micro-biotic [30] scales. Often overlooked in the human literature (although see [31]), strepsirrhines feature a multiplicity of chemical signals that have special relevance to our understanding of chemosensory evolution and function.
(a). Design
A dizzying array of scent sources is used in intraspecific chemical communication [20]; strepsirrhine scents derive from excretory products (faeces, urine) and various exudates (sweat, saliva and specialized glandular secretions; figure 2). Some of these secretions (e.g. palmar, axial, genital and perianal) are commonly expressed across species and by both sexes (figure 2a,b). Nevertheless, in keeping with female dominance [35,36], particularly when hormonally mediated [37,38], certain scent glands are unusually prominent in females (figure 2b), their elaboration being reflected in signal design (§3b,c). These peculiarities draw our attention to the operation of sexual selection in both sexes. Other secretions (e.g. throat/sternal, head, preputial, antebrachial and brachial; figure 2c–f) are more specialized, species-specific and often restricted to males [20,32,39–43] (§3c).
Figure 2.
Representative strepsirrhine scent glands, including (a) genital and perianal glands of male and (b) female red-fronted lemurs (Eulemur rubriventer), in craniocaudal orientation, (c) active sternal gland of a dominant male Coquerel's sifaka (Propithecus coquereli), (d) head gland of a male red-fronted lemur, (e) preputial gland of a male aye-aye (Daubentonia madagascariensis) and (f) brachial gland of a pygmy slow loris (Nycticebus pygmaeus). The specialized secretions in (e) contribute additional organic compounds to those present in bladder urine [32] and in (f), shown as crystallized exudate, activate a venom when mixed with saliva, producing a poisonous bite [33,34]. Photo (d) courtesy of David Haring, Duke Lemur Center.
This diversity of scent sources is but a fraction of the diversity of volatile and non-volatile organic compounds expressed. Mammalian chemosignals predominantly consist of complex mixtures, consistent with an ‘odour mosaic’ concept [44] or ‘signature’ [45], such that multiple messages are conveyed by different combinations or varying proportions of compounds about which conspecifics can learn [46,47]. These characteristics are in stark contrast to pheromones or single chemicals that elicit an unlearned, functionally specialized and species-specific response [44,45]. Understanding the design features of mammalian chemical signals thus requires identifying biologically relevant compounds [48,49] using various techniques in analytical chemistry, principally including gas chromatography- or liquid chromatography-mass spectrometry (GC-MS and LC-MS, respectively), and high-performance LC [48,50]. Thereafter, differences in the presence, absence, abundance or relative proportions of multiple compounds can be related to signaller traits (using e.g. principal component and linear discriminant analysis, PCA/LDA or non-metric multidimensional scaling) [48]. Ecological indices of diversity (richness, Shannon and Simpson) [51] also can be applied to such datasets to reveal numerical and distributional patterns, again relative to signaller features [52,53].
Because of the sheer number of chemicals expressed, the methods used must be tailored to the species, type of odorant investigated or type of compounds targeted [48,50,54,55]. For example, headspace analysis better captures the highly volatile, low-molecular-weight compounds emanating from urine (figure 3a), whereas solvent-based extraction allows capturing the higher-molecular-weight compounds emanating from certain glandular secretions (figure 3b). The heaviest, even non-volatile or proteinaceous, compounds also likely play a role in olfactory and gustatory communication, but have received little attention. For strepsirrhines, we lack information on urinary proteins that have a direct function in the chemical communication of other mammals [57], possibly including platyrrhines [58]. Preliminary data on lemur glandular proteins, derived from one-dimensional SDS-polyacrylamide gel electrophoresis and peptide identification (via Quadropole Time-of-flight MS), are consistent with a role for glandular proteins in strepsirrhine chemical signals [59].
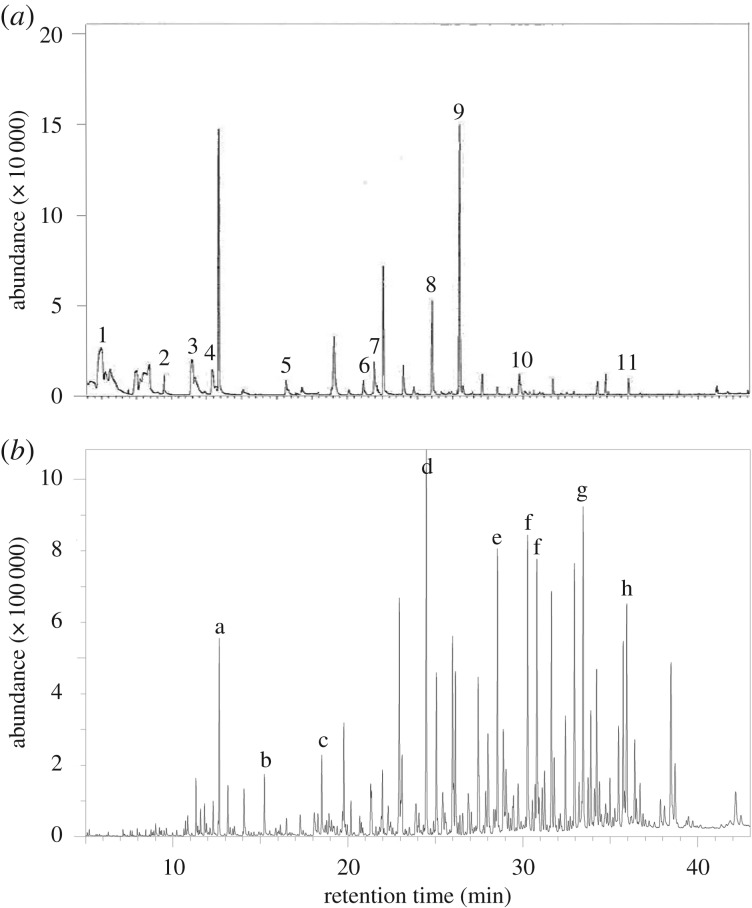
Figure 3.
Representative chromatograms of strepsirrhine excretory and secretory products, deriving from (a) the urine of a female aye-aye (Daubentonia madagascariensis) and (b) the scrotal pockets of a male ring-tailed lemur (Lemur catta). Numbered compounds (1–11) include: acetone; acetic acid; 1-butanol; 3-pentanone; 3-hexanone; 4-heptanone; 3-heptanone; 6-methyl, 3-heptanone; 3-octanone; acetophenone; and decanal; and derive from data generated in delBarco-Trillo et al. [56]. Lettered compounds (a–h) include: n-hexadecanoic acid; octadecanoic acid; octanoic acid, hexadecyl ester; squalene; tetradecanoic acid, hexadecyl ester; and hexadecanoic acid, hexadecyl ester; adapted from Charpentier et al. [52].
The origin of these semiochemicals remains uncertain. A genetic basis to mammalian odorant production [60] is evidenced by the inherited coding of soluble proteins associated with the major histocompatibility complex (MHC; [61,62]; §3c). The MHC codes for proteins essential to the acquired immune system, specifically for recognizing foreign molecules and determining histocompatibility [63] and has a well-known influence over an individual's odour [64]. Otherwise, the fermentation hypothesis posits that symbiotic bacteria play a crucial role in producing volatile organic compounds (VOCs) used in chemical communication [65]. Initially, this hypothesis was assessed using cell culture techniques (e.g. to link specific VOCs to isolated bacterial strains) [66]. More recently, with the advent of high-throughput sequencing of the 16S ribosomal RNA gene, often coupled with analytical chemistry, researchers are conducting studies in various species [67–69], including humans [70] and strepsirrhines [30] (§3d), to identify and quantify the symbiotic bacteria in scent secretions. Whereas sequencing reveals the multitude of site-specific microorganisms, it lacks the specificity of culture techniques for identifying the VOCs produced [71].
With some early exceptions [72,73], the chemical analysis of strepsirrhine scent has been a fairly recent development (table 1 and references therein; §3a–c) and rarely used by field researchers (although see [74,85]), particularly if animal capture is difficult or cold storage is inadequate [48]. When feasible, chemical analysis is often applied to one or two types of odorants, often in only one species; the results thus reflect a mere fraction of the available chemical information expressed by the organism (or its microbiota). Because no single method captures the entire range of potentially important compounds, more ‘complete’ compound detection and quantification would require a logistically challenging combination of tools and techniques [48]. We thus have much to learn in all primates from the continued study of signal design.
Table 1.
A representative subset of olfactory studies on strepsirrhine primates, by genus, in which fixed or transient, condition-dependent chemical signals and cues are inferred from significant differences in patterns of design, delivery and/or perception across multiple odorant types.Repetition of studies horizontally represents single studies using multi-pronged approaches; repetition of studies vertically represents single studies examining multiple, condition-dependent variables. With regard to chemical design relevant to other aspects of transient condition, dietary influences were examined in Propithecus, Eulemur and Lemur [48], age effects were examined in Propithecus, Lemur and Daubentonia [32,74,75] and social group effects were examined in Propithecus [74], but none were supported.
| condition |
taxonomic group [citation] |
||
|---|---|---|---|
| design | Delivery | perception | |
| proposed immutable signals | |||
| species |
— Cheirogaleus [56] Propithecus [56,76] Eulemur [56,77] Lemur [56,76] Varecia [56] Daubentonia [56] Galago [56] Nycticebus [56] Loris [56] |
Microcebus [78] — Propithecus [79] Eulemur [37,80] Lemur [80] — — — — — |
— — — Eulemur [81] — — — — — — |
| phylogenetic relatedness |
Cheirogaleus [56] Propithecus [56] Eulemur [56,77] Lemur [56] Varecia [56] Daubentonia [56] Galago [56] Nycticebus [56] Loris, [56] |
— — — — — — — — — |
— — Eulemur [81] — — — — — — |
| social system |
— Eulemur [77] |
Propithecus [82,83] Eulemur [37] |
— — |
| sex |
Propithecus [76,84,85] Eulemur [77] Lemur [53,76,86] Galago [72] — |
Propithecus [82-84,87] Eulemur [37] Lemur [39,88,89] — — |
Propithecus [84] Eulemur [90] Lemur [74,91–93] Galago [94] Nycticebus [95] |
| individual ID |
— Lemur [86,96] — |
— — Galago [97] |
Eulemur [98] Lemur [96,99,100] Galago [101] |
| heterozygosity | Lemur [52,102] | — | Lemur [103] |
| MHC | Lemur [104,105] | — | Lemur [104] |
| relatedness |
Propithecus [85] Lemur [52,53] |
— — |
— Lemur [103] |
| odorant source |
Eulemur [77] Lemur [86] Daubentonia [32] |
— Lemur [88,89] — |
— Lemur [75, 106] — |
| proposed transient signals | |||
| dominance status |
Microcebus [73] Propithecus [48] — — — |
Microcebus [73] Propithecus [42,79,82,83,107] Eulemur [108] Lemur [35,88,89] — |
Microcebus [73] — — Lemur [75,93,109,10] Nycticebus [95] |
| health/injury | Lemur [111] | — | Lemur [111] |
| reproductive season |
— Propithecus [74,76,84,85] Eulemur [77] Lemur [52,53,86,102] — |
Microcebus [73] Propithecus [79,82–84] Eulemur [80] Lemur [80,88,110] Galago [41] |
— Propithecus [84] — Lemur [75,93,103,110] — |
| ovarian cycle | — — |
Microcebus [73,112] — |
— Galago [94] |
| reproductive success | Propithecus [84] | Propithecus [84] | — |
| proposed transient cues | |||
| pregnancy | Lemur [113] | — | — |
| fetal sex | Lemur [113] | — | — |
| contraception | Lemur [114] | — | Lemur [114] |
Studies of human chemical cues, arising from a broad range of odoriferous sources, are typically focused on diagnostic, forensic, environmental or industrial applications [50,54,115]. In relation to a signal role in intraspecific communication, human chemical studies tend to be focused on axillary VOCs [18,60,116] and their microbial derivation [70,117,118]. This focus is because the axillae are the largest and most active scent sources in humans [19], considered most analogous to the glands of other primates [119]. It might be profitable, however, to broaden the sources (e.g. faeces, urine, breath, saliva, milk and non-axillary skin [50], hands [115] and genitals [120]) studied in humans.
(b). Delivery
Scent signal delivery can be ‘passive’ (as reflected by the natural diffusion of odours from the body [109]), ‘reflexive’ (as evidenced by the involuntary release of VOCs with fear, anxiety or stress [121]) or ‘active’ (as displayed by intentional scent marking or rubbing) [20]. Strepsirrhines use all forms of delivery, but their elaborate scent-marking repertoires, wherein animals adopt specific postures to optimally deploy odorants (figure 4), have garnered the most attention (reviewed in [20,122]; table 1).
Figure 4.
Scent delivery by strepsirrhine primates, showing diverse marking postures, including male (a) sternal marking by a black-and-white ruffed lemur (Varecia rubra) and (b) head marking by a red-fronted lemur (Eulemur rubriventer), or ambisexual (c) genital marking by the ring-tailed lemur (Lemur catta), (d) urogenital marking by the Coquerel's sifaka (Propithecus coquereli), and (e) social marking between crowned lemur (Eulemur coronatus) mates. Terrestrial lemurs (c) use hand stands to deposit genital secretions at nose level, whereas arboreal lemurs (d) use vertical clinging. Photos (a–c) courtesy of David Haring, Duke Lemur Center; photos (d,e) courtesy of Lydia Greene.
Strepsirrhines self-anoint, allomark (figure 4e), deposit marks on fresh surfaces and near or atop existing marks [20], with overmarking being a highly effective form of intraspecific competition (between males [93,95,107]; between females [88]). Scent deposition can also vary quantitatively (in amount and frequency) and qualitatively (in spatial, temporal or seasonal distribution, and in substrate used or topographical detail). Marking can produce visible signs that persist in the environment, including urinary or faecal latrines [123–126] and scent posts created from various gougings [79,127,128] or smears [20]. Sign placement may reflect function, including at territorial boundaries to advertise ownership [129] or in core areas to maintain social bonds [123].
Unlike with transient visual and vocal signals (or with passive and reflexive chemical signals), delivery of an olfactory signal does not depend on the simultaneous presence of a receiver: the distinctive benefits of chemical communication are that it can function over time (i.e. with a delay) and in the absence of social interaction. These characteristics led early researchers to concentrate on solitary, nocturnal species that are dispersed in the environment, rely principally on urine [78,97,130] and would purportedly benefit most from a broadcast approach to communication (reviewed in [6,56,122]). The relevant functions most often proffered for nocturnal species include territorial maintenance (i.e. keeping non-cohabiting animals apart) and long-distance mate attraction (i.e. drawing non-cohabiting animals together). Whereas urinary signals may well attract available mates, including via as yet unidentified pheromones [73], the complex glandular signals of diurnal, social species are known to reveal the quality of potential mates [52,53,94,101].
Paradoxically, therefore, attending to the singular benefits of olfactory communication may underrepresent the complexity and nuance across species, specifically limiting our understanding of the social nature, potential immediacy and varied functions of strepsirrhine chemical signals [73,87,88,93,94,101,131,132]. Indeed, the social complexity of diurnal species may have selected for increased, rather than decreased, complexity in olfactory communication [77] (§3a,b).
Whereas nocturnal species mark cryptically, the highly visual [100], flamboyant displays that characterize glandular scent marking by diurnal species may specifically attract attention (i.e. within seconds [93]; figure 5). Secretions can be delivered alone or as part of a compound unimodal or multimodal signal [88,106,112,124,128,133]. Multimodality typically augments signal detectability, but in ring-tailed lemurs it can do so in real-time [88,128] (figure 5a–c) or with a delay, by introducing fixatives via scent mixing to increase signal longevity [106] (figure 5d–f). Perhaps the impetus to deposit pure scents versus mixtures is to target different audiences by influencing the time-course over which a signal remains functional: an immediate function could be to relay short-lived, condition-dependent messages to one's group members, whereas a deferred function could be to advertise long-term, territorial usage to extra-group conspecifics [106] (figure 5g). These same males can also self-anoint by rubbing both secretions onto their tail to imbue the hairs with a combined odorant ‘cocktail’—creating a composite, unimodal signal—that is then wafted at an opponent or a potential mate during face-to-face ‘stink-fighting’ [35,39] or ‘stink-flirting’ [134], respectively (§3c). Active scent deployment in both cases (fight and flirt) requires a recipient and serves an immediate, evaluative, social function.
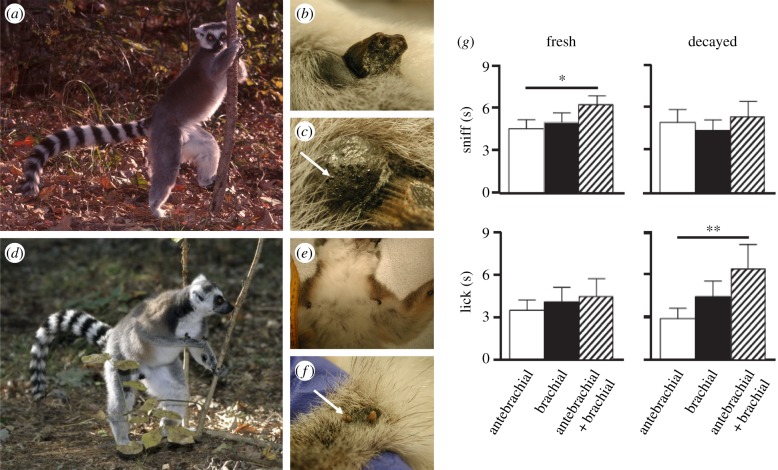
Figure 5.
Multimodal (olfactory, auditory and visual) signalling in the male ring-tailed lemur (Lemur catta) and the immediate or delayed responses such signals generate. During (a) wrist marking, a male permanently and audibly gouges a sapling using (b) spurs on the inner wrists [39,128], depositing (c) clear, highly volatile secretions from the adjacent antebrachial gland [88]. When preceded by (d) shoulder rubbing, whereby the male brings his wrist to (e) the ipsilateral brachial gland in the inner shoulder region, antebrachial secretions are mixed with (f) opaque, greasy, brachial secretions dominated by the fixative squalene [86]. In (g), conspecific response is stronger to mixtures than pure secretions, but changes qualitatively over time. Photos (a,d) provided courtesy of David Haring and reprinted from Charpentier et al. [52], with permission. Figure (g) is modified from Greene et al. [106], with permission.
Like over marking, multimodal or composite unimodal signalling occurs routinely in strepsirrhines, but information about the order of deposition and its consequences [95] is limited. If subjects are difficult to observe, field researchers might collapse potentially discrete categories of behaviour, but the devil may be in these details. Perhaps there is ‘syntactical’ meaning in the delivery sequence of the various components. Further information about the specifics of signal delivery could broaden our functional and mechanistic understanding about a chemical ‘language’.
Whereas active delivery is the best-studied component of chemical communication in strepsirrhines, it is the least-studied component in humans. Signal delivery in humans is generally seen as passive or reflexive, unconsciously changing with an individual's emotional state [135,136]). With few exceptions (e.g. hand shaking [137]), active signal delivery in humans is generally overlooked. Nevertheless, as evidenced by solitary, nocturnal strepsirrhines, scent delivery need not be active or conspicuous for chemical communication to be effective. Moreover, as evidenced by social, diurnal lemurs, scent delivery need not occur asocially. Studies of human chemical communication could thus profit from creative ways of examining the details of signal delivery.
(c). Perception
Strepsirrhine primates perceive odorants both via the olfactory epithelium, leading to the main olfactory bulb (MOB), and via a well-developed functional VNO [138,139], leading to the accessory olfactory bulb (AOB). Although they are particularly well equipped to perceive odours, their endangered status typically precludes them from the invasive procedures often used with laboratory models (although see [140]) and from brain imaging procedures (e.g. fMRI) requiring anaesthesia. Somewhat incongruously, therefore, human chemosignalling studies are far more advanced with regard to the perception component, as one can use powerful approaches for measuring human brain activation in response to odorants [141–143]. In only one comparative study to date has there been direct functional assessment of primate OR sensitivity to various odorants [144]: it included humans, chimpanzees, rhesus macaques and rodents, but not strepsirrhines.
In strepsirrhines, the recipients' perception of chemosignals can be assessed via the physiological (e.g. hormonal [73]) changes induced by exposure. Most commonly, however, perception is inferred from differential behavioural responses to conspecifics, to marks encountered in the environment or to controlled, experimental presentations of odorants (table 1), with sniffing reflecting the detection of VOCs by the MOB and licking or flehmen [91] reflecting the detection of non-volatiles by the AOB. Typically, the responses recorded in bioassays (e.g. investigation, counter marking) bear little resemblance to the function purportedly served (e.g. mate selection). In other words, the perception component is often decoupled from the action component (see [96]).
Important benefits to behavioural bioassays include that they allow distinguishing responses owing to the recipient's state from those owing to the signaller's state [75]. Bioassays testing the perception of and sensitivity to specific chemicals [9,10] or responsiveness to experimentally created, odorant mixtures [106] remain underused, but they have served to confirm multimodal integration (for example, between vocal and olfactory representation of individuality [99]) and to unveil the contribution of different odorants in composite unimodal signals. When presented singly versus in combination, for example, a mixture of secretions generates more investigatory interest from conspecifics than do the individual secretions alone (figure 5g), suggesting a synergistic function to mixing [106]. Moreover, freshly mixed scents are primarily sniffed, whereas decaying signals are primarily licked (figure 5g), implying that animals rely on their nasal epithelium and MOB when encountering fresh scent, but on their VNO and AOB when encountering stale scent. Coupled with the potential for real-time olfactory transmission in lemurs (§2b), this interpretation could suggest that the human MOB may be critical and sufficient for processing immediately available, socially transmitted signals.
Behavioural bioassays are also used to test the socio-reproductive functions of human odorants (reviewed in [19]), multisensory responsiveness [145] or multisensory integration [146], particularly across visual and olfactory domains. The combination of scent, touch and taste might be particularly salient in this regard. Whereas bioassays in non-humans and in preverbal humans [147,148] rely on the overt behavioural discrimination of presented odorants by test subjects, bioassays in verbal humans (e.g. using the ‘T-shirt’ paradigm) rely on self-reporting by test subjects (reviewed in [149,150]). It would be interesting to evaluate the consistency of findings on human responsiveness to odorants across measures.
(d). Integrated studies
Independent study of the design, delivery and perception components of chemical communication is commonplace and informative, but potentially inconclusive. This is because an odorant's chemical composition may be uncoupled from its delivery patterns or may contain information undetectable by recipients. Likewise, the information that is seemingly revealed by patterns of deposition or by response to odorants may not be reflected in chemical design. For example, differences by dominance status occur reliably in scent marking and scent investigation by ring-tailed lemurs [88,89,109,110], but have not been detected in the chemical composition of their signals [86] nor in behavioural bioassays controlling for familiarity between signaller–recipient pairs [75]. These latter discordances suggest that, in some species, dominant animals may be recognized as such not by the chemical encoding of status (although see §3d), but by their learned individual scent signatures [75,96] or the frequency with which their scents are encountered in the environment. Such mismatches might merely influence our interpretation about the mechanism by which a function is served or they could call into question our interpretation about the existence of a purported signal. Consequently, the most powerful approach to studying communication—albeit a logistically challenging one—integrates design, delivery and perception [151] or expression, perception and action [96].
Whereas delivery is often studied in wild primates, design and perception are often studied in captive animals. Nevertheless, multi-pronged approaches from within the same laboratory have been used effectively in strepsirrhines (e.g. figure 6 and table 1). For a given scent type, the studies represented in table 1 show consistency in findings across the three components, supporting an interpretation of functional signals. Sometimes there is redundancy across different odorants in the various conditions encoded, but sometimes not. For example, species identity is strongly encoded in Eulemur genital and perianal secretions [77], but not in their urine [56]. Multi-pronged approaches are likewise powerful in studies of human chemical communication (e.g. [152,153]) and should be adopted more routinely. For example, complementary studies of hand-signal design [115], delivery [137] and perception [154] would be better integrated within a unified framework.
Figure 6.
An integrative, three-pronged study of olfactory communication about reproductive state in the Coquerel's sifaka (Propithecus coquereli). Shown are differences in (a) chemical design, based on principal component and linear discriminant analyses of the chemical compounds identified in female genital secretions, using gas chromatography and mass spectrometry; (b) the frequency of signal delivery in a forest enclosure, based on observed urogenital marking by a representative female; and (c) the perception of or interest in signals across reproductive seasons, based on conspecific investigation, in situ, of freshly deposited marks. Adapted from Greene & Drea [84]. *p < 0.05; ***p < 0.001.
3. Condition-dependent chemosignals
An animal's condition can be affected by various internal (e.g. genetic, physiological) and external (e.g. environmental, pathogenic, social) factors that influence the quality of its signals, and can tilt the balance between reproduction and survival [155,156]. Condition-dependent signals fall into two categories, those that are stable or fixed (such as one's species, sex, individual identity, kinship or genetic quality) and those that are flexible or transient (such as one's diet, age, dominance status, health or reproductive state). Classic studies have promoted visual or vocal modalities in condition-dependent signals [157–160]; however, Penn & Potts [161] extended the rationale to chemical signals, noting that: ‘The scent of a male mouse is the chemical equivalent of a peacock's plumage; they both function to attract females but are costly to produce’ (p. 392). Here, signals of stable and transient condition in strepsirrhines are evaluated in three multi-species comparisons and in focal-species studies, to address (i) urinary signals across six families, (ii) glandular signals within the Eulemur clade of Lemuridae, (iii) glandular (primarily genital) signals in the ring-tailed lemur and (iv) glandular signals in relation to glandular microbiomes within several species of Indriidae (figure 1 and table 1).
The first three reviews derive primarily from work on captive animals housed socially at the Duke Lemur Center (DLC), in Durham, North Carolina; the fourth derives primarily from work on wild animals living near Moramanga, Madagascar. Most of the species at the DLC have access to forest enclosures, where they range semi-free and scent mark freely under naturalistic conditions. Their age, social status and reproductive history are known. They can be unobtrusively monitored at close range to (i) determine the secretory state of their glands, (ii) assess their reproductive state and (iii) observe behavioural details in situ or under controlled bioassays. Many are habituated to handling (without the need for anaesthesia), which facilitates routine sample collection for chemical, genetic, physiological and/or microbial assessment. The wild animals are tracked, annually captured, anaesthetized and sampled as part of a health-monitoring, conservation project [162]. Cold storage is available and samples are kept frozen during transport.
(a). Comparative studies of urinary signals across six strepsirrhine families
Mammalian odorants variably contain a proportion of unknown compounds that, without relevant mass spectrometry data, cannot be matched or compared across conditions [47,163]. Therefore, to facilitate the comparative chemical analyses necessary to address evolutionary or ecological questions, several conditions must be met: (i) secretory samples should derive from the same type of odorant across species, (ii) they must be processed and analysed de novo, using the same methodology and (iii) unknown compounds must be differentiated and included in the dataset [56]. The dearth of comparative chemical studies likely reflects the diversity (and, hence, discordance) of scent sources across unrelated species, as well as the logistical challenges of generating the necessary data. These challenges, however, are not insurmountable.
In a broad comparison of 12 strepsirrhine species from six families (figure 1) that do not share the same types of glands, we used solid-phase microextraction GC-MS to examine the composition and evolution of urinary VOCs [56]. We selected for study six species that mark prominently using urine (urine markers) and six that, instead, mark prominently using glandular secretions (glandular markers). As anticipated, the individual chemical profiles accurately reflected the species' differential reliance on urinary signals: urine markers expressed more urinary VOCs (including putative semiochemicals) than did glandular markers. Interestingly, among the urine markers, the three most social species, including the diurnal sifaka, expressed the greatest diversity of putative semiochemicals. Moreover, whereas the urinary VOCs of confirmed urine markers produced strong species signatures, those of glandular markers produced only weak species signatures.
Such a broad, comparative study also allowed exploring phylogenetic relationships (i.e. phylogenetic distance between pairs of species relative to the chemical distance between their urinary VOCs) [56]. Regardless of the major mode of marking displayed, urinary signals showed gradual (as opposed to saltational) change over time, such that species that are more closely related have more similar urinary profiles than do species that are more distally related. By phylogenetically reconstructing the evolutionary trajectories of putative semiochemicals, we estimate that three compounds (benzaldehyde, nonanal and decanal) occurred at all ancestral nodes, four compounds (acetone, 2-hexanone, 4-heptanone and 2-heptanone) occurred at the ancestral nodes leading to urine markers and only one compound (3-pentanone) occurred at an ancestral node leading primarily to glandular markers. These patterns suggest that urine marking is the ancestral state, whereas glandular marking is derived [56].
These 12 species differ dramatically in their social organization and activity patterns, with urine markers tending to be solitary and nocturnal, and glandular markers tending to be social and diurnal or cathemeral. Because cathemerality (i.e. activity at any time of the day or night) is a rare pattern that challenges sensory adaptations, it remains poorly understood. There has been a long-standing debate about whether cathemeral strepsirrhines descend from nocturnal species recently transitioning to a diurnal lifestyle (e.g. [164]) or from ancient diurnal species reentering a nocturnal niche (e.g. [165]). Using a ‘chemical’ approach to address this debate, we reanalysed the comparative data on urinary VOCs and discovered that urinary compounds covary in relation to the signaller's main activity pattern [166]. The nocturnal and diurnal species are most differentiated in their VOCs, and the intermediary cathemeral species have VOCs more closely aligned with those of diurnal species. Given the gradual rate of signal evolution [56], the similarities shared with diurnal species support cathemerality as an ancient expansion of diurnal animals into a nocturnal niche [165,166]. Through the strength of the phylogenetic approach, these findings are the first to link chemical signals to socioecological variables and evolutionary history [132]. Although much reduced in species diversity, it would be interesting to conduct comparative, chemical studies in the great apes.
(b). Comparative studies of genital and perianal signals across eight Eulemur species
Given the unparalleled diversity of strepsirrhines, the requirement that all subjects possess at least one comparable scent source necessarily constrains the potential for comparative chemical studies of glandular secretions. Here, this challenge is met by examining eight related species from within the relatively specious Eulemur clade. Although some possess species- or sex-specific glands, all clade members of both sexes minimally have genital and perianal glands in common. The selected species differ in their mating systems, which range from monogamous pairs to promiscuous multimale–multifemale groups (reviewed in [77]). Likewise, they differ in their social organization, with most species being characteristically female dominant, while the remainder are rare exceptions in which the sexes are egalitarian (figure 1) [167,168]. We used a three-pronged approach to assess glandular morphology, patterns in the chemical composition of glandular secretions [77] and anogenital scent marking by both sexes [37].
Regardless of the dominance structure of the species, all female Eulemur (relative to male conspecifics) show elaboration of their perianal glands (figure 2a,b) [77]. Moreover, their secretions have stable, condition-dependent chemical patterns (figure 7a) that are body-site specific [77]: despite the close physical proximity of genital and perianal glands (which are often collapsed for study and collectively referred to as ‘anogenital glands’), the secretions from these two areas are chemically distinct (figure 7c), potentially reflecting different functions.
Figure 7.
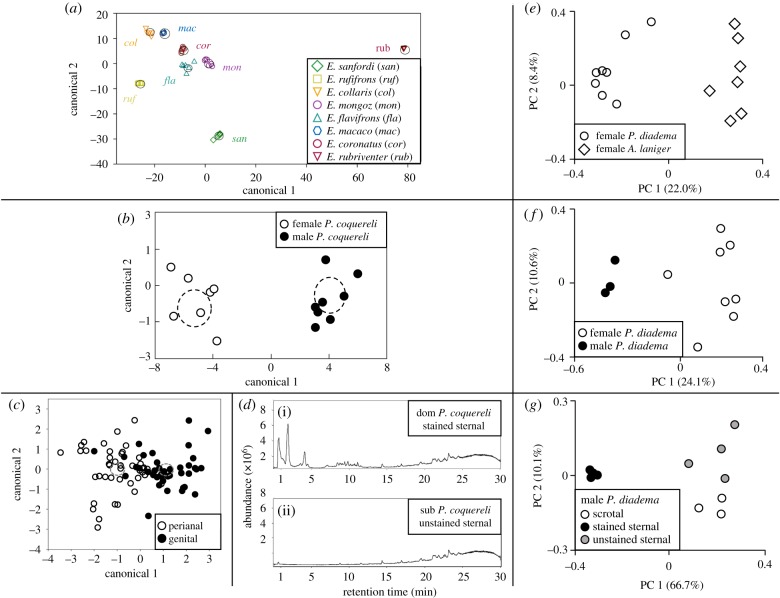
Condition-dependent patterns in the (a–d) chemical compounds and (e–f) microbial communities deriving from several glandular secretions of various (a,c) captive Eulemur species, and (b,d) captive and (e–g) wild indriids. Shown are ‘chemical signatures’ of (a) species (Wilks' λ: p < 0.0001), based on GC-MS and on principal component and linear discriminant analyses (PCA/LDA) of the perianal secretions of Eulemur spp. [77]; (b) sex (Wilks’ λ: p < 0.05), based on GC-MS and PCA/LDA of the genital secretions of Coquerel's sifakas (Propithecus coquereli) [84]; (c) gland (Wilks' λ: p < 0.015), based on GC-MS and LDA of shared compounds between Eulemur genital and perianal secretions [77]; and (d) social status, based on LC-MS of the sternal secretions of a representative dominant (i) and subordinate (ii) male P. coquereli [48]. Also shown are principal coordinates (PCs) of unweighted UniFrac measures for various microbiomes graphed relative to host (e) species, between female woolly lemurs (Avahi laniger) and diademed sifakas (P. diadema); (f) sex, between female and male P. diadema; and (g) gland (scrotal versus sternal) and social status (stained/dominant versus unstained/subordinate sternal) from male P. diadema [30]. Adapted from [30,48,77,84].
These animals’ chemical signals also reflect socio-reproductive, socioecological and phylogenetic differences between species. There are universally strong sex and seasonal differences in the overall chemical composition of both types of secretions, but the sex differences are qualified: ‘promiscuous’ females have richer genital signals than do ‘pair-bonded’ females, and ‘egalitarian’ males have richer genital and perianal signals than do ‘female-dominated’ males. Thus, in the female-dominant species, females express richer signals (as in Lemur; §3c), whereas in the egalitarian species, males express richer signals (as in most other mammals) [77]. Lastly, as with the gradual evolution of urinary signals (over the course of roughly 3–75 Myr) [56], the chemical distances between pairs of Eulemur species (based on each type of secretion) positively covary with pairwise phylogenetic distances, again suggesting gradual, albeit more rapid, evolution (i.e. over the course of roughly 2–9 Myr) [77].
Lastly, difference in Eulemur social organization is also reflected in scent-marking behaviour. Overall, members (particularly females) of female-dominant species engage in more frequent scent marking than do members (particularly females) of egalitarian species. Moreover, there is a tendency for females to mark more often than their male conspecifics in female-dominant species, but for the reverse to be true in egalitarian species [37]. These complementary morphological, chemical and behavioural patterns in females are potentially linked to a suite of hormonally mediated, ‘masculinized’ traits [37,38,169–171] and are consistent with sexual selection operating in females [102].
(c). Focal studies of glandular secretions in ring-tailed lemurs
The ring-tailed lemur is the most socially complex strepsirrhine and the most emblematic olfactory communicator [35] (figures 4c and 5). As the only surviving member of its genus, it is often the subject of focal-species studies (but see [56,76]). As noted for Eulemur, elaboration [172] or ‘masculinization’ [171] of female genital glands in Lemur is consistent with the pronounced chemical richness (or chemical ‘sex-reversal’) of female labial secretions, relative to homologous, male scrotal secretions [52,53,86]. Moreover, chemically distinct brachial, antebrachial, scrotal and labial secretions [86] are matched by the sexes' distinct scent-marking repertoires [39,88,93]. That these signals are differentially deployed and investigated throughout the year [75,93] further suggests effective perception and functional specificity. Research conducted by multiple research groups, including ours, have provided a wealth of information about condition-dependent signals in the ring-tailed lemur, showing that chemical variation by species, gland, sex, reproductive state, social status or individual identity typically corresponds to variation in the signaller's deployment patterns, as well as to the recipient's investigative behaviour in situ or during controlled bioassays (table 1).
Our own in-depth studies of L. catta genital secretions further address condition-dependent signals in relation to genotype and health, and also address cues in relation to reproductive parameters (table 1). With regard to honest signalling of genetic quality and kinship, the chemical composition of male and female genital secretions relates to individual, genome-wide, neutral heterozygosity and pairwise genetic distance between individuals [52,53,102]. For example, the most genetically diverse females show the greatest diversity of fatty acid esters during the breeding season [102]. Moreover, the ‘smell’ of both quality and kinship is salient to conspecifics, as illustrated by females most often associating with the scent of the most genetically diverse females or of unrelated males [103]. Because microsatellite diversity is an accurate predictor of health and survivorship, L. catta genital signals appear to be honest indicators of quality and kinship, functioning as sexually selected ornaments in both sexes.
Neutral heterozygosity differs, however, from functional genetic diversity, so to further examine odour–gene covariance [105], we used next-generation sequencing techniques applied to the MHC-DRB gene of L. catta [173,174]. Genotyping the MHC-DRB in a large number of wild and captive lemurs showed that captive animals have reduced allelic diversity [174]. Nevertheless, their allelic diversity relates significantly to the chemical richness of their genital signals and to the responsiveness of conspecifics encountering their scents for the first time [104]. For example, males that have the most MHC-DRB supertypes also have the greatest chemical richness in their genital secretions—attributes that are detectable by female recipients, who associate most often with the scent of MHC-DRB dissimilar males. In summary, chemicals expressed in the genital secretions of L. catta reliably advertise an individual's genetic quality, whether via neutral, genome-wide heterozygosity or functional, MHC-DRB diversity. They also advertise pairwise kinship or MHC-DRB similarity. Such odour–gene relationships in both sexes facilitate prioritization of agonistic or nepotistic interactions, as well as compatible mate attraction and inbreeding avoidance to increase the immunocompetence of offspring [52,53,102–104]—findings that are functionally relevant to humans and other primates [150,175–177].
With regard to health or wellness, we also uncovered salient effects of injury on signal composition in male and female L. catta [111]. Over the course of a decade, we amassed a set of genital scent samples from naturally ‘injured’ animals, that were or were not treated with antibiotics, and a set of seasonally matched samples from ‘healthy’ animals (both at baseline, prior to injury and following recovery). Relative to healthy animals, both groups of injured animals (i.e. +or−antibiotics) lost signal richness, but with different patterns (revealed via PCA/LDA) in the diversity and composition of their remaining compounds. Injury alone dampens and alters genital signals, consistent with the expensive signal hypothesis (i.e. that chemical signals honestly reflect the signaller's ability to bear the cost of manufacture). Injury coupled with antibiotic treatment likewise dampens, but differentially modifies the signal, consistent with the fermentation hypothesis. Conspecific males are sensitive to the chemical changes owing to injury alone, responding differently, particularly with more competitive overmarking, to the smell of an individual when injured versus when healthy, as if sensing and taking advantage of weakness. Although olfactory-guided predation pressure is not particularly strong in Madagascar, a dampened signal could potentially benefit an injured animal; nevertheless, the proximate mechanism is likely to involve reallocation of energetic resources to processes involved with healing. No comparable effects of injury on scent signals appear to have been investigated in other species, but evidence of health effects on human chemosignals abound [153,178,179].
Lastly, chemical changes associated with female reproductive parameters, including pregnancy [113] and hormonal contraception [114], are considered cues because the benefit to the signaller is either unlikely [114] or remains to be established [180]. Beyond seasonal variation [75,86], evidence of hormonal mediation of female scent derives from the strong chemical indicators of pregnancy status and fetal sex in female L. catta [113]. Notably, preconceptive females express more compounds in their genital secretions than do pregnant females, and pregnant females experience a greater loss in compounds if carrying a fetal son versus a fetal daughter. These findings suggest broader hormonal modification of chemosignals than previously recognized in strepsirrhines, and are consistent with chemical patterns observed in pregnant humans [181].
With regard to contraception using medroxyprogesterone acetate, treatment during the breeding season (when control females have immediate reproductive potential) dramatically alters the chemical richness and composition of female genital secretions, scrambling and degrading olfactory signals of fertility, as well as signals of individuality, genetic quality and relatedness [114]. These sweeping changes are salient (and apparently unappealing) to male conspecifics [114]. Modestly akin to the hormonally mediated effects on male scent as a result of surgical castration [40,41], pronounced effects of hormonal ‘castration’ in females may owe, in part, to diminishing concentrations of naturally occurring sex steroids and, in part, to unknown effects of introducing supra-physiological concentrations of synthetic steroids. These data provide unique insights for better understanding the olfactory ratings of and by hormonally contracepted women [176,182–184], presumably relevant to human mate choice [149]; nevertheless, we await confirmation of contraceptive effects on the composition of human chemical signals.
(d). Comparative studies of the chemicals and microbiomes across members of Indriidae
There is a vast and rapidly growing literature on the gut microbiome, including in strepsirrhines [185–189]; however, the primate literature on other microbiomes (e.g. axial [190], vaginal [191–193]) remains relatively sparse. Although these various microbiomes are potentially related to odour production, the focus of primate microbiome literature is on health. Our final comparative study—the first to describe microbiomes associated with primate scent glands [30]—is instead relevant to the fermentation hypothesis of signal derivation. It explores the microbiota contained in various scent glands of indriids, mostly from the wild (figure 1), to see if any differentiated patterns of community membership concur with typical, condition-dependent patterns observed in the chemical compounds of lemur glandular secretions (§3b,c).
The diurnal and social Coquerel's sifaka (Propithecus coquereli) produces rich urinary signals ([56]; §3a), but also marks prominently using glandular secretions (figures 2c, 4d and 6). Marking behaviour, although rarely observed in Avahi spp. [194], has been well studied in Propithecus spp., both in captivity [84] and in the wild [79,82,83,87] (table 1). Observations typically reveal differences between species and by sex (males > females), by season (breeding > non-breeding) or by status (dominant > subordinate). The status difference in male sternal marking is related to an androgen-mediated ‘bimorphism’ between stained or dominant males (figure 2c) and unstained or subordinate males [42,43]. These behavioural patterns match certain condition-dependent chemical patterns (as determined by GC-MS or LC-MS) in the glandular signals of indriids and other lemurs, including between different species of Eulemur [77] (figure 7a), between the sexes in P. coquereli [84] (figure 7b), between different glands in Eulemur [77] (figure 7c), and between males of different dominance status in P. coquereli [48] (figure 7d).
We selected for microbial study four indriid species (figure 1) that sympatrically occupy the same region of montane rainforest in eastern Madagascar. Whereas the wooly lemur is nocturnal, the other three Propithecus spp. are diurnal; while each has a specific diet, all are generally described as folivorous or frugo-folivorous [30,187,195]. We extracted DNA from samples of their glandular microbiomes, amplified and sequenced the v4 region of the 16S rRNA gene, assigned operational taxonomic units (OTUs) and report on the presence/absence, diversity and differential proportions of OTUs within and across species [30]. These analyses reveal that microbial communities in homologous genital secretions vary by species (figure 7e) and by sex (figure 7f), and microbial communities in male-specific secretions vary by type of gland and by male dominance status (figure 7g).
These broadly comparable patterns in chemical signals (figure 7a–d) and glandular microbial communities (figure 7e–g) are consistent with a bacterial contribution to signal design, and could help explain why mated pairs that have successfully reproduced ‘smell’ more alike than do co-dwelling pairs that have not yet reproduced [84]. Through intimate, long-term co-residency, sexual activity or infant care, certain individuals may more regularly exchange microbiota, eventually converging their microbial community composition, which could ultimately influence their chemical signatures [84]. The same process could occur on a larger scale to influence group microbial signatures [189,196,197] that link to group scent signatures [67,69]. Although we have yet to investigate these various condition-dependent components in a single species, differentiating glandular microbial communities [30] is the first step to relating glandular chemical signals to glandular microbes, as seen in humans [70] and other animals.
4. Summary and conclusion
The proposed sensory trade-off between vision and olfaction, coupled with the emphasis on asocial and delayed benefits to scent marking, contributed to views about primate chemical communication as a secondary or even latent mode of information transfer. To the extent that these historical perspectives do not accurately represent the most odour-reliant of primates—the strepsirrhines—so too may they cloud our understanding of the significance of chemical communication in humans.
The olfactory specialization and diversity of strepsirrhine primates make them excellent models for studies of chemical communication. By integrating methodologies (§2) and adopting a comparative framework (§3), we better appreciate the evolutionary history of chemical signals, their design complexity, the varied modes of dissemination, the wealth of information transferred and the range of functions served. Notably, we have learned that (i) chemical signals, whether excretory or secretory, have a long evolutionary history of gradual change, (ii) species differences in the composition of chemical signals can reveal evolutionary patterns in species diversification, and (iii) sociality may have selected for olfactory complexity in secretory signals (as it does for visual and auditory signals).
This chemical complexity includes condition-dependent information about both stable and flexible traits of the signaller that have many parallels in the human literature. More specifically, (iv) immutable traits that are signalled via scent include species, sex, odorant source, individual identity, kinship and genetic quality (via neutral heterozygosity and MHC-DRB diversity)—information that is salient for targeting intrasexual competition or nepotism, as well as for optimizing mate choice and avoiding inbreeding. Of particular note is that sex differences in glandular anatomy, chemical complexity and marking behaviour among female-dominant strepsirrhines are often reversed, suggesting sexual selection of female olfactory signals. These exceptional findings highlight the importance of the signaller–signal–receiver triad operating bi-directionally in both sexes, at least in the most social of species.
By contrast with stable signals, (v) flexible signals of the condition include information about variable states, such as health or physical condition (e.g. injury), which could likewise influence intraspecific competition, nepotism or mate choice. At an ultimate level, the dampening effect injury has on chemical signals may provide a benefit in terms of minimizing detection by predators, but at a proximate level, it suggests a cost of signal manufacture—one that cannot be born when the animal's condition is compromised. Other transient indicators reflect social status or hormonally mediated reproductive state. Whereas some reproductive indicators, namely those that vary seasonally or with ovarian cycles, likely serve as signals, others, such as pregnancy, fetal sex and hormonal contraception, might serve as cues. Whether evolutionarily selected or a byproduct of endocrinological processes, such chemical indicators may also be salient for intraspecific female–female competition and mate choice.
Lastly, (vi) patterns of microbial communities present in glandular secretions recapitulate both the stable and transient patterns in chemical composition within and between species, suggesting a role for fermentative bacteria in the derivation of VOCs. This relationship, directly relevant to human axillary odours, potentially extends to other human signals also.
Although there is more to learn about the derivation of signals and the role of individual chemical compounds, as well as the relative contributions of the MOB versus the AOB in signal reception and processing, a relevant ‘take-home’ message is that the chemical mixtures identified thus far need not function like pheromones for primate signals to be potent. The potentially misguided search for pheromones in humans [198] may well have been motivated by the early research of Michael & Keverne (e.g. [199]) on the behavioural effects seemingly induced by certain chemical compounds (i.e. ‘copulins’) expressed by female catarrhines. Nevertheless, failure to replicate these findings (e.g. [200]) led Michael's contemporaries to instead attribute the male primates' sexual responsiveness to the effects of associative learning, which is incompatible with the action of pheromones (reviewed in [6]). The continued search for human pheromones thus appears to be motivated more by work in other mammals (including rodents) than by research on primates. Together, recent findings and implications derived from integrative and comparative studies of strepsirrhines suggest a need for refining this broader comparative framework in re-evaluating primate chemical communication. Most notably, however, the uncharacteristic findings about the role of sociality, including intimacy and immediacy, in driving the complexity and functional range of chemical signals in lemurs, better position us to appreciate the critical role that complex odorant mixtures play in the social lives of humans and other primates.
Acknowledgements
I am deeply grateful to former and current laboratory members who conducted the main research described herein, notably M. Boulet, M. J. E. Charpentier, J. C. Crawford, J. delBarco-Trillo, L. K. Greene, K. E. Grogan, R. I. Harris, J. M. A. Petty, C. R. Sacha and E.S. Scordato, and to our collaborators, including G. R. Dubay and P. Silinski, who shared insights, provided training in GC-MS and allowed access to the Duke Chemistry Instrumentation Facility, and T. E. Goodwin and his students, B. A. Burkert, I. H. Harelimana, L. MacDonald and Z. Waldrip, who provided their time and expertise in SPDE/GC-MS. Without them, these projects would not have been feasible. I am also grateful to the laboratory members and collaborators who contributed to discussion, behavioural data and sample collection, anatomical study, video scoring and analyses, notably C. A. Adams, H. Biederman, S. L. Bornbusch, J. Camden, M. Canady, R. Canlas, T. Clarke, S. Cordato, S. Cork, L. Damiani, J. Dexheimer, K. Dimac-Stohl, C. Ditsoga, C. Fitzpatrick, S. R. Gorvetzian, N. Grebe, E. Harris, W. Kesler, S. A. Klager, I. G. Kulahci, B. Kwan, S. G. Kwatra, S. Leclaire, S. Mboumba, G. J. McGinnis, M. Reynolds, J. Rushmore, A. Sandel, A. Starling, A. Stonehill, K. N. Smyth, A. Weil and J. Wisse. I am equally indebted to the Duke Lemur Center (DLC) staff, particularly D. Brewer, S. Combes, E. Ehmke, K. Glenn, J. Hurley, J. Ives, A. Katz, B. Keith, J. Taylor, B. Schopler, C. Stoll, C. Welch, K. Welser, C. V. Williams and S. Zehr, for sharing insights and providing animal care, records, access and assistance; to D. Haring for sharing photographs and; to C. V. Williams and her collaborators R. E. Junge, K. L. Mahefarisoa, V. Mass, T. Rajaonarivelo and H. Rakotondrainibe, for enabling the work on wild animals near Moramanga, Madagascar. For enabling the work on wild animals at Beza Mahafaly Special Reserve, Madagascar, and sharing samples and long-term records, I thank M. L. Sauther and F. P. Cuozzo. Lastly, I thank M. Kwatra, A. Moseley and J. W. Thompson for sharing their expertise in proteomics; J. Pastorini and J. Peeler for providing DNA samples; M. O'Neil for access to lemur specimens; G. Ferrie for sharing information from the Species Survival Plan® for the AZA ring-tailed lemur population; H. Blaylock, M. Campbell, R. Evans, R. Shumaker, T. Roth and L. Villers, at the Indianapolis or Cincinnati Zoos, for providing assistance with and access to animals; and M. Blanco, J. Bollback, J. Clayton, J. W. Finch, C. De Foucault, S. E. Glickman, E. Guevara, A. Hayes, J. Horvath, J. Hurst, P. Klopfer, E. A. McKenney, J. Mercer, D. Nuttal, V. L. Roth, B. Schaal, H. Stapleton, M. Symonds, V. Thomas, R. van Horn, G. Wray and A. Yoder for valuable input over the years. It takes a village. This is DLC publication no. 1453.
Ethics
Our research protocols for captive animals (Protocol Registry nos. MO-4-10-2, A457-99-09, A245-03-07, A156-04-05, A232-06-07, A171-09-06, A102-10-04, A143-12-05 and A111-16-05) abided by the regulations of the United States Department of Agriculture and were approved by the Institutional Animal Care and Use Committee (IACUC) of Duke University. Our research adhered to the American Society of Primatologists principles for the ethical treatment of primates and was approved by the Research Committee of the DLC. The DLC is fully accredited by the American Association for the Accreditation of Laboratory Animal Care. Our research protocols for wild animals (Protocol Registry nos. A028–14-02 and A007-17-01) were also approved by Duke University's IACUC and by Madagascar's Ministère de l'Environnement, de l'Ecologie et des Forêts (MEEF/SG/DGF/DAPT/SCBT.Re permit nos. 197/13; 85/14; 68/15; 38/16; 83/17).
Data accessibility
This article does not contain any additional data.
Competing interests
I declare I have no competing interests.
Funding
External funding was provided by the following: National Science Foundation (NSF) research grants (nos. BCS-0409367, BCS-1749465, IOS-0719003 and IOS-1021633 to C.M.D.), with supplements via NSF Research Experience for Undergraduates (to C.M.D. for CRS, LKG and ESS); NSF DDRIG grants (nos. BCS-1341150, BCS-1232570 and BCS 1749898 to C.M.D. and JMAP, KEG and LKG, respectively); Margot Marsh Biodiversity Foundation grants (to JMAP, KEG, LKG and C.M.D.); Natural Sciences and Engineering Research Council Fellowship (to MB); Outgoing Marie Curie Fellowship (to MJEC); National Evolutionary Synthesis Center Visiting Scholar's Fellowship (NSF EF-0423641), UC Berkeley Chancellor's Fellowship, NSF Graduate Research Fellowship, and Research Council of Norway (to JCC); Spanish Ministry of Economy and Competitiveness (grant no. CGL2012-37423 and Ramón y Cajal fellowship RYC-2011-07943 to Jd-T); Hendrix Odyssey Program, NSF CRUI grant (no. 02–17062), and John and Laura Byrd (to TEG); DLC Director's Fund awards (to SLB, C.M.D., RLH and LKG). Internal funding sources include the following: Duke University Arts and Science Research Council awards (to C.M.D., LKG, SRG); Duke University Center for Science Education and Duke Primate Genomics Initiative (to KEG); Dean's Summer Fellowship (to LKG), Howard Hughes Biology Forum awards (to SGK, MR and ESS), DLC Molly H Glander awards (to JCC, SRG, LKG, SAK, SGK, CRS and ESS), and Duke University Undergraduate Research Support awards (to RC, JCC, SRG, LKG, SAK, CRS and ESS).
References
- 1.Wnuk E, Majid A. 2014. Revisiting the limits of language: the odor lexicon of Maniq. Cognition 131, 125–138. ( 10.1016/j.cognition.2013.12.008) [DOI] [PubMed] [Google Scholar]
- 2.Heritage S. 2014. Modeling olfactory bulb evolution through primate phylogeny. PLoS ONE 9, e113904 ( 10.1371/journal.pone.0113904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liman ER, Innan H. 2003. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc. Natl Acad. Sci. USA 100, 3328–3332. (doi:10.1073pnas.0636123100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilad Y, Wiebe V, Przeworski M, Lancet D, Pääbo S. 2004. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2, e5 ( 10.1371/journal.pbio.0020005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsui A, Go Y, Niimura Y. 2010. Degeneration of olfactory receptor gene repertories in primates: no direct link to full trichromatic vision. Mol. Biol. Evol. 27, 1192–1200. ( 10.1093/molbev/msq003) [DOI] [PubMed] [Google Scholar]
- 6.Drea CM. 2015. D'scent of man: a comparative survey of primate chemosignaling in relation to sex. Horm. Behav. 68, 117–133. ( 10.1016/j.yhbeh.2014.08.001) [DOI] [PubMed] [Google Scholar]
- 7.Smith TD, Siegel MI, Bhatnagar KP. 2001. Reappraisal of the vomeronasal system of catarrhine primates: ontogeny, morphology, functionality, and persisting questions. Anat. Rec. 265, 176–192. ( 10.1002/ar.1152) [DOI] [PubMed] [Google Scholar]
- 8.Charpentier MJ, Mboumba S, Ditsoga C, Drea CM. 2013. Nasopalatine ducts and flehmen behavior in the mandrill: reevaluating olfactory communication in Old World primates. Am. J. Primatol. 75, 703–714. ( 10.1002/ajp.22146) [DOI] [PubMed] [Google Scholar]
- 9.Laska M, Seibt A. 2002. Olfactory sensitivity for aliphatic esters in squirrel monkeys and pigtail macaques. Behav. Brain Res. 134, 165–174. ( 10.1016/S0166-4328(01)00464-8) [DOI] [PubMed] [Google Scholar]
- 10.Laska M, Genzel D, Wieser A. 2005. The number of functional olfactory receptor genes and the relative size of olfactory brain structures are poor predictors of olfactory discrimination performance with enantiomers. Chem. Senses 30, 171–175. ( 10.1093/chemse/bji013) [DOI] [PubMed] [Google Scholar]
- 11.Hill WCO. 1970. Primates, comparative anatomy and taxonomy, vol. 8, Cynopithecinae, Papio, Mandrillus, Theropithecus. Edinburgh, UK: Edinburgh University Press. [Google Scholar]
- 12.Geissmann T, Hulftegger AM. 1994. Olfactory communication in gibbons? In Current primatology, vol. 2: social development, learning and behaviour (eds Roeder JJ, Thierry B, Anderson JR, Herrenschmidt N), pp. 199–206. Strasbourg, France: Université Louis Pasteur. [Google Scholar]
- 13.Feistner AT. 1991. Scent marking in mandrills, Mandrillus sphinx. Folia Primatol. 57, 42–47. ( 10.1159/000156563) [DOI] [Google Scholar]
- 14.Setchell JM, Vaglio S, Moggi-Cecchi J, Boscaro F, Calamai L, Knapp LA. 2010. Chemical composition of scent-gland secretions in an Old World monkey (Mandrillus sphinx): influence of sex, male status, and individual identity. Chem. Senses 35, 205–220. ( 10.1093/chemse/bjp105) [DOI] [PubMed] [Google Scholar]
- 15.Klailova M, Lee PC. 2014. Wild western lowland gorillas signal selectively using odor. PLoS ONE 9, e99554 ( 10.1371/journal.pone.0099554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter RH, Moore JD. 1981. Human kin recognition by olfactory cues. Physiol. Behav. 27, 493–495. ( 10.1016/0031-9384(81)90337-1) [DOI] [PubMed] [Google Scholar]
- 17.Schaal B, Doucet S, Sagot P, Hertling E, Soussignan R. 2006. Human breast areolae as scent organs: morphological data and possible involvement in maternal-neonatal coadaptation. Dev. Psychobiol. 48, 100–110. ( 10.1002/dev.20122) [DOI] [PubMed] [Google Scholar]
- 18.Penn DJ, et al. 2007. Individual and gender fingerprints in human body odour. J. R. Soc. Interface 4, 331–340. ( 10.1098/rsif.2006.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lübke KT, Pause BM. 2015. Always follow your nose: the functional significance of social chemosignals in human reproduction and survival. Horm. Behav. 68, 134–144. ( 10.1016/j.yhbeh.2014.10.001) [DOI] [PubMed] [Google Scholar]
- 20.Schilling A. 1979. Olfactory communication in prosimians. In The study of prosimian behavior (eds Doyle GA, Martin RD), pp. 461–542. New York, NY: Academic Press. [Google Scholar]
- 21.Fleagle JG. 2013. Primate adaptation and evolution, third edition. New York, NY: Academic Press. [Google Scholar]
- 22.Hohenbrink P, Mundy NI, Zimmermann E, Radespiel U. 2013. First evidence for functional vomeronasal 2 receptor genes in primates. Biol. Lett. 9, 20121006 ( 10.1098/rsbl.2012.1006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.dos Reis MD, Gunnell GF, Barba-Montoya J, Wilkins A, Yang Z, Yoder AD. 2018. Using phylogenomic data to explore the effects of relaxed clocks and calibration strategies on divergence time estimation: primates as a test case. Syst. Biol. 67, 594–615. ( 10.1093/sysbio/syy001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maynard Smith J, Harper DGC. 1995. Animal signals: models and terminology. J. Theor. Biol. 177, 305–311. ( 10.1006/jtbi.1995.0248) [DOI] [Google Scholar]
- 25.Hasson O. 1997. Towards a general theory of biological signaling. J. Theor. Biol. 185, 139–156. ( 10.1006/jtbi.1996.0258) [DOI] [PubMed] [Google Scholar]
- 26.Ganzhorn JU, Wright PC, Ratsimbazafy J. 1999. Primate communities: Madagascar. In Primate communities (eds Fleagle JG, Janson CH, Reed K), pp. 75–89. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Vences M, Wollenberg KC, Vieites DR, Lees DC. 2009. Madagascar as a model region of species diversification. Trends Ecol. Evol. 24, 456–465. ( 10.1016/j.tree.2009.03.011) [DOI] [PubMed] [Google Scholar]
- 28.Mittermeier RA, et al. 2008. Lemur diversity in Madagascar. Int. J. Primatol. 29, 1607–1656. ( 10.1007/s10764-008-9317-y) [DOI] [Google Scholar]
- 29.Martin RD. 1972. Review lecture: adaptive radiation and behaviour of the Malagasy lemurs. Phil. Trans. R. Soc. Lond. B 264, 295–352. ( 10.1098/rstb.1972.0013) [DOI] [PubMed] [Google Scholar]
- 30.Greene LK, Bornbusch S, McKenney EA, Harris R, Gorvetzian SR, Yoder AD, Drea CM. 2019. The importance of scale in comparative microbiome research: new insights from the gut and glands of captive and wild lemurs. Am. J. Primatol. 81, e22974 ( 10.1002/ajp.22974) [DOI] [PubMed] [Google Scholar]
- 31.Stoddart DM. 1990. The scented ape: the biology and culture of human odour. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.delBarco-Trillo J, Harelimana IH, Goodwin TE, Drea CM. 2013. Chemical differences between voided and bladder urine in the aye-aye (Daubentonia madagascariensis): implications for olfactory communication studies. Am. J. Primatol. 75, 695–702. ( 10.1002/ajp.22083) [DOI] [PubMed] [Google Scholar]
- 33.Hagey LR, Fry BG, Fitch-Snyder H. 2007. Talking defensively, a dual use for the brachial gland exudate of slow and pygmy lorises. In Primate anti-predator strategies (eds Gursky SL, Nekaris KAI), pp. 253–272. New York, NY: Springer. [Google Scholar]
- 34.Nekaris KAI, Moore RS, Rode EJ, Fry BG. 2013. Mad, bad and dangerous to know: the biochemistry, ecology and evolution of slow loris venom. J. Venom. Anim. Toxins 19, 21 ( 10.1186/1678-9199-19-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolly A. 1966. Lemur behaviour: a Madagascar field study. Chicago, IL: University of Chicago Press. [Google Scholar]
- 36.Richard AF, Nicoll ME. 1987. Female social dominance and basal metabolism in a Malagasy primate, Propithecus verreauxi. Am. J. Primatol. 12, 309–314. ( 10.1002/ajp.1350120308) [DOI] [PubMed] [Google Scholar]
- 37.Petty JM, Drea CM. 2015. Female rule in lemurs is ancestral and hormonally mediated. Sci. Rep. 5, 9631 ( 10.1038/srep09631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grebe NM, Fitzpatrick C, Sharrock K, Starling A, Drea CM. 2019. Organizational and activational androgens, lemur social play, and the ontogeny of female dominance. Horm. Behav. 115, 104554 ( 10.1016/j.yhbeh.2019.07.002) [DOI] [PubMed] [Google Scholar]
- 39.Evans CS, Goy RW. 1968. Social behaviour and reproductive cycles in captive ring-tailed lemurs (Lemur catta). J. Zool. 156, 181–197. ( 10.1111/j.1469-7998.1968.tb05928.x) [DOI] [Google Scholar]
- 40.Dixson AF. 1976. Effects of testosterone on the sternal cutaneous glands and genitalia of the male greater galago (Galago crassicaudatus crassicaudatus). Folia Primatol. 26, 207–213. ( 10.1159/000155751) [DOI] [PubMed] [Google Scholar]
- 41.Bullard SC. 1984. Effects of testosterone upon the chest-rubbing behavior of Galago crassicaudatus umbrosus. Folia Primatol. 42, 70–75. ( 10.1159/000156145) [DOI] [PubMed] [Google Scholar]
- 42.Lewis RJ, van Schaik CP. 2007. Bimorphism in male Verreaux's sifaka in the Kirindy Forest of Madagascar. Int. J. Primatol. 28, 159–182. ( 10.1007/s10764-006-9107-3) [DOI] [Google Scholar]
- 43.Lewis RJ. 2009. Chest staining variation as a signal of testosterone levels in male Verreaux's sifaka. Physiol. Behav. 96, 586–592. ( 10.1016/j.physbeh.2008.12.020) [DOI] [PubMed] [Google Scholar]
- 44.Johnston RE. 2003. Chemical communication in rodents: from pheromones to individual recognition. J. Mammal. 84, 1141–1162. ( 10.1644/BLe-010) [DOI] [Google Scholar]
- 45.Wyatt TD. 2010. Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J. Comp. Physiol. A 196, 685–700. ( 10.1007/s00359-010-0564-y) [DOI] [PubMed] [Google Scholar]
- 46.Albone ES. 1984. Mammalian semiochemistry: the investigation of chemical signals between mammals. New York, NY: John Wiley & Sons. [Google Scholar]
- 47.Burger BV. 2005. Mammalian semiochemicals. Top. Curr. Chem. 240, 231–278. ( 10.1007/b98318) [DOI] [Google Scholar]
- 48.Drea CM, Boulet M, delBarco-Trillo J, Greene LK, Sacha CR, Goodwin TE, Dubay GR. 2013. The ‘secret’ in secretions: methodological considerations in deciphering primate olfactory communication. Am. J. Primatol. 75, 621–642. ( 10.1002/ajp.22143) [DOI] [PubMed] [Google Scholar]
- 49.Charpentier MJ, Barthes N, Proffit M, Bessière JM, Grison C. 2012. Critical thinking in the chemical ecology of mammalian communication: roadmap for future studies. Funct. Ecol. 26, 769–774. ( 10.1111/j.1365-2435.2012.01998.x) [DOI] [Google Scholar]
- 50.de Lacy Costello B, Amann A, Al-Kateb H, Flynn C, Filipiak W, Khalid T, Osborne D, Ratcliffe NM. 2014. A review of the volatiles from the healthy human body. J. Breath Res. 8, 014001 ( 10.1088/1752-7155/8/1/014001) [DOI] [PubMed] [Google Scholar]
- 51.McCune B, Grace JB. 2002. Analysis of ecological communities. Gleneden Beach, OR: MJM Software Design. [Google Scholar]
- 52.Charpentier MJE, Boulet M, Drea CM. 2008. Smelling right: the scent of male lemurs advertises genetic quality and relatedness. Mol. Ecol. 17, 3225–3233. ( 10.1111/j.1365-294X.2008.03831.x) [DOI] [PubMed] [Google Scholar]
- 53.Boulet M, Charpentier MJE, Drea CM. 2009. Decoding an olfactory mechanism of kin recognition and inbreeding avoidance in a primate. BMC Evol. Biol. 9, 281 ( 10.1186/1471-2148-9-281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dormont L, Bessière JM, Cohuet A. 2013. Human skin volatiles: a review. J. Chem. Ecol. 39, 569–578. ( 10.1007/s10886-013-0286-z) [DOI] [PubMed] [Google Scholar]
- 55.Kücklich M, Möller M, Marcillo A, Einspanier A, Weiß BM, Birkemeyer C, Widdig A. 2017. Different methods for volatile sampling in mammals. PLoS ONE 12, e0183440 ( 10.1371/journal.pone.0183440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.delBarco-Trillo J, Burkert BA, Goodwin TE, Drea CM. 2011. Night and day: the comparative study of strepsirrhine primates reveals socioecological and phylogenetic patterns in olfactory signals. J. Evol. Biol. 24, 82–98. ( 10.1111/j.1420-9101.2010.02145.x) [DOI] [PubMed] [Google Scholar]
- 57.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. 2001. Individual recognition in mice mediated by major urinary proteins. Nature 414, 631 ( 10.1038/414631a) [DOI] [PubMed] [Google Scholar]
- 58.Belcher AM, Epple G, Greenfield KL, Richards LE, Küderling I, Smith AB. 1990. Proteins: biologically relevant components of the scent marks of a primate (Saguinus fuscicollis). Chem. Senses 15, 431–446. ( 10.1093/chemse/15.4.431) [DOI] [Google Scholar]
- 59.Kwatra SG, Drea CM. 2007. Proteomic analysis of ringtailed lemur scent gland secretions: glandular- and individual-specific protein profiles. Am. J. Primatol. 69(Suppl. 1), 108–109. [Google Scholar]
- 60.Kuhn F, Natsch A. 2008. Body odour of monozygotic human twins: a common pattern of odorant carboxylic acids released by a bacterial aminoacylase from axilla secretions contributing to an inherited body odour type. J. R. Soc. Interface 6, 377–392. ( 10.1098/rsif.2008.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyse EA, Beauchamp GK, Yamazaki K. 1987. The genetics of body scent. Trends Genet. 3, 97–102. ( 10.1016/0168-9525(87)90192-2) [DOI] [Google Scholar]
- 62.Willse A, Belcher AM, Preti G, Wahl JH, Thresher M, Yang P, Yamazaki K, Beauchamp GK. 2005. Identification of major histocompatibility complex-regulated body odorants by statistical analysis of a comparative gas chromatography/mass spectrometry experiment. Anal. Chem. 77, 2348–2361. ( 10.1021/ac048711t) [DOI] [PubMed] [Google Scholar]
- 63.Piertney SB, Oliver MK. 2006. The evolutionary ecology of the major histocompatibility complex. Heredity 96, 7–21. ( 10.1038/sj.hdy.6800724) [DOI] [PubMed] [Google Scholar]
- 64.Penn D, Potts W. 1998. How do major histocompatibility complex genes influence odor and mating preferences. Adv. Immunol. 69, 411–435. [DOI] [PubMed] [Google Scholar]
- 65.Albone ES, Perry GC. 1976. Anal sac secretion of the red fox, Vulpes vulpes; volatile fatty acids and diamines: implications for a fermentation hypothesis of chemical recognition. J. Chem. Ecol. 2, 101–111. ( 10.1007/BF00988029) [DOI] [Google Scholar]
- 66.Albone ES, Elinton G, Walker JM, Ware GC. 1974. The anal sac secretion of the red fox (Vulpes vulpes); its chemistry and microbiology. A comparison with the anal sac secretion of the lion (Panthera leo). Life Sci. 14, 387–400. ( 10.1016/0024-3205(74)90069-1) [DOI] [PubMed] [Google Scholar]
- 67.Theis KR, Venkataraman A, Dycus JA, Koonter KD, Schmitt-Matzen EN, Wagner AP, Holekamp KE, Schmidt TM. 2013. Symbiotic bacteria appear to mediate hyena social odors. Proc. Natl Acad. Sci. USA 110, 19 832–19 837. ( 10.1073/pnas.1306477110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li D, et al. 2016. The musk chemical composition and microbiota of Chinese forest musk deer males. Sci. Rep. 6, 18975 ( 10.1038/srep18975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leclaire S, Jacob S, Greene LK, Dubay GR, Drea CM. 2017. Social odours covary with bacterial community in the anal secretions of wild meerkats. Sci. Rep. 7, 3240 ( 10.1038/s41598-017-03356-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Troccaz M, Gaïa N, Beccucci S, Schrenzel J, Cayeux I, Starkenmann C, Lazarevic V. 2015. Mapping axillary microbiota responsible for body odours using a culture-independent approach. Microbiome 3, 3 ( 10.1186/s40168-014-0064-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Citron CA, Rabe P, Dickschat JS. 2012. The scent of bacteria: headspace analysis for the discovery of natural products. J. Nat. Prod. 75, 1765–1776. ( 10.1021/np300468h) [DOI] [PubMed] [Google Scholar]
- 72.Crewe RM, Burger BV, Le Roux M, Katsir Z. 1979. Chemical constituents of the chest gland secretion of the thick-tailed galago (Galago crassicaudatus). J. Chem. Ecol. 5, 861–868. ( 10.1007/BF00986569) [DOI] [Google Scholar]
- 73.Schilling A, Perret M. 1987. Chemical signals and reproductive capacity in a male prosimian primate (Microcebus murinus). Chem. Senses 12, 143–158. ( 10.1093/chemse/12.1.143) [DOI] [Google Scholar]
- 74.Hayes RA, Morelli T, Wright P. 2006. Volatile components of lemur scent secretions vary throughout the year. Am. J. Primatol. 68, 1202–1207. ( 10.1002/ajp.20319) [DOI] [PubMed] [Google Scholar]
- 75.Scordato ES, Drea CM. 2007. Scents and sensibility: information content of olfactory signals in the ringtailed lemur, Lemur catta. Anim. Behav. 73, 301–314. ( 10.1016/j.anbehav.2006.08.006) [DOI] [Google Scholar]
- 76.Hayes RA, Morelli TL, Wright PC. 2004. Anogenital gland secretions of Lemur catta and Propithecus verreauxi coquereli: a preliminary chemical examination. Am. J. Primatol. 63, 49–62. ( 10.1002/ajp.20038) [DOI] [PubMed] [Google Scholar]
- 77.delBarco-Trillo J, Sacha CR, Dubay GR, Drea CM. 2012. Eulemur, me lemur: the evolution of scent-signal complexity in a primate clade. Phil. Trans. R. Soc. B 367, 1909–1922. ( 10.1098/rstb.2011.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schilling A. 1980. The possible role of urine in territoriality of some nocturnal prosimians. Symp. Zool. Soc. Lond. 45, 165–193. [Google Scholar]
- 79.Patel E. 2012. Acoustic and olfactory communication in eastern sifakas (Propithecus sp.) and rhesus macaques (Macaca mullata sic). Doctoral thesis, Cornell University; (https://hdl.handle.net/1813/29296). [Google Scholar]
- 80.Gould L, Overdorff DJ. 2002. Adult male scent-marking in Lemur catta and Eulemur fulvus rufus. Int. J. Primatol. 23, 575–586. ( 10.1023/A:1014921701106) [DOI] [Google Scholar]
- 81.Harrington JE. 1979. Responses of Lemur fulvus to scents of different subspecies of L. fulvus and to scents of different species of lemuriformes. Z. Tierpsychol. 49, 1–9. ( 10.1111/j.1439-0310.1979.tb00270.x) [DOI] [Google Scholar]
- 82.Pochron ST, Morelli TL, Scirbona J, Wright PC. 2005. Sex differences in scent marking in Propithecus edwardsi of Ranomafana National Park, Madagascar. Am. J. Primatol. 66, 97–110. ( 10.1002/ajp.20130) [DOI] [PubMed] [Google Scholar]
- 83.Pochron ST, et al. 2005. Patterns of male scent-marking in Propithecus edwardsi of Ranomafana National Park, Madagascar. Am. J. Primatol. 65, 103–115. ( 10.1002/ajp.20102) [DOI] [PubMed] [Google Scholar]
- 84.Greene LK, Drea CM. 2014. Love is in the air: sociality and pair bondedness influence sifaka reproductive signalling. Anim. Behav. 88, 147–156. ( 10.1016/j.anbehav.2013.11.019) [DOI] [Google Scholar]
- 85.Morelli T, Hayes RA, Nahrung H, Goodwin T, Harelimana I, MacDonald L, Wright P. 2013. Relatedness communicated in lemur scent. Naturwissenschaften 100, 769–777. ( 10.1007/s00114-013-1074-x) [DOI] [PubMed] [Google Scholar]
- 86.Scordato ES, Dubay G, Drea CM. 2007. Chemical composition of scent marks in the ringtailed lemur (Lemur catta): glandular differences, seasonal variation, and individual signatures. Chem. Senses 32, 493–504. ( 10.1093/chemse/bjm018) [DOI] [PubMed] [Google Scholar]
- 87.Lewis RJ. 2006. Scent marking in sifaka: no one function explains it all. Am. J. Primatol. 68, 622–636. ( 10.1002/ajp.20256) [DOI] [PubMed] [Google Scholar]
- 88.Drea CM, Scordato ES. 2008. Olfactory communication in the ringtailed lemur (Lemur catta): form and function of multimodal signals. In Chemical signals in vertebrates 11 (eds Hurst JL, Beynon RJ, Roberts SC, Wyatt T), pp. 91–102. New York, NY: Springer. [Google Scholar]
- 89.Kappeler PM. 1990. Social status and scent-marking behaviour in Lemur catta. Anim. Behav. 40, 774–776. ( 10.1016/S0003-3472(05)80706-7) [DOI] [Google Scholar]
- 90.Harrington JE. 1977. Discrimination between males and females by scent in Lemur fulvus. Anim. Behav. 25, 147–151. ( 10.1016/0003-3472(77)90077-X) [DOI] [PubMed] [Google Scholar]
- 91.Bailey K. 1978. Flehmen in the ring-tailed lemur (Lemur catta). Behaviour 65, 309–319. ( 10.1163/156853978X00666) [DOI] [Google Scholar]
- 92.Dugmore SJ, Bailey K, Evans CS. 1984. Discrimination by male ring-tailed lemurs (Lemur catta) between the scent marks of male and those of female conspecifics. Int. J. Primatol. 5, 235–245. ( 10.1007/BF02735759) [DOI] [Google Scholar]
- 93.Kappeler PM. 1998. To whom it may concern: the transmission and function of chemical signals in Lemur catta. Behav. Ecol. Sociobiol. 42, 411–421. ( 10.1007/s002650050455) [DOI] [Google Scholar]
- 94.Clark AB. 1982. Scent marks as social signals in Galago crassicaudatus I. Sex and reproductive status as factors in signals and responses. J. Chem. Ecol. 8, 1133–1151. ( 10.1007/BF00986984) [DOI] [PubMed] [Google Scholar]
- 95.Fisher HS, Swaisgood RR, Fitch-Snyder H. 2003. Countermarking by male pygmy lorises (Nycticebus pygmaeus): do females use odor cues to select mates with high competitive ability? Behav. Ecol. Sociobiol. 53, 123–130. ( 10.1007/s00265-002-0552-5) [DOI] [Google Scholar]
- 96.Palagi E, Dapporto L. 2006. Beyond odor discrimination: demonstrating individual recognition by scent in Lemur catta. Chem. Senses 31, 437–443. ( 10.1093/chemse/bjj048) [DOI] [PubMed] [Google Scholar]
- 97.Charles-Dominique P. 1977. Urine marking and territoriality in Galago alleni (Waterhouse, 1937 — Lorisoidea, Primates) — a field study by radio-telemetry. Z. Tierpsychol. 43, 113–138. ( 10.1111/j.1439-0310.1977.tb00063.x) [DOI] [PubMed] [Google Scholar]
- 98.Harrington JE. 1976. Discrimination between individuals by scent in Lemur fulvus. Anim. Behav. 24, 207–212. ( 10.1016/S0003-3472(76)80117-0) [DOI] [PubMed] [Google Scholar]
- 99.Kulahci IG, Drea CM, Rubenstein DI, Ghazanfar AA. 2014. Individual recognition through olfactory–auditory matching in lemurs. Proc. R. Soc. B 281, 20140071 ( 10.1098/rspb.2014.0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mertl AS. 1975. Discrimination of individuals by scent in a primate. Behav. Biol. 14, 505–509. ( 10.1016/S0091-6773(75)90684-7) [DOI] [PubMed] [Google Scholar]
- 101.Clark AB. 1982. Scent marks as social signals in Galago crassicaudatus II. Discrimination between individuals by scent. J. Chem. Ecol. 8, 1153–1165. ( 10.1007/BF0098698) [DOI] [PubMed] [Google Scholar]
- 102.Boulet M, Crawford J, Charpentier MJE, Drea CM. 2010. Honest olfactory ornamentation in a female-dominant primate. J. Evol. Biol. 23, 1558–1563. ( 10.1111/j.1420-9101.2010.02007.x) [DOI] [PubMed] [Google Scholar]
- 103.Charpentier MJE, Crawford JC, Boulet M, Drea CM. 2010. Message ‘scent’: lemurs detect the genetic relatedness and quality of conspecifics via olfactory cues. Anim. Behav. 80, 101–108. ( 10.1016/j.anbehav.2010.04.005) [DOI] [Google Scholar]
- 104.Grogan KE, Harris R, Boulet M, Drea CM. 2019. Genetic variation at MHC class II loci influences both olfactory signals and scent discrimination in ring-tailed lemurs. BMC Evol. Biol. 19, 171 ( 10.1186/s12862-019-1486-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Knapp LA, Robson J, Waterhouse JS. 2006. Olfactory signals and the MHC: a review and a case study in Lemur catta. Am. J. Primatol. 68, 568–584. ( 10.1002/ajp.20253) [DOI] [PubMed] [Google Scholar]
- 106.Greene LK, Grogan KE, Smyth KN, Adams CA, Klager SA, Drea CM. 2016. Mix it and fix it: functions of composite olfactory signals in ring-tailed lemurs. R. Soc. open sci. 3, 160076 ( 10.1098/rsos.160076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Norscia I, Antonacci D, Palagi E. 2009. Mating first, mating more: biological market fluctuation in a wild prosimian. PLoS ONE 4, e4679 ( 10.1371/journal.pone.0004679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fornasieri I, Roeder J-J. 1992. Marking behaviour in two lemur species (L. fulvus and L. macaco): relation to social status, reproduction, aggression and environmental change. Folia Primatol. 59, 137–148. ( 10.1159/000156651) [DOI] [PubMed] [Google Scholar]
- 109.Palagi E, Telara S, Tarli SB. 2003. Sniffing behavior in Lemur catta: seasonality, sex, and rank. Int. J. Primatol. 24, 335–350. ( 10.1023/A:1023001316988) [DOI] [Google Scholar]
- 110.Palagi E, Telara S, Tarli SB. 2004. Reproductive strategies in Lemur catta: balance among sending, receiving, and countermarking scent signals. Int. J. Primatol. 25, 1019–1031. ( 10.1023/B:IJOP.0000043349.23082.c0) [DOI] [Google Scholar]
- 111.Harris R, Boulet M, Grogan KE, Drea CM. 2018. Costs of injury for scent signalling in a strepsirrhine primate. Sci. Rep. 8, 9882 ( 10.1038/s41598-018-27322-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buesching CD, Heistermann M, Hodges JK, Zimmermann E. 1998. Multimodal oestrus advertisement in a small nocturnal prosimian, Microcebus murinus. Folia Primatol. 69(Suppl. 1), 295–308. ( 10.1159/000052718) [DOI] [Google Scholar]
- 113.Crawford JC, Drea CM. 2015. Baby on board: olfactory cues indicate pregnancy and fetal sex in a non-human primate. Biol. Lett. 11, 20140831 ( 10.1098/rsbl.2014.0831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crawford JC, Boulet M, Drea CM. 2011. Smelling wrong: hormonal contraception in lemurs alters critical female odour cues. Proc. R. Soc. B 278, 122–130. ( 10.1098/rspb.2010.1203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cuzuel V, Cognon G, Rivals I, Sauleau C, Heulard F, Thiébaut D, Vial J. 2017. Origin, analytical characterization, and use of human odor in forensics. J. Forensic Sci. 62, 330–350. ( 10.1111/1556-4029.13394) [DOI] [PubMed] [Google Scholar]
- 116.Preti G, Cutler WB, Christensen CM, Lawley H, Huggins GR, Garcia CR. 1987. Human axillary extracts: analysis of compounds from samples which influence menstrual timing. J. Chem. Ecol. 13, 717–731. ( 10.1007/BF01020154) [DOI] [PubMed] [Google Scholar]
- 117.Natsch A, Gfeller H, Gygax P, Schmid J, Acuna G. 2003. A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. J. Biol. Chem. 278, 5718–5727. ( 10.1074/jbc.M210142200) [DOI] [PubMed] [Google Scholar]
- 118.James AG, Austin CJ, Cox DS, Taylor D, Calvert R. 2013. Microbiological and biochemical origins of human axillary odour. FEMS Microbiol. Ecol. 83, 527–540. ( 10.1111/1574-6941.12054) [DOI] [PubMed] [Google Scholar]
- 119.Wysocki CJ, Preti G. 2004. Facts, fallacies, fears, and frustrations with human pheromones. Anat. Rec. A 281, 1201–1211. ( 10.1002/ar.a.20125) [DOI] [PubMed] [Google Scholar]
- 120.Huggins GR, Preti G. 1976. Volatile constituents of human vaginal secretions. Am. J. Obstet. Gynecol. 126, 129–136. ( 10.1016/0002-9378(76)90477-4) [DOI] [PubMed] [Google Scholar]
- 121.Watson SL, Ward JP, Davis KB, Stavisky RC. 1999. Scent-marking and cortisol response in the small-eared bushbaby (Otolemur garnettii). Physiol. Behav. 66, 695–699. ( 10.1016/S0031-9384(99)00005-0) [DOI] [PubMed] [Google Scholar]
- 122.Colquhoun IC. 2011. A review and interspecific comparison of nocturnal and cathemeral strepsirhine primate olfactory behavioural ecology. Int. J. Zool. 2011, 1–11. ( 10.1155/2011/362976) [DOI] [Google Scholar]
- 123.Dröscher I, Kappeler PM. 2014. Maintenance of familiarity and social bonding via communal latrine use in a solitary primate (Lepilemur leucopus). Behav. Ecol. Sociobiol. 68, 2043–2058. ( 10.1007/s00265-014-1810-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eppley TM, Ganzhorn JU, Donati G. 2016. Latrine behaviour as a multimodal communicatory signal station in wild lemurs: the case of Hapalemur meridionalis. Anim. Behav. 111, 57–67. ( 10.1016/j.anbehav.2015.10.012) [DOI] [Google Scholar]
- 125.Ganzhorn JU, Kappeler PM. 1996. Lemurs of the Kirindy forest. In Ecology and economy of a tropical dry forest in Madagascar (eds Ganzhorn JU, Sorg J-P), pp. 257–274. Göttingen, Germany: Erich Goltze GmbH and Co. [Google Scholar]
- 126.Irwin MT, Samonds KE, Raharison J-L, Wright PC. 2004. Lemur latrines: observations of latrine behavior in wild primates and possible ecological significance. J. Mammal. 85, 420–427. ( 10.1644/1383937) [DOI] [Google Scholar]
- 127.Rasoloharijaona S, Randrianambinina B, Joly-Radko M. 2010. Does nonnutritive tree gouging in a rainforest-dwelling lemur convey resource ownership as does loud calling in a dry forest-dwelling lemur? Am. J. Primatol. 72, 1062–1072. ( 10.1002/ajp.20865) [DOI] [PubMed] [Google Scholar]
- 128.Schilling A. 1974. A study of marking behaviour in Lemur catta. In Prosimian biology (eds Martin RD, Doyle GA, Walker AC), pp. 347–362. Pittsburgh, PA: University of Pittsburgh Press. [Google Scholar]
- 129.Mertl-Millhollen AS. 2006. Scent marking as resource defense by female Lemur catta. Am. J. Primatol. 68, 605–621. ( 10.1002/ajp.20255) [DOI] [PubMed] [Google Scholar]
- 130.Andrew RJ, Klopman RB. 1974. Urine-washing: comparative notes. In Prosimian biology (eds Martin RD, Doyle GA, Walker AC), pp. 303–312. Pittsburgh, PA: University of Pittsburgh Press. [Google Scholar]
- 131.Braune P, Schmidt S, Zimmermann E. 2005. Spacing and group coordination in a nocturnal primate, the golden brown mouse lemur (Microcebus ravelobensis): the role of olfactory and acoustic signals. Behav. Ecol. Sociobiol. 58, 587–596. ( 10.1007/s00265-005-0944-4) [DOI] [Google Scholar]
- 132.delBarco-Trillo J, Drea CM. 2014. Socioecological and phylogenetic patterns in the chemical signals of strepsirrhine primates. Anim. Behav. 97, 249–253. ( 10.1016/j.anbehav.2014.07.009) [DOI] [Google Scholar]
- 133.Palagi E, Norscia I. 2009. Multimodal signaling in wild Lemur catta: economic design and territorial function of urine marking. Am. J. Phys. Anthropol. 139, 182–192. ( 10.1002/ajpa.20971) [DOI] [PubMed] [Google Scholar]
- 134.Walker-Bolton AD, Parga JA. 2017. ‘Stink flirting’ in ring-tailed lemurs (Lemur catta): male olfactory displays to females as honest, costly signals. Am. J. Primatol. 79, e22724 ( 10.1002/ajp.22724) [DOI] [PubMed] [Google Scholar]
- 135.de Groot JH, Smeets MA, Kaldewaij A, Duijndam MJ, Semin GR. 2012. Chemosignals communicate human emotions. Psychol. Sci. 23, 1417–1424. ( 10.1177/0956797612445317) [DOI] [PubMed] [Google Scholar]
- 136.de Groot JH, Smeets MA, Rowson MJ, Bulsing PJ, Blonk CG, Wilkinson JE, Semin GR. 2015. A sniff of happiness. Psychol. Sci. 26, 684–700. ( 10.1177/0956797614566318) [DOI] [PubMed] [Google Scholar]
- 137.Frumin I, et al. 2015. A social chemosignaling function for human handshaking. Elife 4, e05154 ( 10.7554/eLife.05154.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hohenbrink P, Radespiel U, Mundy NI. 2012. Pervasive and ongoing positive selection in the vomeronasal-1 receptor (V1R) repertoire of mouse lemurs. Mol. Biol. Evol. 29, 3807–3816. ( 10.1093/molbev/mss188) [DOI] [PubMed] [Google Scholar]
- 139.Smith TD, Muchlinski MN, Bhatnagar KP, Durham EL, Bonar CJ, Burrows AM. 2015. The vomeronasal organ of Lemur catta. Am. J. Primatol. 77, 229–238. ( 10.1002/ajp.22326) [DOI] [PubMed] [Google Scholar]
- 140.Aujard F. 1997. Effect of vomeronasal organ removal on male socio-sexual responses to female in a prosimian primate (Microcebus murinus). Physiol. Behav. 62, 1003–1008. ( 10.1016/S0031-9384(97)00206-0) [DOI] [PubMed] [Google Scholar]
- 141.Lundström JN, Olsson MJ. 2010. Functional neuronal processing of human body odors. Vitam. Horm. 83, 1–23. ( 10.1016/S0083-6729(10)83001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pause BM, Krauel K. 2000. Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int. J. Psychophysiol. 36, 105–122. ( 10.1016/S0167-8760(99)00105-1) [DOI] [PubMed] [Google Scholar]
- 143.Prehn-Kristensen A, Wiesner C, Bergmann TO, Wolff S, Jansen O, Mehdorn HM, Ferstl R, Pause BM. 2009. Induction of empathy by the smell of anxiety. PLoS ONE 4, e5987 ( 10.1371/journal.pone.0005987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adipietro KA, Mainland JD, Matsunami H. 2012. Functional evolution of mammalian odorant receptors. PLoS Genet. 8, e1002821 ( 10.1371/journal.pgen.1002821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Semin GR, De Groot JH. 2013. The chemical bases of human sociality. Trends Cogn. Sci. 17, 427–429. ( 10.1016/j.tics.2013.05.008) [DOI] [PubMed] [Google Scholar]
- 146.Regenbogen C, Axelsson J, Lasselin J, Porada DK, Sundelin T, Peter MG, Lekander M, Lundström JN, Olsson MJ. 2017. Behavioral and neural correlates to multisensory detection of sick humans. Proc. Natl Acad. Sci. USA 114, 6400–6405. ( 10.1073/pnas.1617357114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Porter R, Winberg J. 1999. Unique salience of maternal breast odors for newborn infants. Neurosci. Biobehav. Rev. 23, 439–449. ( 10.1016/S0149-7634(98)00044-X) [DOI] [PubMed] [Google Scholar]
- 148.Schaal B, Montagner H, Hertling E, Bolzoni D, Moyse A, Quichon R. 1980. Les stimulations olfactives dans les relations entre l'enfant et la mère. Reprod. Nutr. Dévelop. 20(3B), 843–858. [PubMed] [Google Scholar]
- 149.Alvergne A, Lummaa V. 2010. Does the contraceptive pill alter mate choice in humans? Trends Ecol. Evol. 25, 171–179. ( 10.1016/j.tree.2009.08.003) [DOI] [PubMed] [Google Scholar]
- 150.Havlíček J, Roberts SC. 2009. MHC-correlated mate choice in humans: a review. Psychoneuroendocrinology 34, 497–512. ( 10.1016/j.psyneuen.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 151.Endler J. 1993. Some general comments on the evolution and design of animal communication systems. Phil. Trans. R. Soc. Lond. B 340, 215–225. ( 10.1098/rstb.1993.0060) [DOI] [PubMed] [Google Scholar]
- 152.Curran AM, Prada PA, Furton KG. 2010. The differentiation of the volatile organic signatures of individuals through SPME-GC/MS of characteristic human scent compounds. J. Forensic Sci. 55, 50–57. ( 10.1111/j.1556-4029.2009.01236.x) [DOI] [PubMed] [Google Scholar]
- 153.Olsson MJ, et al. 2014. The scent of disease: human body odor contains an early chemosensory cue of sickness. Psychol. Sci. 25, 817–823. ( 10.1177/0956797613515681) [DOI] [PubMed] [Google Scholar]
- 154.Wallace P. 1977. Individual discrimination of humans by odor. Physiol. Behav. 19, 577–579. ( 10.1016/0031-9384(77)90238-4) [DOI] [PubMed] [Google Scholar]
- 155.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 156.Gonzalez G, Sorci G, Møller AP, Ninni P, Haussy C, De Lope F. 1999. Immunocompetence and condition-dependent sexual advertisement in male house sparrows (Passer domesticus). J. Anim. Ecol. 68, 1225–1234. ( 10.1046/j.1365-2656.1999.00364.x) [DOI] [Google Scholar]
- 157.Bakker TC, Künzler R, Mazzi D. 1999. Sexual selection: condition-related mate choice in sticklebacks. Nature 401, 234 ( 10.1038/45727) [DOI] [Google Scholar]
- 158.Hamilton WD, Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387. ( 10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 159.Møller AP, Petrie M. 2002. Condition dependence, multiple sexual signals, and immunocompetence in peacocks. Behav. Ecol. 13, 248–253. ( 10.1093/beheco/13.2.248) [DOI] [Google Scholar]
- 160.Scheuber H, Jacot A, Brinkhof MW. 2003. Condition dependence of a multicomponent sexual signal in the field cricket Gryllus campestris. Anim. Behav. 65, 721–727. ( 10.1006/anbe.2003.2083) [DOI] [Google Scholar]
- 161.Penn D, Potts WK. 1998. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 13, 391–395. ( 10.1016/S0169-5347(98)01473-6) [DOI] [PubMed] [Google Scholar]
- 162.Junge RE, Williams CV, Rakotondrainibe H, Mahefarisoa KL, Rajaonarivelo T, Faulkner C, Mass V. 2017. Baseline health and nutrition evaluation of two sympatric nocturnal lemur species (Avahi laniger and Lepilemur mustelinus) residing near an active mine site at Ambatovy, Madagascar. J. Zoo Wildlife Med. 48, 794–803. ( 10.1638/2016-0261.1) [DOI] [PubMed] [Google Scholar]
- 163.Novotny MV, Soini HA. 2008. Volatile mammalian chemosignals: structural and quantitative aspects. In Chemical signals in vertebrates 11 (eds Hurst JL, Beynon RJ, Roberts SC, Wyatt TD), pp. 13–23. New York, NY: Springer. [Google Scholar]
- 164.van Schaik CP, Kappeler PM. 1996. The social systems of gregarious lemurs: lack of convergence with anthropoids due to evolutionary disequilibrium? Ethology 102, 915–941. ( 10.1111/j.1439-0310.1996.tb01171.x) [DOI] [Google Scholar]
- 165.Griffin RH, Matthews LJ, Nunn CL. 2012. Evolutionary disequilibrium and activity period in primates: a Bayesian phylogenetic approach. Am. J. Phys. Anthropol. 147, 409–416. ( 10.1002/ajpa.22008) [DOI] [PubMed] [Google Scholar]
- 166.Drea CM, Goodwin TE, delBarco-Trillo J. 2019. P-mail: the information highway of nocturnal, but not diurnal or cathemeral, strepsirrhines. Folia Primatol. 90, 422–438. ( 10.1159/000495076) [DOI] [PubMed] [Google Scholar]
- 167.Kappeler PM. 1993. Variation in social structure: the effects of sex and kinship on social interactions in three lemur species. Ethology 93, 125–145. ( 10.1111/j.1439-0310.1993.tb00984.x) [DOI] [Google Scholar]
- 168.Pereira ME, Kaufman R, Kappeler PM, Overdorff DJ. 1990. Female dominance does not characterize all of the Lemuridae. Folia Primatol. 55, 96–103. ( 10.1159/000156505) [DOI] [PubMed] [Google Scholar]
- 169.Drea CM. 2007. Sex and seasonal differences in aggression and steroid secretion in Lemur catta: are socially dominant females hormonally ‘masculinized’? Horm. Behav. 51, 555–567. ( 10.1016/j.yhbeh.2007.02.006) [DOI] [PubMed] [Google Scholar]
- 170.Drea CM. 2011. Endocrine correlates of pregnancy in the ring-tailed lemur (Lemur catta): implications for the masculinization of daughters. Horm. Behav. 59, 417–427. ( 10.1016/j.yhbeh.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 171.Drea CM, Weil A. 2008. External genital morphology of the ring-tailed lemur (Lemur catta): females are naturally ‘masculinized’. J. Morphol. 269, 451–463. ( 10.1002/jmor.10594) [DOI] [PubMed] [Google Scholar]
- 172.Montagna W, Yun JS. 1962. The skin of primates. X. The skin of the ring-tailed lemur (Lemur catta). Am. J. Phys. Anthropol. 20, 95–117. ( 10.1002/ajpa.1330200211) [DOI] [PubMed] [Google Scholar]
- 173.Grogan KE, McGinnis GJ, Sauther ML, Cuozzo FP, Drea CM. 2016. Next-generation genotyping of hypervariable loci in many individuals of a non-model species: technical and theoretical implications. BMC Genomics 17, 204 ( 10.1186/s12864-016-2503-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Grogan KE, Sauther ML, Cuozzo FP, Drea CM. 2017. Genetic wealth, population health: major histocompatibility complex variation in captive and wild ring-tailed lemurs (Lemur catta). Ecol. Evol. 7, 7638–7649. ( 10.1002/ece3.3317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ober C, Weitkamp LR, Cox N, Dytch H, Kostyu D, Elias S. 1997. HLA and mate choice in humans. Am. J. Hum. Genet. 61, 497–504. ( 10.1017/S0003480097006532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wedekind C, Seebeck T, Bettens F, Paepke AJ. 1995. MHC-dependent mate preferences in humans. Proc. R. Soc. Lond. B 260, 245–249. ( 10.1098/rspb.1995.0087) [DOI] [PubMed] [Google Scholar]
- 177.Winternitz J, Abbate JL, Huchard E, Havlíček J, Garamszegi LZ. 2016. Patterns of MHC-dependent mate selection in humans and non-human primates: a meta-analysis. Mol. Ecol. 26, 668–688. ( 10.1111/mec.13920) [DOI] [PubMed] [Google Scholar]
- 178.Kim KH, Jahan SA, Kabir E. 2012. A review of breath analysis for diagnosis of human health. Trends Analyt. Chem. 33, 1–8. ( 10.1016/j.trac.2011.09.013) [DOI] [Google Scholar]
- 179.Shirasu M, Touhara K. 2011. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J. Biochem. 150, 257–266. ( 10.1093/jb/mvr090) [DOI] [PubMed] [Google Scholar]
- 180.Allen C, Cobey KD, Havlíček J, Singleton FP, Hahn AC, Moran CN, Roberts SC. 2019. Preparation for fatherhood: a role for olfactory communication during human pregnancy? Physiol. Behav. 206, 175–180. ( 10.1016/j.physbeh.2019.03.030) [DOI] [PubMed] [Google Scholar]
- 181.Vaglio S, Minicozzi P, Bonometti E, Mello G, Chiarelli B. 2009. Volatile signals during pregnancy: a possible chemical basis for mother–infant recognition. J. Chem. Ecol. 35, 131–139. ( 10.1007/s10886-008-9573-5) [DOI] [PubMed] [Google Scholar]
- 182.Kuukasjärvi S, Eriksson CJP, Koskela E, Mappes T, Nissinen K, Rantala MJ. 2004. Attractiveness of women's body odors over the menstrual cycle: the role of oral contraceptives and receiver sex. Behav. Ecol. 15, 579–584. ( 10.1093/beheco/arh050) [DOI] [Google Scholar]
- 183.Miller G, Tybur JM, Jordan BD. 2007. Ovulatory cycle effects on tip earnings by lap dancers: economic evidence for human estrus? Evol. Hum. Behav. 28, 375–381. ( 10.1016/j.evolhumbehav.2007.06.002) [DOI] [Google Scholar]
- 184.Roberts SC, Gosling LM, Carter V, Petrie M. 2008. MHC-correlated odour preferences in humans and the use of oral contraceptives. Proc. R. Soc. B 275, 2715–2722. ( 10.1098/rspb.2008.0825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bennett G, et al. 2016. Host age, social group, and habitat type influence the gut microbiota of wild ring-tailed lemurs (Lemur catta). Am J. Primatol. 78, 883–892. ( 10.1002/ajp.22555) [DOI] [PubMed] [Google Scholar]
- 186.Greene LK, McKenney EA, O'Connell TM, Drea CM. 2018. The critical role of dietary foliage in maintaining the gut microbiome and metabolome of folivorous sifakas. Sci. Rep. 8, 14482 ( 10.1038/s41598-018-32759-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Greene LK, Clayton JB, Rothman RS, Semel BP, Semel MA, Gillespie TR, Wright PC, Drea CM. 2019. Local habitat, not phylogenetic relatedness, predicts gut microbiota better within folivorous than frugivorous lemur lineages. Biol. Lett. 15, 20190028 ( 10.1098/rsbl.2019.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McKenney EA, Rodrigo A, Yoder AD. 2015. Patterns of gut bacterial colonization in three primate species. PLoS ONE 10, e0124618 ( 10.1371/journal.pone.0124618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Perofsky AC, Lewis RJ, Abondano LA, Di Fiore A, Meyers LA. 2017. Hierarchical social networks shape gut microbial composition in wild Verreaux's sifaka. Proc. R. Soc. B 284, 20172274 ( 10.1098/rspb.2017.2274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Council SE, Savage AM, Urban JM, Ehlers ME, Skene JP, Platt ML, Dunn RR, Horvath JE. 2016. Diversity and evolution of the primate skin microbiome. Proc. R. Soc. B 283, 20152586 ( 10.1098/rspb.2015.2586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Miller EA, Livermore JA, Alberts SC, Tung J, Archie EA. 2017. Ovarian cycling and reproductive state shape the vaginal microbiota in wild baboons. Microbiome 5, 8 ( 10.1186/s40168-017-0228-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Uchihashi M, Bergin IL, Bassis CM, Hashway SA, Chai D, Bell JD. 2015. Influence of age, reproductive cycling status, and menstruation on the vaginal microbiome in baboons (Papio anubis). Am. J. Primatol. 77, 563–578. ( 10.1002/ajp.22378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yildirim S, Yeoman CJ, Janga SC, Thomas SM, Ho M, Leigh SR, Primate Microbiome Consortium, White BA, Wilson BA, Stumpf RM. 2014. Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. ISME J. 8, 2431 ( 10.1038/ismej.2014.90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Norscia I, Borgognini-Tarli S. 2008. Ranging behavior and possible correlates of pair-living in southeastern avahis (Madagascar). Int. J. Primatol. 29, 153–171. ( 10.1007/s10764-007-9219-4) [DOI] [Google Scholar]
- 195.Irwin MT. 2006. Ecologically enigmatic lemurs: the sifakas of the eastern forests (Propithecus candidus, P. diadema, P. edwardsi, P. perrieri, and P. tattersalli). In Lemurs: ecology and adaptation (eds Gould L, Sauther M), pp. 305–326. Boston, MA: Springer. [Google Scholar]
- 196.Theis KR, Schmidt TM, Holekamp KE. 2012. Evidence for a bacterial mechanism for group-specific social odors among hyenas. Sci. Rep. 2, 615 ( 10.1038/srep00615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Leclaire S, Nielsen JF, Drea CM. 2014. Bacterial communities in meerkat anal scent secretions vary with host sex, age, and group membership. Behav. Ecol. 25, 996–1004. ( 10.1093/beheco/aru074) [DOI] [Google Scholar]
- 198.Wyatt TD. 2015. The search for human pheromones: the lost decades and the necessity of returning to first principles. Proc. R. Soc. B 282, 20142994 ( 10.1098/rspb.2014.2994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Michael RP, Keverne EB. 1968. Pheromones in the communication of sexual status in primates. Nature 218, 746–749. ( 10.1038/218746a0) [DOI] [PubMed] [Google Scholar]
- 200.Goldfoot DA, Kravetz MA, Goy RW, Freeman SK. 1976. Lack of effect of vaginal lavages and aliphatic acids on ejaculatory responses in rhesus monkeys: behavioral and chemical analyses. Horm. Behav. 7, 1–27. ( 10.1016/0018-506X(76)90001-5) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any additional data.