Abstract
Across diverse lineages, animals communicate using chemosignals, but only humans communicate about chemical signals. Many studies have observed that compared with other sensory modalities, communication about smells is relatively rare and not always reliable. Recent cross-cultural studies, on the other hand, suggest some communities are more olfactorily oriented than previously supposed. Nevertheless, across the globe a general trend emerges where olfactory communication is relatively hard. We suggest here that this is in part because olfactory representations are different in kind: they have a low degree of embodiment, and are not easily expressed as primitives, thereby limiting the mental manipulations that can be performed with them. New exploratory data from Dutch children (9–12 year-olds) and adults support that mental imagery from olfaction is weak in comparison with vision and audition, and critically this is not affected by language development. Specifically, while visual and auditory imagery becomes more vivid with age, olfactory imagery shows no such development. This is consistent with the idea that olfactory representations are different in kind from representations from the other senses.
This article is part of the Theo Murphy meeting issue ‘Olfactory communication in humans’.
Keywords: language, mental imagery, olfaction, cross-cultural, developmental, embodiment
1. Introduction
The body mediates between the world and the mind. It also serves as a way to conceptualize and communicate about aspects of the world. Take terms that refer to body parts—face, back and hand. These terms pick out parts of our own bodies; they also structure our understanding of other entities—from spatial locations to emotions. Face is used to refer to a part of my head, but also to locate an entity (e.g. The car faces the building), and a way to talk about emotions (e.g. She didn't want to lose face). At every instance, from infancy to adulthood, the body is the first line of information used in thinking and reasoning about the world outside it. Could body odours also play such a role in human cognition?
The renowned Australianist Nick Evans has noted that for many indigenous Australians there is a recurrent emphasis that the smell of sweat conveys the essence of person [1]. Traditionally, it was customary for indigenous people to seek permission to use resources or enter land and sea from custodians of that land. A person's identity and right to be on that land could be recognized in part by a person's scent. In order for an individual to travel safely through land that was not his customary territory, the traveller might be anointed by a custodian's sweat. That is, the smell of sweat communicates between individuals; it is also recognized by the land. For the Dalabon—one such indigenous Australian people—the importance of this custom is reflected in their language. The term ngenbun means: ‘(custodian) put their sweat on a new person to protect them as they enter the former's country' [2]. Communication is happening through chemosignals and about chemosignals.
The example cited above seems to bely the widely touted claim that the language of smell is impoverished [3–5]. Olfactory notions can, in fact, be lexicalized in sophisticated ways that reflect how people in distinct niches have come to use and think about odours. Compare the Dalabon with the Kapsiki of Cameroon [6]. In this society, when a person dies, it is customary for the blacksmiths (who are also undertakers) to dress the corpse in clothes and other accoutrement, and then dance with the corpse for days while the body putrefies in the tropical heat. On the first day, the close kinsfolk begin the ritual mourning that the blacksmiths have prepared, on the second the whole village joins, and by the third day, the surrounding villages also perform mourning dances with the corpse. This funereal ritual is important so that everyone who knew the deceased can witness for themselves that the person is dead; and for the deceased to see all those they knew when alive (for other odorous rituals at death, see [7]). While the blacksmiths do not think the smell of the putrefying corpse is worth mentioning, non-blacksmiths think it the most vile of odours, and refer to ‘the smell of putrefaction, the smell of a corpse' with the term ndalèke. This term is one of 14 in this language that refers to various sorts of culturally relevant odours [6].
Worldwide, distinct communities show an orientation to bodily smells—sweat, urine, faeces, menstrual blood, foul breath, foot and genital odours [8,9]. In Cantonese, for example, jyun1 refers to an extreme stink, which includes as prototypical exemplars strong farts, athlete's foot and corpse; suk1 can indicate sweat odours; and ngaat3 urine odour [10]. For the hunter–gatherer !Xóõ of Botswana, terminology abounds for genital odours—e.g. unpleasant genital odours (e.g. |nuɁā and !gáɁba), unwashed vagina smell (gūhɁu), semen smell (!ɢūã)—urine smells, i.e. ‘regular' (ǁgúɁa) versus ‘stale and pungent' (góhɁlo) and excrement smells (e.g. |gkxɁáa and |gàhɁa) [11]. Chemical communication is not restricted to bodily odours, however. Different languages have developed lexicons to communicate about odours that are of relevance to their cultural and ecological niche [12,13].
While diverse cultures have been documented each with its own rituals, social practices and linguistic expressions for odours, only hunter–gatherer communities to date have been shown to communicate about smells with the same efficiency as visual entities under experimental conditions [14–16]. In one study, the hunter–gatherer Semaq Beri were compared with their closely related neighbours the swidden-horticulturalist Semelai [16]. Both live in the tropical rainforest of the Malay Peninsula, and speak closely related languages, but differ in subsistence and concomitant cultural practices. When tested with standardized colours (visual stimuli) and odours (delivered via sniffin' sticks, marker pens filled with an odorant [17]), the non-hunter–gatherer Semelai showed a marked asymmetry, with high agreement for how they talked about colours, but low agreement for odours. By contrast, the hunter–gatherer Semaq Beri demonstrated equal performance for colours and odours. In a fully enculturated and embodied context, such as exemplified with the Dalabon and Kapsiki examples, the odour is part of a multimodal package. Myriad situational cues scaffold the odour concept. But when presented in isolation, as in the experimental paradigm described here, it seems challenging for most people to name odours. In various empirical studies across diverse communities, people find it harder to name odours than shapes, colours, sounds, textures or tastes [14], and when comparing everyday language use, olfactory talk is infrequent in comparison with the other senses [18–20]. There are notable exceptions, of course, like the Semaq Beri and others [15,20]—and we come back to these in the general discussion—but there is also a general trend that calls for explanation. Why is communication about smells difficult?
Various proposals have been put forward [5]. According to some, odour naming is hard because of the underlying neural architecture: either olfactory areas of the brain are too weakly connected to language areas [21], or too directly connected [22], or the cortical resources that process odours and language hamper one another [23]. Here, we propose a different line of argumentation, and suggest odour naming is hard partially because of the nature of the underlying representations (see [24] for a similar conclusion but for different reasons). Namely, compared with other perceptual modalities odour representations are more weakly represented because:
-
(1)
there are differences in the embodiment of the olfactory sense compared with other senses (i.e. the capacity to literally take advantage of the information provided by the body, as well as the ability to recreate sensory information with the body or secondarily with tools);
-
(2)
there are differences in access to phenomenal sensory primitives (i.e. abstract and concrete descriptors that can be used to communicate sensory stimuli both to others and to oneself).
If correct, then mental imagery emerges as one interesting avenue to explore these ideas, specifically to test the nature of the underlying (olfactory) representations. In this opinion paper, we expand on these theoretical ideas and present a first exploratory study that develops some of these themes. Critically, there is a reciprocity between mental imagery and language where mental imagery shapes as well as is shaped by language, but to what extent and how depends largely on the language and sensory modality [25,26]. Specifically, we propose that differential affordances between the senses based on the availability of (1) and (2) above—i.e. degree of embodiment and availability of sensory primitives—lead to an asymmetry between the senses. That is, olfactory representations, and by extension olfactory imagery, should be weaker than imagery of the other senses owing to differences in the opportunities to train olfactory-specific simulation across the lifespan. To elaborate on this argumentation, we first present some necessary background to mental imagery. We then exemplify the differences in the embodied and conceptual representations of sensory modalities that we believe impact mental imagery. Finally, we provide empirical evidence for this asymmetry by demonstrating that language development does not shape odour imagery as would be predicated if imagery were only dependent on generic language functions.
2. Mental imagery of odours
Numerous studies report that olfactory imagery is the least vivid of all senses, followed by touch and taste sensations, with visual and auditory imagery being the most vivid [27–30]. Conversely, only a very small portion of people are unable to conjure visual images [31], but many more report they cannot simulate olfactory sensations at will [27,28]. Parallel cross-cultural data on imagery are lacking, but one study found that even though there was some evidence for the cross-cultural malleability of imagery, olfactory images were nevertheless systematically rated as the least vivid in different communities [32]. This suggests that odour representations are generally more elusive than their visual or auditory counterparts. For the last five decades, a substantial body of research has targeted the mechanisms underlying mental imagery across sensory modalities [33–35]. So it is surprising that only a fraction of these studies have focused on why differences between modalities emerge in the first place, and why odour images are so much harder to conjure.
One thing that makes odour images particularly difficult to conjure is that odour perception is inherently multimodal in nature, as alluded to earlier. It depends on context [36], interacts with other senses [37,38], including the trigeminal system [39], and is critical to flavour perception [40]. Even if it was possible to remove all of these complexities, and ask people to imagine monomolecular odours they had recently smelled, the vividness of those images pales in comparison with imagery of other sensory modalities. It has been proposed that limited neocortical resources make conscious processing of mental representations of odours difficult, if not impossible [41].
At the same time, others have noted that mental imagery is possible, but that the differences between odour imagery and imagery for the other senses may lie in the underlying relationships between perception and cognition. Imagery appears to be partially dependent on modality-specific working memory systems [42,43]. This is problematic for the olfactory image [28] because—in contrast to vision [44], audition [42] and somatosensation [45]—working memory capacity for odours is limited, and as a consequence appears to recruit language [46–48]. Studies have shown a correlation between olfactory imagery and proficiency in naming odours [49], with odours that are easy to name being easier to imagine, and vice versa [50]. While this evidence is usually taken to imply that odour imagery relies on language, this seems at odds with the corollary that odour naming is difficult in the first place. Instead, it is likely that some third factor underlies both ease of odour imagery and odour naming, namely how deeply entrenched the underlying odour representation is in the first place.
Consistent with this, olfactory experts (such as perfumers), who are much better at odour imagery, undergo considerable perceptual training and show both cortical and functional changes [51,52]. Critically, the cortical changes seem to be restricted to primary and secondary olfactory areas, but not areas involved in semantic processing. In fact, the functional brain architecture during odour imagery in expert perfumers suggests a negative association between the level of odour expertise and involvement of semantic memory networks [52]. In a similar vein, it has been shown that for people with acquired anosmia the duration of olfactory loss affects brain activity during odour imagery, with longer periods of being without the sense of smell being associated with increased activation in areas underlying episodic memory [53].
3. Embodiment of the mental image
Embodied theories state that mental imagery cannot be studied as an internal process alone, but that it—like real perception—must be considered in a framework that involves the body and goal-directed sensory-motor actions within specific environments [34,54,55]. However, the importance of the literal physicality of the body in providing both reference stimuli and volitional sensory feedback during the process of mental imagery has not been fully appreciated. We believe that this notion is important for understanding the observed asymmetry between the senses. Importantly we show this asymmetry—grounded initially in the body and then extending outside of it—limits the possibilities for rehearsing odour representations.
(a). Degrees of embodiment
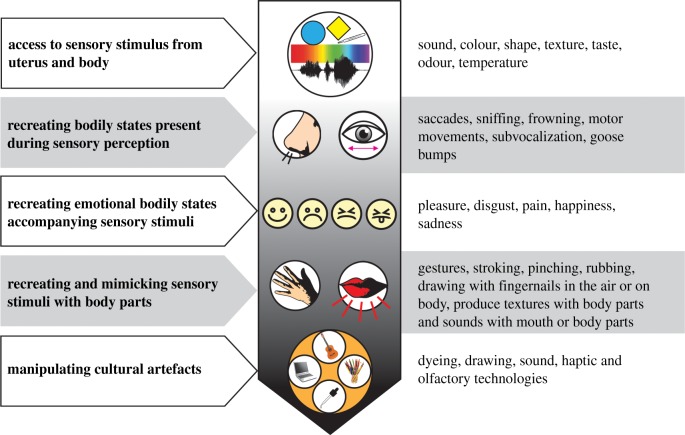
At its most basic form the only perceptual information present to a human at all times is the one provided by the body—the shapes, colours, sounds, textures, temperatures, tastes and smells that the body has at any given moment. In utero, the fetus is a passive recipient of stimuli from the external world, notably from the chemical senses via the mother's ingestion [56], and through audition particularly language and music [57]. In infancy, the child begins to develop the capacity to voluntarily control and manipulate its sensory input, but the affordances available to do that for each modality are not equivalent. With its body alone, the infant can produce different sounds, and view and touch different parts of its body. Differential input provides opportunity to distinguish, compare, and contrast the corresponding sensory stimulus; to practise and consolidate the underlying representations. But the opportunity for controlled, differential chemosensory input from the body is limited. This means that the opportunities to consolidate smell and taste representations from the body alone are also curtailed. Differential opportunities to manipulate sensory stimuli continue into adulthood, with many more technologies enabling visual and acoustic manipulation than olfactory (figure 1).
Figure 1.
Degrees of embodiment of sensory stimuli. From top to bottom of the figure, there is increasing embodiment. Weak embodiment is exemplified by the re-creation of bodily states present during perception (e.g. sniffing). Strong embodiment, by contrast, involves direct simulation of the stimulus by the body—with or without tools. For example, it is possible to mimic visual objects by gestures, drawing in the air or literally on the skin by scratching with the fingernails or by using cultural artefacts like a pencil. It is also relatively easy to recreate auditory information using the vocal cords or by using other body parts to mimic a sound, or to create touch sensations, like pain, temperature and texture by pinching, stroking or rubbing.
We propose that for senses that have strong embodiment (e.g. vision and audition) there will be a direct connection between a sensory image and the body. To put it another way, the image should to some extent be possible to reproduce with the body both directly (e.g. mimicking sounds) and indirectly (e.g. creating sounds with instruments). The important notion is not that this process should result in a perfect reconstruction of the primary sensory stimulus in the real world, but simply that there is the possibility to reproduce it. The possibility is the key.
Conversely, for weak embodiment, there is no direct connection between an image and the body. The only link is indirect and related to other factors (i.e. states) that could be present during actual perception of the sensory stimuli (e.g. eye-saccades for vision [58] or sniffing for odour perception [59,60]), or the simulation of bodily emotional states (e.g. goose bumps or facial expression). Whereas weak embodiment results in fewer opportunities to train the mental image, high embodiment allows frequent, and also incidental opportunities for practice of imagery. So, for olfaction, early chemosensory communication between the fetus and the mother in combination with post-birth continuous exposure to one's own body odour and self-sampling [61] will provide the first reference stimulus that can be used for volitional olfactory imagery.
(b). Phenomenal sensory primitives in odour imagery and directed communication
Our second argument comes not from the asymmetry in embodiment, but from the asymmetry in the mental tools available to think about the various senses. Mental imagery can in many regards be considered a pure act of memory, where perceptual information is retrieved from long-term memory and manipulated with the help of working memory [33]; but mental imagery is also used to imagine things never before experienced [62]. For the latter, the creation of a mental image does not rely on a specific episodic memory, but can be derived from the manipulation of sensory ‘primitives'. For vision and audition, this feat is relatively straight-forward. A visual entity can be decomposed into many phenomenal dimensions (e.g. shape, colour and texture) each with its own set of dimensions. On this, the physical and phenomenological dimensions should not be confused (e.g. light wavelength versus colour category), and although they can correlate, this is not necessary [63,64]. For vision and audition, the phenomenal sensory primitives can be used to decompose and reassemble holistic images from a specific semantic category (e.g. the sight and sound of a dog), and they can also be used to create new complex sounds and images with no specific referent in memory.
What about odours? They do not seem to lend themselves to the same decomposition [65]. The quest for odour primitives has long been intimately connected to the development and categorization of semantic descriptors for odours [66–69]. These descriptors, often mapped onto odour wheels, contain both abstract (e.g. pungent) and concrete (e.g. fish) terms [66,70]. Odour terms like fish or orange can help a person access a ‘fish' or ‘orange' odour from memory (although see [71]), and enable mental mixtures of the two, even if the mixture is perceptually novel [72]. However, it is harder to envisage how a person who has never smelled a fish or an orange odour could simulate them. What phenomenal sensory primitives from the odour wheel would a fish or orange odour be decomposed into? An alternative approach to the odour wheel is provided by Yeshurun & Sobel [73], who propose that the dimensionality of odours should be constrained to its pleasantness. Here, the mental imagery of a specific smell is simply the mental imagery of the odour's hedonic valence.
In visual imagery, an understanding of shape primitives such as those given by geons [74] (e.g. circles, cones and cylinders) is sufficient to enable mental simulation of a wide range of shapes and objects. Importantly, the geons could be used to communicate shapes to a person who has no prior knowledge about a specific novel object. Is the olfactory equivalent of geons—‘odons' (i.e. odour primitives)—possible? Perhaps not. Neither odour wheels, nor the pleasantness of an odour can be considered geon-like. If we turn to the natural vocabularies in languages other than English, even for those with dedicated olfactory lexicons, the odour terms are typically complex packages of information that do not readily lend themselves to an analysis in terms of odons. More generally, as odour perception is inherently multimodal in nature, and depends on context as well as on the interaction with other senses, an odon-like structure seems unlikely.
Figure 2 provides the reader with examples to illustrate some ways in which visual, but not odour images, can be readily communicated. The first example is a thought experiment that can be used to illustrate the qualitative differences between the modalities. Read steps 1–7 below before looking at the last picture in the sequence in panel figure 2a.
1. Imagine a white two-dimensional surface.
2. Imagine a rectangle on this surface so that the longest sides are parallel to the horizontal plane.
3. Next, imagine two identical circles each with a diameter one-fourth the length of the long-side of the rectangle. Put these two circles on the top horizontal line of the rectangle so they do not touch each other. The circles should be in contact with, but not overlap the rectangle.
4. Next, imagine a new rectangle half the length and width of the first rectangle. Place this rectangle directly underneath the first one, so it is centred with respect to it, with its long side on the horizontal plane touching, but not overlapping, the big rectangle's length along the bottom.
5. The final image should have two circles on the top, a big rectangle in the middle, and a small rectangle at the bottom.
6. What do you see?
7. Turn it 180°, what do you see now?
Figure 2.
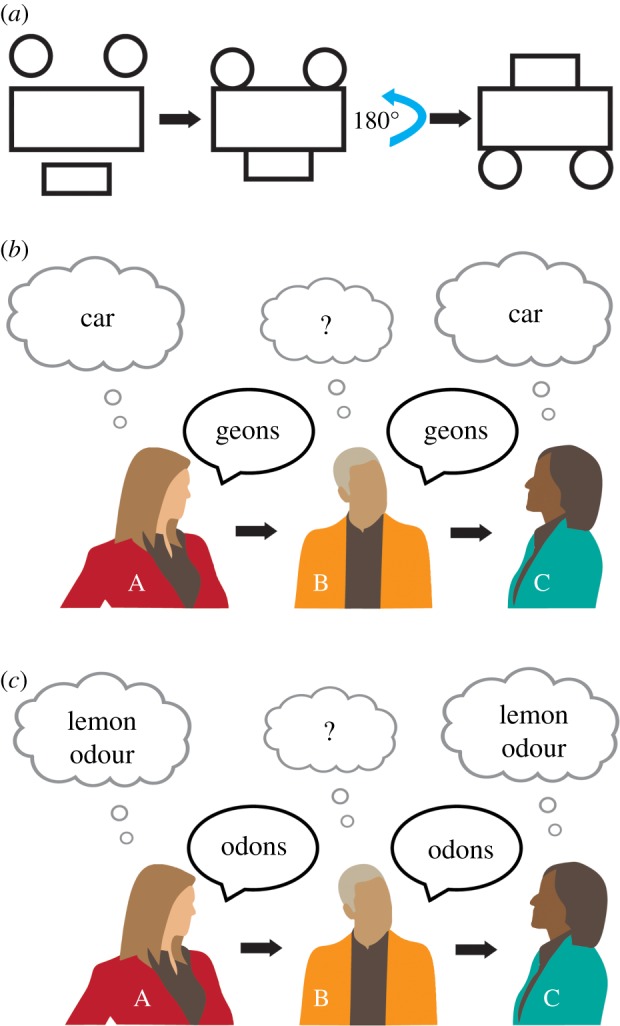
Directed communication and simulation. (a) Illustration of how the manipulation of primitives (in this case rotation) can shift an abstract mental image into a representation of a semantic object (e.g. when this thought exercise is run on undergraduate students informally in class by the first author, the majority of students imagine a car only after rotation). (b) In this scenario, person A has semantic knowledge of cars (i.e. including perceptual facts, ideas and beliefs about cars). Person A uses her knowledge about the visual representation of cars to decompose a car into visual primitives, such as geons, and expresses these and their spatial relationships to person B. Importantly, person B has knowledge only of the primitives, but no semantic knowledge of cars. However, person B can still simulate features of the object and communicate it to person C who has the appropriate primitives and semantic knowledge and thus can simulate and recreate the original object. (c) Can equivalent communication be envisaged for olfaction? If so what kind of odons would be required to do so? We believe this type of task would be difficult to conduct with olfaction.
4. Language development does not shape olfactory sensory simulation
The combination of low embodiment and little access to sensory primitives, taken together, give rise to few opportunities to train the olfactory image. By contrast, the visual and auditory modalities lend themselves to multiple—volitional and incidental—opportunities to further reify the underlying representations. This predicts that visual and auditory modalities, for example, would give rise to stronger mental imagery than olfaction, as has been found previously. It is not clear what implications this has for development exactly—particularly with respect to olfaction, which is our main focus here—so we conducted an exploratory study to unpack this further. To the best of our knowledge, there are currently no data on how the vividness of simulated sensory stimuli change as a function of biological and cognitive development. Here, we took a first step towards redressing this gap, and compared children's sensory imagery with that of adults.
If some form of odour imagery is possible in childhood, it could be linked to perceptual training, but not necessarily involve language or semantic memory networks. One could alternatively argue that the construction of an odour image is initially dependent on the ability to verbalize odours (to access it from memory), but with proficiency the olfactory image gets less dependent on semantic feedback [27]. Both perspectives would predict that olfactory imagery becomes more vivid over development, although not necessarily linearly. Alternatively, there is a fundamental asymmetry in the senses—as outlined above—which means the odour image is consistently weak across development. As a first step towards addressing these questions, we tested children in middle-childhood between the ages of 9 and 12 years. In this age range, children are still acquiring language, and doing so at a remarkable rate. Average 9 year-olds have a vocabulary of around 11 000 words, by 11 they know 20 000 words, and by the time they graduate from high school, they will have adult-like mastery of around 60 000 words [75,76]. This suggests that middle-childhood is a good time window to study the development of imagery. In line with earlier results, we predicted that for adults, vision and audition would give rise to more vivid imagery, and smell the least vivid imagery. In this exploratory study, we ask whether the same pattern holds for children, and whether there are any clear developmental trajectories in imagery abilities, particularly for smell.
5. Sensory imagery in children and adults
(a). Method
(i). Participants
Sixty-one adult participants (46 women and 15 men) were tested, age range 18–52 (mean = 22.39) at Radboud University, Nijmegen and were recruited through the university SONA system (a participant database) on a voluntary basis. In addition, we tested 9 year-olds (n = 38; 21 girls and 17 boys), 10 year-olds (n = 52; 29 girls and 23 boys), 11 year-olds (n = 72; 41 girls and 31 boys), and 12 year-olds (n = 27; 11 girls, 16 boys) from three local schools with comparable socio-economic background near to Nijmegen, whose parents and teachers consented to their participating (see electronic supplementary material, table S1.1 for age distributions). This convenience sample meant there were unequal age groups. All participants were native Dutch speakers. Adult participants were paid for their participation, while children received a certificate and stickers.
(ii). Materials
We adapted the short version of the Plymouth Sensory Imagery Questionnaire (Psi-Q) [77] for use in our study, which was conducted in Dutch. The original questionnaire contained 21 items in English tapping vision, sound, touch, taste,1 smell, bodily sensations and emotion; but we focused on the first five perceptual modalities only. The final questionnaire consisted of 15 items, three targeting each perceptual modality, as in the short form of the Psi-Q [77]: vision (a bonfire, a sunset, a cat climbing a tree); sound (the sound of a car horn, hands clapping in applause, an ambulance siren); touch (fur, warm sand, a soft towel); taste (black pepper, lemon, mustard); smell (newly cut grass, burning wood, a rose). The original questionnaire was translated into Dutch and back-translated to verify its closeness to the original text. Note, while it is typical to distinguish ‘taste' from ‘flavour' in scientific terminology, the Dutch term smaak encompasses both, and we do not distinguish them within this experiment. The imagery questionnaire was compiled in two different orders: people were asked to provide imagery ratings for vision, sound, smell, taste and touch in one questionnaire; and vision, touch, taste, smell and sound in the other. Questionnaire order was counterbalanced across subjects. To simplify for use with children, we reduced the Likert scale from the original 11-point scale to a 5-point scale.
The questionnaire began with a practice example asking participants to imagine seeing a banana, and asked them to indicate on a 5-point scale how clear the image was with 1 being the lowest rating and 5 the highest. With this first example, there was also accompanying text to help clarify the scale: (1) I cannot form an image of the item clearly, I can only think about it; (2) I can form the image of the item vaguely; (3) I can form the image of the item a little bit; (4) I can form the image of the item pretty clearly; and (5) I can form the image of the item clearly, as vivid as in real life. Also accompanying this first practice example was a picture of a banana under each number on the scale, depicting the banana: under (5) the banana was a clear sharp picture, which faded in steps under numbers (4)–(2) until there was an empty box under (1). Participants were asked to indicate how clear a banana was for them under the corresponding number on the scale. For the experimental items, participants were only given the numerical scale ranging from 1 to 5 with no accompanying text or pictorial aid.
(iii). Procedure
Adult participants were tested individually and given a printed questionnaire which they filled in themselves in a self-paced manner. Children were tested in small groups. The experimenter first explained the questionnaire to them. Children were then handed individual questionnaires to fill in by themselves; in addition, individual questions were read out by the experimenter to aid comprehension. Children were encouraged to ask questions if anything was unclear. Additional experimenters were on hand to answer individual questions.
(b). Results
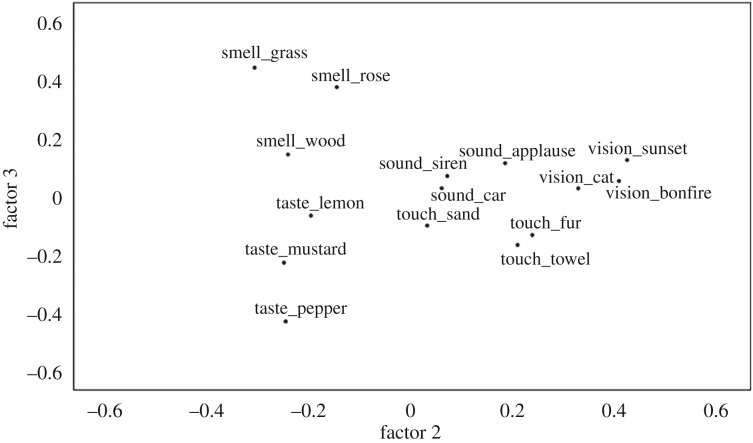
Overall, internal consistency for the 15 questionnaire items was high (Cronbach's α = 0.811); the sampling adequacy was good (KMO = 0.822), with anti-image correlations in the range 0.736–0.886. A factor analysis using maximum-likelihood extraction identified four factors with an eigenvalue higher than 1 and which captured 54.6% of the cumulative variance; goodness of fit test: p = 0.023. All items loaded positively on the first factor, indicating a single factor of general imagery vividness. Factor 2 distinguishes vision from sound and touch, and then smell and taste; factor 3 distinguishes smell from taste (figure 3; electronic supplementary material, table S1.2 and figure S1.1 for all factors and factor loadings).
Figure 3.
Factor analysis of imagery items. A plot of the second and third factors extracted from a factor analysis with maximum-likelihood extraction. Factor 2 distinguishes items loading on vision, touch and sound, and then smell and taste. Factor 3 distinguishes most sharply between smell and taste items.
In order to compare the ability to imagine each sensory modality across development, we calculated an average score per modality based on an individual's responses to items. Altogether there were 34 missing data points: one 12 year-old did not provide a response for imagining the sight of the sunset, one adult did not respond to any touch item, and 18 children and adults did not provide a response for the taste of pepper and another 12 for the taste of mustard. Where there was either no response or only a single value for a modality, we imputed the mean of the group for that individual. A 5 (modality: vision, sound, touch, taste, smell) by 5 (age: 9 year-olds, 10 year-olds, 11 year-olds, 12 year-olds and adult) ANOVA, with modality as a within-participant factor and age group as a between-participant factor, was performed. There was a main effect of modality (F4,980 = 74.38, p < 0.001, and no effect of age (F4,245 = 1.32, p = 0.26, but there was a significant interaction between modality and age (F16,980 = 2.46, p = 0.001, ), indicating that some sensory modalities give rise to more vivid imagery with age, but others do not (figure 4; electronic supplementary material, table S1.3).
Figure 4.
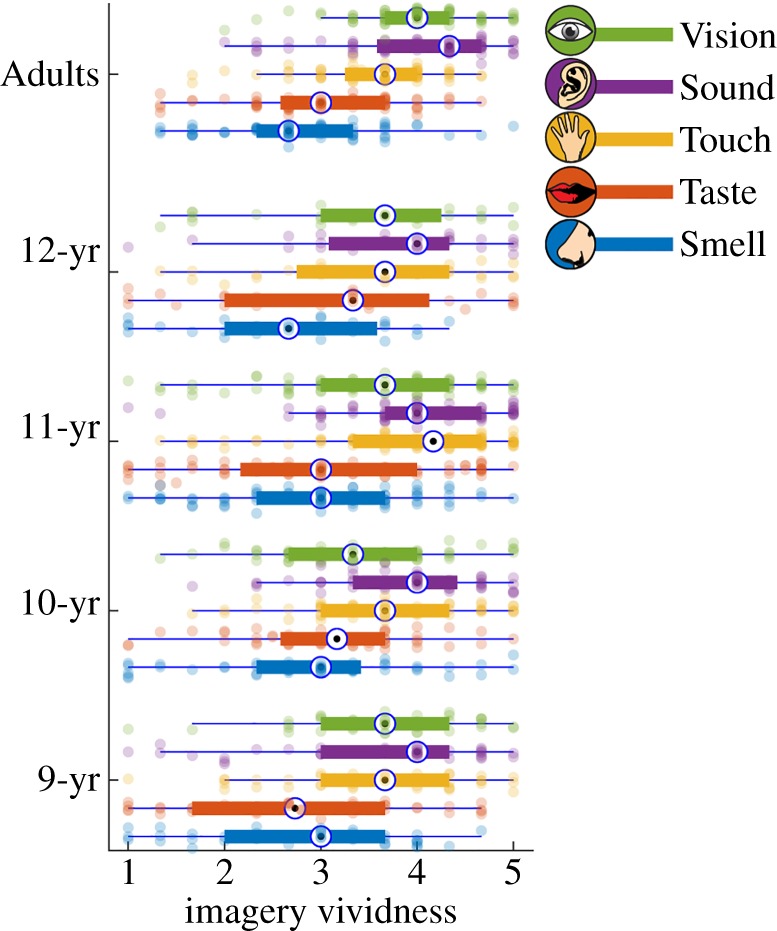
Vividness of mental imagery across age groups, with 1 indicating 'cannot form an image' and 5 indicating 'can form an image as vivid as real life'. Boxes indicate the lower quartile (the left horizontal line), median (bullet circle) and upper quartile (the right horizontal line). Left whiskers indicate the maximum value of the variable located within a distance of 1.5 times the inter-quartile range below the 25th percentile; right whiskers indicate the corresponding distance to the 75th percentile value.
Comparing the relative ease of imaging each of the sense modalities, we see a consistent pattern—the chemical senses are the most difficult to imagine. Using pairwise comparisons with least significant difference adjustment for each age group, we see no difference in adults between vision and sound (p = 0.96), but people reported visual images as more vivid than all the other modalities, i.e. touch (p < 0.001), taste (p < 0.001) and smell (p < 0.001). In turn, sound was also easier to imagine than touch (p < 0.001), taste (p < 0.001) and smell (p < 0.001). Touch was easier than taste (p = 0.001), and smell (p < 0.001), whereas there was no difference in imagery for smell and taste (p = 0.15). For the children, we see a very similar pattern for smell and taste, which are always the least vividly imagined, and never differ from one another. There was some more variation in how vividly the other senses were imagined at each age, with vision, sound and touch vying for the top spot.
For 12 year-olds, there was no difference between vision and sound (p = 0.07), touch (p = 0.60), or taste (p = 0.08), but visual images were more vivid than smell (p < 0.001). Sound imagery was no more vivid than touch (p = 0.19), but was more vivid than taste (p < 0.001) and smell (p < 0.001). Touch was more vivid than taste (p = 0.018) and smell (p < 0.001), but smell and taste did not differ significantly (p = 0.07).
For 11 year-olds, visual imagery was less vivid than sound (p < 0.001) and touch (p < 0.001), but like the adults visual images were more vivid than taste (p < 0.001) and smell (p < 0.001). Sound imagery was no more vivid than touch (p = 0.48), but was more vivid than taste (p < 0.001) and smell (p < 0.001). Touch was more vivid than taste (p < 0.001) and smell (p < 0.001), and smell and taste did not differ (p = 0.24).
For 10 year-olds, visual imagery was also less vivid than sound (p < 0.001) and touch (p < 0.05), not different from taste (p = 0.08), but more vivid than smell (p < 0.004). Sound imagery was no more vivid than touch (p = 0.20), but was more vivid than taste (p < 0.001) and smell (p < 0.001). Touch was more vivid than taste (p < 0.001) and smell (p < 0.001), and smell and taste did not differ (p = 0.24).
For 9 year-olds, there was no difference in imagery between vision and sound (p = 0.40) or touch (p = 0.51), but visual images were more vivid than taste (p < 0.001) and smell (p < 0.001). Sound imagery was no more vivid than touch (p = 0.86), but was more vivid than taste (p < 0.001), and smell (p < 0.001). Touch was also more vivid than taste (p < 0.001) and smell (p < 0.001), and smell and taste did not differ significantly (p = 0.96).
If we look, instead, at each modality separately, we see there is no difference between children and adults in imagery for smell (F4,245 = 0.95, p = 0.44, ) or taste (F4,245 = 0.51, p = 0.73,); there was a marginal effect of touch (F4,245 = 2.34, p = 0.056, ); and clear evidence of a developmental change for vision (F4,245 = 4.01, p = 0.004, ) and sound (F4,245 = 2.49, p = 0.04, ). Pairwise comparisons with least significant difference adjustment showed that 10, 11 and 12 year-olds had less vivid visual imagery than adults (all p < 0.003), with 9 year-olds not differing significantly (p = 0.077). For sound, only 9 year-olds differed and had less vivid imagery than adults, (p = 0.01), and for touch, only 11 year-olds differed significantly from adults (p = 0.01). We did not have a hypothesis about gender differences in imagery across age groups but this information can be found in electronic supplementary material, table S1.4.
Overall, then, smell and taste imagery remains equally poor for adults and children, whereas within the same age-range, we see evidence of a developmental change, with more vivid imagery for sight and hearing especially coming into adulthood.
6. Discussion
Our data show that odour and taste images are the least vivid sensory images during development and importantly do not change from middle-childhood. This is noteworthy, because imagery in other modalities (e.g. vision, sound) does appear to change in the same time window. This tells us two things. First, differences in perceptual experience (middle-childhood to adulthood) do not seem to matter for the vividness of odour images. Second, the changes in cognitive capacity that emerge through development, specifically in relation to language acquisition [75,76] and working memory [78], also do not seem to significantly impact olfactory imagery. This is important because, as we discussed earlier, all three (perceptual experience, language acquisition and working memory) have been implicated in ease of odour imagery in adults.
We conclude that opportunities to practise olfactory imagery early in life are more limited than for visual or auditory imagery. One reason for this developmental asymmetry in training may lie in the asymmetry between sensory modalities in degrees of embodiment and sensory primitives, as outlined above. At this moment, we do not know which one of these two factors is the most critical, and whether they work singly or in combination to affect practice opportunities. It is likely that it is a combination. For future work, studying the use of sensory primitives in mental imagery by children is likely to be challenging, but the study of embodied practices is more tractable. For example, representational gestures such as object- and event-related hand movements depicting referents start as early as 12 months of age [79,80], and the possibility of sound imitation is present at birth [81] (e.g. during auditory–oral matching, when the infant reproduces mouth movements necessary to produce sounds it has just heard). At an early stage, then, children are able to use volitional sensory mimicry (e.g. sound) as sensory feedback (i.e. perceptual training) to build up the to-be-imaged stimulus and to facilitate recollection of a specific sensory stimulus from long-term memory [82]. Olfaction does not lend itself to the same embodied possibilities. While in some cultures we see that bodily odours can be expressed in language, the body itself does not lend itself to the manipulation of these signals in the same way as for visual or auditory signals. One could speculate that imagery of body odours is different from that of other odours, since children may be able to manipulate body odours to some extent. Although, to the best of our knowledge there are no data on mental imagery of body odours, this special issue presents novel data that humans regularly sniff themselves to sample their own odour [61]. This fascinating observation could suggest that body odours are different in kind. Much like body parts—such as hands—are used to conceptualize and communicate about the world, people may unconsciously use body odours to anchor and organize their olfactory cognition, including odour imagery, memory and naming. Importantly our data suggest that mere sensory exposure is not enough to enhance simulation; something else is required. We propose that cases where olfactory exposure significantly affects imagery likely occur only occur after deliberate practice, as found with adult professionals (e.g. perfumers, but see [83]); although it has to be said that, even in adult professionals with extensive training, mental imagery for olfaction remains relatively poor [52,65,84].
(a). Limitations
The present study is exploratory and as such the empirical findings ought to be replicated in a larger sample and in different populations. The reported statistics were not corrected for multiple comparisons, which may lead to type I errors, and so should be treated with appropriate caution. This is likely, for example, in the apparent significantly weaker visual than sound imagery for 10 and 11 year-olds, where we do not find differences between these two modalities for 9 and 12 year-olds or adults. This apparent difference is not grounded in any developmentally plausible theory. In addition, children were tested in groups, while adults were tested individually. It is possible this led to weaker imagery ratings from children, although the results showed no main effect of age on vividness of imagery, only a modality by age interaction. It seems unlikely that group testing would specifically have given rise to weaker imagery for the chemical senses, as we find in this study, but further testing is required to rule this out. As another consideration, the use of a visual example as the practice trial could have biased our data. We applied this approach because using a picture with vivid versus faded depictions of a banana was a practical and a concrete way to illustrate what was meant by imagery vividness for young children. However, we do not believe this fact can account for the pattern of data we see in our study because visual imagery was not the most vivid for children across the board. Finally, we believe it is unlikely that potential differences in olfactory threshold between children and adults underlie these results. Although studies have found prepubescent children are less sensitive than adults for some types of odours, particularly sweaty and musk-like odorants (e.g. androstenone, pentadecanolide and oxahexadecanolide) [85–87], for most odours relevant for our study, i.e. associated with common odours (e.g. eugenol; PEA, rose odour; R-(+)-carvone, chewing-gum odour), the weight of evidence suggests no age difference [88–92], with one study even showing that children are more sensitive than adults to fishy odour (i.e. trimethylamine) [93]. More importantly, even extreme changes in odour capacity—such as that observed in anosmics—have only moderate effects on subjective vividness ratings of odour imagery [53]. With all these caveats in mind, we believe that our data nevertheless are clear in demonstrating that in all groups smell and taste give rise to less vivid imagery than vision and sound.
(b). Future directions
The evidence presented here comes from a Western society with no real cultural or linguistic elaboration in the olfactory domain. It remains an open question as to whether communities with developed cultural practices and linguistic resources in this domain would show the same patterns in imagery. Though previous evidence suggests that olfactory imagery is difficult across cultures [32], no targeted comparison with established olfactorily oriented cultures has been conducted as of yet. It is possible, for example, that in hunter–gatherer communities, the use, interaction and embodiment of odours in everyday life shapes olfactory cognition deeply, even at a representational level. For example, the fact that some cultures monitor and manipulate odours to avert sickness suggests a level of conscious awareness beyond what is displayed by lay people in the West. Similarly, we saw that many communities have developed lexicons for smells emitted by different parts of the body. If this is something children are being enculturated to early in life, perhaps it deepens the degree of embodiment.
One could question whether the low imagery vividness for odours is due to the choice of items used. This seems unlikely. To the best of our knowledge, odour imagery has systematically been rated as the least vivid perceptual modality across all published studies comparing sensory modalities, even in those where the same object (e.g. wine) is rated for its visual versus olfactory appearance [94]. With that said, future studies could use items that have been matched for familiarity across modalities and participant groups (e.g. children and adults). It should also be noted that although questionnaires, such as the Psi-Q, are without question the most common way to assess imagery, this approach is far from ideal as it is subjective. Importantly, questionnaires, and especially those that use labels to elicit an odour image, are problematic as they cannot separate the odorant from the odour [95]. They also miss other complexities that separate olfaction from other senses such as vision. For example, whereas visual phenomena have been analysed as being more objective, olfaction is said to be constrained, and not processed as an autonomous stimulus but as highly dependent on the experiential context [96]. Even if there are better ways to assess odour imagery, such as comparing imagined and real odour mixtures [72], these approaches are unfortunately too complex for children. However, using more objective measures of odour imagery might capture developmental aspects that are otherwise hidden. One way to target this could be to use imagery-triggered salivation with a naturalistic multimodal procedure commonly employed in consumer research, for example, pictures of food in odour imagery and non-imagery trials [97]. These are questions for the future.
In conclusion, whatever the underlying neural architecture [21–23,41], differences in the degree of embodiment and underlying sensory primitives impact mental imagery, and possibly, indirectly, odour communication.
Supplementary Material
Acknowledgements
We thank Josje de Valk, Ewelina Wnuk, John Huisman, Ilja Croijmans and Ilona Plug for help with data collection; Jan van Baren-Nawrocka and Sanne Dekker at WKRU, the project leaders of ‘Scientific breakthroughs in the classroom'; and the schools and teachers for making data collection possible—in particular, Katinka van Kempen, Marleen Nieuwhart, Maartje Poos, Rachel de Hair, Petra Laport, Kim van Zeben and Wilma Nefkens.
Endnote
Dutch does not make a linguistic distinction between taste and flavour. Both are referred to with smaak, which is the term used in this study. We follow the Psi-Q convention of referring to ‘taste’ in this article, although the test items tested clearly tap into the multisensory experience of flavour.
Ethics
Ethical consent was obtained through the Ethics Assessment Committee Humanities (EAChumanities), of the Faculty of Arts at Radboud University and adult participants signed a consent form prior to participating. For children, additional permissions were sought from the local schools where testing was conducted, as well as parents, prior to testing.
Data accessibility
Data are provided in the electronic supplementary material.
Authors' contributions
The concept for this paper arose from A.A. and A.M. The experiment reported here was conceptualized and designed by A.A. and A.M.; P.M. and A.M. acquired the data; P.M. prepared data for analysis and conducted pre-analyses; and A.M. and A.A. conducted analyses. A.A. and A.M. co-wrote the paper. All authors approve the final version to be published.
Competing interests
We declare we have no competing interests.
Funding
This work is part of the research programme Human olfaction at the intersection of language, culture and biology with project no. 277-70-011, which was financed by the Netherlands Organisation for Scientific Research (NWO), Wetenschaps Knooppunt Radboud Universiteit (WKRU); and A.A. was supported by the Swedish Research Council grant no. (2018-01603). A.A. was supported by the Swedish Research Council grant no. (2018-01603) and the Swedish Foundation for Humanities and Social Sciences (M14-0375:1).
References
- 1.Evans N. 2017. Sweat, spirit and place: the semantics of smell in Australian languages. Marysville, WA, Australia: Australian Languages Workshop. [Google Scholar]
- 2.Evans N, Merlan F, Tukumba M. 2004. A first dictionary of Dalabon (Ngalkbon). Maningrida, NT, Australia: Bawinanga Aboriginal Corporation. [Google Scholar]
- 3.Sperber D. 1975. Rethinking symbolism. (transl. AL Morton). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Buck CD. 1949. A dictionary of selected synonyms in the principal Indo-European languages: a contribution to the history of ideas. Chicago, IL: University of Chicago Press. [Google Scholar]
- 5.Levinson SC, Majid A. 2014. Differential ineffability and the senses. Mind Lang. 29, 407–427. ( 10.1111/mila.12057) [DOI] [Google Scholar]
- 6.van Beek WEA. 1992. The dirty smith: smell as a social frontier among the Kapsiki/Higi of North Cameroon and North-Eastern Nigeria. Africa 62, 38–58. ( 10.2307/1160063) [DOI] [Google Scholar]
- 7.Howes D. 1987. Olfaction and transition: an essay on the ritual uses of smell. Can. Rev. Sociol. 24, 398–416. ( 10.1111/j.1755-618X.1987.tb01103.x) [DOI] [Google Scholar]
- 8.Boisson C. 1997. La dénomination des odeurs: variations et régularités linguistiques [The denomination of odours: linguistic variations and regularities]. Intellectica 1, 29–49. [In French.] [Google Scholar]
- 9.Classen C, Howes D, Synnott A. 1994. Aroma: the cultural history of smell. London, UK: Routledge. [Google Scholar]
- 10.de Sousa H. 2011. Changes in the language of perception in Cantonese. Senses Soc. 6, 38–47. ( 10.2752/174589311x12893982233678) [DOI] [Google Scholar]
- 11.Demolin D, Traill A, Sicard G, Hombert J-M. 2016. Odour terminology in Xóõ. In Lone Tree – scholarship in service of the Koon (eds Vossen R, Haacke WHG), pp. 107–118. Cologne: Rüdiger Köppe. [Google Scholar]
- 12.Burenhult N, Majid A. 2011. Olfaction in Aslian ideology and language. Senses Soc. 6, 19–29. ( 10.2752/174589311X12893982233597) [DOI] [Google Scholar]
- 13.O'Meara C, Kung SS, Majid A. 2019. The challenge of olfactory ideophones: reconsidering ineffability from the Totonac-Tepehua perspective. Int. J. Am. Linguist. 85, 173–212. ( 10.1086/701801) [DOI] [Google Scholar]
- 14.Majid A, et al. 2018. Differential coding of perception in the world's languages. Proc. Natl Acad. Sci. USA 115, 11 369–11 376. ( 10.1073/pnas.1720419115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majid A, Burenhult N. 2014. Odors are expressible in language, as long as you speak the right language. Cognition 130, 266–270. ( 10.1016/j.cognition.2013.11.004) [DOI] [PubMed] [Google Scholar]
- 16.Majid A, Kruspe N. 2018. Hunter-gatherer olfaction is special. Curr. Biol. 28, 409–413. ( 10.1016/j.cub.2017.12.014) [DOI] [PubMed] [Google Scholar]
- 17.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 22, 39–52. ( 10.1093/chemse/22.1.39) [DOI] [PubMed] [Google Scholar]
- 18.San Roque L, et al. 2015. Vision verbs dominate in conversation across cultures, but the ranking of non-visual verbs varies. Cogn. Linguist. 26, 31–60. ( 10.1515/cog-2014-0089) [DOI] [Google Scholar]
- 19.Winter B, Perlman M, Majid A. 2018. Vision dominates in perceptual language: English sensory vocabulary is optimized for usage. Cognition 179, 213–220. ( 10.1016/j.cognition.2018.05.008) [DOI] [PubMed] [Google Scholar]
- 20.Floyd S, San Roque L, Majid A. 2018. Smell is coded in grammar and frequent in discourse: Cha'palaa olfactory language in cross-linguistic perspective. J. Linguist. Anthropol. 28, 175–196. ( 10.1111/jola.12190) [DOI] [Google Scholar]
- 21.Engen T. 1987. Remembering odors and their names. Am. Scient. 75, 497–503. [Google Scholar]
- 22.Olofsson JK, Gottfried JA. 2015. The muted sense: neurocognitive limitations of olfactory language. Trends Cogn. Sci. 19, 314–321. ( 10.1016/j.tics.2015.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorig TS. 1999. On the similarity of odor and language perception. Neurosci. Biobehav. Rev. 23, 391–398. ( 10.1016/S0149-7634(98)00041-4) [DOI] [PubMed] [Google Scholar]
- 24.Young BD. 2019. Smell's puzzling discrepancy: gifted discrimination, yet pitiful identification. Mind Lang. 35, Pages 90–114 ( 10.1111/mila.12233) [DOI] [Google Scholar]
- 25.Vigliocco G, Vinson DP, Woolfe T, Dye MWG, Woll B. 2005. Language and imagery: effects of language modality. Proc. R. Soc. B 272, 1859–1863. ( 10.1098/rspb.2005.3169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa S, Keysar B. 2018. Using a foreign language reduces mental imagery. Cognition 173, 8–15. ( 10.1016/j.cognition.2017.12.010) [DOI] [PubMed] [Google Scholar]
- 27.Arshamian A, Larsson M. 2014. Same but different: the case of olfactory imagery. Front. Psychol. 5, 34 ( 10.3389/fpsyg.2014.00034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson RJ, Case TI. 2005. Olfactory imagery: a review. Psychon. Bull. Rev. 12, 244–264. ( 10.3758/BF03196369) [DOI] [PubMed] [Google Scholar]
- 29.White KD, Ashton R, Brown RMD. 1977. The measurement of imagery vividness: normative data and their relationship to sex, age, and modality differences. Br. J. Psychol. 68, 203–211. ( 10.1111/j.2044-8295.1977.tb01576.x) [DOI] [Google Scholar]
- 30.Lawless HT. 1997. Olfactory psychophysics. In Tasting and smelling (eds Beauchamp GK, Bartoshuk L), pp. 125–174. San Diego, CA: Academic Press. [Google Scholar]
- 31.Kosslyn SM, Thompson WL, Ganis G. 2006. The case for mental imagery. New York, NY: Oxford University Press. [Google Scholar]
- 32.Marsella AJ, Quijano WY. 1974. A comparison of vividness of mental imagery across different sensory modalities in Filipinos and Caucasian-Americans. J. Cross Cult. Psychol. 5, 451–464. ( 10.1177/002202217400500406) [DOI] [Google Scholar]
- 33.Kosslyn SM, Ganis G, Thompson WL. 2001. Neural foundations of imagery. Nat. Rev. Neurosci. 2, 635–642. ( 10.1038/35090055) [DOI] [PubMed] [Google Scholar]
- 34.Barsalou LW. 2008. Grounded cognition. Annu. Rev. Psychol. 59, 617–645. ( 10.1146/annurev.psych.59.103006.093639) [DOI] [PubMed] [Google Scholar]
- 35.Fodor JA. 1975. The language of thought. Cambridge, MA: Harvard University Press. [Google Scholar]
- 36.Djordjevic J, Lundstrom JN, Clément F, Boyle JA, Pouliot S, Jones-Gotman M. 2008. A rose by any other name: would it smell as sweet? J. Neurophysiol. 99, 386–393. ( 10.1152/jn.00896.2007) [DOI] [PubMed] [Google Scholar]
- 37.Gottfried JA, Dolan RJ. 2003. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron 39, 375–386. ( 10.1016/S0896-6273(03)00392-1) [DOI] [PubMed] [Google Scholar]
- 38.Jadauji JB, Djordjevic J, Lundström JN, Pack CC. 2012. Modulation of olfactory perception by visual cortex stimulation. J. Neurosci. 32, 3095–3100. ( 10.1523/JNEUROSCI.6022-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frasnelli J, Schuster B, Hummel T. 2006. Interactions between olfaction and the trigeminal system: what can be learned from olfactory loss. Cereb. Cortex 17, 2268–2275. ( 10.1093/cercor/bhl135) [DOI] [PubMed] [Google Scholar]
- 40.Stevenson RJ. 2014. Object concepts in the chemical senses. Cogn. Sci. 38, 1360–1383. ( 10.1111/cogs.12111) [DOI] [PubMed] [Google Scholar]
- 41.Stevenson RJ, Attuquayefio T. 2013. Human olfactory consciousness and cognition: its unusual features may not result from unusual functions but from limited neocortical processing resources. Front. Psychol. 4, 819 ( 10.3389/fpsyg.2013.00819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baddeley A. 2012. Working memory: theories, models, and controversies. Annu. Rev. Psychol. 63, 1–29. ( 10.1146/annurev-psych-120710-100422) [DOI] [PubMed] [Google Scholar]
- 43.Tong F. 2013. Imagery and visual working memory: one and the same? Trends Cogn. Sci. 17, 489–490. ( 10.1016/j.tics.2013.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luck SJ, Vogel EK. 2013. Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends Cogn. Sci. 17, 391–400. ( 10.1016/j.tics.2013.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haegens S, Osipova D, Oostenveld R, Jensen O. 2010. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum. Brain Mapp. 31, 26–35. ( 10.1002/hbm.20842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jönsson FU, Møller P, Olsson MJ. 2011. Olfactory working memory: effects of verbalization on the 2-back task. Mem. Cognit. 39, 1023–1032. ( 10.3758/s13421-011-0080-5) [DOI] [PubMed] [Google Scholar]
- 47.Zelano C, Montag J, Khan R, Sobel N. 2009. A specialized odor memory buffer in primary olfactory cortex. PLoS ONE 4, e4965 ( 10.1371/journal.pone.0004965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmiero M, Matteo RD, Belardinelli MO. 2013. The representation of conceptual knowledge: visual, auditory, and olfactory imagery compared with semantic processing. Cogn. Process 15, 143–157. ( 10.1007/s10339-013-0586-9) [DOI] [PubMed] [Google Scholar]
- 49.Tomiczek C, Stevenson RJ. 2009. Olfactory imagery and repetition priming: the effect of odor naming and imagery ability. Exp. Psychol. 56, 397–408. ( 10.1027/1618-3169.56.6.397) [DOI] [PubMed] [Google Scholar]
- 50.Stevenson RJ, Case TI, Mahmut M. 2007. Difficulty in evoking odor images: the role of odor naming. Mem. Cogn. 35, 578–589. ( 10.3758/BF03193296) [DOI] [PubMed] [Google Scholar]
- 51.Delon-Martin C, Plailly J, Fonlupt P, Veyrac A, Royet J-P. 2013. Perfumers' expertise induces structural reorganization in olfactory brain regions. Neuroimage 68, 55–62. ( 10.1016/j.neuroimage.2012.11.044) [DOI] [PubMed] [Google Scholar]
- 52.Plailly J, Delon-Martin C, Royet J-P. 2012. Experience induces functional reorganization in brain regions involved in odor imagery in perfumers. Hum. Brain Mapp. 33, 224–234. ( 10.1002/hbm.21207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flohr ELR, Arshamian A, Wieser MJ, Hummel C, Larsson M, Mühlberger A, Hummel T. 2014. The fate of the inner nose: odor imagery in patients with olfactory loss. Neuroscience 268, 118–127. ( 10.1016/j.neuroscience.2014.03.018) [DOI] [PubMed] [Google Scholar]
- 54.Thomas NJT. 2019. Mental imagery. In The Stanford encyclopedia of philosophy (ed. Zalta EN.), pp. 1–55. Stanford, CA: Metaphysics Research Lab, Stanford University. [Google Scholar]
- 55.Varela FJ, Thompson E, Rosch E. 2017. The embodied mind: cognitive science and human experience. Cambridge, MA: MIT Press. [Google Scholar]
- 56.Schaal B, Marlier L, Soussignan R. 2000. Human foetuses learn odours from their pregnant mother's diet. Chem. Senses 25, 729–737. ( 10.1093/chemse/25.6.729) [DOI] [PubMed] [Google Scholar]
- 57.Ullal-Gupta S, Vanden Bosch der Nederlanden CM, Tichko P, Lahav A, Hannon EE. 2013. Linking prenatal experience to the emerging musical mind. Front. Syst. Neurosci. 7, 48 ( 10.3389/fnsys.2013.00048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laeng B, Teodorescu D-S. 2002. Eye scanpaths during visual imagery reenact those of perception of the same visual scene. Cogn. Sci. 26, 207–231. ( 10.1207/s15516709cog2602_3) [DOI] [Google Scholar]
- 59.Bensafi M, et al. 2003. Olfactomotor activity during imagery mimics that during perception. Nat. Neurosci. 6, 1142–1144. ( 10.1038/nn1145) [DOI] [PubMed] [Google Scholar]
- 60.Arshamian A, Olofsson JK, Jönsson FU, Larsson M. 2008. Sniff your way to clarity: the case of olfactory imagery. Chem. Percept. 1, 242–246. ( 10.1007/s12078-008-9035-z) [DOI] [Google Scholar]
- 61.Perl O, Mishor E, Ravia A, Ravreby I, Sobel N. 2020. Are humans constantly but subconsciously smelling themselves? Phil. Trans. R. Soc. B 375, 20190372 ( 10.1098/rstb.2019.0372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jankowska DM, Karwowski M. 2015. Measuring creative imagery abilities. Front. Psychol. 6, 1591 ( 10.3389/fpsyg.2015.01591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gallistel CR. 1990. The organization of learning: learning, development, and conceptual change. Cambridge, MA: MIT Press. [Google Scholar]
- 64.Gärdenfors P. 2004. Conceptual spaces as a framework for knowledge representation. Mind Matter 2, 9–27. ( 10.1017/S0140525X04280098) [DOI] [Google Scholar]
- 65.Royet J-P, Delon-Martin C, Plailly J. 2013. Odor mental imagery in non-experts in odors: a paradox? Front. Hum. Neurosci. 7, 87 ( 10.3389/fnhum.2013.00087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gutiérrez ED, Dhurandhar A, Keller A, Meyer P, Cecchi GA. 2018. Predicting natural language descriptions of mono-molecular odorants. Nat. Commun. 9, 4979 ( 10.1038/s41467-018-07439-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wise PM, Olsson MJ, Cain WS. 2000. Quantification of odor quality. Chem. Senses 25, 429–443. ( 10.1093/chemse/25.4.429) [DOI] [PubMed] [Google Scholar]
- 68.Kaeppler K, Mueller F. 2013. Odor classification: a review of factors influencing perception-based odor arrangements. Chem. Senses 38, 189–209. ( 10.1093/chemse/bjs141) [DOI] [PubMed] [Google Scholar]
- 69.Dravnieks A. 1982. Odor quality: semantically generated multidimensional profiles are stable. Science 218, 799–801. ( 10.1126/science.7134974) [DOI] [PubMed] [Google Scholar]
- 70.Noble AC, Arnold RA, Masuda BM, Pecore SD, Schmidt JO, Stern PM. 1984. Progress towards a standardized system of wine aroma terminology. Am. J. Enol. Vitic. 35, 107–109. [Google Scholar]
- 71.Speed LJ, Majid A. In press. Grounding language in the neglected senses of touch, taste, and smell. Cogn. Neuropsychol. ( 10.1080/02643294.2019.1623188) [DOI] [PubMed] [Google Scholar]
- 72.Algom D, Cain WS. 1991. Remembered odors and mental mixtures: tapping reservoirs of olfactory knowledge. J. Exp. Psychol. Hum. Percept. Perform. 17, 1104 ( 10.1037/0096-1523.17.4.1104) [DOI] [PubMed] [Google Scholar]
- 73.Yeshurun Y, Sobel N. 2010. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu. Rev. Psychol. 61, 219–241. ( 10.1146/annurev.psych.60.110707.163639) [DOI] [PubMed] [Google Scholar]
- 74.Biederman I. 1987. Recognition-by-components: a theory of human image understanding. Psychol. Rev. 94, 115 ( 10.1037/0033-295X.94.2.115) [DOI] [PubMed] [Google Scholar]
- 75.Anglin JM, Miller GA, Wakefield PC. 1993. Vocabulary development: a morphological analysis. Monogr. Soc. Res. Child Dev. 58, i+iii+v−vi+1–186. ( 10.2307/1166112) [DOI] [Google Scholar]
- 76.Bloom P, Markson L. 1998. Capacities underlying word learning. Trends Cogn. Sci. 2, 67–73. ( 10.1016/S1364-6613(98)01121-8) [DOI] [PubMed] [Google Scholar]
- 77.Andrade J, May J, Deeprose C, Baugh S-J, Ganis G. 2014. Assessing vividness of mental imagery: the Plymouth Sensory Imagery Questionnaire. Br. J. Psychol. 105, 547–563. ( 10.1111/bjop.12050) [DOI] [PubMed] [Google Scholar]
- 78.Isbell E, Fukuda K, Neville HJ, Vogel EK. 2015. Visual working memory continues to develop through adolescence. Front. Psychol. 6, 696 ( 10.3389/fpsyg.2015.00696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acredolo L, Goodwyn S. 1988. Symbolic gesturing in normal infants. Child Dev. 59, 450–466. ( 10.2307/1130324) [DOI] [PubMed] [Google Scholar]
- 80.Crais ER, Watson LR, Baranek GT. 2009. Use of gesture development in profiling children's prelinguistic communication skills. Am. J. Speech Lang. Pathol. 18, 95–108. ( 10.1044/1058-0360(2008/07-0041)) [DOI] [PubMed] [Google Scholar]
- 81.Chen X, Striano T, Rakoczy H. 2004. Auditory–oral matching behavior in newborns. Dev. Sci. 7, 42–47. ( 10.1111/j.1467-7687.2004.00321.x) [DOI] [PubMed] [Google Scholar]
- 82.Schulze K, Vargha-Khadem F, Mishkin M. 2012. Test of a motor theory of long-term auditory memory. Proc. Natl Acad. Sci. USA 109, 7121–7125. ( 10.1073/pnas.1204717109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bensafi M, Tillmann B, Poncelet J, Przybylski L, Rouby C. 2013. Olfactory and gustatory mental imagery: modulation by sensory experience and comparison to auditory mental imagery. In Multisensory imagery (eds Lacey S, Lawson R), pp. 77–91. New York, NY: Springer. [Google Scholar]
- 84.Gilbert AN, Crouch M, Kemp SE. 1998. Olfactory and visual mental imagery. J. Ment. Imag. 22, 137–146. [Google Scholar]
- 85.Koelega HS, Köster EP. 1974. Some experiments on sex differences in odor perception. Ann. NY Acad. Sci. 237, 234–246. ( 10.1111/j.1749-6632.1974.tb49859.x) [DOI] [PubMed] [Google Scholar]
- 86.Koelega HS. 1994. Prepubescent children may have specific deficits in olfactory sensitivity. Percept. Mot. Skills 78, 191–199. ( 10.2466/pms.1994.78.1.191) [DOI] [PubMed] [Google Scholar]
- 87.Hummel T, Krone F, Lundström JN, Bartsch O. 2005. Androstadienone odor thresholds in adolescents. Horm. Behav. 47, 306–310. ( 10.1016/j.yhbeh.2004.10.007) [DOI] [PubMed] [Google Scholar]
- 88.Cain WS, Stevens JC, Nickou CM, Giles A, Johnston I, Garcia-Medina MR. 1995. Life-span development of odor identification, learning, and olfactory sensitivity. Perception 24, 1457–1472. ( 10.1068/p241457) [DOI] [PubMed] [Google Scholar]
- 89.Lehrner J, Glück J, Laska M. 1999. Odor identification, consistency of label use, olfactory threshold and their relationships to odor memory over the human lifespan. Chem. Senses 24, 337–346. ( 10.1093/chemse/24.3.337) [DOI] [PubMed] [Google Scholar]
- 90.Hummel T, Bensafi M, Nikolaus J, Knecht M, Laing D, Schaal B. 2007. Olfactory function in children assessed with psychophysical and electrophysiological techniques. Behav. Brain Res. 180, 133–138. ( 10.1016/j.bbr.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 91.Chalouhi C. 2005. Olfactory evaluation in children: application to the CHARGE syndrome. Pediatrics 116, e81–e88. ( 10.1542/peds.2004-1970) [DOI] [PubMed] [Google Scholar]
- 92.Monnery-Patris S, Rouby C, Nicklaus S, Issanchou S. 2009. Development of olfactory ability in children: sensitivity and identification. Dev. Psychobiol. 51, 268–276. ( 10.1002/dev.20363) [DOI] [PubMed] [Google Scholar]
- 93.Solbu EH, Jellestad FK, Strætkvern KO. 1990. Children's sensitivity to odor of trimethylamine. J. Chem. Ecol. 16, 1829–1840. ( 10.1007/BF01020497) [DOI] [PubMed] [Google Scholar]
- 94.Croijmans IM, Speed LJ, Arshamian A, Majid A. 2019. Measuring the multisensory imagery of wine: the vividness of wine imagery questionnaire (VWIQ). Multisens. Res. 32, 179–195. ( 10.1163/22134808-20191340) [DOI] [PubMed] [Google Scholar]
- 95.Dubois D, Rouby C. 2002. Names and categories for odors: the veridical label. In Olfaction, taste, and cognition (eds Rouby C, Schaal B, Dubois D, Gervais R, Holley A), pp. 47–66. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 96.Dubois D. 2000. Categories as acts of meaning: the case of categories in olfaction and audition. Cogn. Sci. Q. 1, 35–68. [Google Scholar]
- 97.Krishna A, Morrin M, Sayin E. 2013. Smellizing cookies and salivating: a focus on olfactory imagery. J. Consum. Res. 41, 18–34. ( 10.1086/674664) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided in the electronic supplementary material.