Abstract
Odours can have a significant influence on the outcome of social interactions. However, we have yet to characterize the chemical signature of any specific social cue in human body odour, and we know little about how changes in social context influence odour chemistry. Here, we argue that adoption of emerging analytical techniques from other disciplines, such as atmospheric chemistry, might become game-changing tools in this endeavour. First, we describe the use of online chemical ionization time-of-flight mass spectrometry to sensitively measure many hundreds of gas-phase volatile organic compounds in real time. By analysing ambient air emanating from undisturbed individuals or groups, the technique enables a continuous recording of an instantaneous odour change in response to external stimuli and changing social context. This has considerable advantages over the traditional approach of periodic sampling for analysis by gas chromatography. We also discuss multivariate statistical approaches, such as positive matrix factorization, that can effectively sift through this complex datastream to identify linked groups of compounds that probably underpin functional chemosignals. In combination, these innovations offer new avenues for addressing outstanding questions concerning olfactory communication in humans and other species, as well as in related fields using odour, such as biometrics and disease diagnostics.
This article is part of the Theo Murphy meeting issue ‘Olfactory communication in humans’.
Keywords: odour, chemosignal, smell, pheromone, volatilome, olfaction
1. Introduction
Odour constitutes a critical medium for information exchange across most mammals, whereby chemical cues associated with a given attribute or state in one individual (the sender) may elicit a subsequent change in behaviour of another individual (the receiver). Social odours are often extremely chemically diverse, having potential to reveal multiple forms of information. In mice, for example, receivers can discriminate and remember individuals from their urinary odour, as well as detecting and responding to cues of sex, nutritional state, health, stress, fertility, relatedness and social status [1–3]. They can also flexibly weight the importance of different cues during social decision-making even when they are processed simultaneously [4].
We know the chemical basis of such olfactory information in several mammalian species. These include a mammary signal eliciting suckling behaviour in rabbit pups [5], sex steroid derivatives facilitating mating in pigs [6] and volatile compounds associated with sexual receptivity in antelopes and elephants [7,8]. In mice, quantitative differences exist between several volatile organic compounds (VOCs) emitted from dominant and subordinate males, which change rapidly after status reversals [9,10]. Relative genetic dissimilarity used in mate choice decisions by female mice may also be coded for by varying ratios of VOCs [11], and individual identity is revealed by differences in the expression of major urinary proteins and their ligands [12]. Mouse urinary odour also contains most of the pheromones that have been documented across mammals. Pheromones are typically defined as specific chemical signals that have evolved for the purpose of communicating between conspecifics and that exert repeatable behavioural or physiological responses in receivers [13,14]. These include murine urinary compounds that regulate pregnancy block, oestrus synchronization and mate attraction [15,16].
Progress in unravelling the chemical basis of animal chemosignals stands in stark contrast to the same endeavour in human odours [17]. It has become evident in recent decades that a similarly broad range of social cues lie hidden somewhere within the human volatilome, the entirety of the complex cocktail of VOCs emanating from human skin and breath that together constitute an individual's distinct odour. However, the chemical mechanism for any such cue has yet to be identified [17,18]. We can thus picture the human social volatilome as an unknown language: we know that it has potential to communicate important social information, but we do not yet know how to decode it.
In this paper, we first briefly outline the potential for social communication in human odours and describe some of the main constraints facing efforts to characterize the chemical basis of specific social messages. We then introduce two new approaches that help overcome these constraints and in so doing provide new ways of answering outstanding questions. The first is an analytical tool, online time-of-flight (TOF) chemical ionization mass spectrometry (CIMS), which is designed for sensitive, real-time measurement of VOC changes in ambient air. The second is a multivariate statistical approach which enables the deconvolution of the type of complex odour mixtures used in mammalian social communication. Finally, we describe how the key advantages of these two approaches might be harnessed in coming years to address outstanding questions in chemical communication.
2. Complexity of the human volatilome
Excluding extrinsic compounds associated with artificially fragranced products, the human volatilome is remarkably diverse, containing at least 2000 known VOCs produced from breath and the skin [19,20]. Different people produce unique VOC combinations at variable concentrations, thus generating individually distinct odour profiles, or ‘signatures’, which are shaped by both genes and environmental factors. For example, twins have similar VOC signatures [21], suggesting a genetic component, while potent environmental factors include aromatic compounds in diet and disease-related metabolites [22], as well as other markers of social condition [23].
Research also demonstrates that at least some of this variation is perceptible by the human nose. More comprehensive reviews of such studies can be found elsewhere (e.g. [23]). Briefly, however, we know from laboratory testing that people are better able to match odours of monozygotic than dizygotic twins [24], can discriminate between odours of individuals [25], and recognize and prefer body odours of relatives [26,27] or partners [28]. Such abilities are present from birth: human infants learn and prefer the odour of their mother over those of other breastfeeding women [29,30]. Furthermore, adults demonstrate preferences for odours of unfamiliar individuals who possess socially relevant traits, such as high fertility [31], dominance [32], attractiveness and confidence [33,34] as well as genetic complementarity [35] (but see [36]). They can discriminate between odours of others who are in a fearful or relaxed state [37–39], and between ‘infected’ and healthy samples of the same people [40,41].
We can thus picture an individual's volatilome as being constituted of three distinct layers. At the core are compounds that are genetically encoded and temporally robust, enabling the discrimination of biological sex, kinship and relative genetic dissimilarity (e.g. major histocompatibility complex-associated cues [35,36]). The second layer contains temporally labile, environmentally influenced constituents such as current health and nutritional status, social or reproductive status and emotional state. These two layers capture an individual's natural odour and contain VOCs produced directly from the body or released by commensal microflora [42–44]. The proportion of these VOCs that is implicated in some form of social communication is unknown, but it seems likely that there is a (potentially large) fraction of non-communicatory VOCs within these two layers. Finally, a third layer is represented by VOCs of extrinsic origin. In humans, this includes compounds in breath from recently ingested food or tobacco smoking and those applied to the skin via hygiene products and perfumes.
3. Current constraints in human chemosignalling research
The complex and distinctive nature of human body odour provides a potential medium for exchange of social information, as in other species. If receivers can discern differences in odour between groups of individuals who share traits in common, or can discriminate between samples of the same individual in different states, then these perceptual assessments must necessarily be underpinned by the differences in the pattern or abundance of VOCs within the volatilome of the sender(s). However, to our knowledge, no study to date has definitively identified the chemical markers responsible for any of the above-described observable behavioural effects. This stands at odds with progress in defining the chemical basis of social odours in other species, particularly mice. We think there are at least three main reasons.
A primary reason arises from inherent difficulties in working with humans as study participants. Perhaps the most immediate of these is the pervasive nature of extrinsic compounds in the third layer of the individual's volatilome. Humans have used perfumes since at least ancient Mesopotamian and Egyptian civilizations, and it is now a multi-billion dollar industry [45]. Artificial fragrances, as well as those from household and laundry products, may mix with an individual's natural odour, contributing to distinctive odour blends [46,47]. These influences can be minimized by inviting study participants to refrain from their use, but even so, suspension of product use probably triggers a corollary odour change over at least several days as microbial populations on the skin surface readapt.
Another consideration is the relative lack of experimental tractability when studying humans. Environmental factors affecting odour (e.g. diet, temperature, humidity, activity level and social context) are all readily controlled in animal studies, but each introduces significant variability in human studies. Comparing odours between individuals thus presents unavoidable challenges. Although researchers often aim to compare odours of two (or more) groups of individuals, where individuals within each group share some factor in common and groups differ in that factor, profound individual differences in odour may well obscure the characteristic of interest. This problem is reduced, though not eliminated, by using approaches where several samples are collected from the same individual at different times.
A third reason for lack of progress has been a significant technical problem, for which, as we argue in the remainder of this paper, there is now a realistic solution. Those few studies (e.g. [21]) that have attempted to describe the underlying chemical basis and individual variability of human odour have collected, stored and analysed samples from a single time point. For example, this would be typically achieved by collecting swabs from a specific body region on a person at a given time for subsequent analysis by gas chromatography–mass spectrometry (GC–MS). While this can be very informative, sample collection can be done only periodically. This is necessarily disruptive to the source of odour production; for example, an axillary skin swab will probably alter the microbial population at the skin surface and effect changes in later samples. Similarly, it also involves disruption to the participant; while this may be trivial if intersample interval is long (e.g. days and weeks), it could be critical in investigations of very short-term odour change. In either case, collected swabs are typically frozen until analysis can be performed. This may further result in the loss of highly volatile species and alteration of the odour to be measured.
In consequence, GC–MS data have not yet enabled us to identify chemical signatures of any perceivable social cue (see §2). We also lack answers to other questions that are highly relevant in the context of chemical communication, such as how individual odour profiles change in response to social circumstances. That is, periodic sampling does not permit the satisfactory measurement of changes in odour that might occur when an individual's emotional state alters, or when it meets a competitor or a mate. We also do not know how rapidly such changes might occur. However, as we describe in the next section, techniques to measure rapid odour change from the ambient air surrounding undisturbed individuals are now available, meaning that answers to these questions are now within our reach.
4. Tracking rapid change in odour chemistry
(a). Technical considerations
There are several types of online CIMSs capable of tracking rapid changes in odour. However, the approach most widely used for VOCs over the last 20 years is proton transfer reaction–mass spectrometry (PTR-MS) [48,49]. This analytical method can measure VOCs in ambient air in real time (e.g. 10 Hz), with the exception of a small number of low proton affinity molecules such as methane, short saturated alkanes [50] and, importantly, the major constituents of air (e.g. N2, O2, Ar and CO2). The PTR-MS instrument consists of three distinct regions: an ion source, a reaction chamber (or drift tube) and a detector (figure 1). Water vapour passes through a high voltage hollow cathode to form hydronium (H3O+) reagent ions. These are accelerated from the ion source into the drift tube where they softly transfer protons to neutral VOCs which are directly sampled from ambient air. The protonated VOCs (VOCH+) are subsequently detected by a mass spectrometer (MS). The soft ionization provided by the transfer of protons ensures that there is little fragmentation of the target VOCs, allowing for the majority of compounds to be sensitively detected on their protonated parent ion mass. This is in stark contrast to many GC–MS systems, where high energy electron impact ionization frequently results in the fragmentation of the molecule of interest and thus adds a further level of complexity to the interpretation of the measured mass spectra.
Figure 1.
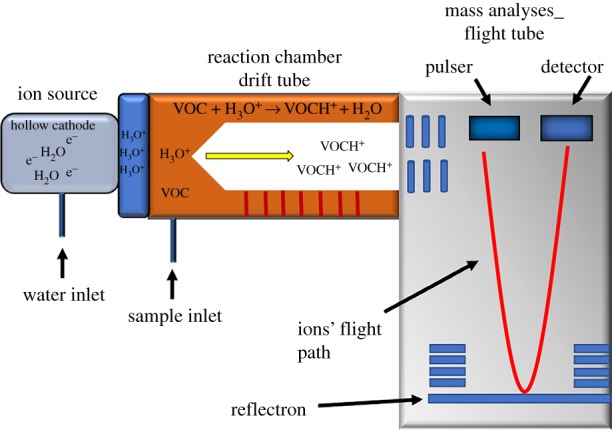
Schematic of a PTR-TOF-MS. In the flight tube (right), the red line represents the flight path of the ions from the pulser to the detector.
In early versions of PTR-MS, VOCH+s reached the MS via a quadrupole mass filter. The quadrupole restricts the flow of ions that pass through to the detector to those with a pre-defined, unit mass resolution (e.g. 1 Da). This places two distinct limitations on the technique. First, compounds with the same nominal mass cannot be distinguished. For example, isoprene (protonated m/z 69.070), a metabolite found in breath, could not be distinguished from furan (protonated m/z 69.034) because, at a unit mass resolution, both would be detected as m/z 69. Second, individual ions of interest have to be preselected and sampled sequentially, which limits the temporal resolution of the technique when multiple ions of interest are to be detected.
These limitations have recently been overcome through the introduction of TOF-MS. Rather than passing through a quadrupole, protonated ions are pulsed into a low-pressure flight tube where they separate based on their molecular weight: small ions travel faster than larger and heavier compounds. This development [51] enables simultaneous measurement of all the VOCH+s in the drift tube, without need for any preselection. The time with which ions traverse the flight chamber is proportional to their mass-to-charge ratio (m/z), making it possible to determine the exact mass of individual ions within 0.001 Da. With this level of resolution, not only can multiple ions detected at the same nominal m/z be resolved, but the number of different elements (C, H, N, O, S, F, Cl, Br, etc.) contained in each molecule can be determined to give its chemical formula. However, it is still not possible to determine the exact chemical structure of a compound to explicitly identify the molecule. An example of this limitation can be found when considering terpenoid compounds, which are found in many fragrances and personal care products. Although there are many different types of C10H16 terpenoid compounds (e.g. limonene, α-pinene and β-pinene), they are all detected on the same protonated parent ion mass of 137.133 Da. They are only distinguishable by the structural arrangement of their elements, which is what gives these molecules their individually distinct scents. Therefore, while the sum of these structural isomers is detected by online MS, their structural speciation can only be resolved using GC. Online MS, therefore, offer chemical formula level identification and are a compromise between a very fast time response and absolute compound selectivity.
The most recent versions of PTR-TOF-MS, such as PTR-Qi-TOF-MS [52], PTR3 [53] and VOCUS [54], are extremely sensitive and enable sub-parts per trillion (ppt) detection limits for hundreds or even thousands of ions. As a result, the PTR-TOF-MS technique has quickly found favour in many diverse research areas, including atmospheric chemistry [55], human VOC emissions in the aspect of indoor chemistry [56,57], medicine [58] and, more recently, in the measurement of chemical signals from humans in a psychological context [59].
(b). Tracking rapid odour changes in the axilla
The axilla is known to emit hundreds of VOCs, some of which might be important for chemical communication. However, accessing this rich source of information is not a trivial task. Emissions from the axilla accumulate under clothing which means that before sampling can commence, a steady state must be achieved following the insertion of a sampling tube in the axilla. This steady state can be easily disturbed if the subject moves and disrupts the flow of air under the arm, or if the sample inlet becomes blocked between arm and body. Furthermore, measuring the concentration of VOCs from the axillary region does not guarantee that the observed changes in concentrations are a result of changes in the emission rate from the skin. Rather, they may simply reflect changes in the room odour and can be further confounded by external factors, including odours from the subject's own clothing or unrelated bodily functions.
Here, we propose an alternative approach in which the emission rate of VOCs from the skin is measured directly using a flow-through sampling cuvette. Figure 2 shows a custom-built cuvette made from the chemically inert material polyether ether ketone (PEEK). The cuvette has two ports: an inflow for VOC-free air from a platinum catalyst and an outflow for analysis by online PTR-TOF. The cuvette is placed under the axilla (or at other body regions) with the 10 mm diameter opening facing the subject's body. A strap secures the cuvette in place and ensures that an air-tight seal is made against the subject's skin. The emission rate of VOCs, Fχ, from the skin is then calculated as follows:
where Q is the flow rate through the cuvette, χin and χout are concentrations of VOCs in the air before and after passing through the cuvette, respectively, and S is the surface area of skin covered by the cuvette. In practice, χin is calculated as a linear interpolation between two measurements made before and after sampling from a subject when the opening of the cuvette is sealed using a PEEK plate. Figure 2b shows example emission rates of acetone and butanone, measured from the axilla of a volunteer watching neutral and scary video scenes. Emissions of acetone show a general downwards trend with a rapid increase in emission rates observed within the first minute of the scary scenes. By contrast, butanone tends to increase under neutral conditions and decreases rapidly during the scary scene, indicating that the two metabolites are anti-correlated for this individual.
Figure 2.
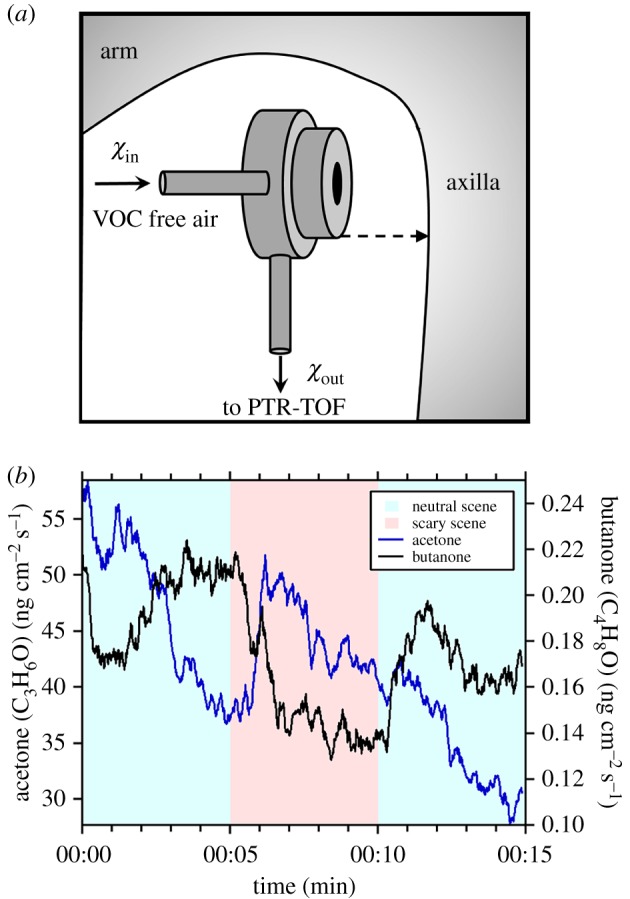
Measurement of odour change from the axilla. (a) A 10 mm diameter axilla cuvette (12 mm deep), which when pressed against the skin, can be used to directly determine the emission rate of VOCs by measuring the difference between the incoming VOC-free air (χin) and that exiting the cuvette (χout). (b) An example time series of emissions of acetone and butanone from the axilla, while a volunteer watched neutral and scary video clips (electronic supplementary material).
5. Decoding complex odour signals
Online TOF-MS can quantify many of the individual compounds emitted in real time from the human body in response to physical or social cues. This presents the opportunity to probe the temporal dynamics of the human volatilome and to help us better understand the role of odours in communication between humans. However, it also presents a challenge in terms of interpreting the significant volume of data that the technique generates. For example, the instrument returns a mass spectral profile of the ions contained in air ranging from m/z of 21 to over 500, at a rate of up to 10 Hz. In a typical sample of air from the axilla, this range may include over 1000 individual ions, making the analysis of the collected data a highly time-consuming process. Rather than examining these ions individually, an alternative approach is to analyse the mass spectral dataset holistically. This may identify groups of compounds that share similar temporal trends and thus may be co-emitted from the body in response to a specific stimulus.
This type of approach is common in atmospheric chemistry, as just one example, where MS has long been used to measure the composition of fine particulate matter pollution (PM1 < 1 µm) in the atmosphere. In this context, the total mass spectrum of PM measured in the atmosphere represents the sum of many different sources. These may be primary, such as from traffic, cooking or biomass burning, or secondary, formed in the atmosphere as low vapour pressure VOCs condense to form new particles. Unravelling the contribution of these individual sources to the total measured PM concentration in ambient air is made possible through multivariate statistical techniques such as positive matrix factorization (PMF) [60,61] or principal component analysis (PCA) [62]. Since 2007, these approaches have been routinely applied to measurements of PM [63–68], with the results contributing to a comprehensive library of the mass spectral profiles [63] of the major PM sources. The atmospheric community has further established tools and technical guidelines for performing and interpreting results from PMF analyses [65], including the verification of solutions by comparison with ancillary measurements.
As we describe below, such techniques can be extended to the analysis of VOCs measured from human skin or breath, with the goal of identifying mass spectral profiles that are representative of specific social cues. Although PMF has been routinely applied to measurements of PM, there are relatively few examples of its application to measurements of VOCs in ambient air (but see [69–71]). This may relate to the physico-chemical properties of VOCs compared to that of PM. For example, the chemical lifetime of VOCs with respect to the major atmospheric oxidants, hydroxyl (OH), ozone (O3) and nitrate (NO3−), can vary hugely between compounds [72], lasting a matter of seconds for compounds like sesquiterpenes [73], to days or even weeks for others (e.g. acetone [74]). These differences mean that temporal patterns in VOC concentrations in ambient air are influenced not just by changes in the sources of emission, but also by their reactivity, which can confound factor analysis techniques that are focused on grouping compounds based on their temporal similarities. Of the few examples where PMF has been applied to VOC data, most have focused on longer-lived compounds known to be characteristic of specific sources, such as acetonitrile (biomass burning) and benzene (traffic), and have been combined with PM1 datasets to aid in the identification of PM1 source factors [75,76].
Focusing on VOCs measured close to the skin or from human breath would allow for a broader range of compounds to be included in an analysis while not confounding the solution with extraneous processes such as chemical kinetics and volatility. For example, measuring the air directly above the emission source (e.g. skin and breath) and sampling into the instrument within seconds greatly reduce the influence of atmospheric chemistry in augmenting the measured signature and ensure temporal patterns in the measured concentrations relate solely to changes in emission rather than to the effects of chemistry.
(a). Positive matrix factorization
The PMF algorithm, its uncertainties and the interpretation of solutions have all been described in detail by Paatero et al. [60,61,77,78], Ulbrich et al. [63] and Canonaco et al. [64]. Therefore, here we present only the basic concepts and how they might be applied in the context of measuring VOCs from human skin or breath. The PMF algorithm is a receptor-only, bilinear model that assumes the total mass spectrum of a quantity measured over time represents the linear combination of a number of individual sources or ‘factors’, F, each with a distinct chemical signature (or mass spectral profile) that does not change over time, such that [55]
| 5.1 |
In the case of VOCs measured from the axilla, X represents the total axilla odour in the form of a two-dimensional matrix, where rows (i) are the individual mass spectra and columns (j) are the time series of the concentration of individual ions. F represents the characteristic mass spectral profile that best describes the odour associated with individual factors (for example, in a study of emotion, these factors might include joy, fear or anxiety), and G represents their relative contribution to the total odour, X, over the course of the measurement period. Accordingly, any change in overall axilla odour (X) is a consequence of the varying contributions of the individual emotions (factors) over time plus some residual, E. As implied by the name, each of the quantities X, G and F are subject to positive constraints, meaning that all data must be non-negative. This is the key feature that separates PMF from other factorization methods such as PCA. The number of factors, p, used in the analysis is determined by the user, but no a priori assumptions are made by the model about the mass spectral profile of each factor or how their contribution to the overall odour might change over time. Instead, F and G are calculated iteratively using a least-squares regression to minimize the quantity Q, which represents the sum of the squared residuals relative to their respective uncertainties:
| 5.2 |
Here, m and n are the dimensions of the residual matrix which has elements eij and σij. These are the uncertainties associated with the individual elements of X. The uncertainties are typically calculated as the signal-to-noise ratio of the individual measurements [64].
(b). Example of positive matrix factorization
To illustrate the concept of PMF, a simple experiment was conducted to measure the axillary odours from three volunteers simultaneously using a PTR-Qi-TOF instrument (figure 3). The volunteer's axillary odour is unique to them, and thus each volunteer represents a discrete emission source, F. For that reason, by measuring the combined axillary odour of the volunteers as X, we can manipulate the contribution of each source G, by removing or adding volunteers from the sampling system, and ultimately identify which individuals were connected to the instrument across the sampling period. By comparison of figure 3b, and 3c, it is clear that the derived solution is broadly able to reflect the presence or absence of each of the volunteers. However, upon closer inspection, it appears that some minor erroneous contributions are detected from persons B and C in the first measurement period and from persons A and B in the last measurement period. It is interesting that during these periods, the volunteers were not directly connected to the sample inlet but they were still present in the room and their odour continued to influence the background odour profile of the room. Remarkably, the PTR-Qi-TOF and PMF algorithms were still able to distinguish the volunteer's presence despite no longer directly sampling from their axilla. This simple experiment therefore serves to highlight the use of PMF analysis to convincingly resolve the contribution of distinct odour sources to a total measured volatilome.
Figure 3.
Use of PTR-TOF and PMF to discriminate odour of different individuals. (a) The total measured odour spectrum (X) from a group of three volunteers (black) and the mass spectral profiles identified by the PMF algorithm for a three-factor solution. (b) The time series of the measured odour (black) and the relative contributions of factor 1 (green), factor 2 (blue) and factor 3 (red) (G). (c) Which volunteers were connected to the instrument during the five-sampling period (electronic supplementary material).
(c). Limitations of positive matrix factorization
The power of factor analysis techniques to resolve complex mixtures of odours is evident, but it is also important to be aware of the limitations. Considering the example above, we could add a fourth volunteer, person D. If person D's participation mirrored exactly that of person A, then the PMF algorithm would simply recognize their combined odour as a single source (A + D) and their individual contributions would remain unresolved. Furthermore, solutions derived by PMF may not always be unique. That is to say, there may be a number of possible combinations of F and G that equal X within the non-negative constraints. This problem, termed rotational ambiguity, is discussed in detail by Paatero et al. [78] and Canonaco et al. [64], along with methods to assess the uncertainty of a given solution. Ultimately, determining the most appropriate solution requires reducing the degrees of freedom by applying further constraints either within the model or more typically by comparison to external factors. Returning to the example of particulate matter pollution, derived solutions are typically assessed by (i) comparing the derived factor profiles to the library of profiles previously measured in the atmosphere and (ii) comparing the time series of a factor profile to some ancillary measurement representative of that factor. For example, when assessing a factor thought to be representative of traffic, the time series could be compared to traffic counts or measurements of oxides of nitrogen (NOx) that are emitted in vehicle exhaust. In the case of human odours, we do not yet have an understanding of the characteristic profiles of analogous social cues from which to compare and constrain our results, but we can employ ancillary measurements that might guide the interpretation of PMF solutions. For example, tracking physiological parameters during measurements (e.g. measures of sympathetic neuronal activation, particularly electrodermal activity) may provide vital supplementary information to help determine specific cognitive states and constrain PMF solutions. Finally, the PMF solution is always based on the number of factors directed by the user, but unlike the data shown in figure 3, the exact number of factors present is typically unknown. In practice, this means that any PMF analysis is an iterative process where multiple solutions are viewed for a varying number of factors and interpreted relative both to supporting measurements and the model residuals. In consequence, any PMF solution usually has an element of subjectivity which should be made clear when presenting the results.
6. Addressing outstanding questions in odour communication
The technical capabilities of online CIMS instruments and multivariate statistical techniques such as PMF introduce novel opportunities for researchers aiming to decode the complex human volatilome and to determine its role in social communication. These opportunities fall into several interconnected domains: they enable the measurement of the time course of odour changes in response to social context (§6a), they may significantly facilitate the characterization of the chemical basis of specific social cues (§6b) and they open doors to new experimental designs and paradigms (§6c) that can be used to address outstanding theoretical questions (§6d). Beyond this, the ability to monitor a rapid change in human chemosignals may also have practical implications (§6e).
(a). Time course of response
A primary benefit introduced by these techniques is the ability to determine the time course of odour changes in response to social stimuli (figure 2). Whereas periodic sampling might allow us to conclude that changes occur within any given sampling interval, we can now plot precisely the latency to specific changes occurring, the shape of the response over ensuing time and the point at which the relevant response returns to baseline.
Understanding the temporal shape of the response is a critical step for researchers in determining the parameters for social communication. For example, consider how a fearful situation might be communicated by odour. If odour changes associated with fear are to play a role in producing adaptive responses in others nearby—for example, by triggering an increase in adrenaline ready for taking flight, or increasing alertness and vigilance—then these responses must occur within seconds of the sender becoming aware of the danger. If responses are much slower than this, then they are unlikely to usefully function as fear signals. We might also expect the return to baseline levels to occur relatively quickly once the sender has assessed the level of imminent danger and taken appropriate action or judged it to be passed. Such a temporal pattern would be in contrast with one associated with a long-term state, such as chronic anxiety. Here, we would not expect selection to have shaped such a brief latency to onset of response, but would predict a sustained, longer-term change in odour profile. The differences in the expected time course of acute and sustained responses, owing to variable neural and hormonal mechanisms, probably means that they will involve disparate regions of the volatilome with different involved VOCs that have appropriate reactivity and volatility.
(b). Characterization of chemical markers
Dynamic VOC changes in response to any given social stimulus can be measured by online techniques and then correlated with concurrent behavioural or physiological responses, without disturbance to either the participant or the odour source. The task of identifying behaviourally relevant compounds is facilitated because the technique samples only those chemical markers emanating from individuals in the surrounding airspace (or from specific parts of the body, if using localized sensors such as the cuvette described in §4b). This means that the analysis involves only those VOCs that would be expected to reach the nose of other individuals, in contrast with skin swab samples which also contain a substantial component of non-volatile compounds. Furthermore, it enables researchers to essentially ignore the complex of invariant compounds, including those linked to incidental differences in environment or to extrinsic factors, because they can be assumed to be uninvolved in the relevant social response. Focus can then be applied instead on the fraction of the odour profile that co-varies with time-resolved social stimuli.
Perhaps, the clearest example of this new capability lies in efforts to characterize odour changes induced by variable emotional states, particularly those related to alarm or fear. These emotions are a promising starting point because they are expected to be critically important to human survival and thus subject to intense selection pressure. Indeed, chemosignals associated with alarm are known to occur widely in invertebrates, fishes and mammals, including mice [79]. In humans too, there is evidence that fearful state elicits odour changes which can be detected by others. In laboratory studies, people can discriminate between the odours of others that were collected while watching either stressful or benign films [37,80]. Exposure to odours of individuals collected while in fearful or anxious emotional states (e.g. students before an examination and participants in fear of falling from a height) induce changes in brain activation, mood and cognition [81–84]. These studies suggest the possibility that odour may communicate emotional state between individuals.
Studies are now beginning to uncover the chemical basis of these odour changes. Using PTR-TOF-MS, Williams et al. [85] measured changes in airborne compounds emanating from audiences in a cinema theatre. They observed spikes in certain airborne compounds as the audience simultaneously experienced specific emotions elicited by events portrayed in the film. Suspenseful scenes (including those associated with injury and chases) were associated with higher emission of several compounds known to occur in human odours, particularly in breath. Figure 2 further illustrates from our pilot data that these changes also likely occur specifically in axillary odour, over very short timescales.
While negative emotions such as fear have received more attention, particularly in relation to discrimination of fear by odour receivers, it is possible that other emotions may also influence odour. This is illustrated by odour from cinema audiences also being marked by emission of a further set of compounds in scenes associated with comedy [85]. In another study, similar spikes in breath-associated compounds were found in air from crowds of football spectators when a goal was scored, although the extent to which these are directly associated with emotion as opposed to movement (e.g. standing up) remains unresolved [86].
A final point here is stimulated by the approach used in the cinema and football studies [85,86]. The ability to measure changes in VOC concentrations in ambient air extends the range of sampling opportunities from individuals to larger groups and crowds [87]. This is potentially useful in identifying markers of given chemosignals because it provides a composite odour trace that should consistently contain a target signature, even a subtle one, while effectively averaging out non-target variation in individual odours. A useful ‘reverse-engineering’ approach might thus initially incorporate a group-based study to identify a set of candidate VOC markers for a putative chemosignal, perhaps facilitated by PMF, which can then be explored and tested in individual odours.
(c). Implications for experimental approaches
These advantages also offer new avenues for empirically testing odour responses to given social stimuli. A principal aim would be to investigate whether predicted changes in specified chemical signatures are observed in appropriate social contexts. Such changes would be predicted if they actively communicate a sender's current state.
For example, when people are placed within a fearful situation, we might expect to observe upregulation (or downregulation) of identified compounds or compound mixtures relating to fear (as suggested in figure 2), but no change in other parts of the odour profile. Based on previous work [37,80], we could expose experimental participants to a range of scenes varying in emotional salience, expecting to see the fear signature only in those scenes that elicit a fearful response. Variation in the intensity of the response might match individual differences in the degree of subjective fear, as assessed by self-report or by measures of physiological arousal. Nonetheless, we would expect a relatively uniform response across individuals, because a reliable cue of emotional state must be consistent if it is to be learned and thus to elicit appropriate responses. If these predictions are upheld, it would then be possible to examine corresponding (but time-shifted) odour changes in receivers, in a way that might be expected if odour contributes to emotional contagion [81,88,89].
This kind of approach is very appropriate to the rapid changes expected under variable emotional state, but also might be potentially extended to explore context-dependent changes for a range of other putative chemosignals. For example, because we know that perceived differences in individual axillary odours correlate with social dominance [32], a study investigating these relevant markers might have participants view scenes depicting strong competition (e.g. same-sex individuals engaged in a competitive task) or non-competitive scenes (e.g. the same individuals co-operating). Alternatively, given that odours of women are most attractive at the fertile phase of their menstrual cycle [31,90], researchers might assess the expression of candidate chemical markers of current fertility when the experience of attraction or sexual desire is elicited (e.g. viewing segments of scenes with attractive others), compared with when the same women view matched control scenes (e.g. scenes depicting less attractive others).
(d). Theoretical advances
In turn, these new approaches hold significant potential for advances relating to a core theoretical issue in communication: the signal versus cue debate. The distinction between an odour signal and a cue relates to whether a given trait's chemical signature has evolved specifically as a result of selective pressure to convey information about that trait (a signal) or, rather, is information that exists and may be used by others in drawing social inferences, but has not evolved for that purpose (a cue). A good illustration of this distinction comes from how mosquitoes locate human hosts, by the smell of carbon dioxide (CO2). The CO2 plume is a reliable indication of the presence of a host, but the host would prefer the information not to be made available—in this context, it is therefore a cue and not a signal. On the other hand, the compound bombykol, produced by females of the silkworm moth, attracts males for mating. This is to their mutual benefit and appears to be a true signal [91].
Whether information available in human odour are cues or signals is contentious. However, one possible approach is to look at patterns of context-dependent upregulation of specific components of an individual's volatilome. If upregulation of any candidate chemosignal occurs in a specific and relevant context, but not in other contexts, this could provide evidence that it is a signal in the true sense. Investigations of this kind become feasible through the real-time monitoring of chemical changes enabled by online CIMS.
For example, it has been suggested that perceivable peri-ovulatory changes in women's odour represent a signal of sexual receptivity [92]. By contrast, others have argued that while men's behaviour may be sensitive to these changes, the changes may more likely be cues, as they appear to be simple by-products of cyclical changes in steroid hormone levels that regulate the menstrual cycle—that is, they are useful but did not evolve for that purpose [93]. Separating these competing ideas is not straightforward. However, if we were able to identify current fertility markers within the odour of ovulating women, and then demonstrate that they are upregulated when women meet attractive men but not when they meet unattractive men, we might be more inclined to interpret these markers as signals than cues.
We concede that even specific upregulation of odour components according to a given context is not definitive or conclusive evidence of a true signal. It could still be argued that such changes occur as a by-product of other physiological responses, and we would also need to address whether and how receivers respond. Nonetheless, we suggest that a robust and reliable increase in production, at the precise time and in the precise context in which it is most beneficial, would probably be compelling evidence for a signal in any other sensory modality. The reason this issue may be so contentious when considering odour signals may lie with their relative inconspicuousness, at least in our human minds, when compared with visual and acoustic signals. Perhaps, if we were less accustomed to thinking about odour as being slow to change, being poorly detected, and having only subtle effects on our behaviour, then we would be more willing to consider context-specific increases in emission rates of specific VOC signatures as clear signals. In this light, and assuming of course that such changes did occur, they could be an olfactory equivalent of a peacock dramatically unfolding its train and rattling it in the direction of a passing peahen.
(e). Applications and impact
In addition to insights into chemical communication per se, we can anticipate a further range of practical uses to which the introduction of online monitoring of human chemical signals might be applied. Such possibilities arise from the technical ability to sample individual odour non-invasively and to detect rapid odour change. Exploring the variety of potential applications is beyond the scope of this paper, but we may briefly consider some possibilities arising specifically from the example of emotional markers, discussed above.
For example, Williams et al. [85] demonstrated how different emotional states could be tracked in real time by measuring air surrounding cinema audiences, and that certain scene types were associated with specific suites of chemical markers. This might create possibilities for using such data to produce an objective benchmark for the recommended age classification of new films [94], or to objectively assess emotional reaction to new films (or indeed other products). In a similar vein, an ability to identify extreme anxiety from the odour of people rapidly and remotely might be applied to identify individuals of interest while they queue to pass through security checks (e.g. at airports). Finally, the technique could also be used to ascertain an emotional state (whether positive or negative) in individuals who are otherwise unable to communicate, such as in coma patients or those with locked-in syndrome, or in very young infants.
7. Conclusion
Drawing on insights gained from their application in atmospheric and environmental chemistry, we have described two new approaches for the measurement and analysis of real-time change in human odour chemistry. Future studies could use these tools to resolve the odour changes induced in individuals in response to particular social or environmental situations. The capacity to focus on specific odour changes linked to concurrent situational cues, in ambient air emanating from specific body regions, offers significant advantages over many previously used approaches, facilitating the endeavour of identifying the mass spectral profiles characteristic of specific social cues. These techniques could become increasingly important tools in future efforts to decode the human social volatilome.
Supplementary Material
Acknowledgements
We thank Katarzyna Marciniak, Benoist Schaal and two anonymous reviewers for their comments on an earlier version of this manuscript. We also thank James Cash for help with data collection.
Ethics
Work contained in the paper was approved by the University of Stirling General University Ethics Panel (project no. 616-2019).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
All authors contributed to the ideas and writing of the manuscript. B.L. led the data collection and analysis of pilot data contained herein.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Hurst JL. 2009. Female recognition and assessment of males through scent. Behav. Brain Res. 200, 295–303. ( 10.1016/j.bbr.2008.12.020) [DOI] [PubMed] [Google Scholar]
- 2.Coombes HA, Stockley P, Hurst JL. 2018. Female chemical signalling underlying reproduction in mammals. J. Chem. Ecol. 44, 851–873. ( 10.1007/s10886-018-0981-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosling LM, Roberts SC. 2001. Scent-marking by male mammals: cheat-proof signals to competitors and mates. Adv. Stud. Behav. 30, 169–217. ( 10.1016/S0065-3454(01)80007-3) [DOI] [Google Scholar]
- 4.Roberts SC, Gosling LM. 2003. Genetic similarity and quality interact in mate choice decisions by female mice. Nat. Genet. 35, 103–106. ( 10.1038/ng1231) [DOI] [PubMed] [Google Scholar]
- 5.Schaal B, Coureaud G, Langlois D, Ginies C, Semon E, Perrier G. 2003. Chemical and behavioural characterisation of the rabbit mammary pheromone. Nature 424, 68–72. ( 10.1038/nature01739) [DOI] [PubMed] [Google Scholar]
- 6.Melrose DR, Reed HC, Patterson RL. 1971. Androgen steroids associated with boar odour as an aid to the detection of oestrus in pig artificial insemination. Br. Vet. J. 127, 497–502. ( 10.1016/s0007-1935(17)37337-2) [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen LEL, Lee TD, Roelofs WL, Zhang A, Daves GD. 1996. Insect pheromone in elephants. Nature 379, 684 ( 10.1038/379684a0) [DOI] [PubMed] [Google Scholar]
- 8.Archunan G, Rajagopal T. 2013. Detection of estrus in Indian blackbuck: behavioural, hormonal and urinary volatiles evaluation. Gen. Comp. Endocrinol. 181, 156–166. ( 10.1016/j.ygcen.2012.11.012) [DOI] [PubMed] [Google Scholar]
- 9.Harvey S, Jemiolo B, Novotny M. 1989. Pattern of volatile compounds in dominant and subordinate male mouse urine. J. Chem. Ecol. 15, 2061–2072. ( 10.1007/BF01207438) [DOI] [PubMed] [Google Scholar]
- 10.Novotny M, Harvey S, Jemiolo B. 1990. Chemistry of male dominance in the house mouse, Mus domesticus. Experientia 46, 109–113. ( 10.1007/bf01955433) [DOI] [PubMed] [Google Scholar]
- 11.Singer AG, Beauchamp GK, Yamazaki K. 1997. Volatile signals of the major histocompatibility complex in male mouse urine. Proc. Natl Acad. Sci. USA 94, 2210–2214. ( 10.1073/pnas.94.6.2210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DHL, Cavaggioni A, Beynon RJ. 2001. Individual recognition in mice mediated by major urinary proteins. Nature 414, 631–634. ( 10.1038/414631a) [DOI] [PubMed] [Google Scholar]
- 13.Karlson P, Luscher M. 1959. Pheromones: new term for a class of biologically active substances. Nature 183, 55–56. ( 10.1038/183055a0) [DOI] [PubMed] [Google Scholar]
- 14.Wyatt TD. 2014. Pheromones and animal behaviour. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Brennan PA, Binns EK. 2005. Vomeronasal mechanisms of mate recognition in mice. Chem. Senses 30, i148–i149. ( 10.1093/chemse/bjh157) [DOI] [PubMed] [Google Scholar]
- 16.Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. 2010. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male's odour. BMC Biol. 8, 75 ( 10.1186/1741-7007-8-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberles SD. 2014. Mammalian pheromones. Ann. Rev. Physiol. 76, 151–175. ( 10.1146/annurev-physiol-021113-170334). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyatt TD. 2015. The search for human pheromones: the lost decades and the necessity of returning to first principles. Proc. R. Soc. B 282, 20142994 ( 10.1098/rspb.2014.2994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lacy Costello B, Amann A, Al-Kateb H, Flynn C, Filipiak W, Khalid T, Osborne D, Ratcliffe N. 2014. A review of the volatiles from the healthy human body. J. Breath Res. 8, 014001 ( 10.1088/1752-7155/8/1/014001) [DOI] [PubMed] [Google Scholar]
- 20.Amann A, Costello B, Miekisch W, Schubert J, Buszewski B, Pleil J, Ratcliffe N, Risby T. 2014. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 8, 034001 ( 10.1088/1752-7155/8/3/034001) [DOI] [PubMed] [Google Scholar]
- 21.Sommerville BA, Green MA, Gee DJ. 1990. Using chromatography and a dog to identify some of the compounds in human sweat which are under genetic influence. In Chemical signals in vertebrates (eds Macdonald DW, Muller-Schwarze D, Natynczuk SE), pp. 634–639. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Fialová J, Roberts SC, Havlíček J. 2016. Consumption of garlic positively affects hedonic perception of axillary body odour. Appetite 97, 8–15. ( 10.1016/j.appet.2015.11.001) [DOI] [PubMed] [Google Scholar]
- 23.Havlíček J, Fialová J, Roberts SC. 2017. Individual variation in body odor. In The Springer handbook of odor (ed. Buettner A.), pp. 949–961. Berlin, Germany: Springer. [Google Scholar]
- 24.Roberts SC, Gosling LM, Spector TD, Miller P, Penn DJ, Petrie M. 2005. Body odor similarity in noncohabiting twins. Chem. Senses 30, 651–656. ( 10.1093/chemse/bji058) [DOI] [PubMed] [Google Scholar]
- 25.Russell MJ. 1976. Human olfactory communication. Nature 260, 520–522. ( 10.1038/260520a0) [DOI] [PubMed] [Google Scholar]
- 26.Ferdenzi C, Schaal B, Roberts SC. 2010. Family scents: developmental changes in the perception of kin body odor? J. Chem. Ecol. 36, 847–854. ( 10.1007/s10886-010-9827-x). [DOI] [PubMed] [Google Scholar]
- 27.Weisfeld GE, Czilli T, Phillips KA, Gall JA, Lichtman CM. 2003. Possible olfaction-based mechanisms in human kin recognition and inbreeding avoidance. J. Exp. Child Psychol. 85, 279–295. ( 10.1016/s0022-0965(03)00061-4) [DOI] [PubMed] [Google Scholar]
- 28.Hold B, Schleidt M. 1977. The importance of human odour in non-verbal communication. Z. Tierpsychol. 43, 225–238. ( 10.1111/j.1439-0310.1977.tb00072.x) [DOI] [PubMed] [Google Scholar]
- 29.Schaal B. 1988. Olfaction in infants and children: developmental and functional perspectives. Chem. Senses 13, 145–190. ( 10.1093/chemse/13.2.145) [DOI] [Google Scholar]
- 30.Schaal B, Saxton TK, Loos H, Soussignan R, Durand K. 2020. Olfaction scaffolds the developing human from neonate to adolescent and beyond. Phil. Trans. R. Soc. B 375, 20190261 ( 10.1098/rstb.2019.0261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Havlicek J, Dvorakova R, Bartos L, Flegr J. 2006. Non-advertized does not mean concealed: body odour changes across the human menstrual cycle. Ethology 112, 81–90. ( 10.1111/j.1439-0310.2006.01125.x) [DOI] [Google Scholar]
- 32.Havlicek J, Roberts SC, Flegr J. 2005. Women's preference for dominant male odour: effects of menstrual cycle and relationship status. Biol. Lett. 1, 256–259. ( 10.1098/rsbl.2005.0332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornhill R, Gangestad SW. 1999. The scent of symmetry: a human sex pheromone that signals fitness? Evol. Hum. Behav. 20, 175–201. ( 10.1016/S1090-5138(99)00005-7) [DOI] [Google Scholar]
- 34.Roberts SC, Kralevich A, Ferdenzi C, Saxton TK, Jones BC, DeBruine LM, Little AC, Havlicek J. 2011. Body odor quality predicts behavioral attractiveness in humans. Arch. Sex. Behav. 40, 1111–1117. ( 10.1007/s10508-011-9803-8) [DOI] [PubMed] [Google Scholar]
- 35.Havlicek J, Roberts SC. 2009. MHC-correlated mate choice in humans: a review. Psychoneuroendo 34, 497–512. ( 10.1016/j.psyneuen.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 36.Havlíček J, Winternitz J, Roberts SC. 2020. Major histocompatibility complex-associated odour preferences and human mate choice: near and far horizons. Phil. Trans. R. Soc. B 375, 20190260 ( 10.1098/rstb.2019.0260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ackerl K, Atzmueller M, Grammer K. 2002. The scent of fear. Neuroendocrinol. Lett. 23, 79–84. [PubMed] [Google Scholar]
- 38.de Groot JHB, Kirk PA, Gottfried JA. 2020. Encoding fear intensity in human sweat. Phil. Trans. R. Soc. B 375, 20190271 ( 10.1098/rstb.2019.0271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pause BM, Storch D, Lübke KT. 2020. Chemosensory communication of aggression: women's fine-tuned neural processing of male aggression signals. Phil. Trans. R. Soc. B 375, 20190270 ( 10.1098/rstb.2019.0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regenbogen C, Axelsson J, Lasselin J, Porada DK, Sundelin T, Peter MG, Lekander M, Lundström JN, Olsson MJ. 2017. Behavioral and neural correlates to multisensory detection of sick humans. Proc. Natl Acad. Sci. USA 114, 6400–6405. ( 10.1073/pnas.1617357114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarolidou G, Axelsson J, Kimball BA, Sundelin T, Regenbogen C, Lundström JN, Lekander M, Olsson MJ. 2020. People expressing olfactory and visual cues of disease are less liked. Phil. Trans. R. Soc. B 375, 20190272 ( 10.1098/rstb.2019.0272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savelev SU, et al. 2008. Individual variation in 3-methylbutanal: a putative link between human leukocyte antigen and skin microflora. J. Chem. Ecol. 34, 1253–1257. ( 10.1007/s10886-008-9524-1) [DOI] [PubMed] [Google Scholar]
- 43.Rennie PJ, Gower DB, Holland KT, Mallet AI, Watkins WJ. 1990. The skin microflora and the formation of human axillary odor. Int. J. Cosm. Sci. 12, 197–207. ( 10.1111/j.1467-2494.1990.tb00535.x) [DOI] [PubMed] [Google Scholar]
- 44.Misztal PK, et al. 2018. Emission factors of microbial volatile organic compounds from environmental bacteria and fungi. Environ. Sci. Technol. 52, 8272–8282. ( 10.1021/acs.est.8b00806) [DOI] [PubMed] [Google Scholar]
- 45.Havlíček J, Roberts SC. 2013. The perfume-body odour complex: an insightful model for culture-gene coevolution? In Chemical signals in vertebrates 12 (eds East M, Dehnhard M), pp. 185–195. Berlin, Germany: Springer. [Google Scholar]
- 46.Allen C, Havlíček J, Roberts SC. 2015. Effect of fragrance use on discrimination of individual body odor. Front. Psychol. 6, 1115 ( 10.3389/fpsyg.2015.01115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenochová P, Vohnoutová P, Roberts SC, Oberzaucher E, Grammer K, Havlíček J. 2012. Psychology of fragrance use: perception of individual odor and perfume blends reveals a mechanism for idiosyncratic effects on fragrance choice. PLoS ONE 7, e33810 ( 10.1371/journal.pone.0033810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindinger W, Hansel A, Jordan A. 1998. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Proc. 173, 191–241. ( 10.1016/S0168-1176(97)00281-4) [DOI] [Google Scholar]
- 49.de Gouw J, Warneke C. 2007. Measurements of volatile organic compounds in the Earth's atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev. 26, 223–257. ( 10.1002/mas.20119) [DOI] [PubMed] [Google Scholar]
- 50.Amador-Muñoz O, Misztal PK, Weber R, Worton DR, Zhang H, Drozd G, Goldstein AH. 2016. Sensitive detection of n-alkanes using a mixed ionization mode proton-transfer-reaction mass spectrometer. Atmos. Meas. Tech. 9, 5315–5329. ( 10.5194/amt-9-5315-2016) [DOI] [Google Scholar]
- 51.Jordan A, Haidacher S, Hanel G, Hartungen E, Märk L, Seehauser H, Schottkowsky R, Sulzer P, Märk T. 2009. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 286, 122–128. ( 10.1016/j.ijms.2009.07.005) [DOI] [Google Scholar]
- 52.Sulzer P, et al. 2014. A proton transfer reaction-quadrupole interface time-of-flight mass spectrometer (PTR-QiTOF): high speed due to extreme sensitivity. Int. J. Mass Spectrom. 368, 1–5. ( 10.1016/j.ijms.2014.05.004) [DOI] [Google Scholar]
- 53.Breitenlechner M, Fischer L, Hainer M, Heinritzi M, Curtius J, Hansel A. 2017. PTR3: an instrument for studying the lifecycle of reactive organic carbon in the atmosphere. Anal. Chem. 89, 5824–5831. ( 10.1021/acs.analchem.6b05110) [DOI] [PubMed] [Google Scholar]
- 54.Riva M, et al. 2019. Evaluating the performance of five different chemical ionization techniques for detecting gaseous oxygenated organic species. Atmos. Meas. Tech. 12, 2403–2421. ( 10.5194/amt-12-2403-2019) [DOI] [Google Scholar]
- 55.Hewitt C, Hayward S, Tani A. 2003. The application of proton transfer reaction-mass spectrometry (PTR-MS) to the monitoring and analysis of volatile organic compounds in the atmosphere. J. Environ. Monit. 5, 1–7. ( 10.1039/b204712h) [DOI] [PubMed] [Google Scholar]
- 56.Tang X, Misztal PK, Nazaroff WW, Goldstein AH. 2016. Volatile organic compound emissions from humans indoors. Environ. Sci. Technol. 50, 126 86–12 694. ( 10.1021/acs.est.6b04415) [DOI] [PubMed] [Google Scholar]
- 57.Stönner C, Edtbauer A, Williams J. 2018. Real-world volatile organic compound emission rates from seated adults and children for use in indoor air studies. Indoor Air 28, 164–172. ( 10.1111/ina.12405) [DOI] [PubMed] [Google Scholar]
- 58.Buszewski B, Rudnicka J, Ligor T, Walczak M, Jezierski T, Amann A. 2012. Analytical and unconventional methods of cancer detection using odor. Trends Anal. Chem. 38, 1–12. ( 10.1016/j.trac.2012.03.019) [DOI] [Google Scholar]
- 59.Wicker J, Krauter N, Derstorff B, Stönner C, Bourtsoukidis E, Klüpfel T, Williams J, Kramer S. 2015. Cinema data mining: the smell of fear. In Proc. 21th ACM SIGKDD Int. Conf. on Knowledge Discovery and Data Mining, pp. 1295–1304. New York, NY: ACM. [Google Scholar]
- 60.Paatero P, Tapper U. 1994. Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5, 111–126. ( 10.1002/env.3170050203) [DOI] [Google Scholar]
- 61.Paatero P. 1997. Least squares formulation of robust non-negative factor analysis. Chemometr. Intell. Lab. Syst. 37, 23–35. ( 10.1016/S0169-7439(96)00044-5) [DOI] [Google Scholar]
- 62.Wold S, Esbensen K, Geladi P. 1987. Principal component analysis. Chemometr. Intell. Lab. Syst. 2, 37–52. ( 10.1016/0169-7439(87)80084-9) [DOI] [Google Scholar]
- 63.Ulbrich IM, Canagaratna MR, Zhang Q, Worsnop DR, Jimenez JL. 2009. Interpretation of organic components from positive matrix factorization of aerosol mass spectrometric data. Atmos. Chem. Phys. 9, 2891–2918. ( 10.5194/acp-9-2891-2009) [DOI] [Google Scholar]
- 64.Canonaco F, Crippa M, Slowik JG, Baltensperger U, Prévôt ASH. 2013. SoFi, an IGOR-based interface for the efficient use of the generalized multilinear engine (ME-2) for the source apportionment: ME-2 application to aerosol mass spectrometer data. Atmos. Meas. Tech. 6, 3649–3661. ( 10.5194/amt-6-3649-2013) [DOI] [Google Scholar]
- 65.Crippa M, et al. 2014. Organic aerosol components derived from 25 AMS data sets across Europe using a consistent ME-2 based source apportionment approach. Atmos. Chem. Phys. 14, 6159–6176. ( 10.5194/acp-14-6159-2014) [DOI] [Google Scholar]
- 66.Lanz VA, et al. 2008. Source attribution of submicron organic aerosols during wintertime inversions by advanced factor analysis of aerosol mass spectra. Environ. Sci. Technol. 42, 214–220. ( 10.1021/es0707207) [DOI] [PubMed] [Google Scholar]
- 67.Jimenez JL, et al. 2009. Evolution of organic aerosols in the atmosphere. Science 326, 1525–1529. ( 10.1126/science.1180353) [DOI] [PubMed] [Google Scholar]
- 68.Lanz VA, Alfarra MR, Baltensperger U, Buchmann B, Hueglin C, Prévôt ASH. 2007. Source apportionment of submicron organic aerosols at an urban site by factor analytical modelling of aerosol mass spectra. Atmos. Chem. Phys. 7, 1503–1522. ( 10.5194/acp-7-1503-2007) [DOI] [Google Scholar]
- 69.Zhou X, Li Z, Zhang T, Wang F, Wang F, Tao Y, Zhang X, Wang F, Huang J. 2019. Volatile organic compounds in a typical petrochemical industrialized valley city of northwest China based on high-resolution PTR-MS measurements: characterization, sources and chemical effects. Sci. Total Environ. 671, 883–896. ( 10.1016/j.scitotenv.2019.03.283) [DOI] [PubMed] [Google Scholar]
- 70.Buzcu B, Fraser MP. 2006. Source identification and apportionment of volatile organic compounds in Houston, TX. Atmos. Environ. 40, 2385–2400. ( 10.1016/j.atmosenv.2005.12.020) [DOI] [Google Scholar]
- 71.Lanz VA, Hueglin C, Buchmann B, Hill M, Locher R, Staehelin J, Reimann S. 2008. Receptor modeling of C-2-C-7 hydrocarbon sources at an urban background site in Zurich, Switzerland: changes between 1993–1994 and 2005–2006. Atmos. Chem. Phys. 8, 2313–2332. ( 10.5194/acp-8-2313-2008) [DOI] [Google Scholar]
- 72.Atkinson R. 2000. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 34, 2063–2101. ( 10.1016/S1352-2310(99)00460-4) [DOI] [Google Scholar]
- 73.Shu Y, Atkinson R. 1995. Atmospheric lifetimes and fates of a series of sesquiterpenes. J. Geophys. Res. Atmos. 100, 7275–7281. ( 10.1029/95jd00368) [DOI] [Google Scholar]
- 74.Gierczak T, Burkholder JB, Bauerle S, Ravishankara AR. 1998. Photochemistry of acetone under tropospheric conditions. Chem. Phys. 231, 229–244. ( 10.1016/S0301-0104(98)00006-8) [DOI] [Google Scholar]
- 75.Slowik JG, Vlasenko A, McGuire M, Evans GJ, Abbatt JPD. 2010. Simultaneous factor analysis of organic particle and gas mass spectra: AMS and PTR-MS measurements at an urban site. Atmos. Chem. Phys. 10, 1969–1988. ( 10.5194/acp-10-1969-2010) [DOI] [Google Scholar]
- 76.Vlasenko A, et al. 2009. Measurements of VOCs by proton transfer reaction mass spectrometry at a rural Ontario site: sources and correlation to aerosol composition. J. Geophys. Res. Atmos. 114, D21305 ( 10.1029/2009jd012025) [DOI] [Google Scholar]
- 77.Paatero P, Hopke PK. 2009. Rotational tools for factor analytic models. J. Chemometr. 23, 91–100. ( 10.1002/cem.1197) [DOI] [Google Scholar]
- 78.Paatero P, Hopke PK, Song XH, Ramadan Z. 2002. Understanding and controlling rotations in factor analytic models. Chemometr. Intell. Lab. Syst. 60, 253–264. ( 10.1016/S0169-7439(01)00200-3) [DOI] [Google Scholar]
- 79.Brechbühl J, Klaey M, Broillet MC. 2008. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science 321, 1092 ( 10.1126/science.1160770) [DOI] [PubMed] [Google Scholar]
- 80.Chen D, Haviland-Jones J. 2000. Human olfactory communication of emotion. Perc. Motor Skills 91, 771–781. ( 10.2466/pms.2000.91.3.771) [DOI] [PubMed] [Google Scholar]
- 81.Albrecht J, et al. 2011. Smelling chemosensory signals of males in anxious versus nonanxious condition increases state anxiety of female subjects. Chem. Senses 36, 19–27. ( 10.1093/chemse/bjq087) [DOI] [PubMed] [Google Scholar]
- 82.Mujica-Parodi L, Strey H, Frederick B, Savoy R, Cox D, Botanov Y, Tolkunov D, Rubin D, Weber J. 2009. Chemosensory cues to conspecific emotional stress activate amygdala in humans. PLoS ONE 4, e6415 ( 10.1371/journal.pone.0006415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prehn-Kristensen A, Wiesner C, Bergmann T, Wolff S, Jansen O, Mehdorn H, Ferstl R, Pause B. 2009. Induction of empathy by the smell of anxiety. PLoS ONE 4, e5987 ( 10.1371/journal.pone.0005987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pause B, Ohrt A, Prehn A, Ferstl R. 2004. Positive emotional priming of facial affect perception in females is diminished by chemosensory anxiety signals. Chem. Senses 29, 797–805. ( 10.1093/chemse/bjh245) [DOI] [PubMed] [Google Scholar]
- 85.Williams J, Stönner C, Wicker J, Krauter N, Derstroff B, Bourtsoukidis E, Klüpfel T, Kramer S. 2016. Cinema audiences reproducibly vary the chemical composition of air during films, by broadcasting scene specific emissions on breath. Sci. Rep. 6, 25464 ( 10.1038/srep25464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stönner C, Williams J. 2016. Goals change crowd air chemistry. Nature 535, 355 ( 10.1038/535355a) [DOI] [PubMed] [Google Scholar]
- 87.Williams J, Pleil J. 2016. Crowd-based breath analysis: assessing behavior, activity, exposures, and emotional response of people in groups. J. Breath Res. 10, 032001 ( 10.1088/1752-7155/10/3/032001) [DOI] [PubMed] [Google Scholar]
- 88.Hatfield E, Cacioppo J, Rapson R. 1993. Emotional contagion. Curr. Dir. Psychol. Sci. 2, 96–100. ( 10.1111/1467-8721.ep10770953) [DOI] [Google Scholar]
- 89.Barsade SG. 2002. The ripple effect: emotional contagion and its influence on group behavior. Admin. Sci. Quart. 47, 644–675. ( 10.2307/3094912) [DOI] [Google Scholar]
- 90.Singh D, Bronstad PM. 2001. Female body odour is a potential cue to ovulation. Proc. R. Soc. B 268, 797–801. ( 10.1098/rspb.2001.1589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wyatt TD. 2011. Pheromones and behavior. In Chemical communication in crustaceans (eds Breithaupt T. & Thiel M.), pp. 23–38. New York, NY: Springer. [Google Scholar]
- 92.Miller SL, Maner JK. 2010. Scent of a woman: men's testosterone responses to olfactory ovulation cues. Psychol. Sci. 21, 276–283. ( 10.1177/0956797609357733) [DOI] [PubMed] [Google Scholar]
- 93.Havlíček J, Cobey KD, Barrett L, Klapilová K, Roberts SC. 2015. The spandrels of Santa Barbara? A new perspective on the peri-ovulation paradigm. Behav. Ecol. 26, 1249–1260. ( 10.1093/beheco/arv064) [DOI] [Google Scholar]
- 94.Stönner C, Edtbauer A, Derstroff B, Bourtsoukidis E, Klüpfel T, Wicker J, Williams J. 2018. Proof of concept study: testing human volatile organic compounds as tools for age classification of films. PLoS ONE 13, e0203044 ( 10.1371/journal.pone.0203044) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.



