Abstract
Interactions relating to human chemical signalling, although widely acknowledged, are relatively poorly characterized chemically, except for human axillary odour. However, the extensive chemical ecology of insects, involving countless pheromone and other semiochemical identifications, may offer insights into overcoming problems of characterizing human-derived semiochemicals more widely. Current techniques for acquiring insect semiochemicals are discussed, particularly in relation to the need for samples to relate, as closely as possible, to the ecological situation in which they are naturally deployed. Analysis is facilitated by chromatography coupled to electrophysiological preparations from the olfactory organs of insects in vivo. This is not feasible with human olfaction, but there are now potential approaches using molecular genetically reconstructed olfactory preparations already in use with insect systems. There are specific insights of value for characterizing human semiochemicals from advanced studies on semiochemicals of haematophagous insects, which include those involving human hosts, in addition to wider studies on farm and companion animals. The characterization of the precise molecular properties recognized in olfaction could lead to new advances in analogue design and a range of novel semiochemicals for human benefit. There are insights from successful synthetic biology studies on insect semiochemicals using novel biosynthetic precursors. Already, wider opportunities in olfaction emerging from in silico studies, involving a range of theoretical and computational approaches to molecular design and understanding olfactory systems at the molecular level, are showing promise for studying human semiochemistry.
This article is part of the Theo Murphy meeting issue ‘Olfactory communication in humans’.
Keywords: semiochemical, pheromone, chemical signalling, olfaction, human, insect
1. Introduction
Studies on insect chemical ecology receive more attention than human semiochemistry because of their impact as pests and also, more recently, with the growing concern for raising the value of insects in ecosystem services such as pollination. However, vertebrate and particularly mammal semiochemistry is more sophisticated in terms of the behaviours evoked, presumably on account of the highly evolved interactions with the central nervous system of these animals, especially true for human interactions. Here, some insights are offered from studies on insects that may be of value in chemically characterizing the cues underlying olfactory communication in human beings and other vertebrates. The first step in characterizing semiochemicals involves capturing samples for confirmation as being biologically active. For insects, there is usually an emphasis on such samples being derived directly from the insect itself and its own exocrine secretory system. Although bacteria living superficially on the cuticle of insects or at sites of excretion have provided some insights, for human studies there is more value in studying bacterial and other parasite-associated semiochemistry for non-invasive disease detection and relating directly to human interactions. The paper in this issue by Andreas Natsch and Roger Emter [1] describes the production of highly impacting human semiochemicals from the conversion of essential precursors by human commensal bacteria. Semiochemicals from insects are preferably captured during a situation ecologically relevant to the signalling process under study, e.g. mating or host location. There is often a specific chemical composition of the pheromones for individual insect species, although there can be some intraspecific variation. However, human beings and other mammals often produce particular pheromonal signatures that differentiate individuals.
For insects, we can access the olfactory signal recognition system by insertion of microelectrodes into the olfactory organs, i.e. antennal electrophysiology. This allows direct monitoring of chromatographically separated components of complex mixtures comprising semiochemicals, and obviates the need to identify individual components for behavioural testing by allowing early pinpointing of biologically active compounds. It is also possible genetically to modify insect olfactory systems, for example, recent advances in the neurophysiology of the fruit fly Drosophila melanogaster to investigate more sophisticated aspects of insect semiochemistry, in addition to chemical characterization. This raises new prospects for studying human semiochemistry, with the potential for using overexpressed human molecular recognition genetics in non-sentient animal systems. However, while we await advances towards this approach, we can still exploit the insect system, particularly where insects have an evolutionary dependence on human semiochemistry, and we already have evidence for biting flies detecting not only human attractants but semiochemicals that might relate directly to human interactions.
The rational design of better-performing insect semiochemical analogues is more difficult than for drug and pesticide discovery because of the substantially more precise nature of semiochemical recognition, but some opportunities are now being realized by synthetic biological techniques. We may also have new theoretical approaches to molecular design, which could be applied to human semiochemical development.
2. Capturing semiochemicals from insects
An early dispute over the natural composition of a honeybee pheromone involved in foraging and swarming behaviour was resolved using samples taken directly from the site of pheromone generation. This was achieved by injecting, and then retracting, minute volumes of inert solvent (figure 1) from the closed secretory Nasonov gland known to produce the pheromone [2]. This approach established that aerial oxidation did not produce artefactual components, as had been claimed, but that these were generated biosynthetically during release [3]. This pheromonal composition was then successfully mimicked in the field by a polymeric slow-release formulation [4] and is currently a commercially valuable tool in apiculture, e.g. Swarm Commander. This approach may not appear to offer the direct application in human semiochemical elucidation, but a sampling of regions of human skin, and particularly organs within the epidermis, would comprise an analogous approach (figure 2). For studies on human and other vertebrate pheromones, many solvents cause irritation, whereas for insects, there is much higher tolerance of solvent contact. Even inter-tergite regions of insects can accept hydrocarbon solvents without trauma. Instead of using solvents, polymeric absorbents, e.g. those for reverse-phase liquid chromatography [5], can be used to extract human skin volatiles directly, and then either be eluted with solvent or the volatiles desorbed by a flow of inert gas for analysis. For identifying insect pheromones as they are released into the air above an insect, entrainment onto a porous polymer is most effective and can accurately measure the naturally released pheromone composition [6]. A very simple system employs solid-phase microextraction (SPME) such as by a retractable absorbent polymer fibre, which is exposed to the headspace above the pheromone-releasing insect and combined as a syringe device for direct insertion for gas chromatographic (GC) analysis [7]. This approach has the disadvantage of a relatively small part of the headspace being sampled and the sample is directly eluted into the GC apparatus, precluding multiple analysis, for example, of the chemical composition and biological activity [7]. The most successful approach is by the entrainment of air from above the biological sample (figure 3) and by drawing the air through a porous polymer and eluting with a purified solvent, to obtain a liquid sample for multiple types of analysis [6]. For long-term storage, the sample must be sealed in a glass ampoule under an inert gas, preferably nitrogen. Insects can be held in a glass vessel through which a stream of clean air is passed. If the system is not sealed prior to the airflow being analysed, a positive pressure should be established to ensure no contamination enters the system. This can be readily applied to the study of mammal semiochemicals and has been used for studies on human beings [8,9] and farm [10] and companion animals [11]. Here, polyester or other non-contaminating polymeric bags are used to enclose parts of the body to be sampled selectively, and to exclude regions producing extraneous volatiles. Thus, volatiles from the head and thereby breath can be obviated [8] (figure 2). Such a procedure led to the identification of a group of simple compounds from children, comprising short-chain oxo-substituted alkanes (figure 4), which in the context of other human volatile compounds increased attraction of mosquitoes vectoring the causative pathogens of malaria [9].
Figure 1.
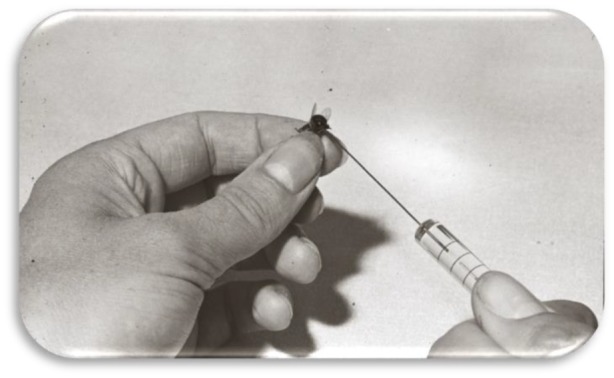
The Nasonov pheromone of the honeybee is sampled by placing the tip of a blunted syringe needle into the closed Nasonov gland minimizing aerial oxidation or other perturbation of composition. (Courtesy Ingrid Williams, Rothamsted Research).
Figure 2.

Sampling air from over a particular region of human skin for isolation of volatile semiochemicals. (Courtesy Jetske de Boer, Netherlands Institute of Technology). (Online version in colour.)
Figure 3.

Portable apparatus for entrainment of volatiles from over biological samples, under positive pressure of purified air, by absorption on porous polymer. (Courtesy Irene Castellan, Cardiff University). (Online version in colour.)
Figure 4.
Human volatile compounds, associated with infection by the causative pathogen of malaria and increased attractiveness to the mosquito vector, isolated from children by air entrainment and identified by GC-mass spectrometry following GC-electroantennography studies.
For insects, most pheromones are emitted away from waste excretory sites. However, as with human semiochemistry, there are exceptions. Thus, an aggregation pheromone, and faecal or detritus-related semiochemicals, can be entrained from the air above cellulosic substrates acting as refuges for the common bed bug, Cimex lectularius, and show maximal aggregation activity [12]. Indeed, socks worn by human subjects function maximally to capture, directly, human-related semiochemicals for behavioural assays with haematophagous insects [13]. However, for GC-coupled electrophysiological and chemical analysis, entrainment onto porous polymers, such as Porapak Q or Tenax TA (figure 3), from air having passed over the feet of individuals, is employed [9]. In more sophisticated investigations, for example on the subtle inherited differences of human semiochemical profiles, volatile samples are isolated directly by entrainment of air from over human hands [8,14].
3. Capturing semiochemicals from the insect environment, including on the host
Insects can alter their pheromone composition or response when releasing on or near their host, e.g. sex pheromones released when the insect is on its host and thus indicating a food supply for ensuing larvae, particularly by synergizing the pheromone response [15,16]. Entrainment of the semiochemicals via porous polymers, with desorption by inert gas stripping, can allow the analysis of compounds normally emerging with the solvent from the chromatograph, again using positive air pressure in glass vessels or polyester bags surrounding the host or part of the host (figure 3) [7]. When insects feed on their hosts, this can reduce the attractiveness of the host to subsequent attack, particularly by that insect species, thus avoiding competition. For plant hosts, this has been recently reviewed [17] and also discussed for vertebrate hosts [18,19], particularly in terms of the contextual relationship with feeding stress and non-host-related repellents. The analogy with human and other vertebrate semiochemical studies is that we can study signalling under stress to identify semiochemical markers of pathogen or parasite development [9], which can be useful in early diagnosis and may, perhaps, offer remedies for psychological problems.
4. Exploitation of haematophagous insects
Insects that have vertebrate hosts, particularly for the consumption of blood, not only determine the suitability of potential hosts by taxonomic recognition via semiochemicals but can also avoid closely related animals which are less suitable hosts. This involves the insect detecting the presence, or heightened levels, of certain compounds which, in the context of normal host semiochemicals, cause avoidance or even repulsion. This phenomenon is an example of contextual repellency [18,19]. Thus, the tsetse flies, Glossina species, comprising the vectors of trypanosomes causing animal trypanosomiasis or nagana in sub-Saharan Africa, are known to avoid the non-host waterbuck, Kobus ellipsiprymnus defassa, although the livestock cattle hosts are in the same family (Bovidae). The semiochemicals identified as effecting this avoidance (i.e. pentanoic acid, 2-methoxyphenol, 6-propyltetrahydro-2H-2-one, (E)-6,10-dimethylundeca-5,9-dien-2-one) are used to protect cattle in the field against infection by trypanosomes [20]. Even within a breed of cattle [10] and within the human species, variation in the semiochemistry of individuals causes contextual inhibition of potential host characteristics [8]. This provides repellents for experimental treatments on human arms to repel mosquitoes, including Anopheles gambiae, the vector of the causative pathogens (Plasmodium species) of malaria [21], in favourable comparison with DEET, the ‘gold standard’ of mosquito repellents.
From these studies, it is evident that haematophagous insects can detect semiochemicals underpinning host choice relating to human physiology, in addition to the manipulation by Plasmodium species of human semiochemistry causing attraction of the vector An. gambiae. There is evidence that even changes in human physiology, e.g. throughout the menstrual cycle, can be detected behaviourally by the yellow fever mosquito, Aedes aegypti (JG Logan and CM Woodcock 2000, unpublished). This means that, while the development of non-invasive molecular tools directly for neurophysiological detection of human semiochemicals is awaited, live recordings comprising electrophysiological preparations from the antennae of haematophagous insects can be used to detect human semiochemicals unrelated, directly, to insect behaviour. Not only electroantennogram preparations of entire antennae but also single neuron preparations can be coupled to high-resolution GC for human semiochemical identification studies and, as a technique for studying mosquito olfaction, has been available for over two decades [22].
The close relationship between human volatile production and olfactory recognition by haematophagous insects suggests that human pheromones may be detected by these insects. Thus, insect electrophysiology could be used in the identification of human pheromone components. Furthermore, olfactory organs of insects, specifically the ‘empty’ neuron of the fruit fly, D. melanogaster, can be modified to express functional olfactory proteins heterologously from other insects [23] to understand the mechanism, at the molecular level, that helps to discover largely unrecognized olfactory proteins and receptors. More speculatively, the opportunity may eventually arise for expressing human olfactory receptor proteins in these electrophysiologically more compliant organisms for direct identification studies of human semiochemistry. The fundamental differences between insect and mammalian olfactory systems are well understood (see §5a) and so, for this speculative approach to be realized, the amino acid sequences for molecular recognition by the proteins involved in human olfaction would need to be genetically expressed within the functional background of the insect olfactory system.
5. Rational design of better-performing semiochemical analogues
(a). Biosynthetic approaches
In learning from insect semiochemistry, there are well-defined differences in the olfactory processes of insects compared with mammals. Insects have a family of odorant-gated ion channels comprising a highly conserved co-receptor (Orco), specifically functioning as the odorant-gated ion channel, and a divergent odorant receptor (OR) that confer chemical specificity and chemical signal amplification. In mammals, this is delivered by G-protein-coupled ORs [24,25]. Both are bathed in an aqueous lumen, with transport to the OR from the air mediated by odorant-binding proteins (OBPs) or lipocalin-like proteins, respectively. However, the molecular recognition systems in both, although insufficiently well understood for the predictable design of analogues, appear to be essentially similar. The rational design of semiochemical analogues (i.e. synthetic chemicals with biological activities related to those of known insect semiochemicals but with improved properties, such as resistance to oxidation or isomerization under sunlight) would be an obvious way forward. However, the molecular recognition of semiochemicals, as for all olfactory recognition systems, as well as being poorly understood is extremely selective because of the need to discriminate semiochemicals from the vast range of other volatile compounds in the environment [24].
For drugs and pesticides, the recognition system is protected by the blood/brain barrier and other physiological filters, making the task of designing biologically active analogues a much more rational process than for semiochemicals. However, some opportunities are now being realized for insect semiochemicals by a synthetic biological approach, which produces novel analogues by biosynthesis. In this approach, novel non-natural precursors of semiochemical ligands, from the late stages of the biosynthesis route, are fed to the final synthase enzymes in the biosynthesis of the semiochemical. This strategy has tested the hypothesis that, if the enzyme accepts the novel precursor and a semiochemical-like product ensues, the product will have a sufficiently similar chemical ‘space’ to the natural semiochemical to show related biological activity. This hypothesis has tested true for the phytophagous insects, aphids, by which analogues of the natural plant stress-related sesquiterpene (S)-germacrene D have been generated that are highly active [26]. This work is currently being further developed for pest control in a push-pull system, by which the natural repellent (S)-germacrene D (push), and novel analogues, e.g. (S)-15-methylgermacrene, together with a novel attractant, (S)-14,15-dimethylgermacrene D (pull), are tested in the field. New semiochemicals have been targeted for related studies, for example, 7-epizigiberene [27], a powerful whitefly repellent from wild tomato plants which is produced by a related biosynthetic pathway with similar potential late-stage biosynthetic precursors. This approach could be applied to human semiochemistry once new work has identified the specific enzymology by which some human semiochemicals are biosynthesized, including terpenoids related to the plant semiochemical above, in which homozygous (identical) human twins are analysed for mosquito attraction and the correlation investigated for associated semiochemicals and genetics already known to exist [14].
(b). In silico approaches
Currently, we do not have sufficient understanding of the interaction between olfactory ligands and olfactory recognition proteins for the predictable design of novel synthetic semiochemicals. However, for drug and pesticide design, considerable knowledge is available and the synthesis of highly active analogues of natural and synthetic lead structures for molecular design is routine. In this process, computer-based (in silico) molecular design is categorized as either ligand-based or structure-based. Ligand-based (in silico) strategies are used from the existing knowledge of ligand structures, which for this example would be known semiochemicals. This technique is particularly useful when the experimental three-dimensional structure of the recognition protein is not available, which is the situation with olfaction. Indeed, historically, ligand-based methods have been used in semiochemical studies (see §5b(i)). However, without information on a direct protein interaction, we can potentially use an approach called homology modelling if coordinates from a related system are available (see §5b(ii)). This would then make available structure-based studies for olfaction by a virtual approach.
A major problem in earlier attempts to design novel semiochemicals in olfaction research is the perceived need to have relatively low molecular weights, and thereby boiling points, to provide sufficient volatility to reach the olfactory apparatus of insects. However, these essential physico-chemical restrictions do allow for some higher molecular weights, provided the compounds are sufficiently lipophilic for a positive vapour pressure. For example, although (5R,6S)-6-acetoxy-5-hexadecanolide contains four oxygen atoms with the molecular weight of 312.45 Da, it is the oviposition pheromone for Culex spp. mosquitos, where the response is via olfaction.
As described in §5a, semiochemicals are perceived by insects in association with OBPs which, when bound together, are recognized by ORs [24,25]. Furthermore, when studied in vitro, OBPs are apparently highly promiscuous and many ORs are not apparently selective, although certain ORs do selectively recognize pheromones. However, when this arrangement is complete in vivo, molecular recognition is spectacularly selective and sensitive. Additionally, insect ORs are only active when forming a heteromeric complex with the co-expressed Orco (also known as ‘tuning’ receptor subunits [28–31]) and OR neurons also play a mediating role in signal transduction [32,33]. This complex molecular recognition system poses difficulties, of course, even in ligand-based modelling.
(i). Ligand-based approaches
The first ligand-based studies in olfaction involved structure–activity relationships (SARs), i.e. the relationships between the chemical structure of pheromones and other semiochemicals and their olfactory activities in insects. For further advances, these relationships were studied quantitatively by measuring biological activity against different but related chemical structures, termed quantitative structure–activity relationships (QSARs). In this, QSAR provides a mathematical model derived from a series of similar molecules possessing known physiological activities, correlated with their physico-chemical properties. For insects, the physiological activity can readily be derived from the electrophysiological activity (see §4). However, for human physiological activity, this would be more difficult to measure unless, in the future, it proves possible to express human molecular olfactory recognition in insect systems as proposed speculatively earlier (see §4). For physico-chemical properties in QSAR of semiochemicals, predicted volatility from molecular parameters [34] would need to be added to other components of physico-chemical properties, normally used in the study of QSARs for drugs and pesticides [35,36]. In practical terms, a series of semiochemicals and analogues possessing experimental olfactory activity is divided into training and test sets. The training set is used to derive a QSAR model, and the test set is used to determine the quality of the model. For semiochemicals, the advanced QSAR modelling technique, 3D-QSAR, developed using the three-dimensional properties of chemical structures, including molecular shape, electrostatic and hydrophobic properties calculated in three-dimensional space, could be useful in developing new semiochemicals.
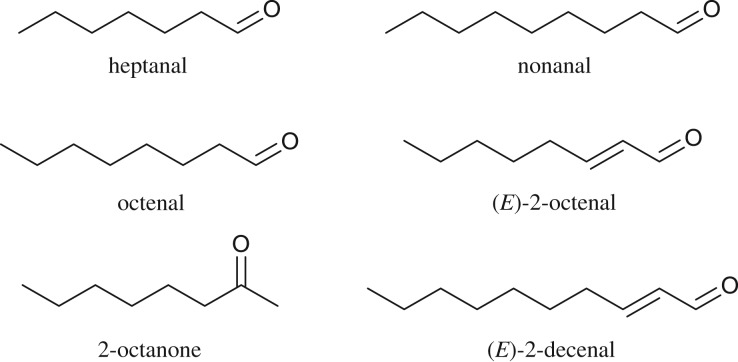
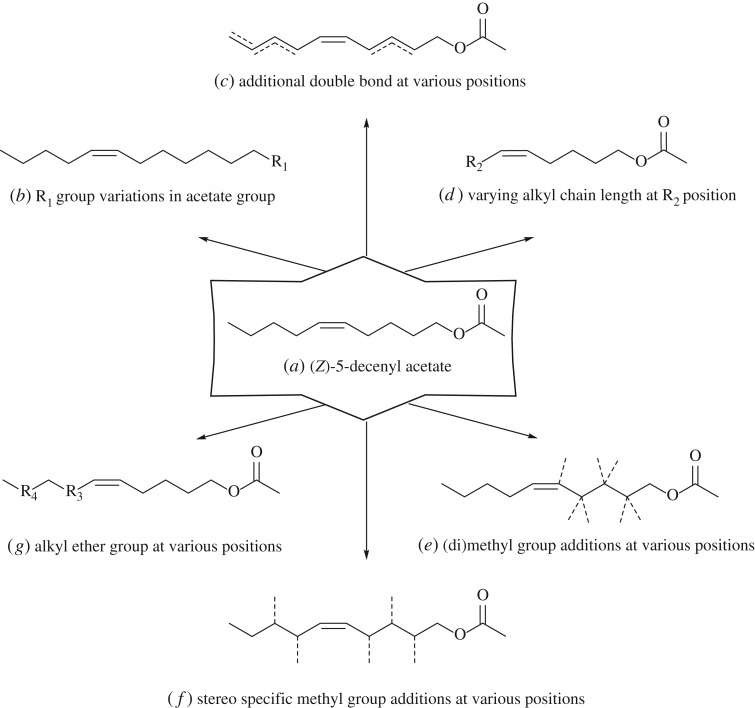
For insect olfaction, SAR studies were initiated with the identification of (Z)-7-dodecenyl acetate and other minor components, including (Z)-5-decenyl acetate, as the sex pheromone produced by the female turnip moth, Agrotis segetum [37]. Synthetic analogues of (Z)-7-dodecenyl acetate led to SARs emphasizing the importance of the acetate group, along with strict requirements for the polar functional group for pheromonal activity in A. segetum [38]. The SAR study was extended to using (Z,E)-dienic analogues of the minor pheromonal component (Z)-5-decenyl acetate, which identified a new role for E double bonds in conferring pheromonal activity [39]. Varying the alkyl chain length among the analogues of (Z)-5-decenyl acetate led to an extended SAR, suggesting that the terminal alkyl chain requires a specific chain length for interaction with a hydrophobic part of the protein target [40]. Subsequently, the introduction of methyl groups into the carbon chain of (Z)-5-decenyl acetate [41] showed discrimination among the ensuing optical isomers [42]. This investigation provided the first SAR example (figure 5) of a systematic variation in chemical structures and impact on pheromonal activity. Although this was an interesting initiation to the SAR study of semiochemicals, the lack of improved activity suggested further refinements for the approach, and so the data from 49 analogues were used for 3D-QSAR modelling [43]. Although this 3D-QSAR provided three-dimensional contour maps of the potential binding pocket of the target protein, it again did not lead to the production of new semiochemicals and pointed to the need for further experiments.
Figure 5.
Development of analogues of the insect sex pheromone from the female moth Agrotis segetum for (a) minor component (Z)-5-decenyl acetate analogues; (b) major component (Z)-7-dodecenyl acetate analogues by varying group R1; (c) analogues introduced with one or more E double bonds; (d) analogues with varying carbon chain length at R2; (e) methyl analogues with increased branching; (f) methyl analogues with defined chirality; (g) analogues incorporating ether functionality at R3 or R4 and having varying alkyl chain lengths.
Human olfaction. An early attempt to study human semiochemistry by QSAR targeted the olfactorily active components of ambergris. Although ambergris is a naturally produced lipoidal secretion known for its fixative properties in perfumery [44], the principal component, a triterpene alcohol, ambrein, oxidized into a range of compounds with human odorant properties. A QSAR study on 98 of these compounds was made, in which physiological activities were measured using a panel of human volunteers trained on a series of 10 semantic odour descriptors (i.e. different types of smells including earthy, woody, camphor, fruity, rosy, marine, sandalwood, musky and cedarwood) [45]. This was correlated with a set of physico-chemical properties expressing descriptors of molecular characteristics, including chirality and other topological elements and atomic connectivity, calculated with the help of a range of computer software tools [45]. This type of modelling could be used to derive synthetic ambergris and other fragrances, depending upon the availability of a wide range of natural analogues.
An artificial intelligence approach has been made to correlate physico-chemical parameters of human semiochemicals with their olfactory perception. This involved establishing a crowdsourcing non-profit community working through contributors across the research spectrum, including researchers from universities, technology companies, not-for-profit agencies, and biotechnology and pharmaceutical companies, entitled the DREAM Olfaction Prediction Consortium. The objective was to develop machine-learning algorithms that could differentiate 476 structurally diverse odorant molecules [46]. For this, the physiological parameters, determined according to 21 semantic odorant descriptors, were derived initially from the individual perceptions of a panel of 49 volunteers. An additional 19 semantic odorant descriptors were derived by averaging the population perceptions, also from the 49 volunteers. The artificial intelligence approach allowed a greater number of physico-chemical properties than normally available to QSAR to be correlated with the olfactory properties of the odorant molecules [47]. Among the dataset from the 476 molecular structures, 338 were given to 19 participating teams at the international level, while the omitted 69 were used in qualifying tests. Of the participating 19 teams, one team was found to be the most effective in developing machine-learning in silico models, specialized in predicting individual perceptions. A second team was found to be most effective in developing in silico predictive models, specialized in population perception [47,48]. These two separate artificial machine-learning models were then used for differentiating structurally diverse molecules from the 376, based on odour intensity and pleasantness, and could also correctly predict 8 among 19 semantic odour descriptors, specifically garlic, fish, sweet, fruit, burnt, spices, flower and sour.
Future ligand-based directions. Use of simple QSAR and 3D-QSAR based approaches to semiochemical analogue production could be more valuable if vastly more molecular structures were analysed, also employing the apparent advantages of a machine-learning approach [47,48]. Because QSAR studies usually do not use entirely random libraries, it is essential to include structural diversity so as not to miss unexpected SARs, particularly important for semiochemical invention where there are structurally unrelated compounds with similar biological activity. In silico searches of vast theoretical libraries could provide virtual hits to be followed by synthesis, but this objective would not be served by the use of combinatorial libraries which, by definition, lack diversity. Indeed, for semiochemical analogue discovery, the creation of specifically diverse semiochemical-related libraries would be valuable. Commercially available virtual libraries, such as Enamine-REAL [49] and ZINC [50], containing millions of molecular structures, could be assessed for the availability of semiochemical-like molecules. This could be achieved by searching natural semiochemical structures, eliminating non-volatile compounds and those likely to interact pharmacologically with the insect recognition processes.
(ii). Structure-based approaches
Many aspects of the mechanisms and physiology of olfactory systems, including the key ORs, remain unknown. There are nearly 400 human ORs identified but no X-ray, nuclear magnetic resonance or even cryogenic electron microscopy (cryo-EM) based structures have been reported [51,52]. Since these ORs are relatively high molecular weight transmembrane proteins and difficult to crystallize, this limits olfactory research and the identification of human pheromones. A cryo-EM structure for an insect Orco (a co-expressed co-receptor), which is essential for the functioning of insect ORs (see §5a), has recently been reported, but only at a low resolution [24].
Essentially, for advancing human olfactory research, it is important to identify the functionality and molecular mechanisms of G-protein-coupled ORs. Therefore, in the absence of experimental structural data, we can use homology modelling, involving structure-based computational biology in conjunction with chemical biology. Homology models of ORs can be built using computational approaches in multiple steps, sequentially starting from protein sequence alignment search tools [53,54], for identifying similar protein or protein domain structures from the protein data banks [55,56]. The best matches can then be used as templates for the construction of the homology model and there are well developed methods available for this general purpose [57,58]. However, it is crucial to assess the quality of the newly constructed homology model by means of various, equally well established, techniques [57–59]. This homology model can be tested in silico using relevant semiochemical structures and further refinements made to the homology model. The model is then tested experimentally to access discrimination between semiochemical structures.
The putative human odorant receptors OR5AN1 and OR1A1, for musk-related odorants used in perfumery, have been modelled to derive virtual three-dimensional homology models [60]. In this process, an X-ray crystal structure of a specific muscarinic acetylcholine receptor was used as a template. This receptor comprises the closest homologue protein for which there is a high-grade crystal structure, and which is defined by interaction with the alkaloid muscarine. The homology models of OR5AN1 and OR1A1 were used to identify the binding specificity of 35 musk-related odorants, including all three classes of aromatic nitro-, polycyclic- and macrocyclic-musk odorants. Computer-based studies of molecular interactions showed how these musk odorants differentially interacted with the homology models of OR5AN1 and OR1A1. This was experimentally confirmed using a human cell line (Hana3A) to express, heterologously, the OR5AN1 and OR1A1 proteins. In addition, the amino acid residues identified as essential for molecular recognition in the homology model were confirmed experimentally by directed mutation studies, Tyr260 to Phe in OR5AN1, and Tyr251 to Phe and Tyr258 to Phe in OR1A1 proteins [60].
In another study, a homology model of OR7D4 was developed from the template protein structure of a β2-adrenergic receptor [61]. This model was then used to investigate the differential perception of androstenone as being either foul-smelling or pleasant. This work related to the natural mutant variants in the human OR, OR7D4-WM, confirming in silico that the mutations Arg88 to Typ and Thr133 to Met should change the perception of androstenone from foul to pleasant. As with the previous study, the amino acids identified as crucial for olfactory discrimination were confirmed by experimental mutation studies, with a success rate of 75% [61].
6. Conclusion
As the growing need for exploiting insect semiochemistry becomes even more apparent, there will perhaps be more insights gained in the quest to overcome the many challenges associated with further understanding human chemical signalling networks, and associated molecular modulators, at the physiological level. The identification of insect semiochemical structures is rendered simple, in principle, by use of electrophysiological preparations from the insect olfactory recognition apparatus. However, with the eventual possibility of transferring, by heterologous gene expression into the insect recording systems, crucial gene products from human and other vertebrate olfactory recognition systems, the analytical convenience of insect electrophysiological analysis could be made available. As has been done for many insects, the specific semiochemicals with which each OR interacts, at physiologically relevant stimulus concentrations, need to be identified to advance this aspect of human semiochemistry research. We have the task of exploring new opportunities beyond those from insect semiochemistry research and should try, as some are doing in other sections of this issue [1], to research ways for advancing human wellbeing with mood enhancers and better therapies. As the exploitation of insect semiochemistry impacts more on food sustainability, and also on farm animal and human health, we can point to the social and economic value of olfaction research and development in this field. Although it would perhaps be specious to believe that we could manipulate human behaviour in quite the same dramatic and predictable way as can be achieved with insects, the value of insights from insect semiochemistry may provide opportunities to expand the understanding of human semiochemistry and help move towards innovation in human health and wellbeing.
Data accessibility
This article has no additional data.
Authors' contributions
A.R. and J.A.P. both have equal contribution to writing this review.
Competing interests
We declare we have no competing interests.
Funding
This review was supported by BBSRC grants nos. BB/P018017/1, BB/M023729/1, BB/N012526/1 and BB/R019681/1, and an MRC grant held at the London School of Hygiene and Tropical Medicine, UK (grant no. MR/P021972).
References
- 1.Natsch A, Emter R. 2020. The specific biochemistry of human axilla odour formation viewed in an evolutionary context. Phil. Trans. R. Soc. B 375, 20190269 ( 10.1098/rstb.2019.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickett JA, Williams IH, Martin AP, Smith MC. 1980. Nasonov pheromone of the honey bee, Apis mellifera L. (Hymenoptera, Apidae). Part 1. Chemical characterization. J. Chem. Ecol. 6, 425–434. ( 10.1007/Bf01402919) [DOI] [PubMed] [Google Scholar]
- 3.Pickett JA, Williams IH, Smith MC, Martin AP. 1981. Nasonov pheromone of the honey bee, Apis mellifera L. (Hymenoptera, Apidae). Part 3. Regulation of pheromone composition and production. J. Chem. Ecol. 7, 543–554. ( 10.1007/Bf00987702) [DOI] [PubMed] [Google Scholar]
- 4.Kigatiira IK, Beament JWL, Free JB, Pickett JA. 1986. Using synthetic pheromone lures to attract honeybee colonies in Kenya. J. Apic. Res. 25, 85–86. ( 10.1080/00218839.1986.11100698) [DOI] [Google Scholar]
- 5.Gikonyo NK, Hassanali A, Njagi PGN, Gitu PM, Midiwo JO. 2002. Odor composition of preferred (buffalo and ox) and nonpreferred (waterbuck) hosts of some Savanna tsetse flies. J. Chem. Ecol. 28, 969–981. ( 10.1023/A:1015205716921) [DOI] [PubMed] [Google Scholar]
- 6.Harvey DJ, et al. 2018. Environmentally vulnerable noble chafers exhibit unusual pheromone-mediated behaviour. PLoS ONE 13, e0206526 ( 10.1371/journal.pone.0206526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agelopoulos NG, Pickett JA. 1998. Headspace analysis in chemical ecology: effects of different sampling methods on ratios of volatile compounds present in headspace samples. J. Chem. Ecol. 24, 1161–1172. ( 10.1023/A:1022442818196) [DOI] [Google Scholar]
- 8.Logan JG, Birkett MA, Clark SJ, Powers S, Seal NJ, Wadhams LJ, Mordue AJ, Pickett JA. 2008. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J. Chem. Ecol. 34, 308–322. ( 10.1007/s10886-008-9436-0) [DOI] [PubMed] [Google Scholar]
- 9.Robinson A, et al. 2018. Plasmodium-associated changes in human odor attract mosquitoes. Proc. Natl. Acad. Sci. USA 115, E4209–E4218. ( 10.1073/pnas.1721610115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkett MA, et al. 2004. The role of volatile semiochemicals in mediating host location and selection by nuisance and disease-transmitting cattle flies. Med. Vet. Entomol. 18, 313–322. ( 10.1111/j.0269-283X.2004.00528.x) [DOI] [PubMed] [Google Scholar]
- 11.Borges LMF, de Oliveira JG, Ferreira LL, Louly CCB, Pickett JA, Birkett MA. 2015. Identification of non-host semiochemicals for the brown dog tick, Rhipicephalus sanguineus sensu lato (Acari: Ixodidae), from tick-resistant beagles, Canis lupus familiaris. Ticks Tick-Borne Dis. 6, 676–682. ( 10.1016/j.ttbdis.2015.05.014) [DOI] [PubMed] [Google Scholar]
- 12.Weeks ENI, Logan JG, Birkett MA, Pickett JA, Cameron MM. 2013. Tracking bed bugs (Cimex lectularius): a study of the effect of physiological and extrinsic factors on the response to bed bug-derived volatiles. J. Exp. Biol. 216, 460–469. ( 10.1242/jeb.074930) [DOI] [PubMed] [Google Scholar]
- 13.Verhulst NO, Andriessen R, Groenhagen U, Kiss GB, Schulz S, Takken W, van Loon JJA, Schraa G, Smallegange RC. 2010. Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS ONE 5, e15829 ( 10.1371/journal.pone.0015829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Grandon GM, Gezan SA, Armour JAL, Pickett JA, Logan JG. 2015. Heritability of attractiveness to mosquitoes. PLoS ONE 10, e0122716 ( 10.1371/journal.pone.0122716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson GW, Griffiths DC, Pickett JA, Wadhams LJ, Woodcock CM. 1987. Plant-derived synergists of alarm pheromone from turnip aphid, Lipaphis (Hyadaphis) erysimi (Homoptera, Aphididae). J. Chem. Ecol. 13, 1663–1671. ( 10.1007/Bf00980207) [DOI] [PubMed] [Google Scholar]
- 16.Dickens JC, Smith JW, Light DM. 1993. Green leaf volatiles enhance sex attractant pheromone of the tobacco budworm, Heliothis virescens (Lep.: Noctuidae). Chemoecology 4, 175–177. ( 10.1007/bf01256553) [DOI] [Google Scholar]
- 17.Pickett JA, Khan ZR. 2016. Plant volatile-mediated signalling and its application in agriculture: successes and challenges. New Phytol. 212, 856–870. ( 10.1111/nph.14274) [DOI] [PubMed] [Google Scholar]
- 18.Pickett JA, Birkett MA, Dewhirst SY, Logan JG, Omolo MO, Torto B, Pelletier J, Syed Z, Leal WS. 2010. Chemical ecology of animal and human pathogen vectors in a changing global climate. J. Chem. Ecol. 36, 113–121. ( 10.1007/s10886-010-9739-9) [DOI] [PubMed] [Google Scholar]
- 19.Pickett JA, Barasa S, Birkett MA. 2014. Vertebrate pheromones and other semiochemicals: the potential for accommodating complexity in signalling by volatile compounds for vertebrate management. Biochem. Soc. Trans. 42, 846–850. ( 10.1042/Bst20140134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saini RK, Orindi BO, Mbahin N, Andoke JA, Muasa PN, Mbuvi DM, Muya CM, Pickett JA, Borgemeister CW. 2017. Protecting cows in small holder farms in East Africa from tsetse flies by mimicking the odor profile of a non-host bovid. PLoS Neglect. Trop. Dis. 11, e0005977 ( 10.1371/journal.pntd.0005977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan JG, Stanczyk NM, Hassanali A, Kemei J, Santana AEG, Ribeiro KAL, Pickett JA, Mordue AJ. 2010. Arm-in-cage testing of natural human-derived mosquito repellents. Malaria J. 9, 239 ( 10.1186/1475-2875-9-239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickett JA, Woodcock CM. 1996. The role of mosquito olfaction in oviposition site location and in the avoidance of unsuitable hosts. Ciba Found. Symp. 200, 109–123. ( 10.1002/9780470514948.ch9) [DOI] [PubMed] [Google Scholar]
- 23.Dobritsa AA, van Naters WvdG, Warr CG, Steinbrecht RA, Carlson JR. 2003. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37, 827–841. ( 10.1016/S0896-6273(03)00094-1) [DOI] [PubMed] [Google Scholar]
- 24.Butterwick JA, Del Marmol J, Kim KH, Kahlson MA, Rogow JA, Walz T, Ruta V. 2018. Cryo-EM structure of the insect olfactory receptor Orco. Nature 560, 447–452. ( 10.1038/s41586-018-0420-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silbering AF, Benton R. 2010. Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design’? EMBO Rep. 11, 173–179. ( 10.1038/embor.2010.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Touchet S, Chamberlain K, Woodcock CM, Miller DJ, Birkett MA, Pickett JA, Allemann RK. 2015. Novel olfactory ligands via terpene synthases. Chem. Commun. 51, 7550–7553. ( 10.1039/c5cc01814e) [DOI] [PubMed] [Google Scholar]
- 27.Avila CA, Marconi TG, Viloria Z, Kurpis J, Del Rio SY. 2019. Bactericera cockerelli resistance in the wild tomato Solanum habrochaites is polygenic and influenced by the presence of Candidatus Liberibacter solanacearum. Sci. Rep.-UK 9, 14031 ( 10.1038/s41598-019-50379-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714. ( 10.1016/j.neuron.2004.08.019) [DOI] [PubMed] [Google Scholar]
- 29.Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Stortkuhl K, Hatt H. 2005. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 8, 15–17. ( 10.1038/nn1371) [DOI] [PubMed] [Google Scholar]
- 30.Benton R, Sachse S, Michnick SW, Vosshall LB. 2006. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, e20 ( 10.1371/journal.pbio.0040020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. 2008. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006. ( 10.1038/nature06850) [DOI] [PubMed] [Google Scholar]
- 32.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. 2007. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 10, 1474–1482. ( 10.1038/nn1976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo SX, Axel R, Abbott LF. 2010. Generating sparse and selective third-order responses in the olfactory system of the fly. Proc. Natl Acad. Sci. USA 107, 10 713–10 718. ( 10.1073/pnas.1005635107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen E, Nielsen F. 2001. Predicting vapour pressures of organic compounds from their chemical structure for classification according to the VOC-directive and risk assessment in general. Molecules 6, 370–389. ( 10.3390/60400370) [DOI] [Google Scholar]
- 35.Gramatica P. 2007. Principles of QSAR models validation: internal and external. QSAR Comb. Sci. 26, 694–701. ( 10.1002/qsar.200610151) [DOI] [Google Scholar]
- 36.Tropsha A. 2010. Best practices for QSAR model development, validation, and exploitation. Mol. Inform. 29, 476–488. ( 10.1002/minf.201000061) [DOI] [PubMed] [Google Scholar]
- 37.Lofstedt C, Vanderpers JNC, Lofqvist J, Lanne BS, Appelgren M, Bergstrom G, Thelin B. 1982. Sex-pheromone components of the turnip moth, Agrotis segetum—chemical identification, electro-physiological evaluation and behavioral activity. J. Chem. Ecol. 8, 1305–1321. ( 10.1007/Bf00987764) [DOI] [PubMed] [Google Scholar]
- 38.Liljefors T, Thelin B, Van Der Pers JN. 1984. Structure-activity relationships between stimulus molecule and response of a pheromone receptor cell in turnip moth, Agrotis segetum: modifications of the acetate group. J. Chem. Ecol. 10, 1661–1675. ( 10.1007/BF00987353) [DOI] [PubMed] [Google Scholar]
- 39.Bengtsson M, Liljefors T, Hansson BS. 1987. Dienic analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth, Agrotis segetum: synthesis, conformational analysis and structure-activity relationships. Bioorg. Chem. 15, 409–422. ( 10.1016/0045-2068(87)90036-8) [DOI] [Google Scholar]
- 40.Bengtsson M, Liljefors T, Hansson BS, Lofstedt C, Copaja SV. 1990. Structure-activity relationships for chain-shortened analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth, Agrotis segetum. J. Chem. Ecol. 16, 667–684. ( 10.1007/BF01016478) [DOI] [PubMed] [Google Scholar]
- 41.Jonsson S, Liljefors T, Hansson BS. 1992. Introduction of methyl groups to acetate substituted chain of (Z)-5-decenyl acetate, a pheromone component of turnip moth, Agrotis segetum: synthesis, single-sensillum recordings, and structure-activity relationships. J. Chem. Ecol. 18, 637–657. ( 10.1007/BF00987825) [DOI] [PubMed] [Google Scholar]
- 42.Jonsson S, Malmstrom T, Liljefors T, Hansson BS. 1993. Enantiomers of methyl substituted analogs of (Z)-5-decenyl acetate as probes for the chirality and complementarity of its receptor in Agrotis segetum 1: synthesis and structure-activity relationships. J. Chem. Ecol. 19, 459–484. ( 10.1007/BF00994319) [DOI] [PubMed] [Google Scholar]
- 43.Norinder U, Gustavsson A-L, Liljefors T. 1997. A 3D-QSAR study of analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth, Agrotis segetum. J. Chem. Ecol. 23, 2917–2934. ( 10.1023/A:1022531531072) [DOI] [Google Scholar]
- 44.Rowland SJ, Sutton PA, Knowles TDJ. 2019. The age of ambergris. Nat. Prod. Res. 33, 3134–3142. ( 10.1080/14786419.2018.1523163) [DOI] [PubMed] [Google Scholar]
- 45.Kovatcheva A, Golbraikh A, Oloff S, Xiao YD, Zheng W, Wolschann P, Buchbauer G, Tropsha A. 2004. Combinatorial QSAR of ambergris fragrance compounds. J. Chem. Inf. Comput. Sci. 44, 582–595. ( 10.1021/ci034203t) [DOI] [PubMed] [Google Scholar]
- 46.Keller A, Vosshall LB. 2016. Olfactory perception of chemically diverse molecules. BMC Neurosci. 17, 55 ( 10.1186/s12868-016-0287-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller A, et al. 2017. Predicting human olfactory perception from chemical features of odor molecules. Science 355, 820–826. ( 10.1126/science.aal2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Panwar B, Omenn GS, Guan Y. 2018. Accurate prediction of personalized olfactory perception from large-scale chemoinformatic features. Gigascience 7, gix127 ( 10.1093/gigascience/gix127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enamine. See https://enamine.net/library-synthesis/real-compounds/real-space-navigator.
- 50.Sterling T, Irwin JJ. 2015. ZINC 15—ligand discovery for everyone. J. Chem. Inf. Model. 55, 2324–2337. ( 10.1021/acs.jcim.5b00559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Block E. 2018. Molecular basis of mammalian odor discrimination: a status report. J. Agric. Food Chem. 66, 13 346–13 366. ( 10.1021/acs.jafc.8b04471) [DOI] [PubMed] [Google Scholar]
- 52.Massberg D, Hatt H. 2018. Human olfactory receptors: novel cellular functions outside of the nose. Physiol. Rev. 98, 1739–1763. ( 10.1152/physrev.00013.2017) [DOI] [PubMed] [Google Scholar]
- 53.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. ( 10.1093/nar/25.17.3389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boratyn GM, Schaffer AA, Agarwala R, Altschul SF, Lipman DJ, Madden TL. 2012. Domain enhanced lookup time accelerated BLAST. Biol. Direct. 7, 12 ( 10.1186/1745-6150-7-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berman H, Henrick K, Nakamura H. 2003. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 10, 980–980 ( 10.1038/nsb1203-980) [DOI] [PubMed] [Google Scholar]
- 56.Burley SK, et al. 2019. RCSB Protein Data Bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 47, D464–D474. ( 10.1093/nar/gky1004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muhammed MT, Aki-Yalcin E. 2019. Homology modeling in drug discovery: overview, current applications, and future perspectives. Chem. Biol. Drug Des. 93, 12–20. ( 10.1111/cbdd.13388) [DOI] [PubMed] [Google Scholar]
- 58.Orry AJW, Abagyan R. 2012. Homology modeling. Totowa, NJ: Humana Press. [Google Scholar]
- 59.Ramachandran GN, Ramakrishnan C, Sasisekharan V. 1963. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 7, 95–99. ( 10.1016/s0022-2836(63)80023-6) [DOI] [PubMed] [Google Scholar]
- 60.Ahmed L, et al. 2018. Molecular mechanism of activation of human musk receptors OR5AN1 and OR1A1 by (R)-muscone and diverse other musk-smelling compounds. Proc. Natl Acad. Sci. USA 115, E3950–E3958. ( 10.1073/pnas.1713026115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de March CA, Topin J, Bruguera E, Novikov G, Ikegami K, Matsunami H, Golebiowski J. 2018. Odorant receptor 7D4 activation dynamics. Angew. Chem. Int. Edn 57, 4554–4558. ( 10.1002/anie.201713065) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.