Abstract
Background
Ozone injection around Gasserian ganglion (OIAGG) has been reported to be an effective treatment for trigeminal neuralgia (TN); however, there remain areas for improvement. To overcome one of these limitations, a multicenter examination of application would be extremely helpful.
Objective
The goal of this report was to assess the efficacy of OIAGG for refractory TN across multiple centers and to explore factors predictive of successful treatment.
Design
A multicenter, retrospective study.
Setting
The study was conducted across 3 pain centers across China.
Patients and Methods
A total of 103 subjects from 3 pain centers were enrolled in the study. An ozone-oxygen mixture gas at a concentration of 30 µg/mL was injected into the area around the Gasserian ganglion performed under C-arm X-ray guidance. Primary outcome measures included a pain assessment using a visual analog scale (VAS) and the Barrow Neurological Institute (BNI) pain intensity scale. Clinical assessment of patients for these outcome measures was performed at pretreatment, post-treatment, 6 months, 1 year and 2 years after the OIAGG.
Results
Successful pain relief was defined as a score within BNI grades I–IIIa. The pain relief rates at post-treatment, 6 months, 1 year and 2 years after the procedure were 88.35%, 86.87%, 84.46% and 83.30%, respectively. The VAS at each observation time point was significantly different from the preoperative levels (P<0.05). Logistic regression analysis showed that previous nerve damage had a significant effect on the treatment results. No significant complications or side effects were found during or after treatment.
Conclusion
This multicenter research confirms our previous single center results that OIAGG is both effective and safe for patients with TN.
Keywords: trigeminal neuralgia, trigeminal post-herpetic neuralgia, ozone therapy, Gasserian ganglion
Introduction
Trigeminal neuralgia (TN) is a type of neuropathic pain characterized by brief, sudden, and recurrent severe pain in the distribution of the trigeminal nerve. It primarily affects the middle and old-ages and is more commonly seen in women.1–3 Some studies have showed that TN severely affects the lives and work of patients.4 A variety of treatment options are available for the management of pain for TN patients, such as anti-epileptic drugs, radiofrequency thermocoagulation, chemical (alcohol and glycerol) injections, microvascular decompression (MVD), gamma knife radiosurgery (GKRS) and percutaneous balloon compression (PBC).5–12 However, each of these therapies has its own unique disadvantage. Long-term use of anti-epileptic drugs can cause various side effects, such as dizziness, nausea and vomiting. Following radiofrequency thermocoagulation, chemical (alcohol and glycerol) injection and percutaneous balloon compression can develop a variety of complications, such as facial numbness, headaches and diplopia. For patients with neurovascular symptoms, MVD is the first-choice for treatment. Unfortunately, this procedure requires a craniotomy and posterior fossa exploration; further, there is a significant recurrence rate. Additionally, this type of surgery may not be suitable for the elderly or those in poor health.13
In recent years, oxygen-ozone therapy has been widely recommended for the treatment of various diseases, especially neuropathic pain such as lumbar disc herniation and post-herpetic neuralgia.14–22 The advantages of ozone procedures include minimal trauma, fewer complications and simplicity. Since 2013, patients with TN have been treated by ozone injection at our pain center. Moreover, our team has published our experience on the treatment of TN with ozone injection.15 However, one small sample size. Therefore, we expanded the scope of our previous retrospective review to include two other pain centers which are also using ozone treatment for TN.
Patients and Methods
Study Design
This research was an expanded retrospective review. The study sample consisted of patients treated with OIAGG across three pain centers from June 2015 to October 2018. This study was approved by the Institutional Review Board of the Aviation General Hospital of China Medical University (approval number HK2018-0820) and complied with Declaration of Helsinki. The application for the waiver of informed consent from the study was approved by the institutional review committee of aviation general hospital of China medical university. The rights and health of the patient were not adversely affected by the waiver of consent. All patient identifiers were removed from the data set at the time of initial collection. Data were collected and analyzed retrospectively for all patients from existing medical records.
Patients
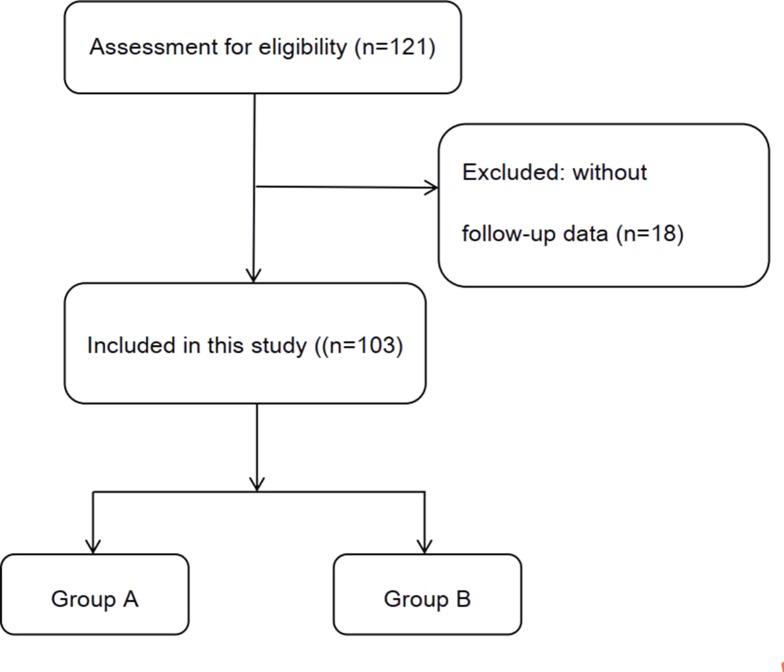
Inclusion criteria: (1) definitely diagnosed as TN; (2) age ≥18-year-old; (3) visual analog scale (VAS) pain scores ≥4; Exclusion criteria include the following: (1) age < 18 years; (2) local infection in puncture area; (3) coagulation disorder or hemorrhagic disease; (4) mental illness and inability to cooperate; (5) serious heart, brain, lung, or liver diseases. The flow chart of the entry and exit of patients is shown in Figure 1.
Figure 1.
Flow chart of inclusion of trigeminal neuralgia patients.
Surgical Procedure
Patients were placed in a supine position on the C-arm bed. Vital parameters including pulse rate, electrocardiogram (ECG), noninvasive blood pressure, and oxygen saturation were monitored during the procedure. Meanwhile, intravenous access was obtained. Procedures were performed by one attending physician and one assistant at each center.
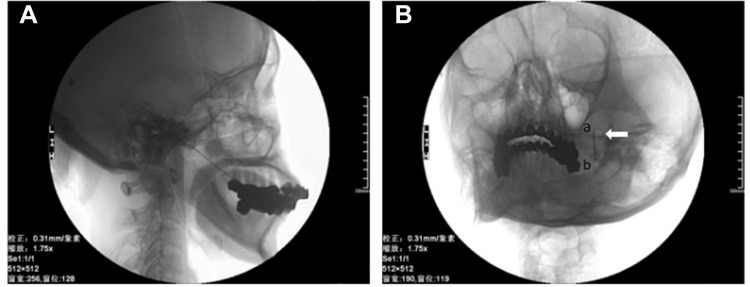
All patients in three hospitals were injected with ozone around Gasserian ganglion guided by C-arm. The puncture point was located about 2.5–3 cm outside the corner of the mouth on the affected side. After routine disinfection and draping, local infiltration anesthesia was performed with 1% lidocaine. Using Hartel anterior route, the puncture was performed toward the around the Gasserian ganglion. Then, a 22 G needle (30 mm) was used to puncture along the established angle and path to the area around the foramen ovale (Figure 2A). A C-arm scan image was obtained to confirm the position of the foramen ovale and needle (Figure 2B). The angle and depth of the needle were adjusted under C-arm guidance. The location of the needle was examined in coaxial and lateral view by C-arm to make sure the depth of the needle tip did not enter the foramen ovale. The needle does not enter the foramen ovale, which effectively prevents the occurrence of pneumocephalus. After confirming that the tip was around the foramen ovale, the O2–O3 (3–5mL) mixture was slowly injected at a concentration of 30 µg/mL. The O2–O3 mixture was generated by the ozone therapy devices (Ozomed Basic; Kastner-Praxisbedarf GmbH, Rastatt, Germany). Since ozone gas reacts with itself and rapidly decomposes to oxygen, it was required to be produced immediately by ozone generators. The process was carried out at room temperature. All patients were observed for at least 30 minutes after injection. After the vital signs were stable, the patient was sent back to the ward.
Figure 2.
C-arm X-ray scans were performed to confirm the correct localization .
Notes: (A) C-arm image showing the trajectory of the needle after insertion. (B) C-arm scan showed that the tip of the trocar was located around foramen ovale. “a” was the point of the needle, “b” was the tail of the needle, and the arrow was the foramen ovale.
Data Collection
The following demographic and clinical characteristics were collected from the medical records: age, sex, branch affected, severity and duration of pain, skin temperature, past medical history, tactile sensory and follow-up records. The Barrow Neurological Institute (BNI) scale and visual analogue scale (VAS) were employed to evaluate pain degree. Complications and recurrence of pain were also recorded. The Barrow Neurological Institute (BNI)23 Score is a composite scale assessing TN patients’ pain intensity and medication use. The BNI pain intensity scale was used as follows: I (no pain, no medication), II (occasional pain, not requiring medication), III (some pain, controlled with medication), IV (some pain, not controlled with medication) and V (severe pain, no pain relief with medication). The visual analogue scale (VAS) is a widely used measure of pain severity. The 0 point represents the painless state and 10 points represent severe pain.
Facial skin temperature (FST) was evaluated through the use of the non-contact infrared thermometer (Berrcom, model JTB183, Guangzhou, China). Using a series of von Frey fairs (Stoelting, Chicago, IL, USA), we evaluated the tactile responses of facial trigeminal branches. The test was performed in a quiet area, requiring the patient to close their eyes, thereby obstructing the patient’s vision. Facial tactile assessments were performed in six areas (three branches of the trigeminal nerves on both sides). A series of calibrated von Frey hairs was applied perpendicular to the facial surface. When the patients responded to the stimulus, the corresponding von Frey date was recorded. If the patient does not respond to the stimulus, select the next largest monofilament and repeat the process.15 FST and tactile sensation should be carried out before the first treatment and after the last treatment.
Follow-Up
The patients were followed up 6 months, 1 year and 2 years after the operation. The patients were followed up by non-operative staff. During the follow-up, an evaluation of pain using a BNI and VAS were conducted. Side-effects and complications were also included in the follow-up.
Statistical Analysis
All statistical analyses were performed using SPSS Version 24.0 (IBM Corporation, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviations, and categorical data were presented as numbers (percentages). The independent samples t-test was used to analyze continuous data between two groups. Paired t-tests were used for comparing differences before and after treatment. The χ2 and the Mann−Whitney U-test were used for comparing the categorical data. Logistic regression analysis was performed to identify the possible impact factors for outcome, such as age, gender and history of underlying disease. Moreover, all significant variables (p < 0.05) in the univariate analysis were included in a multivariate logistic regression analysis. P < 0.05 was considered to be statistically significant.
Results
As a result, a total of 103 patients were available for analysis, including 48 males and 55 females. They ranged in age from 23 to 89 years (mean, 62.44 ± 11.06 years). The course of the disease ranged from 0.5 to 420 months. Forty-seven patients had pain on the left side, 54 on the right and 2 suffered from bilateral pain. Among the patients, 11 had first-branch (I) TN, 22 second-branch (II), 14 third-branch (III), 16 I+II, 3 I+III, 31II+III, and 6 I+II+III. Before moving to our pain center, 11 patients had received MVD, 14 had undergone pulse radiofrequency (PRF), 2 had been treated with GKRS and 18 had accepted destructive procedures. The demographic and clinical data of the patients are presented in Table 1.
Table 1.
Patient Demographics and Clinical Data
| Characteristics | Baseline (Mean ± SD) |
|---|---|
| Gender(n) | |
| Male | 48 |
| Female | 55 |
| Age, year | |
| Mean ± SD | 62.440 ± 11.060 |
| Range | 23–89 |
| Pain Duration, Month | |
| Median | 18 |
| Range | 0.5–480 |
| Pain Side(n) | |
| Right | 54 |
| Left | 47 |
| Both | 2 |
| Branches Affected(n) | |
| V1 | 11 |
| V2 | 22 |
| V3 | 14 |
| V1+v2 | 16 |
| V1+V3 | 3 |
| V2+V3 | 31 |
| V1+V2+V3 | 6 |
| Preoperative VAS | 7.19±1.38 |
| Type of Prior Procedure(n) | |
| MVD | 11 |
| PRF | 14 |
| GKRS | 2 |
| Destructive procedures | 18 |
Abbreviations: VAS, visual analogue scale; MVD, microvascular decompression; PRF, pulsed radiofrequency; GKRS, gamma knife radiosurgery.
There were 58 patients (56.3%) with classical trigeminal neuralgia (group A), while 45 patients (43.7%) with trigeminal neuropathy (group B) caused by post-herpetic neuralgia among all patients in the study. Between group A and group B, there were no statistically significant differences in sex, gender, VAS score, BNI score or pain distribution. The difference of duration of disease between the two groups was statistically significant (P <0.001).
Forty-five patients in this study had undergone other surgical procedures prior to the ozone injection. Out of forty-five patients, thirty-six had a recurrence after surgery and nine failed after MVD, respectively. Among thirty-six patients, twenty-seven achieved pain relief. Moreover, six of the nine cases responded to ozone injection.
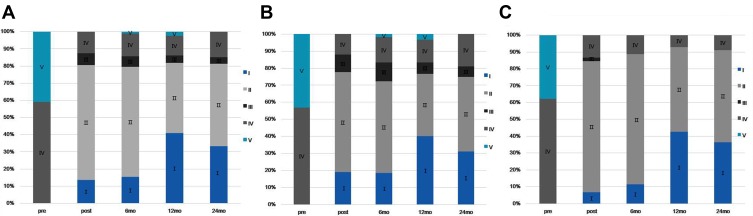
Response according to the BNI Pain Intensity Scoring System is shown in Table 2. A total of 91 patients (88.35%) experienced initial pain relief (BNI score ≤ IIIa) after OIAGG. The 6-month, 1 year, and 2-year pain relief rates (BNI pain scores I-IIIa) in the A and B group were 83.64%, 74%, 72%, and 90.91%, 87.67%, 83.41%, respectively. Post-operative group A and B BNI scores were both significantly improved compared with their respective preoperative BNI scores (P<0.01). In addition, there was no statistically significant difference in BNI scores between the two groups before and after treatment. The detailed distribution of the BNI scores is shown in Figure 3.
Table 2.
Barrow Neurological Institute (BNI) Pain Intensity Scale
| Score | Description |
|---|---|
| I | No pain, no medication |
| II | Occasional pain, not requiring medication |
| IIIa | No pain, completely controlled with medication |
| IIIb | Some pain, adequately controlled with medication |
| IV | Some pain, not adequately controlled with medication |
| V | Severe pain, no pain relief |
Figure 3.
Details of the BNI score.
Notes: (A) Bar chart showing the BNI score at follow-up in patients with total trigeminal neuralgia. (B) Bar chart showing the BNI score at follow-up in patients with classical trigeminal neuralgia. (C) Bar chart showing the BNI score at follow-up in patients with post-herpetic trigeminal neuralgia.
Abbreviation: BNI, Barrow Neurological Institute.
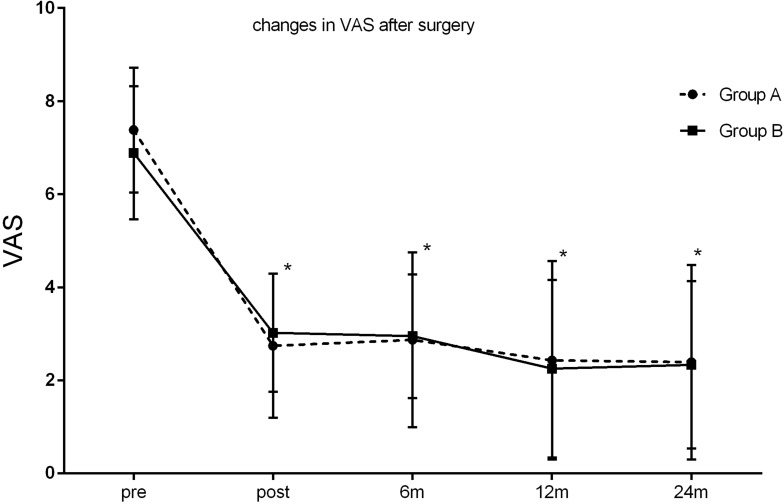
In Group A, the mean VAS score was 7.38 ± 1.34 at pretreatment, 2.74 ± 1.55 at post-treatment, 2.87 ± 1.88 at 6 months, 2.43 ± 2.13 at 1 year, and 2.27± 1.25 at 2 years. In Group B, the mean VAS score was 6.89 ± 1.43 at pretreatment, 3.02 ± 1.27 at posttreatment, 2.95 ± 1.33 at 6months, 2.25 ± 1.91 at 1 year, and 2.23 ± 1.80 at 2 years. The VAS pain scores were significantly reduced in both groups after the procedure (P < 0.05). There was no significant difference in VAS scores between group A and group B at any time follow-up time period (P>0.05) (Figure 4).
Figure 4.
Changes in VAS.
Notes: There was a significant decrease in pain intensity after the ozone injection in both groups (*P < 0.05), and this was even maintained for up to 24 months of the follow-up. No difference was found in the follow-up VAS scores between two groups (P > 0.05).
Abbreviation: VAS, visual analog scale.
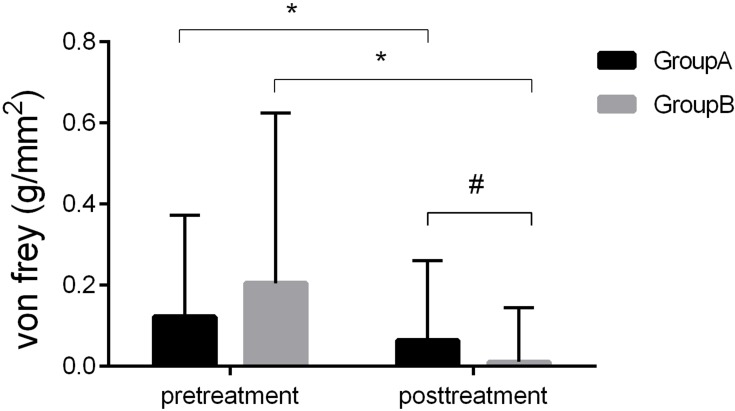
Before the procedure, the von Frey of groups A and B were 0.122±0.251 and 0.205 ± 0.420g/mm2, respectively. In group A, the von Frey was 0.064 ± 0.197 g/mm2, and in group B, the von Frey was 0.011 ± 0.134 g/mm2 after the treatment. The von Frey measurements were improved in both groups. In the post-operative period, von Frey measurements decreased in both groups, and the differences were significantly different when compared with preoperative levels (*P < 0.05). Tactile detection assessed by the von Frey pre-treatment showed no difference between groups A and B. There was also a significant difference in von Frey measurements of group A and group B after the treatment (#P<0.05) as shown in Figure 5. No complications were observed in any of the patients after treatment, such as FST, abnormalities or facial tactile loss.
Figure 5.
Changes in von Frey.
Notes: Tactile detection: There was no difference in von Frey of group A and group B before the treatment (P > 0.05). After the ozone therapy, there was a significant decrease in von Frey (*P < 0.05) of group A and group B. Meanwhile, the von Frey of group A and group B have a significant difference after the ozone therapy (#P<0.05).
The patients were divided into two groups: ≤65 years old and >65 years old. Pain duration was considered a potential predictor and was classified as acute or sub-acute (≤6 months) or chronic (>6 months). Destructive procedures refer to the destruction of nerves that includes chemotherapy (ethanol and adriamycin), radiofrequency thermocoagulation, PBC and partial sensory rhizotomy. We also classified the patient’s diabetes mellitus (DM) status, including the presence or absence of DM and the presence or absence of hypertension (HTN). Age, gender, and duration of pain did not independently predict clinically successful outcomes at 6 months (P > 0.05). Multivariate regression analysis showed that DM history (OR: 0.087, 95% CI: 0.021–0.368, P=0.001) and destructive procedures (OR: 0.129, 95% CI: 0.027–0.623, P=0.014) had a significant effect at the 6 months evaluation (P < 0.05). More details are provided in Tables 3 and 4. Interestingly, through statistical analysis, DM history was not a significant prognostic factor for treatment effectiveness at the 2-year evaluation (P>0.05). However, the history of neurological damage still was independently associated with a successful response 2 years after ozone injection (OR: 0.019; 95% CI: 0.000–0.935; P=0.046). Further details are listed in Tables 5 and 6.
Table 3.
Univariate Analysis of Possible Outcome Predictors for Injection Effectiveness After 6 Months
| Characteristics | Effective | Not Effective | P-value |
|---|---|---|---|
| Gender | 0.985 | ||
| Male | 39 | 6 | |
| Female | 46 | 7 | |
| Age(years) | 0.162 | ||
| <65 | 48 | 10 | |
| >65 | 37 | 3 | |
| Duration | 0.580 | ||
| ≤6months | 30 | 3 | |
| >6months | 55 | 10 | |
| DM | 0.001 | ||
| Yes | 13 | 7 | |
| No | 72 | 6 | |
| Hypertension | 0.577 | ||
| Yes | 23 | 2 | |
| No | 62 | 11 | |
| PRF | 0.263 | ||
| Yes | 10 | 3 | |
| No | 75 | 10 | |
| MVD | 0.752 | ||
| Yes | 7 | 2 | |
| No | 78 | 11 | |
| Destructive procedures | 0.038 | ||
| Yes | 10 | 5 | |
| No | 75 | 8 |
Abbreviations: DM, diabetes mellitus; MVD, microvascular decompression; PRF, pulsed radiofrequency.
Table 4.
Multiple Logistic Regression Analysis of Possible Outcome Predictors for Injection Effectiveness After 6 Months
| Characteristics | OR | 95% CI | P-value |
|---|---|---|---|
| DM | 0.087 | 0.021–0.368 | 0.001 |
| Destructive procedures | 0.129 | 0.027–0.623 | 0.011 |
| Constant | 26.764 | 0.000 |
Abbreviations: DM, diabetes mellitus; OR, odds ratio; CI, confidence interval.
Table 5.
Patient Characteristics at 2 Years Follow-Up
| Pt. No. | TN Classification | Age | Gender | Side | Pre-Operative BNI | 2 Years Followed-Up BNI | Pre-Operative VAS | 2 Years Followed-Up VAS | DM History |
Destructive Procedures History | Pre-Von Frey | Post-Von Frey |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Primary | 68 | F | Right | 5 | 1 | 8 | 0 | N | N | 0.008 | 0.008 |
| 2 | Primary | 68 | M | Right | 5 | 1 | 7 | 1 | N | N | 0.008 | 0.008 |
| 3 | Primary | 46 | M | Left | 4 | 2 | 9 | 2 | N | Y | 0.008 | 0.008 |
| 4 | Primary | 68 | M | Right | 4 | 1 | 10 | 1 | Y | N | 0.6 | 0.008 |
| 5 | Primary | 65 | M | Bilateral | 5 | 1 | 8 | 0 | N | N | 0.008 | 0.008 |
| 6 | Secondary | 64 | F | Left | 4 | 4 | 7 | 7 | Y | Y | 0.008 | 0.008 |
| 7 | Secondary | 48 | F | Left | 4 | 2 | 7 | 1 | N | N | 0.008 | 0.008 |
| 8 | Secondary | 59 | M | Left | 5 | 1 | 8 | 1 | N | N | 0.07 | 0.008 |
| 9 | Secondary | 70 | F | Left | 4 | 2 | 8 | 2 | N | Y | 0.008 | 0.008 |
| 10 | Primary | 62 | M | Left | 4 | 2 | 8 | 2 | N | N | 0.008 | 0.008 |
| 11 | Primary | 69 | F | Right | 4 | 4 | 8 | 8 | Y | Y | 0.07 | 0.008 |
| 12 | Primary | 66 | F | Left | 4 | 1 | 8 | 1 | N | N | 0.008 | 0.008 |
| 13 | Primary | 81 | F | Left | 5 | 3 | 9 | 4 | N | N | 0.008 | 0.008 |
| 14 | Primary | 63 | M | Left | 5 | 3 | 6 | 4 | N | Y | 0.008 | 0.008 |
| 15 | Primary | 73 | M | Right | 5 | 2 | 8 | 2 | Y | Y | 0.6 | 0.6 |
| 16 | Primary | 60 | F | Right | 4 | 2 | 8 | 3 | N | N | 0.008 | 0.008 |
| 17 | Primary | 75 | F | Right | 4 | 1 | 8 | 1 | N | N | 0.008 | 0.008 |
| 18 | Secondary | 51 | M | Left | 4 | 1 | 5 | 2 | N | N | 0.008 | 0.008 |
| 19 | Secondary | 74 | F | Right | 5 | 1 | 8 | 2 | N | N | 0.008 | 0.008 |
| 20 | Primary | 80 | M | Right | 4 | 2 | 6 | 2 | N | Y | 0.02 | 0.008 |
| 21 | Primary | 23 | F | Left | 4 | 2 | 7 | 6 | N | N | 0.008 | 0.008 |
| 22 | Primary | 60 | F | Left | 5 | 4 | 8 | 2 | N | N | 0.008 | 0.008 |
| 23 | Primary | 74 | F | Left | 5 | 2 | 8 | 2 | N | N | 0.2 | 0.2 |
| 24 | Secondary | 67 | F | Right | 5 | 2 | 8 | 2 | N | N | 0.008 | 0.008 |
| 25 | Secondary | 64 | F | Left | 5 | 1 | 9 | 2 | N | N | 0.4 | 0.008 |
| 26 | Secondary | 81 | F | Right | 4 | 2 | 9 | 2 | N | N | 1 | 0.008 |
| 27 | Primary | 38 | M | Right | 4 | 2 | 7 | 3 | N | N | 0.008 | 0.008 |
Abbreviations: TN, trigeminal neuralgia; F, female; M, male; BNI, Barrow Neurological Institute; VAS, visual analogue scale; DM, diabetes mellitus; Y, yes; N, no.
Table 6.
Multiple Logistic Regression Analysis of Possible Outcome Predictors for Injection Effectiveness After 2 Years
| Characteristics | OR | 95% CI | P-value |
|---|---|---|---|
| DM | 0.079 | 0.002–2.680 | 0.158 |
| Destructive procedures | 0.019 | 0.000–0.935 | 0.046 |
| Gender | 0.085 | 0.002–3.297 | 0.186 |
| Age | 1.050 | 0.936–1.179 | 0.406 |
| Constant | 17.000 | 0.404 |
Abbreviations: DM, diabetes mellitus; OR, odds ratio; CI, confidence interval.
Pain Recurrence
Six patients suffered pain recurrence between 2 months and 1 year after the procedure. It is worth noting that two patients were triggered by eating irritating food. Among the six patients, 5 people were in group A and one person belonged to group B. The postoperative recurrence rate was 5.83% (6/103). Three of the patients received ozone therapy again. Reassuringly, the pain VAS scores of the three relapse patients significantly decreased after the second ozone injection treatment. In addition, one of the patients underwent nerve root resection and the pain was not alleviated. The other two patients did not receive any other treatment.
Side Effects and Complications
Three cases of facial swelling and one case of nerve root pain returned to normal within one day. No other serious complications or side effects were observed. In addition, no complications were noticed in patients during follow-up.
Discussion
TN is a disorder of the trigeminal nerve that results in intense episodic pain, which impacts the quality of life.2 The pain can be so intense that in some literature TN has been called the “suicide disease”. The treatment of patients with TN is always a challenge in clinical practice. To date, there is no an ideal treatment method that is suitable for all patients under all situations. Obviously, new and innovative treatment options are warranted. Currently, ozone therapy is one of the minimally invasive treatments available.
Over the past several years, our team has carried out a large number of examinations on the treatment of TN with ozone injection. Fortunately, considerable progress has been made in our research. As reported in our previous single-center study, OIAGG has been shown to be a safe and effective treatment modality. But it is regrettable that the research15 had some shortcomings, including the fact that as a single center we had small sample sizes. In previous research, we used computed tomography (CT)-guided ozone injection around Gasserian ganglion for the treatment of TN. However, we identified the disadvantages associated with CT guidance such as high cost, low efficiency, and large amounts of radiation. Therefore, in this study, we used a C-arm to guide the placement of our catheter, which effectively avoids the above shortcomings. Meanwhile, this study also confirmed the feasibility of C-arm guided ozone injection around Gasserian ganglion for treatment. In addition, Chang et al researches indicated that ultrasound can be used to guide the injection of trigeminal neuroglia.24,25 An ultrasound device is portable and easy to operate without radiation, saving manpower and material resources. The ultrasound-guided ozone injection around the Gasserian ganglion for the treatment of TN might be a worthwhile method in the future.
This multicenter research results have extended our previous research results and documented that our original work is both duplicated and extended. In previous study, after the treatment, VAS scores were significantly decreased in both groups when compared with the preceding treatment (P < 0.05). As for Von frey data, group B has shown an obvious decline after the treatment. However, there was no significant difference between the two groups before and after the therapy (P > 0.05); in this study, the results of the clinical measures such as VAS, Von Frey and facial skin temperature, are consistent with previous single-center study. The difference is von Frey test results, there was a significant decrease in group A and group B after the ozone injection in this research. Meanwhile, group A and group B after the procedure have a significant difference compared with that before the procedure in the test. Using our von Frey data, we found that the current ozone injection has a protective effect on tactile sensitivity. In other words, ozone treatment can maintain normal facial touch and avoid facial numbness. The VAS and von Frey data results of this study show that ozone injection can relieve the pain of TN and improve the patient’s tactile sensitivity.
Compared to single-center study, we have added new comprehensive assessment measures for TN. The BNI score was an assessment of trigeminal patients from both pain intensity and drug use. The results of BNI were consistent with the VAS. After the treatment, the trend of BNI and VAS scores was decreasing. The result indicated that the patient pain intensity and medication used have improved after the ozone therapy.
Notably, the pain relief rate in group B was better than that in group A. Moreover, the patients who recurrent were mainly from group A and only one patient in group B recurrent. This phenomenon was same with previous research results. Despite many therapeutic options are available, post-herpetic neuralgia (PHN) is still a very difficult disease. An important reason is that the varicella-zoster virus can persist in the ganglion for long time and persistent neural injuries.26 In this research, ozone was injected around the ganglion. We assume that ozone’s anti-inflammatory and antiviral may play a role in the treatment.
Single-center study had suggested that gender, age and pain duration were not associated with the treatment effectiveness. Similarly, the results of this study also showed that influencing factors, such as age, pain duration, and gender, did not affect treatment outcomes. In addition, we found that history of diabetes mellitus had a significant effect on the treatment results at 6 months followed-up. Furthermore, the history of neurological damage was an independent factor affecting 6 months and 2 years treatment outcome. It can cause damage to tissues throughout the body, particularly in the nervous system. Diabetes is a chronic disease characterized by hyperglycemia. It can cause damage to tissues throughout the body, particularly in the nervous system. Different degrees of neuropathy caused by hyperglycemia might be the reason that affects the therapeutic effect. Besides, at 2 years of follow-up, diabetes was no longer influencing factor and may be associated with better glucose control. In addition, destructive treatment makes it difficult to repair damaged nerves. This may be the reason why the treatment has been affected.
Ozone could improve the microcirculation and supply the oxygen and nutrients. Therefore, ozone can eliminate accumulated inflammatory factors, improve blood circulation, and thus reduce pain.27,28 The analgesic mechanism of ozone injection in TN was not completely clear. Fortunately, there was an experimental animal29 had shown that ozone can repair the facial nerve that is being crushed. The experimental results provide a theoretical basis for our research. However, this experiment used intraperitoneal injection, which is systemic administration. Currently, there are no reports on the experimental studies of local drug administration.
We used ozone injection of the Gasserian ganglion for the treatment of TN in previous study. During the treatment, place the needle into the foramen ovale. However, two cases of suspected pneumocephalus occurred during previous treatment. Thus, we improved the operation process by placing the needle around the foramen ovale instead of placing the needle into the foramen ovale, which is safer than before. Moreover, in the current study, there was no occurrence of pneumocephalus. In addition, we think that is safer for patients to use liquid ozone in the future.
Limitations
This study has several mentionable limitations. First and consistent with the retrospective nature of this study, some data were missing or incomplete. Second, in this study, the concentration and dose of ozone were fixed and there was no dose-response examined. Thus, further well-designed, controlled, double-blinded randomized, trials are needed.
Conclusion
In this multicenter retrospective analysis, the examined outcomes proved that OIAGG was a safe, effective and non-injurious method for the treatment of TN patients. Thus, we recommend that the use of ozone therapy is considered before any surgical intervention or when surgery is not possible. Moreover, directed ozone injection using a C-arm system is both safe and feasible. In addition, ozone injection around the Gasserian ganglion avoids the occurrence of pneumocrania; however, further studies are needed to define the optimal dose and concentration for ozone treatment.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81671076) and Chaoyang District Commission of Science and Technology of Beijing Municipality (SYSF1830).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yadav YR, Nishtha Y, Sonjjay P, et al. Trigeminal neuralgia. Asian J Neurosurg. 2017;12:585–597. doi: 10.4103/ajns.AJNS_67_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zakrzewska JM, Wu J, Mon-Williams M, et al. Evaluating the impact of trigeminal neuralgia. Pain. 2017;158:1166–1174. doi: 10.1097/j.pain.0000000000000853 [DOI] [PubMed] [Google Scholar]
- 3.Maarbjerg S, Di Stefano G, Bendtsen L. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia. 2017;37:648–657. doi: 10.1177/0333102416687280 [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, He M, Li C. A research on quality of life score (QOLS) of patients with trigeminal neuralgia (TN). J Infect Public Health. 2019;12:690–694. doi: 10.1016/j.jiph.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 5.Montano N, Conforti G, Di Bonaventura R, et al. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015;11:289–299. doi: 10.2147/TCRM.S37592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens JL, Beaudoin DA. The challenges of treating patients with trigeminal neuralgia. Gen Dent. 2018;66:20–23. [PubMed] [Google Scholar]
- 7.Qin Z, Xie S, Mao Z, et al. Comparative efficacy and acceptability of antiepileptic drugs for classical trigeminal neuralgia: a Bayesian network meta-analysis protocol. BMJ Open. 2018;8:e017392. doi: 10.1136/bmjopen-2017-017392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nie F, Su D, Shi Y, et al. A prospective study of X-ray imaging combined with skin stimulation potential-guided percutaneous radiofrequency thermocoagulation of the Gasserian ganglion for treatment of trigeminal neuralgia. Pain Med. 2014;15:1464–1469. doi: 10.1111/pme.12359 [DOI] [PubMed] [Google Scholar]
- 9.Ding Y, Yao P, Li H, et al. CT-Guided stellate ganglion pulsed radiofrequency stimulation for facial and upper limb postherpetic neuralgia. Front Neurosci. 2019;13:170. doi: 10.3389/fnins.2019.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karki P, Yamagami M, Takasaki K, et al. Microvascular decompression in patients aged 30 years or younger. Asian J Neurosurg. 2019;14:111–117. doi: 10.4103/ajns.AJNS_266_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhople AA, Adams JR, Maggio WW, et al. Long-term outcomes of Gamma Knife radiosurgery for classic trigeminal neuralgia: implications of treatment and critical review of the literature. Clinical article. J Neurosurg. 2009;111:351–358. doi: 10.3171/2009.2.JNS08977 [DOI] [PubMed] [Google Scholar]
- 12.Wang JY, Bender MT. Percutaneous procedures for the treatment of trigeminal neuralgia. Neurosurg Clin N Am. 2016;27:277–295. doi: 10.1016/j.nec.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 13.Wang DD, Raygor KP, Cage TA, et al. Prospective comparison of long-term pain relief rates after first-time microvascular decompression and stereotactic radiosurgery for trigeminal neuralgia. J Neurosurg. 2018;128:68–77. doi: 10.3171/2016.9.JNS16149 [DOI] [PubMed] [Google Scholar]
- 14.Murphy K, Elias G, Steppan J, et al. Percutaneous treatment of herniated lumbar discs with ozone: investigation of the mechanisms of action. J Vasc Interv Radiol. 2016;27:1242–1250. doi: 10.1016/j.jvir.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 15.An JX, Liu H, Chen RW, et al. Computed tomography-guided percutaneous ozone injection of the Gasserian ganglion for the treatment of trigeminal neuralgia. J Pain Res. 2018;11:255–263. doi: 10.2147/JPR.S140369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SY, Zhang SZ, An JX, et al. The effect of ultrasound-guided percutaneous ozone injection around cervical dorsal root ganglion in zoster-associated pain: a retrospective study. J Pain Res. 2018;11:2179–2188. doi: 10.2147/JPR.S163340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crockett MT, Moynagh M, Long N, et al. Ozone-augmented percutaneous discectomy: a novel treatment option for refractory discogenic sciatica. Clin Radiol. 2014;69:1280–1286. doi: 10.1016/j.crad.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 18.Paoloni M, Di Sante L, Cacchio A, et al. Intramuscular oxygen-ozone therapy in the treatment of acute back pain with lumbar disc herniation: a multicenter, randomized, double-blind, clinical trial of active and simulated lumbar paravertebral injection. Spine (Phila Pa 1976). 2009;34:1337–1344. doi: 10.1097/BRS.0b013e3181a3c18d [DOI] [PubMed] [Google Scholar]
- 19.Oder B, Loewe M, Reisegger M, et al. CT-guided ozone/steroid therapy for the treatment of degenerative spinal disease–effect of age, gender, disc pathology and multi-segmental changes. Neuroradiology. 2008;50:777–785. doi: 10.1007/s00234-008-0398-2 [DOI] [PubMed] [Google Scholar]
- 20.Hu B, Zheng J, Liu Q, et al. The effect and safety of ozone autohemotherapy combined with pharmacological therapy in postherpetic neuralgia. J Pain Res. 2018;11:1637–1643. doi: 10.2147/JPR.S154154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautam S, Rastogi V, Jain A. Comparative evaluation of oxygen-ozone therapy and combined use of oxygen-ozone therapy with percutaneous intradiscal radiofrequency thermocoagulation for the treatment of lumbar disc herniation. Pain Pract. 2011;11:160–166. doi: 10.1111/j.1533-2500.2010.00409.x [DOI] [PubMed] [Google Scholar]
- 22.Beyaz SG. Six-month results of cervical intradiscal oxygen-ozone mixture therapy on patients with neck pain: preliminary findings. Pain Physician. 2018;21:E449–E456. [PubMed] [Google Scholar]
- 23.Rogers CL, Shetter AG, Fiedler JA, et al. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47:1013–1019. doi: 10.1016/S0360-3016(00)00513-7 [DOI] [PubMed] [Google Scholar]
- 24.Chang KV, Wu WT, Huang KC, et al. Limb muscle quality and quantity in elderly adults with dynapenia but not sarcopenia: an ultrasound imaging study. Exp Gerontol. 2018;108:54–61. doi: 10.1016/j.exger.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 25.Allam AE, Khalil AAF, Eltawab BA, et al. Ultrasound-guided intervention for treatment of trigeminal neuralgia: an updated review of anatomy and techniques. Pain Res Manag. 2018;2018:5480728. doi: 10.1155/2018/5480728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahalingam R, Wellish M, Wolf W, et al. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med. 1990;323:627–631. doi: 10.1056/NEJM199009063231002 [DOI] [PubMed] [Google Scholar]
- 27.Clavo B, Santana-Rodriguez N, Gutierrez D, et al. Long-term improvement in refractory headache following ozone therapy. J Altern Complement Med. 2013;19(5):453–458. doi: 10.1089/acm.2012.0273 [DOI] [PubMed] [Google Scholar]
- 28.Rahimi-Movaghar V. The major efficient mechanisms of ozone therapy are obtained in intradiscal procedures. Pain Physician. 2012;15:E1007–8. [PubMed] [Google Scholar]
- 29.Ozbay I, Ital I, Kucur C, et al. Effects of ozone therapy on facial nerve regeneration. Braz J Otorhinolaryngol. 2017;83:168–175. doi: 10.1016/j.bjorl.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]