Abstract
Background:
This study examined brain tissue integrity in sites that controls cognition (prefrontal cortices; PFC) and its relationships to glycemic outcomes in adults with type 2 diabetes mellitus (T2DM).
Methods:
We examined 28 T2DM patients (median age 57.1 years; median BMI 30.6 kg/m2 ;11 males) and 47 healthy controls (median age 55.0 years; median BMI 25.8 kg/m2 ; 29 males) for cognition (Montreal Cognitive Assessment [MoCA]), glycemic control (hemoglobin A1c [HbA1c]), and PFC tissue status via brain magnetic resonance imaging (MRI). High-resolution T1-weighted images were collected using a 3.0-Tesla MRI scanner, and PFC tissue changes (tissue density) were examined with voxel-based morphometry procedures.
Results:
Reduced PFC density values were observed in T2DM patients compared to controls (left, 0.41±0.02 mm3/voxel vs. 0.44±0.02 mm3/voxel, p<0.001; right, 0.41±0.03 mm3/voxel vs. 0.45±0.02 mm3/voxel, p<0.001). PFC density values were positively correlated with cognition; left PFC region (r= 0.53, p=0.005) and right PFC region (r= 0.56, p=0.003), with age and sex as covariates. Significant negative correlations were found between PFC densities and HbA1c values; left PFC region (r= −0.39, p=0.049) and right PFC region (r=−0.48, p=0.01), with age and sex as covariates.
Conclusions:
T2DM patients showed PFC brain tissue damage, which is associated with cognitive deficits and poor glycemic control. Further research is needed to identify causal relationships between HbA1c, cognition, and brain changes in T2DM and to evaluate the impact of interventions to prevent brain tissue injury or neuroregeneration in this high-risk patient population, to eventually preserve or enhance cognition and improve glucose outcomes.
Keywords: HbA1c, cognition, prefrontal cortex, brain injury, magnetic resonance imaging
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic and progressive metabolic disorder, characterized by insulin resistance and hyperglycemia. Over 30 million (9.4%) adults in the US are estimated to have T2DM, and 1.5 million individuals are newly-diagnosed each year.1, 2 The greatest incidence of T2DM in adults occurs between the ages 45–64 (an estimated 13 million people).2 Because chronic hyperglycemia is the primary cause of T2DM complications that lead to high morbidity and mortality, T2DM treatments are aimed at preventing hyperglycemia.3–5 A core component of achieving an excellent glycemic control is patients’ self-care, which includes following prescribed medical regimens and performing various glucose management activities, such as exercise, diet, medication adherence, and self-monitoring of blood glucose.6–8
T2DM self-care tasks are complex and challenging, and require multi-domain cognitive skills and processes, including memory, attention, planning, and calculation (executive decision-making functions). However, cognitive dysfunction is common in T2DM.9 Both cross-sectional and longitudinal/prospective studies have shown T2DM persons perform less well than non-T2DM controls in several cognitive domains, primarily information-processing speed, memory, attention, verbal fluency and learning, and executive function (includes planning, attention, and problem solving).10–14
Cognition requires intact brain structure and function.15 The prefrontal cortices, located in the anterior frontal lobe, play a major role in high-level cognitive activities, such as executive functions (planning, organizational skills, reasoning, problem solving, decision-making, and abstract thinking).14, 16, 17 Reports on damage to prefrontal cortices in T2DM are few, yet existing studies indicate gray matter changes in prefrontal cortices.18, 19 Whether or not the damage in prefrontal cortices is linked to cognitive function or glucose outcomes in T2DM is unclear, although there are some reports on global brain changes (total brain volume, white matter volume, gray matter volume) and its potential impact on cognition and glycemic control.19, 20, 21 Therefore, the specific aims of this study are to: 1) evaluate prefrontal cortices density (the proportion of prefrontal gray matter relative to other tissue types) in T2DM compared with age-and sex-matched non-diabetic healthy controls; and 2) examine the relationships between prefrontal cortices and cognition and glucose control (hemoglobin A1c [HbA1c]) in T2DM patients.
Methods
Study design and subjects
This study used a cross-sectional correlational research design and studied 28 T2DM patients recruited from the University of California Los Angeles (UCLA) Gonda Diabetes Center and 47 healthy controls recruited from the UCLA campus and Los Angeles community via UCLA Institutional Review Board-approved study flyers and postings. All T2DM subjects were on glucose lowering medications, except two patients. Among 26 patients on medications, 17 patients were on oral medication alone and nine patients were on both oral medications and insulin. No patients were on insulin only. Twelve T2DM patients were taking medications for high blood pressure. Demographic, clinical, and cognitive data are summarized in Table 1. Subjects with a history of stroke, heart failure, diagnosed brain abnormality (i.e., tumors, aneurysm, epilepsy, multiple sclerosis, neuropathy), metallic implants, or body weight more than 129 kg (magnetic resonance imaging [MRI] scanner limitation) were excluded from the study. Control subjects were healthy, did not have diabetes (type 1 or type 2), and were not taking any medications that might alter neural functioning or hemodynamics. None of the study participants had contraindications to the MRI scanner (http://www.mrisafety.com).22 All subjects were fully informed about the study procedure and provided written informed consent prior to the study, and the protocol was approved by the UCLA Institutional Review Board. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Table 1.
Demographics and biophysical variables of T2DM and control subjects
|
Variables |
T2DM n = 28 Median(IQR) |
Controls n = 47 Median(IQR) |
P values |
|---|---|---|---|
| Age (years) | 57.1(51.2–61.4) | 55.0(52.3–60.3) | 0.67 |
| Sex [male] (%) | 11 (39%) | 29 (62%) | 0.06 |
| BMI (kg/m2) | 30.6(24.9–33.7) | 25.8(24.1–27.7) | 0.004* |
| Systolic Blood Pressure (mmHg) | 123.5(113.3–132.8) | 119.0(110.0–129.0) | 0.26 |
| Diastolic Blood Pressure (mmHg) | 72.0(68.3–81.5) | 77.0(70.0–85.0) | 0.22 |
| Duration of T2DM (years, mean ± SD) | 10.2±7.4 | - | - |
| Smoking | None | - | - |
| Exercise (≥ 3 days/week) | 22 (78.6%) | - | - |
| Physical activity ≥ 30 min per day (≥ 5 days/week) |
7 (25%) | - | - |
| Fruits and Vegetables 5 or more servings per day (≥ 5 days/week) | 15 (53.6%) | - | - |
| HbA1c (%) | 7.1(6.3–8.3) | - | - |
| MoCA (total scores) | 25.0 (23.3–27) | 27.0 (26.0–29.0) | 0.006* |
| Prefrontal cortices gray matter density, Left (mm3/voxel, mean ± SD)† | 0.41±0.02 | 0.44±0.02 | <0.001* |
| Prefrontal cortices gray matter density, Right (mm3/voxel, mean ± SD)† | 0.41±0.03 | 0.45±0.02 | <0.001* |
IQR = interquartile range; BMI = body mass index; SD = standard deviation; HbA1c = hemoglobin A1c; MoCA= Montreal Cognitive Assessment
Prefrontal cortices gray matter density corrected for age and sex
indicates statistically significant differences between groups (p < 0.05)
Demographic and clinical data
Demographic and clinical questionnaire was used for age, sex, duration of T2DM, and smoking status. Blood pressure and weight were measured before MRI data collection. Physical activity/exercise level was obtained by two questions based on American Diabetes Association recommendations,23 which asked about days per week engaged in exercise and whether the physical activity was at least 150 minutes per week (30 minutes daily 5 times per week). Diet was assessed by asking frequency of fruit and vegetable consumption per week.24
Cognitive assessment
The Montreal Cognitive Assessment (MoCA) test was used for cognitive evaluation in all subjects. This 30-item test is a screening instrument for mild cognitive impairment (MCI), early Alzheimer’s disease (AD), and dementia, and assesses multiple cognitive domains: attention and concentration, executive functions, memory, language, visuo-constructional skills, conceptual thinking, calculations, and orientation. Concurrent validity of the MoCA established with the Mini-Mental State Exam was reported to be high (r = 0.87) with sensitivity for identifying both AD and MCI patients to be 100% and 90%, respectively.25 The MoCA has high test-retest reliability (r= 0.92) and internal consistency (Cronbach alpha = 0.83). 25 Time to administer the MoCA is approximately 10 minutes. The total possible score is 30 points, and a score of 26 or above is considered normal. 25 The MoCA test has been used in a variety of settings and patient populations, including patients with T2DM. 25
Glycemic measure
Assessment of glycemic control in T2DM patients was based on HbA1c level, which reflects average blood glucose level over the previous three months (indicated by percentage of glycated hemoglobin). 26 Value below 5.7 percent is considered normal, prediabetes 5.7–6.4 percent, and diabetes 6.5 percent or above.26 Higher HbA1c levels indicate poorer blood glucose control, and higher risk of diabetes complications. HbA1c values were measured using point-of-care instrument A1C Now®, 26, 27 which requires a fingerstick sample of fresh blood (using a portable lancet system and approximately 5µL of blood) to be placed into the device. The HbA1c results are available in digital readout and printout in 5 minutes. This device has demonstrated accuracy (on average 99% accuracy compared to certified reference lab) for the assessment of whole blood HbA1c and is certified by the National Glycohemoglobin Standardization Program. 27
Magnetic Resonance Imaging (MRI)
Brain MRI in T2DM and control subjects were performed using a 3.0-Tesla MRI scanner (Siemens, Magnetom Prisma, Erlangen, Germany). Subjects lay supine with foam pads placed on both sides of the head to minimize head motion during data acquisition. Two high-resolution T1-weighted images were collected using the magnetization-prepared rapid acquisition gradient-echo pulse sequence [repetition-time (TR) = 2200 ms; echo-time (TE) = 2.34/2.41 ms; inversion time = 900 ms; flip angle (FA) = 9°; matrix size = 320 × 320; field-of-view (FOV) = 230 × 230 mm2; slice thickness = 0.9 mm)]. Proton-density (PD) and T2-weighted images were collected in the axial plane using a dual-echo turbo spin-echo sequence (TR = 10,000 ms; TE1, 2 = 12, 123/124 ms; FA = 130°; matrix size = 256 × 256; FOV = 230 × 230 mm2; slice thickness = 3.5 mm). T1-, T2-, and PD-weighted images of T2DM and control subjects were visually-assessed for any major pathological changes, such as presence of cystic lesions, infarcts or tumors. High-resolution T1-weighted images were examined for any head motion related or other imaging artifacts.
MRI data processing and Voxel-Based Morphometry (VBM) analyses
The statistical parametric mapping package (SPM12, http://www.fil.ion.ucl.ac.uk/spm/), MRIcroN, 28 and MATLAB-based (The MathWorks Inc., Natick, MA, USA) custom software were used for data processing and analyses. Data processing steps were followed as described in previous publications.29 Two high-resolution T1-weighted images were reoriented to remove any potential variation from head motion and averaged to increase signal-to-noise ratio. The averaged T1-weighted images were partitioned into gray matter, white matter, and cerebrospinal fluid tissue types. The Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra algorithm (DARTEL) toolbox 30 was used to generate the flow fields which are nonlinear deformations applied for warping all the gray matter images to match each other and template images which were implemented for normalization of gray matter maps to Montreal Neurological Institute (MNI) space (voxel size: 1×1×1 mm3). The unmodulated normalized maps were smoothed using a Gaussian filter (kernel, 10 mm).
The average T1-weighted images from each T2DM and control subjects were normalized to MNI space. The normalized images obtained from each subject were averaged to create background images for structural identification.
Statistical Analyses
The Statistical Package for the Social Sciences (SPSS, v24.0, Armonk, NY, USA) was used for assessment and comparison between groups of demographic, clinical, and cognitive variables. The Shapiro-Wilk test of normality was performed on T2DM and control subjects for all continuous data, including age, body mass index (BMI), systolic and diastolic blood pressure, T2DM duration, MoCA total score, and HbA1c. Demographic and clinical variables were evaluated by independent samples t-tests for normally distributed data, Mann-Whitney U test for non-normally distributed data, and categorical variables (e.g., sex) were compared using the Chi-square test. A P-value of <0.05 was considered statistically significant.
The smoothed whole-brain gray matter density maps were compared voxel-by-voxel between T2DM and control subjects using analysis of covariance (ANCOVA, SPM12; covariates, age and sex, family-wise error corrected, p<0.05). Brain regions that showed significant differences in gray matter density between groups were overlaid onto background images for identification of their locations. Region of interest (ROI) analyses were performed on bilateral prefrontal cortices to determine brain region specific gray matter density values and compared between groups (ANCOVA, SPSS; covariates, age and sex). Partial correlation analyses were performed in SPM12 (covariates, age and sex; uncorrected p<0.005) to evaluate the correlations between prefrontal cortices and cognition, and HbA1c levels. Relationships between gray matter density values of prefrontal cortices, cognition, and HbA1c levels were also examined using partial correlation procedures in SPSS software.
Results
Demographics, clinical variables, and cognition
The Shapiro-Wilk test of normality showed that age, BMI, systolic and diastolic blood pressure, MoCA total score of control group, and HbA1c of T2DM were not normally distributed, and therefore, median values are reported for these variables (Table 1). No significant differences in age and sex emerged between T2DM and control subjects as shown in Table 1. However, BMI values were significantly higher in T2DM subjects over controls. Global MoCA scores were significantly lower in T2DM patients compared to controls, with significant differences in language scores [2.0 (1.0–3.0) vs. 3.0 (2.0–3.0); P<0.001]. Median HbA1c in the T2DM subjects was 7.1% and ranged 5.4–10.8%. None of the T2DM subjects smoked currently. Approximately 79% of T2DM subjects exercised at least 3 days per week and 25% were engaged in at least 30 minutes of daily physical activity 5 or more times per week. Approximately 54% of T2DM subjects reported eating daily five or more serving of fruits and vegetables at least 5 days per week.
Prefrontal gray matter density
T2DM patients showed reduced prefrontal cortices gray matter density as compared to control subjects (left 0.41±0.02 mm3/voxel vs. 0.44±0.02 mm3/voxel, p<0.001; right 0.41±0.03 mm3/voxel vs. 0.45±0.02 mm3/voxel, p<0.001; Figure 1). None of the brain regions showed increased gray matter density in T2DM patients as compared to controls.
Figure 1.
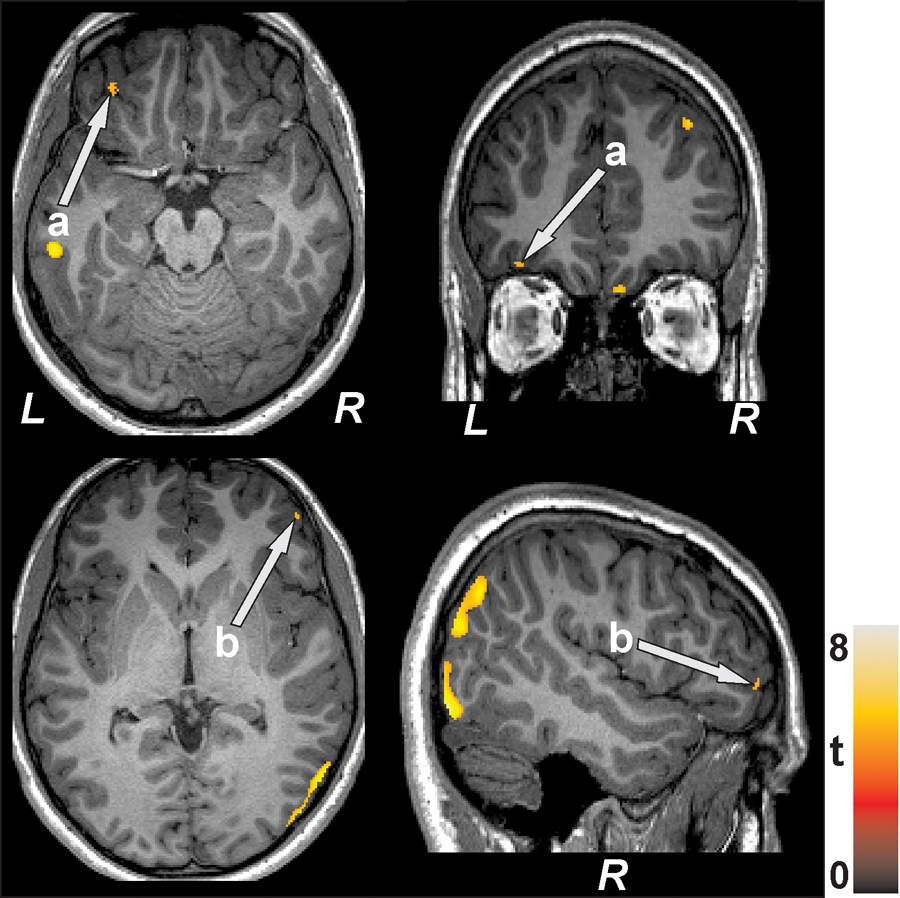
Prefrontal cortices [a, left (axial view, coronal view); b, right (axial view, sagittal view)] with reduced gray matter density in T2DM patients over controls are illustrated. All images are in neurological convention (L = left; R = right). Color bar indicates t-statistic values.
Prefrontal cortex density and cognition (MoCA)
The MoCA total scores had significant positive relationships to both left and right prefrontal cortex density values (left, r=0.58, p=0.001; right, r=0.58, p=0.001), indicating higher overall cognitive functioning in subjects with higher prefrontal cortices density (less damage). When controlled for age and sex (partial correlation), highly significant positive relationships remained for MoCA and left prefrontal regions (r= 0.53, p=0.005) and right prefrontal regions (r= 0.56, p=0.003) (Table 2, Figure 2).
Table 2.
Correlation values between prefrontal cortex volume and cognition and HbA1c (n=28)
| Left Prefrontal Cortex Correlation coefficient (p-value) |
Right Prefrontal Cortex Correlation coefficient (p-value) |
|
|---|---|---|
| MoCA (Pearson’s correlation) | r=0.58 (p=0.001) |
r=0.58 (p=0.001) |
| HbA1c (Pearson’s correlation) | r=−0.25 (p=0.20) |
r=−0.28 (p=0.15) |
| MoCA (Partial correlation; covariates: age and sex) | r=0.53 (p=0.005) |
r=0.56 (p=0.003) |
| HbA1c (Partial correlation; covariates: age and sex) | r=−0.39 (p=0.049) |
r=−0.48 (p=0.01) |
MoCA= Montreal Cognitive Assessment; HbA1c = hemoglobin A1c
Figure 2.
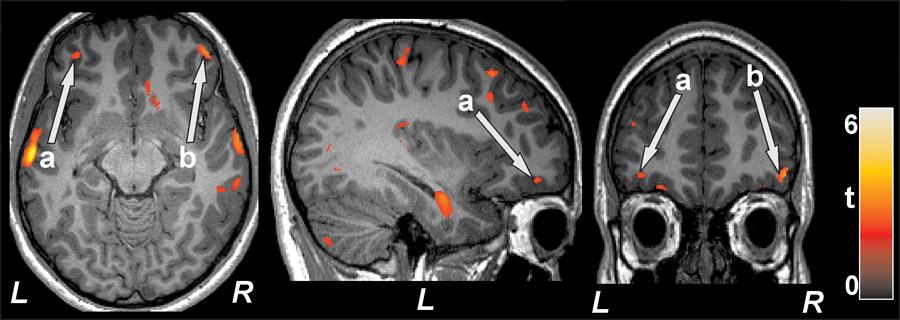
Left prefrontal cortex (a; axial, sagittal) and right prefrontal cortex (b; axial, coronal) showed significant positive correlation with MoCA scores in T2DM subjects. All images are in neurological convention (L = left; R = right). Color bar indicates t-statistic values.
Prefrontal cortex density and glucose control (HbA1c)
Significant negative relationships emerged for HbA1c and left prefrontal regions (r= −0.39, p=0.049) and right prefrontal regions (r=−0.48, p=0.01) when controlled for age and sex (partial correlations), as shown in Table 2 and Figure 3.
Figure 3.
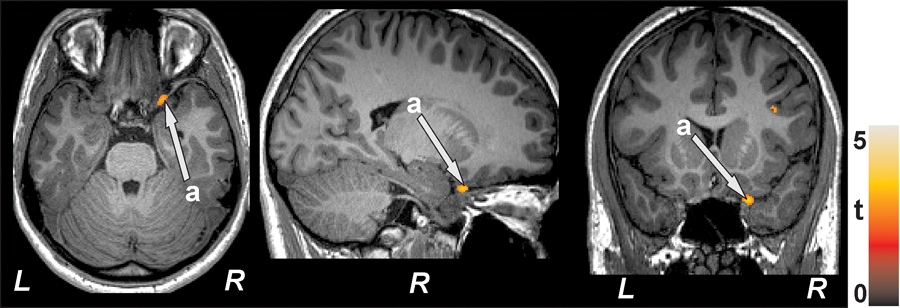
Negative correlations emerged between prefrontal cortex density and HbA1c values in T2DM subjects. The significant cluster appeared in right prefrontal cortex (a; axial, sagittal, coronal). All images are in neurological convention (L = left; R = right). Color bar indicates t-statistic values.
Discussion
The results of the present study demonstrated that T2DM patients had prefrontal cortex damage (reduced gray matter density) and that the damage was associated with lower overall cognition and poorer glucose control. The finding on brain tissue density change is consistent with previous studies that showed reduced regional gray matter in brain sites such as prefrontal cortex, medial temporal, anterior cingulate, and medical frontal lobes, and hippocampal region in T2DM patients as compared with non-T2DM control subjects.18,20, 31
Etiology of the brain damage specifically occurring in prefrontal cortices in T2DM is not fully understood. However, diabetic vasculopathy (arterial stiffness, atherosclerosis, and endothelial dysfunction) and oxidative stress are believed to be important potential underlying mechanisms of T2DM-induced brain injury by causing neuro-inflammation, neurodegeneration, and neuronal apoptosis. Particularly, endothelial dysfunction occurring from decreased nitric oxide (NO), driven by insulin inefficiency or deficiency, is believed to be the root cause of blood vessel damage in T2DM and low NO availability can also induce oxidative stress.32, 33 The brain is vulnerable to oxidative stress because of its highly aerobic nature and low antioxidant defense mechanisms.32 Oxidative stress damages the blood brain barrier (BBB) and brain microvasculature, resulting in the loss of pericytes (contractile cells that wrap around endothelial cells that line blood vessels), and BBB disruption with leak of toxic substances into the brain tissue further damages nervous structures. 34, 35 It is known that BBB damage is associated with brain injury in non-T2DM subjects. Oxidative stress may arise from several mechanisms related to chronic hyperglycemia, including glucose autoxidation with production of reactive oxygen species during accelerated respiration (mitochondrial oxidative metabolism of glucose) and increased formation of advanced glycation end products (AGEs) which result in disturbance of cell behavior/regulations. 36, 37 Moreover, cerebral vascular reactivity is abnormal is T2DM38 and hypertension, which is present in many T2DM patients, is linked to abnormal BBB and cerebrovascular autoregulation.39 Cerebral vascular reactivity is key for maintaining an optimal blood flow environment for neuronal survival39, and alteration in vascular regulations in T2DM may contribute to regional gray matter changes and eventual death of brain cells (manifested as decreased gray matter density).36, 38
Many T2DM patients have coexisting conditions that are associated with diminished NO and vascular damages, such as hypertension and hypercholesterolemia.33 These conditions, along with poor glycemic control, longer duration of T2DM, and presence of diabetic retinopathy, are associated with brain changes.40 Since the detrimental effects of these risk factors on blood vessels add to neurodegeneration in brain tissues and structures in T2DM patients,41 potential interventions for neuroprotection and repair/restoration of damaged brain tissues (neurogenesis) in prefrontal cortices should target these risk factors as well as underlying mechanisms.
Impaired cognition in T2DM patients in our study is consistent with previous studies that showed poor cognitive performance in T2DM patients.9–14 Our findings show that there is a positive correlation between prefrontal cortex density and cognitive function, which are also consistent with previous research that showed that brain volume loss is associated with poor cognitive performance in their T2DM samples.31 Intact cognitive function is critical for optimal self-care in T2DM patients.42, 43 Particularly, executive functions governed by prefrontal cortices are essential for day-to-day self-care planning and decision-making for T2DM management (e.g., identifying/differentiating groups of food/behaviors to limit or increase for glucose control). The positive associations between prefrontal cortex density and impaired cognition found in this study suggest that brain tissue injury in prefrontal cortices may contribute to poor glucose control in T2DM patients.
Significant negative relationships found in the present study between glycemic control and prefrontal cortex density in T2DM subjects are consistent with existing studies that showed associations between hyperglycemia and overall structural brain damage.44–46 One previous study also showed significantly lower gray matter volume primarily in the frontal and temporal (hippocampi location) lobes in association with poor glucose control, and intensive treatment to decrease HbA1c decelerated volume loss in these areas.46 These data suggest that prefrontal cortices may be a particularly vulnerable brain region to hyperglycemia and maintaining optimal glucose control may help to protect and preserve the integrity of this brain site. Protecting prefrontal cortices may be important for long-term T2DM health outcomes because this brain site governs key cognitive functions essential for self-care abilities of T2DM patients to maintain optimal glucose control.
Relationships found in our study between prefrontal cortex density, cognitive function and HbA1c among T2DM patients have important clinical implications: protecting prefrontal cortices from injury and repairing existing damage to the brain region may have a significant potential impact on preserving cognitive functions and improving glycemic control in T2DM patients. In a previous report, disease duration of greater than one year was associated with the greatest differences in brain tissue volume whereas those with T2DM diagnosed less than one year had tissue volume similar to nondiabetic subjects.40 This initial period may be a “window opportunity” for brain protection and to make the greatest clinical impact on T2DM patient care and outcomes. However, it may take time to fully understand the causes and mechanisms of brain injury in T2DM, develop new therapies to treat brain injury (e.g., promote neurogenesis and neuroprotection), and establish their effects on, or related to, HbA1c. Alternatives ways to preserve brain tissue integrity in T2DM may need to be considered as well as continuing with current evidence-based recommendations such as exercise, nutrition (e.g., thiamine supplement), excellent control of blood glucose and CVD risk factors (e.g., hyperglycemia, hypertension).40, 46–49
The present study has some limitations. The small sample size limited our ability to consider important variables in our analyses (such as duration of T2DM, mood, medications, and comorbidities). However, studies of this nature inherently have relatively small numbers of subjects and the magnitude of the differences we observed may compensate for some of the statistical limitations. Another limitation of the study pertains to characteristics of our sample (younger age, relatively well-managed blood pressure), which may not reflect the demographics of the majority of T2DM patients, limiting the generalizability of this study’s findings. Since this study was cross-sectional with correlational design, the findings cannot be interpreted as establishing causal relationships.
In summary, the present study showed prefrontal cortices damage in T2DM patients and its association with impaired cognitive functions and poor glycemic control. Protecting brain tissue integrity in this major cognition regulatory brain region may have an important clinical impact on preserving/enhancing cognition and improving glycemic outcomes. Further research is needed to identify the causal relationships between HbA1c levels, cognition status, and brain changes in T2DM and to examine interventions to enhance brain neurogenesis and its impact on cognition and HbA1c.
Highlights.
T2DM patients have prefrontal cortices changes over non-T2DM healthy individuals.
Prefrontal cortices tissue changes are associated with cognitive deficits in T2DM patients.
Prefrontal cortices damage is associated with poor glycemic control in T2DM.
Acknowledgement:
This work was supported by the National Institutes of Health R01 NR017190. Authors would like to thank Cristina Cabrera-Mino and Luke Ehlert for assistance with data collection.
Footnotes
Disclosure: The authors have no confliects of interest relevant to this manuscript.
References
- 1.American Diabetes Association Fast Facts. 2017.
- 2.National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States; 2017. http://www.diabetes.org/assets/pdfs/basics/cdc-statistics-report-2017.pdf.
- 3.Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care 2018;41 Suppl 1:S4–6. [DOI] [PubMed] [Google Scholar]
- 4.Kent D, D’Eramo Melkus G, Stuart PM, et al. Reducing the risks of diabetes complications through diabetes self-management education and support. Popul Health Manag. 2013;16:74–81. [DOI] [PubMed] [Google Scholar]
- 5.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–71. [DOI] [PubMed] [Google Scholar]
- 6.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561–87. [DOI] [PubMed] [Google Scholar]
- 7.Mehravar F, Mansournia MA, Holakouie-Naieni K, Nasli-Esfahani E, Mansournia N, Almasi-Hashiani A. Associations between diabetes self-management and microvascular complications in patients with type 2 diabetes. Epidemiol Health. 2016;38:e2016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grodstein F, Chen J, Wilson RS, Manson JE, Nurses’ Health S. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. 2001;24:1060–5. [DOI] [PubMed] [Google Scholar]
- 10.Vincent C, Hall PA. Executive Function in Adults With Type 2 Diabetes: A Meta-Analytic Review. Psychosom Med. 2015;77:631–42. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg E, Reijmer YD, de Bresser J, et al. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebady SA, Arami MA, Shafigh MH. Investigation on the relationship between diabetes mellitus type 2 and cognitive impairment. Diabetes Res Clin Pract. 2008;82:305–9. [DOI] [PubMed] [Google Scholar]
- 13.Ruis C, Biessels GJ, Gorter KJ, van den Donk M, Kappelle LJ, Rutten GE. Cognition in the early stage of type 2 diabetes. Diabetes Care. 2009;32:1261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funahashi S. Working Memory in the Prefrontal Cortex. Brain Sci. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manschot SM, Brands AM, van der Grond J, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–13. [DOI] [PubMed] [Google Scholar]
- 16.Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107:471–82. [DOI] [PubMed] [Google Scholar]
- 17.Masterman DL, Cummings JL. Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors. J Psychopharmacol. 1997;11:107–14. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Haroon E, Darwin C, et al. Gray matter prefrontal changes in type 2 diabetes detected using MRI. J Magn Reson Imaging. 2008;27:14–9. [DOI] [PubMed] [Google Scholar]
- 19.Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. 2015;1353:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Liu T, Wang W, et al. Reduced Gray Matter Volume in Patients with Type 2 Diabetes Mellitus. Front Aging Neurosci. 2017;9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray AM, Hsu FC, Williamson JD, et al. Action to Control Cardiovascular Risk in Diabetes Follow-On Memory in Diabetes (ACCORDION MIND) Investigators. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia. 2017;60: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shellock FG. MRI safety; http://www.mrisafety.com/. Accessed August 13, 2019.
- 23.Colberg SR, Sigal RJ, Yardley JE, et al. Physcial activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care. 2019;42:731–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 26.The A1C Test & Diabetes; 2018. https://www.niddk.nih.gov/health-information/diabetes/overview/tests-diagnosis/a1c-test. Accessed August 13, 2019.
- 27.National Glycohemoglobin Standardization Program (NGSP). http://www.ngsp.org/protocol.asp. Assessed August 13, 2019.
- 28.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–8. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner J. VBM Tutorial; 2010. https://www.fil.ion.ucl.ac.uk/~john/misc/VBMclass10.pdf. Accessed August 13, 2019.
- 30.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 31.Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei X, Schneider JG, Shenouda SM, et al. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J Biol Chem. 2011;286:2933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. [DOI] [PubMed] [Google Scholar]
- 34.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkat P, Chopp M, Chen J. Blood-Brain Barrier Disruption, Vascular Impairment, and Ischemia/Reperfusion Damage in Diabetic Stroke. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamed SA. Brain injury with diabetes mellitus: evidence, mechanisms and treatment implications. Expert Rev Clin Pharmacol. 2017;10:409–28. [DOI] [PubMed] [Google Scholar]
- 37.Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. Int J Mol Sci. 2013;14:21525–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Last D, Alsop DC, Abduljalil AM, et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saczynski JS, Siggurdsson S, Jonsson PV, et al. Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care. 2009;32:1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–78. [DOI] [PubMed] [Google Scholar]
- 42.Feil DG, Zhu CW, Sultzer DL. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. J Behav Med. 2012;35:190–9. [DOI] [PubMed] [Google Scholar]
- 43.Tomlin A, Sinclair A. The influence of cognition on self-management of type 2 diabetes in older people. Psychol Res Behav Manag. 2016;9:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strachan MW. R D Lawrence Lecture 2010. The brain as a target organ in Type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med. 2011;28:141–7. [DOI] [PubMed] [Google Scholar]
- 46.Erus G, Battapady H, Zhang T, et al. Spatial patterns of structural brain changes in type 2 diabetic patients and their longitudinal progression with intensive control of blood glucose. Diabetes Care. 2015;38:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson GS, Reger MA, Baker LD, et al. Effects of exercise and nutrition on memory in Japanese Americans with impaired glucose tolerance. Diabetes Care. 2006;29:135–6. [DOI] [PubMed] [Google Scholar]
- 48.Enomoto M, Yoshii H, Mita T, et al. Relationship between dietary pattern and cognitive function in elderly patients with type 2 diabetes mellitus. J Int Med Res. 2015;43:506–17. [DOI] [PubMed] [Google Scholar]
- 49.Zheng F, Yan L, Yang Z, Zhong B, Xie W. HbA1c, diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia. 2018;61:839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


