Abstract
Background:
The en bloc resection of inferior vena cava (IVC) leiomyosarcoma often necessitates IVC reconstruction. The objective of this study is to examine outcomes after IVC reconstruction and determine optimal graft sizing.
Method:
A retrospective review was conducted of all IVC reconstructions after IVC leiomyosarcoma resection at a single institution. Cross sectional dimensions at the IVC resection margins were measured on pre-operative imaging. The tumor location was based on the most superiorly involved region of the IVC and was classified as infrarenal, between hepatic and renal veins, or superior to the hepatic veins. Peri-operative details and long-term outcomes including graft sizing, graft patency, morbidity, and mortality were recorded.
Results:
Between 2007 and 2017, 12 patients (6 females, mean age: 64.5 years, age range: 46–80 years) underwent IVC leiomyosarcoma resection and reconstruction. All reconstructions were performed with ringed polytetrafluoroethylene (PTFE); graft sizes ranged from 12 mm to 16 mm. The tumor location was exclusively infrarenal in seven patients, between the renal and hepatic veins in two patients, and involved multiple segments in three patients. Larger graft sizes were utilized in reconstructing more superior segments of the IVC. Grafts were typically undersized and based on the diameter of the superior resection margin with 12 mm grafts approximately correlating to a 20 mm diameter, 14 mm to 25 mm, and 16 mm to 30 mm. The average undersizing ratio was 0.6. At a mean follow-up time of 43+/−27 months, radiographic graft patency was 92%, overall survival was 83%, and disease-free survival was 25%.
Conclusion:
After en bloc resection of IVC leiomyosarcoma, caval reconstruction with an undersized ringed PTFE has acceptable patency. Grafts sizes should be based on the IVC diameter superior to the tumor and undersizing by approximately 40% appears to be associated with acceptable patency rates. Further multi-institutional studies should be performed to best determine the optimal treatment of this rarely encountered tumor.
Keywords: Inferior Vena Cava Reconstruction, Leiomyosarcoma, Venous Reconstruction
1.0. Introduction
Primary leiomyosarcoma of the inferior vena cava (IVC) is a rare malignancy associated with a poor prognosis. The 5-year disease-free survival ranges from 31%−37% and the recurrence rate from 50–68% at 14–21 months.1–6 Though there are few cases reported in the literature, some aspects of the optimal surgical management are clear. Two large collections of patients, a 1992 registry of 218 patients and a recent 2015 pooled data analysis of 377 patients, have shown that radical surgical resection and grossly negative margins are associated with higher survival rates.1,7 With a goal of grossly negative margins, en bloc resection of the IVC is necessary.
After radical excision of the IVC tumor, there are several options for reconstruction that depend on the extent of resection. For circumferential resections, reconstruction with an interposition graft is frequently performed.7 However, consensus regarding the details of reconstruction including conduit type and sizing is lacking. The graft material used in reconstruction varies greatly. Options that have been used include autologous, homologous, and prosthetic material including Dacron and ring reinforced polytetrafluoroethylene (PTFE). Sizing has a widely reported range varying from 10mm to 22mm, with the principles of graft sizing rarely being described in the literature.4,8–10
The objective of this study is to develop a general guideline for graft sizing during IVC reconstruction after en bloc resection of a primary IVC leiomyosarcoma and report post-operative outcomes on the case series from which the sizing criteria is developed. We hypothesize that significant undersizing of a prosthetic, externally supported graft can safely be used to reconstruct any segment of the IVC after en bloc resection of IVC leiomyosarcoma with acceptable outcomes.
2.0. Material and Methods
This study was approved by the Institutional Review Board of the University of California Los Angeles.
2.1. Study Design.
A retrospective chart review was performed of all patients undergoing primary leiomyosarcoma resection requiring IVC reconstruction at Ronald Reagan University of California Los Angeles Medical Center between 2007 and 2017. Patients were identified by querying the electronic health record for cases associated with the Current Procedure Terminology code 34502 for reconstruction of vena cava, any method. A manual chart review of each case was performed to determine if the case met inclusion criteria. The inclusion criteria were age greater than eighteen, IVC reconstruction with an interposition conduit, and final pathology showing primary leiomyosarcoma of the IVC. Patients with a pathology diagnosis other than primary leiomyosarcoma, those with primary or patch repair, and those with a hypercoagulable disorder were excluded. All IVC reconstructions were performed by a vascular surgeon.
2.2. Data Variables.
Demographic and comorbidity data was collected including age at time of operation, duration from diagnosis to operation, neoadjuvant therapy (radiation, chemotherapy, a combination, or none), presence of chronic kidney disease with an estimated glomerular filtration rate less than 60, and history of prior abdominal surgeries. Although a history of congestive heart failure, myocardial infarction, stroke, and connective tissue disorder was recorded, none of the patients in this group had these conditions. Pre-operative tumor location was classified according to the commonly used IVC segment classification, where the upper IVC is cephalad to the hepatic vein, lower IVC is caudal to the renal veins, and middle IVC is between the two (figure 1).11 When the tumor spanned multiple regions, its location was classified by the most superiorly involved region.
Figure 1:
Tumor location is organized by IVC segments. The distribution of tumor locations is as follows: I only - 7, II only - 2, III only - 0, II & III - 2, I & II & III – 1.
The major and minor axes of the normal IVC cephalad and caudal to the tumor were also measured on pre-operative cross-sectional imaging. An elliptical cross-sectional area was calculated for both margins. A circular cross-sectional area equal to the actual elliptical area was used to calculate the equivalent circular IVC diameter. An equivalent circular IVC diameter facilitates size comparison between the graft and the IVC. Operative details were recorded including operative duration, blood loss, additional organs resected, margin status, and graft size. At our institution, IVC reconstruction with undersized graft is performed on all patients requiring circumferential resection. Since the graft is undersized, anticoagulation or adjunctive inflow procedures such as an arteriovenous fistula are not used to maintain graft patency. Anti-platelet therapy was routinely used post-operatively.
Standard operative details of IVC reconstruction used at our institution have previously been published.10,12 The tumor location determined the patient position and incision. Typically, a transperitoneal midline approach was appropriate for lower and middle IVC tumors, while a right flank incision was appropriate for tumors with lateral extension. For retrohepatic tumors, a right flank or thoracoabdominal incision was selected. Ringed PTFE was the graft of choice in circumferential resections where the surgical field was not contaminated. In a contaminated field, an autologous graft such as a paneled graft would typically be used. However, there were no patients with a contaminated field during the study period. The cephalad anastomosis was performed first to minimize hepatic ischemia time in upper IVC tumors or renal ischemia in middle IVC tumors. After the cephalad anastomosis, the clamp was moved below the anastomosis onto the graft to restore organ outflow. Renal vein re-implantation was routinely used. The PTFE graft rings at the planned anastomosis site were cut to accommodate the renal vein, preserving the rings up to the anastomosis. When the right renal vein was involved, the right renal artery was controlled to prevent organ congestion during the reconstruction. Routinely heparinized saline was used. However, systemic heparin was only used in patients with a history of deep venous thrombosis or with evidence of partial IVC thrombosis.
Post-operative complications within 30 days of discharge were recorded including lower extremity deep venous thrombosis, wound infection, and bleeding requiring reoperation. Primary, primary-assisted, and secondary patency were recorded. The patients underwent regular post-operative clinic visits with cross-sectional imaging for surveillance, allowing for assessment of radiographic patency. Primary patency was defined as the time interval between the initial operation and thrombosis or any intervention performed to maintain or re-establish patency. Primary-assisted patency was the time interval between the initial operation and thrombosis or any intervention performed to re-establish patency. This time interval included interventions intended to maintain patency. Secondary patency was the time interval between the initial operation and thrombosis. This included interventions intended to maintain or re-establish patency. Patients were primarily followed by the oncology team and only followed-up with vascular surgery past a standard post-operative visit when there was concern over graft patency. Overall survival and disease-free survival (survival without local or systemic recurrence of leiomyosarcoma) were recorded.
2.3. Data Analysis.
Continuous variables were described as mean +/− standard deviation (SD) while categorical variables were described using frequencies and percentiles. Categorical variables were analyzed using chi-square test and general linear model regression analysis was used for continuous variables, which can perform a student t-test equivalent for difference in mean for two groups or an ANOVA equivalent for more than two groups. Kaplan-Meier survival analysis was not conducted given the concern for validity in small sample sizes. Instead, survival rates were presented as the proportion of patients that did not experience an event (mortality for overall survival and mortality or disease recurrence for disease-free survival). Statistics were completed using the R statistical package (Version 3.5.1, R Core Team, Vienna, Austria).13
3.0. Results
3.1. Demographics.
Between 2007 and 2017, 12 patients (6 female, mean age: 64.5 years, age range: 46–80 years) with primary IVC leiomyosarcoma underwent IVC resection and reconstruction with an interposition ringed PTFE graft (table I). All patients had pre-operative cross-sectional imaging allowing for evaluation of the IVC. Most patients underwent IVC reconstruction within six months of their diagnosis. Only one patient received neoadjuvant therapy, which occurred prior to surgical consultation.
Table I.
Characteristics of patients undergoing circumferential resection of primary IVC leiomyosarcoma. SD: standard deviation
| Characteristic | n=12 (%) |
|---|---|
| Demographics | |
| Female Sex | 6 (50) |
| Age, years (SD) | 64.5 (10.3) |
| Comorbidities | |
| Diabetes Mellitus | 2 (17) |
| Hypertension | 7 (58) |
| Chronic Kidney Disease ≥ Stage 3 | 2 (17) |
| Prior or current smoking history | 2 (17) |
| Pre-operative Details | |
| Duration of diagnosis prior to surgery <6 months | 10 (83) |
| Neoadjuvant therapy | 1 (8) |
| Past history of malignancy other than leiomyosarcoma | 2 (17) |
| Prior abdominal surgery | 8 (67) |
3.2. Perioperative Details.
A majority of patients had involvement of only their infrarenal IVC. Reconstruction in three (25%) patients involved the suprahepatic IVC along with an additional segment (figure 1). Some patients required concomitant organ resection to achieve appropriate margins while four patients required reimplantation of a renal vein (table II). Eight patients (67%) had a pathologic grade III tumor. Grossly negative margins were achieved in 11 (92%) patients. Margin status did not differ between tumor locations or tumor grades (p= 0.341, 0.385). The only patient with positive resection margins had a grade 3 tumor involving the middle IVC segment, required adrenal gland resection and reconstruction with a 14mm graft plus reimplantation of bilateral renal veins. Mean total operative time for all cases, including oncological resection, was 238.4 +/− 76.9 minutes. Although not reaching significance, longer surgical duration weakly correlated with higher tumor location (p=0.135) with resections of more cephalad tumors lasting longer. Estimated blood loss was not associated with tumor location (p=0.305).
Table II.
Peri-operative details of en bloc IVC leiomyosarcoma resection and subsequent caval reconstruction with prosthetic graft. SD: standard deviation
| Peri-operative Details | n=12 (%) |
|---|---|
| Leiomyosarcoma tumor grade | |
| Grade 2 | 3 (25) |
| Grade 3 | 8 (67) |
| Indeterminant | 1 (8) |
| Resection margin status | |
| R0 – microscopically negative margin | 8 (67) |
| R1 – microscopically positive, grossly negative margin | 3 (25) |
| Cases with additional organs resected | 3 (25) |
| Nephrectomy | 2 (17) |
| Adrenalectomy | 2 (17) |
| Renal vein reimplanted | 4 (33) |
| Polytetrafluoroethylene (PTFE) Graft size | |
| 12mm | 3 (25) |
| 14mm | 7 (58) |
| 16mm | 2 (17) |
| Total operative duration, minutes (SD) | 238(77) |
| IVC Segment I, minutes (SD) | 203 (70) |
| IVC Segment II, minutes (SD) | 263 (89) |
| IVC Segment III, minutes (SD) | 305 (47) |
| Estimated blood loss, mL (SD) | 808 (718) |
| IVC Segment I, mL (SD) | 529 (546) |
| IVC Segment II, mL (SD) | 1200 (1131) |
| IVC Segment III, mL (SD) | 1200 (800) |
| Hospital length of stay, days (SD) | 9.2 (3.9) |
| Patients requiring post-operative ICU admission | 6 (50) |
3.3. Graft Sizing.
Ring-enforced PTFE grafts were used in all cases. Graft sizes ranged from 12mm to 16 mm and all were undersized. The mean IVC area at the superior resection margin was 480.4+/−274.5 mm2 while the mean equivalent IVC diameter was 24.4+/−6.2mm. Inferiorly, the area was 341.5+/−87.6 mm2 and the mean diameter was 21.0+/−2.8mm. The graft sizes were weakly associated with superior margin equivalent diameter (p=0.082) and area (p=0.079) (figure 2). However, graft sizes were not significantly associated with inferior margin IVC diameter (p=0.501) or area (p=0.536). Larger graft sizes were associated with more superior tumor locations (p=0.005) such that all of the 16mm grafts were used in upper IVC reconstructions and all 12mm were used in lower IVC reconstructions. The 14mm grafts were used in 4 lower, 2 middle, and 1 upper IVC reconstructions.
Figure 2:
Mean diameter (A) and cross-sectional area (B) of the IVC at the margin cephalad to the tumor
3.4. Post-operative Outcomes.
Post-operatively, six patients were admitted to the intensive care unit. Although anticoagulation therapy was not standard in this group, two patients required anticoagulation therapy within 30 post-operative days. One patient used anticoagulation pre-operatively and was resumed. Lower extremity deep venous thrombosis occurred in one patient during the initial hospitalization and was treated with anticoagulation. This occurred in a patient with a tumor that involved all three IVC segments, was reconstructed with a 14mm graft, and required nephrectomy plus renal vein reimplantation. There were no occurrences of myocardial infarction, pulmonary embolism, wound infection, bleeding requiring operative management, or acute kidney injury.
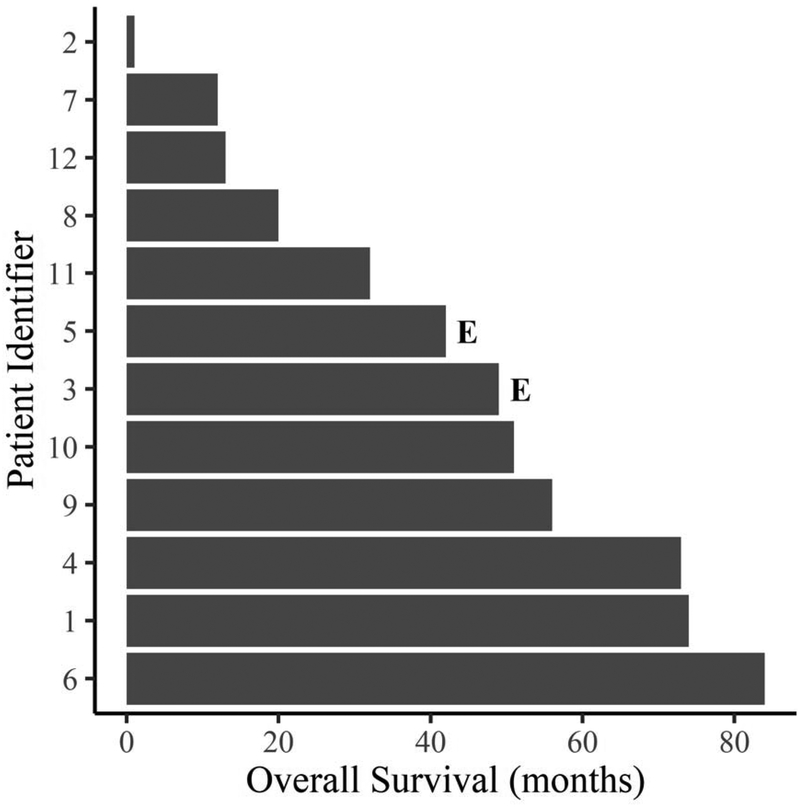
There were no in-hospital or 30-day mortalities in the group. Two late mortalities occurred at 42 and 49 months post-operatively secondary to progression of their oncologic disease (figure 3). All but two patients developed systemic disease recurrence (figure 4). At a mean follow-up time of 43.3 +/−27.3 months, overall survival was 83% while disease free survival was 25%. Over the same mean follow-up time, primary, primary-assisted and secondary graft patency by computed tomography was 92%. There was only one occlusion in the cohort, which occurred during the index hospitalization. This patient had a suprahepatic tumor with the largest pre-operative equivalent diameter of the series and did not achieve negative margin status. Of note, this patient was reconstructed with a 16mm graft, which was a 60% reduction in diameter instead of the average 40% reduction. Although there was radiographic occlusion of the graft, the patient was managed with anticoagulation and did not develop long term clinical signs of IVC occlusion.
Figure 3:
Individual overall survival times in patients with primary IVC leiomyosarcoma requiring circumferential resection. Patients that experienced an event (death from any cause) are labeled with an “E” and unlabeled patients did not experience an event.
Figure 4:
Individual disease-free survival times in patients with primary IVC leiomyosarcoma requiring circumferential resection. Patients that experienced an event (death from any cause or disease recurrence) are labeled with an “E” and unlabeled patients did not experience an event.
4.0. Discussion
Radical resection with grossly negative resection margins is the standard surgical therapy for primary IVC leiomyosarcoma.2,3,6,14 After radical resection, there are a variety of reconstruction options. For non-circumferential vascular resections, the IVC is either repaired primarily or with an autologous or prosthetic patch.2,15,16 With circumferential resection, there are proponents of IVC ligation.17 However, IVC ligation has been associated with lower extremity edema and acute renal failure in 50% of patients.17 Furthermore, IVC ligation is not recommended in situations where collateral vessels are disrupted or in cases of long-segment resection. Reconstruction with an interposition graft is the preferred option in these cases and is performed more frequently than ligation.7 When a prosthetic graft such as PTFE is used, the method of sizing is underreported.
After circumferential resection, IVC reconstruction with an undersized ringed PTFE graft is a viable alternative with favorable outcomes in all segments of the IVC. Graft patency in our patient cohort is consistent with previously published data demonstrating long term graft patency of 63%−100%.8,10,18 The technical challenges presented with segment II and III involvement did not diminish these outcomes. Thoracotomy was unnecessary in this cohort as suprahepatic infradiaphragmatic control of the IVC was possible through a flank incision with the patient in semilateral decubitus position on a hyperextended table. When compared to published groups, the complication rate was comparable and overall survival was higher in this group, which may be a factor of the small sample size.2,3,14 A 2019 small case series used PTFE grafts sized to match the IVC diameter resulting in one-in-four graft thrombosis; they recommended using smaller graft sizes when reconstructing a large IVC.19 It is unclear whether other groups used an undersized graft. Nonetheless, with comparable results it is evident that this method of reconstruction likely does not worsen outcomes.
There are variations in reconstruction methods with an underlying principle geared towards preventing thrombosis. A commonly used technique is creating an arteriovenous fistula to increase flow through the graft thereby preventing thrombosis.2 Graft undersizing achieves this same principle. The amount of undersizing in this cohort of patients is approximately 40% and based on the diameter of the cephalad IVC margin. According to the continuity equation in fluid dynamics, incompressible fluids flow in a manner that conserves mass thereby increasing velocity as cross-sectional area decreases. Although this principle cannot be directly applied to human venous flow, it is plausible that a reduction in diameter would induce an increase in velocity within the graft albeit less so than a fistula. A 40% reduction is also not significant enough to limit flow; the highly compliant venous system has the remarkable ability to accommodate changes in blood volume without a significant variation in pressure.20
An important caveat in graft sizing is taking the cephalad IVC dimension into account more so than the caudal IVC. Although all grafts diameters were smaller than the caudal IVC diameter, sizing the graft per the caudal dimension would certainly cause too severe of an undersizing, especially in reconstructions that involve multiple IVC segments. It is noteworthy that the only patient with graft thrombosis had a 60% diameter reduction, based on the cephalad IVC margin. It is unclear if such a high degree of undersizing would promote thrombosis, but may suggest that there is an optimal reduction ratio in venous reconstruction. Furthermore, this patient was asymptomatic after 2 months despite persistent thrombosis. Presumably, this patient developed venous collaterals and/or recanalized the graft. A useful sizing guide based on superior margin IVC diameter can be deduced from this cohort. A 12mm graft can be used in a 20mm IVC, 14mm graft in a 25mm IVC, and 16mm graft in a 30mm IVC. Although graft sizing in these patients was not done using a formula, the pattern that emerges from the data can be used to replicate results.
These results must also be interpreted in the context of other important details of reconstruction. A ring reinforced PTFE graft was used as it is believed to resist compression and collapse from pressure variations throughout the respiratory cycle, which are thought to contribute to graft thrombosis, while Dacron lacks these mechanical advantages.21–23 Although no direct comparison between Dacron and PTFE graft materials in intra-abdominal venous reconstruction has been done, PTFE is commonly used.24,25 In reconstructing segment III of the IVC, hepatic ischemia time was kept under 30 minutes by performing the cephalic anastomosis first, readjusting the vascular clamp onto the graft distal to the anastomosis, and restoring outflow through the hepatic veins. Lastly, use of post-operative acetylsalicylic acid was routine while use of anticoagulation was not. However, intra-operative systemic anticoagulation was selectively used only in patients with thrombogenic risk factors. Some of these principles of reconstruction have been used for resections of other malignancies including those that invade instead of originate from the IVC wall.9,25
There are several limitations to this study, namely the small sample size and retrospective nature. The small sample size severely limits the number of adverse events limiting appropriateness of useful statistical tools such as a multivariate analysis. Although small, this cohort of patients is homogenous with respect to pathology. It can allow for more direct comparison of future studies. Additionally, the graft sizing criteria presented here are based on a retrospective study; this sizing criteria was not applied pre-operatively. The measurement of IVC diameters on pre-operative computed tomography is subject to dimensional variations throughout the respiratory cycle, which may contribute to large variations in diameters especially in segment III. Given the predominance of segment I involvement in this group, there may be a more optimal criteria that was not evident in this study. A prospective validation of the proposed sizing criteria would be ideal. However, the rarity of the disease makes this type of study challenging.
5.0. Conclusion
After en bloc resection, IVC reconstruction with undersized ringed PTFE graft is safe. It has acceptable post-operative outcomes including patency with a low complication rate. Graft sizes should be based on the cephalad IVC diameter superior to the tumor. Undersizing the graft by approximately 40% is associated with acceptable patency rates. This generally translates to a 12mm graft for a 20mm IVC, 14mm for a 25mm IVC, and 16mm for a 30mm IVC. Reconstruction of more cephalad segments of IVC requires larger grafts and may present a greater operative challenge.
6.0. Acknowledgement
This research was supported by the National Institute of Health (NIDDK 1K08DK107934).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
7.0 References
- [1].Mingoli A, Cavallaro A, Sapienza P, et al. International registry of inferior vena cava leiomyosarcoma: analysis of a world series on 218 patients. Anticancer Res. 1996. October;16(5B):3201–5. [PubMed] [Google Scholar]
- [2].Kieffer E, Alaoui M, Piette J-C, et al. Leiomyosarcoma of the Inferior Vena Cava. Ann Surg. 2006. August;244(2):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hollenbeck ST, Grobmyer SR, Kent KC, et al. Surgical treatment and outcomes of patients with primary inferior vena cava leiomyosarcoma. J Am Coll Surg. 2003. October;197(4):575–9. [DOI] [PubMed] [Google Scholar]
- [4].Mann GN, Mann LV, Levine EA, et al. Primary leiomyosarcoma of the inferior vena cava: a 2-institution analysis of outcomes. Surgery. 2012. February;151(2):261–7. [DOI] [PubMed] [Google Scholar]
- [5].Dew J, Hansen K, Hammon J, et al. Leiomyosarcoma of the inferior vena cava: surgical management and clinical results. Am Surg. 2005. June;71(6):497–501. [DOI] [PubMed] [Google Scholar]
- [6].Ito H, Hornick JL, Bertagnolli MM, et al. Leiomyosarcoma of the inferior vena cava: survival after aggressive management. Ann Surg Oncol. 2007. December;14(12):3534–41. [DOI] [PubMed] [Google Scholar]
- [7].Wachtel H, Gupta M, Bartlett EK, et al. Outcomes after resection of leiomyosarcomas of the inferior vena cava: A pooled data analysis of 377 cases. Surgical Oncology. 2015. March 1;24(1):21–7. [DOI] [PubMed] [Google Scholar]
- [8].Bower TC, Nagorney DM, Cherry KJ, et al. Replacement of the inferior vena cava for malignancy: an update. J Vasc Surg. 2000. February;31(2):270–81. [DOI] [PubMed] [Google Scholar]
- [9].Caldarelli G, Minervini A, Guerra M, et al. Prosthetic replacement of the inferior vena cava and the iliofemoral vein for urologically related malignancies. BJU Int. 2002. September;90(4):368–74. [DOI] [PubMed] [Google Scholar]
- [10].Quinones-Baldrich W, Alktaifi A, Eilber F, et al. Inferior vena cava resection and reconstruction for retroperitoneal tumor excision. J Vasc Surg. 2012. May;55(5):1386–93; discussion 1393. [DOI] [PubMed] [Google Scholar]
- [11].Kulaylat MN, Karakousis CP, Doerr RJ, et al. Leiomyosarcoma of the inferior vena cava: a clinicopathologic review and report of three cases. J Surg Oncol. 1997. July;65(3):205–17. [DOI] [PubMed] [Google Scholar]
- [12].Quinones-Baldrich WJ, Farley S. Techniques for inferior vena cava resection and reconstruction for retroperitoneal tumor excision. J Vasc Surg Venous Lymphat Disord. 2013. January;1(1):84–9. [DOI] [PubMed] [Google Scholar]
- [13].R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2019. Available from: https://www.R-project.org [Google Scholar]
- [14].Hines OJ, Nelson S, Quinones-Baldrich WJ, et al. Leiomyosarcoma of the inferior vena cava: prognosis and comparison with leiomyosarcoma of other anatomic sites. Cancer. 1999. March 1;85(5):1077–83. [PubMed] [Google Scholar]
- [15].Wachtel H, Jackson BM, Bartlett EK, et al. Resection of primary leiomyosarcoma of the inferior vena cava (IVC) with reconstruction: a case series and review of the literature. J Surg Oncol. 2015. March;111(3):328–33. [DOI] [PubMed] [Google Scholar]
- [16].Kyriazi MA, Stafyla VK, Chatzinikolaou I, et al. Surgical challenges in the treatment of leiomyosarcoma of the inferior vena cava: analysis of two cases and brief review of the literature. Ann Vasc Surg. 2010. Aug;24(6):826.e13–17. [DOI] [PubMed] [Google Scholar]
- [17].Daylami R, Amiri A, Goldsmith B, et al. Inferior vena cava leiomyosarcoma: is reconstruction necessary after resection? J Am Coll Surg. 2010. February;210(2):185–90. [DOI] [PubMed] [Google Scholar]
- [18].Kuehnl A, Schmidt M, Hornung H-M, et al. Resection of malignant tumors invading the vena cava: Perioperative complications and long-term follow-up. Journal of Vascular Surgery. 2007. September 1;46(3):533–40. [DOI] [PubMed] [Google Scholar]
- [19].Kalluri AG, Jain AK, Rodriguez HE, et al. Polytetrafluoroethylene Is a Safe and Effective Interposition Conduit for Caval Reconstruction After Resection of Primary Leiomyosarcoma of the Inferior Vena Cava. Annals of Vascular Surgery. 2019. July 1;58:289–94. [DOI] [PubMed] [Google Scholar]
- [20].Gelman S Venous Function and Central Venous PressureA Physiologic Story. Anesthes. 2008. April 1;108(4):735–48. [DOI] [PubMed] [Google Scholar]
- [21].Sarkar R, Eilber FR, Gelabert HA, et al. Prosthetic replacement of the inferior vena cava for malignancy. J Vasc Surg. 1998. July;28(1):75–81; discussion 82–83. [DOI] [PubMed] [Google Scholar]
- [22].Orimo T, Kamiyama T, Yokoo H, et al. Usefulness of artificial vascular graft for venous reconstruction in liver surgery. World J Surg Oncol. 2014. April 23;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nuzzo G, Giordano M, Giuliante F, et al. Complex liver resection for hepatic tumours involving the inferior vena cava. Eur J Surg Oncol. 2011. November;37(11):921–7. [DOI] [PubMed] [Google Scholar]
- [24].Risher WH, Arensman RM, Ochsner JL, et al. Retrohepatic vena cava reconstruction with polytetrafluoroethylene graft. Journal of Vascular Surgery. 1990. September 1;12(3):367–70. [DOI] [PubMed] [Google Scholar]
- [25].Gloviczki P, Pairolero PC, Toomey BJ, et al. Reconstruction of large veins for nonmalignant venous occlusive disease. J Vasc Surg. 1992. November;16(5):750–61. [PubMed] [Google Scholar]