Abstract
Background
Essential tremor (ET) is among the most prevalent neurological diseases. Its environmental determinants are poorly understood. Harmane (1-methyl-9H-pyrido[3,4-b]indole), a dietary tremor-producing neurotoxin, has been linked to ET in a few studies in New York and Madrid. Mercury, also a tremor-producing neurotoxin, has not been studied in ET. The Faroe Islands have been the focus of epidemiological investigations of numerous neurological disorders.
Objective
In this population-based, case-control study, we directly measured blood harmane concentrations [HA] and blood mercury concentrations [Hg] in ET cases and controls.
Methods
1,328 Faroese adults were screened; 26 ET cases were identified whose [HA] and [Hg] were compared to 197 controls.
Results
Although there were no statistically significant differences between diagnostic groups, median [HA] was 2.7x higher in definite ET (4.13 g−10/ml) and 1.5x higher in probable ET (2.28 g−10/ml) than controls (1.53 g−10/ml). Small sample size was a limitation. For definite ET vs. controls, p = 0.126. [Hg] were similar between groups.
Conclusions
We demonstrated marginally elevated [HA] in definite and probable ET. These data are similar to those previously published, and possibly extend etiological links between this neurotoxin and ET to a third locale. The study did not support a link between mercury and ET.
Keywords: environmental epidemiology, population-based design, case-control study, Faroe Islands, essential tremor, neurotoxin, harmane, mercury
Introduction
Essential tremor (ET) is among the most common neurological diseases, with an estimated 7 million people affected in the US (i.e., 2.2% of the entire US population and 2.9% of the US population age 20 and older) [1]. Prevalence rises exponentially with age, and reaches >20% in advanced age groups [2,3]. The underlying causes of ET are unclear, but are thought to be both genetic [4–6] and environmental [7–9]. With numerous studies noting that fewer than one-half of patients report a positive family history of ET [10], and twin studies reporting monozygotic concordance of approximately 60% [11], the role for environmental factors is apparent.
Among the environmental factors most studied is harmane (1-methyl-9H-pyrido[3,4-b]indole), a dietary tremor-producing toxin whose blood [12–15] and brain levels [16] have been demonstrated to be elevated in ET in a small number of studies. These studies have been geographically-restricted to the US (New York) and Spain (Madrid) [12–15]. Given its high concentration in dietary animal protein [15], studies of harmane in populations with high animal protein consumption, such as the Faroe Islands [17], are of particular value.
Other neurotoxins are tremor-producing. Mercury is one of these, and it has long been linked with tremor [18–20]. Yet curiously, we are unaware of any studies directly assessing the links between mercury and ET.
The Faroe Islands, located in the North Atlantic Ocean, between Norway and Iceland, are inhabited by 49,121 individuals of whom 24,154 are age ≥40 (January 1, 2016). Due to the isolated geographic location and homogenous population, they have been the focus of epidemiological investigations of numerous neurological disorders [21–24]. The Faroese diet is high in marine food [17], and high level of exposure to methyl mercury through diet makes it a valuable location to study the effects of this neurotoxicant [25].
In this population-based, case-control study, we directly measured blood concentrations of harmane and mercury in ET cases and controls. The goal was to extend the etiological links between harmane and ET to a third locale and to take the first steps in exploring the etiological links between mercury and ET. Given the extraordinarily prevalence of ET in human populations, the identification of toxicant-risk factors would have considerable public health impact in terms of disease prevention.
Methods
Study Population and Sampling Frame
We used a two-phase, population-based study design [24,26,27]. All study procedures were approved by the local ethical review committee of the Faroe Islands and the Institutional Review Board at Yale University, with participation on a voluntary basis and signed informed consent obtained from each enrollee. As described [24,26,27], the study was undertaken between August 2016 and December 2017. The names and addresses of 4,798 individuals aged ≥40 were obtained from the Faroese Population Registry, which included 24,154 individuals aged ≥40 years living in the Islands. These individuals were selected based on six randomly-selected birthdates - 10th, 16th, 17th, 18th, 22th, and 30th [24]. From this group of 4,798, all 1,155 individuals aged ≥70 years were selected into the screening group, while the remaining 1,845 were selected by random sampling using SPSS [24]. Thus, the screening group comprised 3,000 Faroese individuals aged ≥40 years (Figure 1 in Eliasen et al.[24]).
First Phase Screening
During the first screening phase (September 2016 - December 2016), these 3,000 individuals in the screening group received an invitation letter to participate in a study of lifestyle, diet and neurological conditions [24]. The invitation letter included a screening package (questionnaires, a request for hand-drawn spirals, a return stamped envelope). The questionnaires included demographic and clinical questions, and seven screening questions for tremor [24]. The letter included instructions on drawing two Archimedes spirals with each hand and four blank sheets of paper [24]. A total of 1,334 (44.5%) individuals returned the completed screening package (Figure 1 in Eliasen et al.[24]).
Tremor on each spiral was rated by a senior movement disorders neurologist (E.D.L) using an ordinal clinical rating scale (0 – 3.0). Based on data from the questionnaire and spiral scores, participants were stratified into four groups: (1) high likelihood of having ET, (2) intermediate likelihood of having ET, (3) low-intermediate likelihood of having ET and (4) low likelihood of having ET [24].
Second Phase
In the second phase, a subsample of 282 individuals who had returned the spirals and questionnaire was invited to participate in an in-person clinical evaluation at the Department of Occupational Medicine and Public Health or in their own homes [24]. All 85 individuals in group 1 were invited. Also, a randomly-selected subsample of individuals in group 2 (n = 97), group 3 (n = 32) and group 4 (n = 68) were invited. A total of 227 (80.5%) individuals accepted and completed the in-person clinical evaluation, while 54 declined participation and one had died. The in-person clinical evaluation was conducted by a trained nurse (E.H.E.) and included 1) anthropometric measures (body weight [kilograms] and height [cm]), 2) a questionnaire encompassing data on demographics, socioeconomic status (e.g., education, number of rooms in house), medical comorbidity (i.e., Cumulative Illness Rating Scale[28]), smoking habits, family history of ET or tremor, diet, ethanol consumption, fasting status, and hours since last food consumption, 3) a detailed videotaped tremor examination, and 4) phlebotomy for blood harmane and blood mercury determinations. The videotaped tremor examination included (1) an ET-specific metric comprised of one test for postural tremor and five for kinetic tremor (e.g., pouring, drinking) performed with each arm (12 tests total, total tremor score = 0 – 36), (2) the motor portion of the Unified Parkinson’s Disease Rating Scale (UPDRS) [29] excluding an assessment of rigidity, and (3) an assessment of dystonia.
ET Diagnoses
As described, ET diagnoses (definite, probable, possible) were assigned by E.D.L. by review of questionnaire data and the videotaped neurological examination and based on published diagnostic criteria (moderate or greater amplitude kinetic tremor during three or more activities or a head tremor in the absence of Parkinson’s disease or another known cause [e.g., medication-induced tremor]) [30]. These diagnostic criteria for ET were developed for a population-based genetic study and based on data from approximately 2,000 normal (non-diseased controls)[30] the criteria carefully specify the specific examination maneuvers during which tremor should be present and the severity of tremor that should be evident during these maneuvers to distinguish normal from ET. These criteria have been shown to be both reliable [31] and valid [32], and have been used by tremor investigators in the United States and internationally [33–44]. As described, a diagnosis of ET was assigned to 27 of 227 individuals [24].
Measurement of Blood Harmane Concentration
After phlebotomy, blood harmane was quantified, blinded to clinical information, by a well-established high performance liquid chromatography method used in our previous studies [12,14,16,45]. Concentrations were reported in g−10/ml.
Measurement of Whole Blood Mercury
Analyses were conducted in the laboratory at the Department of Environmental Medicine, University of Southern Denmark, blinded to demographic, clinical and diagnostic information. Total mercury was determined in a volume of 100 μL of whole blood on a Direct Mercury Analyzer system (DMA-80) from Milestone, Sorisole, Italy [46]. The concentration in whole blood was reported in ng/ml. Certified quality control samples from Seronorm Trace Elements (Sero, Norway) were included in all sample series, and the calculated imprecision was less than 5% [46]. The limit of detection was 0.15 ng/ml.
Final Sample
There were 26 ET cases and 196 controls with successful phlebotomy and hence, blood concentrations of harmane and mercury. None of the controls came from the same household as an ET case.
Statistical Analyses
Differences between the ET and non-ET group were tested with Student’s t-test (continuous variables) or chi square test or Fischer’s exact test (categorical variables). Mann-Whitney tests were used when continuous variables were not normally distributed. Neither blood harmane nor blood mercury concentrations were normally distributed (Kolmogorov Smirnov test); hence, non-parametric tests were used to compare blood levels across diagnostic groups, and median values were also reported. As several prior publications report log-transformed harmane concentrations, we also do so here. In our control sample, we assessed the correlation between blood levels and demographic and clinical variables using non-parametric tests (Spearman’s r, Mann-Whitney tests). For these analyses, log-tranformed values were used to minimize the effects of outliers. Statistical analyses were performed using SPSS version 26.0.
Study Power
The study was designed, through screening, to yield 108 ET and to compare these to a similar number of controls. For comparisons of blood harmane concentrations, this number was deemed sufficient, as similar sample sizes (n = 100 – 150 per group) had revealed significant differences in all prior studies [12–15]. There were no prior studies of mercury in ET on which to base sample size.
The screens yielded far fewer ET than expected, as the crude prevalence of ET was only 2.9% among persons age ≥40 [24]. Hence, the final number of ET cases for these analyses was 26, with the resultant power for the harmane analysis at 30%.
Results
The 26 ET cases were similar to the 197 controls in all demographic and clinical variables - age, gender, education, number of rooms in home, body mass index, cigarette smoking, ethanol consumption, Cumulative Illness Rating Scale score, years since most recent hospitalization, fasting status, and hours since last meal or liquid consumption (Table 1). As expected, family history of ET or tremor was more prevalent among ET cases than controls, and the total tremor score was higher in ET cases than controls (Table 1). Definite ET cases were older than controls (Table 1).
Table 1:
Clinical characteristics of 222 study participants by tremor diagnosis
| ET (all) | Definite ET | Probable ET | Possible ET | Control | |
|---|---|---|---|---|---|
| N | 26 | 3 | 12 | 11 | 196 |
| Age in years | 65.7 ± 11.7 [67.0] 1 | 77.7 ± 5.8 [81.0] 1 * | 68.0 ± 11.9 [69.0] 1 | 59.8 ± 9.7 [58.0] 1 | 61.8 ± 12.2 [60.0] |
| Male gender | 17 (65.4) 2 | 2 (66.7) 2 | 8 (66.7) 2 | 7 (63.6) 2 | 105 (53.6) |
| Education # | |||||
| <High School | 8 (33.3) 2 | 2 (100) 2 | 2 (16.7) 2 | 4 (40.0) 2 | 54 (28.0) |
| High School | 1 (4.2) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 7 (3.6) |
| Trade School | 3 (12.5) | 0 (0.0) | 2 (16.7) | 1 (10.0) | 43 (22.3) |
| Associates | 5 (20.8) | 0 (0.0) | 1 (8.3) | 4 (40.0) | 19 (9.8) |
| Bachelors | 5 (20.8) | 0 (0.0) | 4 (33.1) | 1 (10.0) | 57 (29.5) |
| Masters | 1 (4.2) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 11 (5.7) |
| Doctorate | 1 (4.2) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 2 (1.0) |
| Number of rooms in home | 7.4 ± 3.3 [6.0] 1 | 5.3 ± 1.2 [6.0] 1 | 7.6 ± 3.1 [6.5] 1 | 7.8 ± 3.8 [6.0] 1 | 7.8 ± 2.9 [8.0] |
| Body mass index in Kg/m2 | 28.0 ± 4.0 3 | 24.6 ± 4.3 3 | 29.4 ± 4.1 3 | 27.5 ± 3.4 3 | 28.4 ± 4.6 |
| Current cigarette smoker | 8 (30.8) 2 | 1 (33.3) 2 | 2 (16.7) 2 | 5 (45.5) 2 | 38 (19.4) |
| Number of cigarettes smoked per day (including smokers and non-smokers) | 3.1 ± 6.2 [0.0] 1 | 2.3 ± 4.0 [0.0] 1 | 2.3 ± 6.0 [0.0] 1 | 4.1 ± 7.1 [0.0] 1 | 2.2 ± 5.6 [0.0] |
| Number of drinks of ethanol per month | 10.5 ± 12.3 [4.7] 1 | 5.2 ± 5.2 [5.2] 1 | 13.0 ± 16.6 [6.0] 1 | 9.8 ± 9.9 [3.3] 1 | 20.1 ± 34.6 [8.4] |
| Cumulative Illness Rating Scale score | 3.6 ± 2.5 [3.0] 1 | 6.7 ± 3.8 [5.0] 1 | 3.8 ± 2.3 [3.0] 1 | 2.4 ± 1.5 [2.0] 1 | 3.5 ± 3.1 [3.0] |
| Years since most recent hospitalization | 8.2 ± 7.8 [5.8] 1 | 8.0 ± 2.8 [8.0] 1 | 6.1 ± 6.9 [3.0] 1 | 10.3 ± 9.2 [7.5] 1 | 11.8 ± 13.7 [5.0] |
| Fasting # | 3 (12.5) 2 | 0 (0.0) 2 | 1 (9.1) 2 | 2 (18.2) 2 | 19 (9.8) |
| Hours since last liquid intake | 2.9 ± 3.7 [1.8] 1 | 2.8 ± 0.4 [2.8] 1 | 2.6 ± 3.3 [1.5] 1 | 3.2 ± 4.4 [1.0] 1 | 2.1 ± 2.9 [1.0] |
| Hours since last meal | 3.1 ± 3.6 [2.3] 1 | 2.8 ± 0.4 [2.8] 1 | 3.0 ± 3.2 [2.0] 1 | 3.2 ± 4.4 [1.0] 1 | 2.7 ± 3.3 [1.5] |
| Family history of ET or tremor | 10 (38.5) 2 * | 1 (33.3) 2 | 6 (50.0) 2 * | 3 (27.3) 2 | 37 (18.9) |
| Total tremor score | 16.8 ± 3.8 [16.6] 1 *** | 21.8 ± 2.8 [20.3] 1 ** | 16.1 ± 4.4 [18.0] 1 *** | 16.1 ± 2.3 [15.5] 1 *** | 10.3 ± 3.3 [10.3] |
ET = essential tremor.
Values are mean ± standard deviation [median] or number (percentage).
Some data are missing.
Mann-Whitney test compared to controls
Chi square test compared to controls
Student’s t test compared to controls.
p<0.05
p < 0.01
p < 0.001.
Higher blood harmane concentration was marginally correlated with older age and was correlated with higher body mass index but not with any other variables (Table 2). Higher blood mercury concentration was correlated with older age, male gender, lower education and higher body mass index (Table 2). Neither blood harmane nor mercury concentrations were correlated with fasting status or hours since last food or liquid consumption (Table 2).
Table 2:
Correlations between clinical characteristics and harmane and mercury concentrations in controls
| Harmane | Mercury | |
|---|---|---|
| Age in years | r = 0.14, p = 0.053 | r = 0.22, p = 0.002 |
| Gender | ||
| Male | 0.24 ± 0.39 [0.20] | 1.11 ± 0.41 [1.11] |
| Female | 0.25 ± 0.34 [0.18] | 0.92 ± 0.40 [0.94] |
| p = 0.87 1 | p = 0.002 1 | |
| Education | ||
| <Bachelors | 0.25 ± 0.36 [0.19] | 1.06 ± 0.42 [1.09] |
| Bachelors or higher | 0.25 ± 0.38 [0.19] | 0.93 ± 0.38 [0.96] |
| p = 0.82 1 | p = 0.03 1 | |
| Number of rooms in home | r = 0.01, p = 0.86 | r = 0.03, p = 0.66 |
| Body mass index (Kg/m2) | r = 0.15, p = 0.035 | r = 0.14, p = 0.049 |
| Current cigarette smoker | ||
| Yes | 0.30 ± 0.37 [0.28] | 0.96 ± 0.42 [1.00] |
| No | 0.23 ± 0.36 [0.18] | 1.04 ± 0.41 [1.06] |
| p = 0.35 1 | p = 0.46 1 | |
| Number of cigarettes smoked per day (including smokers and non-smokers) | r = 0.03, p = 0.71 | r = −0.06, p = 0.39 |
| Number of drinks of ethanol per month | r = 0.74, p = 0.40 | r = 0.04, p = 0.63 |
| Cumulative Illness Rating Scale score | r = 0.09, p = 0.20 | r = 0.10, p = 0.18 |
| Years since most recent hospitalization | r = −0.01, p = 0.90 | r = 0.047, p = 0.57 |
| Fasting | ||
| Yes | 0.22 ± 0.35 [0.24] | 1.05 ± 0.44 [1.00] |
| No | 0.25 ± 0.37 [0.19] | 1.02 ± 0.41 [1.06] |
| p = 0.96 1 | p = 0.93 1 | |
| Hours since last liquid intake | r = 0.058, p = 0.42 | r = 0.045, p = 0.53 |
| Hours since last meal | r = 0.02, p = 0.80 | r = 0.049, p = 0.50 |
| Family history of ET or tremor | ||
| Yes | 0.17 ± 0.31 [0.13] | 0.92 ± 0.39 [0.91] |
| No | 0.26 ± 0.38 [0.24] | 1.05 ± 0.42 [1.08] |
| p = 0.20 1 | p = 0.07 1 | |
| Total tremor score | r = −0.05, p = 0.50 | r = 0.02, p = 0.75 |
ET = essential tremor.
r = Spearman’s r.
Harmane (in g−10/ml) and mercury levels (in ng/ml) were log-transformed.
Values are r values or mean ± standard deviation [median].
Mann-Whitney test.
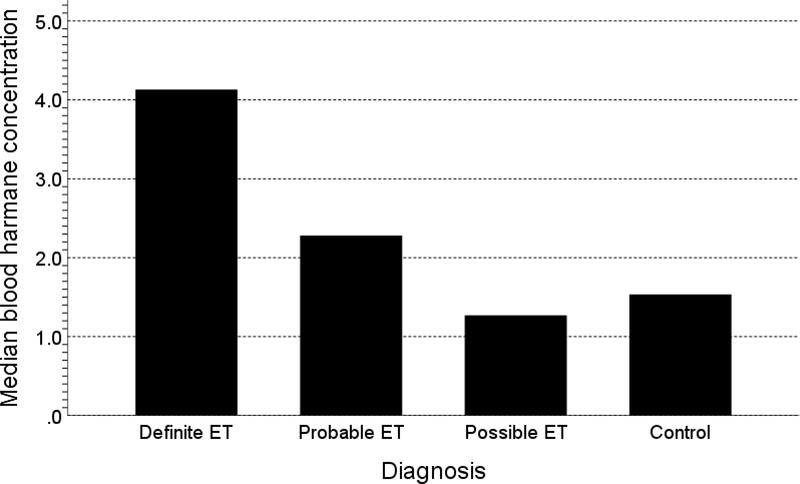
Although there were no statistically significant differences between diagnostic groups (Table 3), median blood harmane concentration was 2.7 times higher in definite ET (4.13 g−10/ml) and 1.5 times higher in probable ET (2.28 g−10/ml) than controls (1.53 g−10/ml). For the comparison of definite ET vs. controls, p = 0.126 (Table 3, Figure 1). Mercury levels were similar between groups (Table 3).
Table 3:
Harmane and mercury concentrations by tremor diagnosis
| ET (all) | Definite ET | Probable ET | Possible ET | Control | |
|---|---|---|---|---|---|
| N | 26 | 3 | 12 | 11 | 196 |
| Harmane | 1.74 [2.32 ± 2.05] p = 0.92 |
4.13 [4.86 ± 3.86] p = 0.126 |
2.28 [2.35 ± 1.82] p = 0.91 |
1.27 [1.60 ± 1.18] p = 0.28 |
1.53 [2.51 ± 2.29] |
| Log Harmane | 0.24 [0.21 ± 0.40] p = 0.92 |
0.62 [0.58 ± 0.40] p = 0.126 |
0.36 [0.23 ± 0.40] p = 0.91 |
0.10 [0.09 ± 0.37] p = 0.28 |
0.19 [0.25 ± 0.37] |
| Mercury | 10.11 [17.30 ± 17.78] p = 0.86 |
11.41 [10.49 ± 8.22] p = 0.64 |
11.30 [21.31 ± 20.77] p = 0.51 |
8.40 [14.80 ± 16.19] p = 0.86 |
11.36 [16.18 ± 17.41] |
ET = essential tremor.
Values are median [mean ± standard deviation].
Harmane was in g−10/ml and mercury was in ng/ml.
All statistical tests are Mann-Whitney test compared to controls.
For comparison of harmane concentration in definite and probable ET (n = 15) vs. 196 controls, p = 0.44 (Mann-Whitney test); for comparison of mercury concentration in definite and probable ET (n = 15) vs. 196 controls, p = 0.70 (Mann-Whitney test).
Figure 1:
Median blood harmane concentrations in g−10/ml
We compared ET cases with a family history of ET or tremor to those without such history, and blood harmane concentrations were similar: 2.38 ± 2.60 g−10/ml [median = 1.85 g−10/ml] vs. 2.28 ± 1.72 g−10/ml [median = 1.71 g−10/ml], Mann Whitney p = 0.78). We compared ET cases with a family history of ET or tremor to those without such history and blood mercury concentrations were similar: 15.09 ± 11.80 [median = 9.96] vs. 18.69 ± 20.93 [median = 10.25], Mann Whitney p = 0.82).
ET cases and controls did not differ with respect to any demographic or clinical variables (Table 1); hence, there were no confounding variables to consider in adjusted models. Neither blood harmane nor mercury concentrations were correlated with fasting status or hours since food or liquid consumption; hence, there was no need to consider these confounding variables in additional analyses either.
Discussion
The contribution of environmental factors to disease etiology has been examined in numerous epidemiological studies of Parkinson’s disease, Alzheimer’s disease and amyotrophic lateral sclerosis [47–53]. Yet the study of toxins in ET lags far behind, with surprisingly few studies [7,8].
Although there were no statistically significant differences between diagnostic groups (Table 3), median blood harmane concentration was 2.7 times higher in definite ET (i.e., 4.13 g−10/ml) and 1.5 times higher in probable ET (2.28 g−10/ml) than controls (i.e., 1.53 g−10/ml). In a prior study in New York [15], harmane concentrations were similarly highest in definite ET, second highest in probable ET and similar in possible ET and controls (Table 4), although in that study, the numbers of definite and probable ET (38 and 53 respectively) were 5 to 10 times higher than the present study and the differences reached statistical significance. The current study, which is a fifth study of blood harmane concentrations in ET [12–15], suggests that the higher levels of harmane extend now to a third locale, the Faroe Islands, and as with a prior study[15], mainly affect those with more severe or more diagnostically definitive ET. In addition, one study demonstrated higher brain harmane concentrations in ET than controls [16].
Table 4:
Studies comparing harmane concentrations in ET cases and controls
| Authors (location) | ET (all) | Definite ET | Probable ET | Possible ET | Control |
|---|---|---|---|---|---|
| Louis et al 2002 [12] (New York) |
5.21 g−10/ml (n = 100) p = 0.005 |
2.28 g−10/ml (n = 100) | |||
| Louis et al 2005 [15] (New York) |
Log harmane = 0.61 ± 0.67 g−10/ml (n = 106) p = 0.035 |
Log harmane = 0.74 ± 0.69 g−10/ml (n = 38) | Log harmane = 0.56 ± 0.67 g−10/ml (n = 53) | Log harmane = 0.48 ± 0.64 g−10/ml (n = 15) | Log harmane = 0.43 ± 0.72 g−10/ml (n = 161) |
| Louis et al 2008 [13] (New York) |
2.61 g−10/ml (n = 150) p = 0.016 |
1.82 g−10/ml (n = 135) | |||
| Louis et al 2013 [14] (Madrid) |
2.90 g−10/ml in familial ET (n = 62) 2.41 g−10/ml in sporadic ET (n = 68) p = 0.049 a |
2.09 g−10/ml (n = 135) | |||
| Current study 2019 (Faroe Islands) |
1.74 g−10/ml (n = 26) p = 0.92 |
4.13 g−10/ml (n = 3) p = 0.126 |
2.28 g−10/ml (n = 12) p = 0.91 |
1.27 g−10/ml (n = 11) p = 0.28 |
1.53 (n = 196) |
Values are median harmane unless otherwise specified as log harmane.
in an adjusted logistic regression model comparing familial ET to controls.
p values show comparisons with controls.
The mechanism for a higher blood harmane concentration in ET is unclear and has been discussed in several or our earlier papers [12–15], although the data most lend support to the notion that it could be the result of a genetically-driven reduction in harmane metabolism. In prior analyses in a cohort in New York, we did not major detect dietary differences in animal protein consumption between ET cases and controls [15] and in the current cohort, we collected detailed data on animal protein consumption and food cooking practices (i.e., level of doneness of meat, which is associated with blood harmane concentrations), and similarly did not detect significant differences between ET cases and controls (data not presented), suggesting that the case-control difference we observed in harmane concentration are not driven by dietary differences.
The study did not support a link between mercury and ET. Action tremor has been a well-described outcome of mercury exposure in a broad and varied range of settings in numerous studies spanning many years [18–20], and further interest in mercury relates to the fact that it is toxic to the Purkinje cell population in the cerebellum [54,55], a population of neurons increasingly linked to the pathophysiology of ET [56–58]. With respect to ET, specifically, however, we are unaware of any study that has directly compared mercury exposures in ET cases vs. controls or that has quantified and compared blood mercury concentrations in ET cases vs. controls. The mercury levels we report are relatively high, and diet, namely the intake of whale meat contaminated with mercury, could account for this. Self-reported whale meat consumption was similar in the two groups (data not shown) as were blood mercury levels.
This study should be interpreted within the context of several limitations. First, despite the large screening sample, the number of ET cases was small (n = 27 and 26 with blood levels of harmane and mercury), thereby limiting the power to detect differences and increasing the possibility of Type II statistical error. Indeed, even robust differences (e.g., difference in harmane concentrations between definite ET and controls or probable ET vs. controls) did not achieve statistical significance. A larger sample of ET cases would achieve greater power, but would require the screening of several thousand more participants. The second issue is that we did not assess fasting blood harmane concentrations; fasting concentrations are less likely to reflect recent food consumption. However, prior data indicated that there was no association between time elapsed from last food or beverage ingestion and blood harmane concentration (Spearman’s r = 0.12, p = 0.34) or HA/HI ratio (Spearman’s r = 0.11, p = 0.41), indicating that blood harmane was not a function of time since last food consumption [59]. Our current analyses confirmed that this was the case (Table 2).
The study had a number of strengths. First, this is the first population-based study of harmane in ET, with all prior studies largely or exclusively sampling cases from clinics [12–14]. Second, it extends studies of this neurotoxin from only two locales [12–15] now to a third locale. Third, it is the first study to directly measure blood mercury concentrations in ET cases. Finally, all ET diagnoses were carefully assigned by a movement disorder neurologist with longstanding interest in ET based on a detailed clinical assessment using stringent, validated diagnostic criteria.
In summary, we demonstrated marginally elevated blood harmane concentrations in probable and definite ET cases. These data seem to extend the etiological links between this neurotoxin and ET to a third locale. The study did not support a link between mercury and ET.
Acknowledgement and Funding
The authors thank the participants of the study for their support. This work was supported by NIH grants R01 NS039422 and NS094607 (Dr. Louis).
References
- 1.Louis ED, Ottman R: How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov (N Y) 2014;4:259. doi: 10.7916/D8TT4P4B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis ED, Ferreira JJ: How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–541. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Thawani SP, Andrews HF: Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology 2009;32:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark LN, Louis ED: Essential tremor. Handb Clin Neurol 2018;147:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan EK, Schapira AH: Hunting for genes in essential tremor. Eur J Neurol 2008;15:889–890. [DOI] [PubMed] [Google Scholar]
- 6.Testa CM: Key issues in essential tremor genetics research: Where are we now and how can we move forward? Tremor Other Hyperkinet Mov (N Y) 2013;3 pii: tre-03–105-1843–1. doi: 10.7916/D8Q23Z0Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis ED: Environmental Epidemiology of Essential Tremor. Neuroepidemiology 2008;31:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong YL, Deng X, Tan EK: Etiologic links between environmental and lifestyle factors and essential tremor. Ann Clin Transl Neurol 2019;6:979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimenez-Jimenez FJ, de Toledo-Heras M, Alonso-Navarro H, Ayuso-Peralta L, Arevalo-Serrano J, Ballesteros-Barranco A, Puertas I, Jabbour-Wadih T, Barcenilla B: Environmental risk factors for essential tremor. Eur Neurol 2007;58:106–113. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Ottman R: How familial is familial tremor? The genetic epidemiology of essential tremor. Neurology 1996;46:1200–1205. [DOI] [PubMed] [Google Scholar]
- 11.Tanner CM, Goldman SM, Lyons KE, Aston DA, Tetrud JW, Welsh MD, Langston JW, Koller WC: Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology. Neurology 2001;57:1389–1391. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, Factor-Litvak P, Parides M: Elevation of blood beta-carboline alkaloids in essential tremor. Neurology 2002;59:1940–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis ED, Jiang W, Pellegrino KM, Rios E, Factor-Litvak P, Henchcliffe C, Zheng W: Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in essential tremor. Neurotoxicology 2008;29:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis ED, Benito-Leon J, Moreno-Garcia S, Vega S, Romero JP, Bermejo-Pareja F, Gerbin M, Viner AS, Factor-Litvak P, Jiang W, Zheng W: Blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentration in essential tremor cases in Spain. Neurotoxicology 2013;34:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P: Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology 2005;65:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis ED, Factor-Litvak P, Liu X, Vonsattel JP, Galecki M, Jiang W, Zheng W: Elevated brain harmane (1-methyl-9H-pyrido[3,4-b]indole) in essential tremor cases vs. controls. Neurotoxicology 2013;38:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weihe P, Grandjean P, Debes F, White R: Health implications for Faroe islanders of heavy metals and PCBs from pilot whales. Sci Total Environ 1996;186:141–148. [DOI] [PubMed] [Google Scholar]
- 18.Roels H, Abdeladim S, Braun M, Malchaire J, Lauwerys R: Detection of hand tremor in workers exposed to mercury vapor: a comparative study of three methods. Environ Res 1989;49:152–165. [DOI] [PubMed] [Google Scholar]
- 19.Fabrizio E, Vanacore N, Valente M, Rubino A, Meco G: High prevalence of extrapyramidal signs and symptoms in a group of Italian dental technicians. BMC Neurol 2007;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields CA, Borak J, Louis ED: Persistence of mercury-induced motor and sensory neurotoxicity: systematic review of workers previously exposed to mercury vapor. Crit Rev Toxicol 2017;47:845–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtzke JF, Hyllested K: Multiple sclerosis in the Faroe Islands: I. Clinical and epidemiological features. Ann Neurol 1979;5:6–21. [DOI] [PubMed] [Google Scholar]
- 22.Wermuth L, Bech S, Petersen MS, Joensen P, Weihe P, Grandjean P: Prevalence and incidence of Parkinson’s disease in The Faroe Islands. Acta Neurol Scand 2008;118:126–131. [DOI] [PubMed] [Google Scholar]
- 23.Joensen P: High prevalence of primary focal dystonia in the Faroe Islands. Acta Neurol Scand 2016;133:55–60. [DOI] [PubMed] [Google Scholar]
- 24.Eliasen EH, Ferrer M, Gaini S, Louis ED, Petersen MS: Prevalence of Essential Tremor in the Faroe Islands: A Population-Based Study. Neuroepidemiology 2019;52:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth S, Zeller D: Mercury, food webs, and marine mammals: implications of diet and climate change for human health. Environ Health Perspect 2005;113:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis ED, Ferrer M, Eliasen EH, Gaini S, Petersen MS: Tremor in normal adults: A population-based study of 1158 adults in the Faroe Islands. J Neurol Sci 2019;400:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis ED, Eliasen EH, Kim CY, Ferrer M, Gaini S, Petersen MS: High Prevalence of Dystonia in the Faroe Islands: A Population-Based Study. Neuroepidemiology 2019:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linn BS, Linn MW, Gurel L: Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–626. [DOI] [PubMed] [Google Scholar]
- 29.Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, LaPelle N: Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord 2007;22:41–47. [DOI] [PubMed] [Google Scholar]
- 30.Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, Hauser WA: The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology 1997;16:124–133. [DOI] [PubMed] [Google Scholar]
- 31.Louis ED, Ford B, Bismuth B: Reliability between two observers using a protocol for diagnosing essential tremor. Mov Disord 1998;13:287–293. [DOI] [PubMed] [Google Scholar]
- 32.Louis ED, Pullman SL: Comparison of clinical vs. electrophysiological methods of diagnosing of essential tremor. Mov Disord 2001;16:668–673. [DOI] [PubMed] [Google Scholar]
- 33.Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, Kaleagasi H, Un S, Louis ED: Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology 2003;61:1804–1806. [DOI] [PubMed] [Google Scholar]
- 34.Inzelberg R, Mazarib A, Masarwa M, Abuful A, Strugatsky R, Friedland RF: Essential tremor prevalence is low in Arabic villages in Israel: door-to-door neurological examinations. J Neurol 2006;253:1557–1560. [DOI] [PubMed] [Google Scholar]
- 35.Gasparini M, Bonifati V, Fabrizio E, Fabbrini G, Brusa L, Lenzi GL, Meco G: Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol 2001;248:399–402. [DOI] [PubMed] [Google Scholar]
- 36.Farrer M, Gwinn-Hardy K, Muenter M, DeVrieze FW, Crook R, Perez-Tur J, Lincoln S, Maraganore D, Adler C, Newman S, MacElwee K, McCarthy P, Miller C, Waters C, Hardy J: A chromosome 4p haplotype segregating with Parkinson’s disease and postural tremor. Hum Mol Genet 1999;8:81–85. [DOI] [PubMed] [Google Scholar]
- 37.Dogu O, Sevim S, Louis ED, Kaleagasi H, Aral M: Reduced body mass index in patients with essential tremor: a population-based study in the province of Mersin, Turkey. Arch Neurol 2004;61:386–389. [DOI] [PubMed] [Google Scholar]
- 38.Gatto EM, Roca MC, Raina G, Micheli F: Low doses of topiramate are effective in essential tremor: a report of three cases. Clin Neuropharmacol 2003;26:294–296. [DOI] [PubMed] [Google Scholar]
- 39.Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Louis ED: Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. J Neurol Sci 2009;287:138–142. [DOI] [PubMed] [Google Scholar]
- 40.Obwegeser AA, Uitti RJ, Turk MF, Strongosky AJ, Wharen RE: Thalamic stimulation for the treatment of midline tremors in essential tremor patients. Neurology 2000;54:2342–2344. [DOI] [PubMed] [Google Scholar]
- 41.Putzke JD, Uitti RJ, Obwegeser AA, Wszolek ZK, Wharen RE: Bilateral thalamic deep brain stimulation: midline tremor control. J Neurol Neurosurg Psychiatry 2005;76:684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seijo-Martinez M, Del Rio MC, Alvarez JR, Prado RS, Salgado ET, Esquete JP, Sobrido-Gomez MJ: Prevalence of Essential Tremor on Arosa Island, Spain: a Community-based, Door-to-Door Survey. Tremor Other Hyperkinet Mov (N Y) 2013;3 pii: tre-03–192-4299–1. doi: 10.7916/D89P30BB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sur H, Ilhan S, Erdogan H, Ozturk E, Tasdemir M, Boru UT: Prevalence of essential tremor: a door-to-door survey in Sile, Istanbul, Turkey. Parkinsonism Relat Disord 2009;15:101–104. [DOI] [PubMed] [Google Scholar]
- 44.Ozel L, Demir R, Ozdemir G, Ozyildirim E, Avsar U, Ulvi H, Aygul R: Investigation of the prevalence of essential tremor in individuals aged 18–60 in Erzurum. Acta Neurol Belg 2013;113:127–131. [DOI] [PubMed] [Google Scholar]
- 45.Zheng W, Wang S, Barnes LF, Guan Y, Louis ED: Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal Biochem 2000;279:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim BM, Choi AL, Ha EH, Pedersen L, Nielsen F, Weihe P, Hong YC, Budtz-Jorgensen E, Grandjean P: Effect of hemoglobin adjustment on the precision of mercury concentrations in maternal and cord blood. Environ Res 2014;132:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ: Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology 1997;48:650–658. [DOI] [PubMed] [Google Scholar]
- 48.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ: The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 1998;50:1346–1350. [DOI] [PubMed] [Google Scholar]
- 49.Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS: Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology 2001;56:8–13. [DOI] [PubMed] [Google Scholar]
- 50.Dick FD: Parkinson’s disease and pesticide exposures. Br Med Bull 2006;79–80:219–231. [DOI] [PubMed] [Google Scholar]
- 51.Baldereschi M, Inzitari M, Vanni P, Di Carlo A, Inzitari D: Pesticide exposure might be a strong risk factor for Parkinson’s disease. Ann Neurol 2008;63:128. [DOI] [PubMed] [Google Scholar]
- 52.Morahan JM, Yu B, Trent RJ, Pamphlett R: Genetic susceptibility to environmental toxicants in ALS. Am J Med Genet B Neuropsychiatr Genet 2007;144:885–890. [DOI] [PubMed] [Google Scholar]
- 53.Shcherbatykh I, Carpenter DO: The role of metals in the etiology of Alzheimer’s disease. J Alzheimers Dis 2007;11:191–205. [DOI] [PubMed] [Google Scholar]
- 54.Manto M: Toxic agents causing cerebellar ataxias. Handb Clin Neurol 2012;103:201–213. [DOI] [PubMed] [Google Scholar]
- 55.Sorensen FW, Larsen JO, Eide R, Schionning JD: Neuron loss in cerebellar cortex of rats exposed to mercury vapor: a stereological study. Acta Neuropathol 2000;100:95–100. [DOI] [PubMed] [Google Scholar]
- 56.Louis ED: Essential tremor and the cerebellum. Handb Clin Neurol 2018;155:245–258. [DOI] [PubMed] [Google Scholar]
- 57.Louis ED, Kerridge CA, Chatterjee D, Martuscello RT, Diaz DT, Koeppen AH, Kuo SH, Vonsattel JG, Sims PA, Faust PL: Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach. Acta Neuropathol 2019;138:859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimaldi G, Manto M: Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord 2013;28:1759–1761. [DOI] [PubMed] [Google Scholar]
- 59.Louis ED, Jiang W, Gerbin M, Mullaney MM, Zheng W: Relationship between blood harmane and harmine concentrations in familial essential tremor, sporadic essential tremor and controls. Neurotoxicology 2010;31:674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]