Abstract
Objective:
Postmortem brains of subjects diagnosed with human immunodeficiency virus-1 (HIV) associated neurocognitive disorders (HAND) exhibit loss of dendrites. However, the mechanisms by which synapses are damaged are not fully understood.
Design:
Dendrite length and remodeling occurs via microtubules (MTs) the dynamics of which are regulated by microtubule binding proteins, including MT associated protein 2 (MAP2). The HIV protein gp120 is neurotoxic and interferes with neuronal MTs. We measured MAP2 concentrations in human cerebrospinal fluid (CSF) and MAP2 immunoreactivity in rat cortical neurons exposed to HIV and gp120.
Methods:
First, we examined whether HIV affects MAP2 levels by analyzing the CSF of 27 persons living with HIV (PLH) whose neurocognitive performance had been characterized. We then used rat cortical neurons to study the mechanisms of HIV-mediated dendritic loss.
Results:
PLH who had HAND had greater MAP2 concentrations within the CSF than cognitive normal PLH. In cortical neurons, the deleterious effect of HIV on MAP2 positive dendrites occurred through a gp120-mediated mechanism. The neurotoxic effect of HIV was blocked by a CCR5 antagonist and prevented by Helix-A, a peptide that displaces gp120 from binding to MTs, conjugated to a nanolipoprotein particle delivery platform.
Conclusions:
Our findings support that HIV at least partially effects its neurotoxicity via neuronal cytoskeleton modifications and provide evidence of a new therapeutic compound that could be used to prevent the HIV-associated neuropathology.
Keywords: HIV, HAND, DAPTA, dendrites, gp120, Helix-A peptide, neuronal microtubules
Introduction
Although antiretroviral treatment (ART) reduces HIV replication, HIV remains present and active in the central nervous system (CNS), where it can cause cognitive and mental health disorders, including HIV-associated neurocognitive disorders (HAND) [1]. HAND is a heterogeneous syndrome that has been linked to several biological mechanisms and clinical risk factors. These include neuroinflammation [2], comorbid conditions [3], aging [4], addictive drugs [5], and viral proteins such as gp120 and Tat [6–8].
Common features of HIV-associated neuropathology include abnormal dendrites with recurving of distal segments, short-segment branching, focal dendritic swellings and marked spine loss [9]. Viral replication appears to drive synaptodendritic degeneration because ART ameliorates HIV-mediated neurodegeneration [10] but does not abolish brain atrophy [11]. Since neurons are not infected by HIV, the molecular mechanisms that underlie HIV-mediated dendritic pathology remain uncertain.
The envelope protein gp120 reproduces dendritic injury seen in HAND [12–16]. Gp120 is endocytosed into neurons [17, 18] by a chemokine receptor-dependent mechanism [19]. Once inside neurons, it forms a complex with mannose binding lectin [20] and associates with microtubules (MTs) [21], most likely by binding to tubulin β III (TUBB3) [22]. Endocytosed gp120 alters the transport of mitochondria in axons [23], as well as synaptic vesicles along somatodendritic compartments, which may explain why the envelope protein experimentally reproduces HIV-mediated synaptic pruning. Because mitochondria, lysosomes, and other cargo are trafficked along axons and dendrites by MTs, HIV may damage dendrites via gp120-mediated alteration of MT dynamics.
Changes in the integrity or dynamics of MTs are sufficient to alter neuronal function and produce neuronal atrophy [24–27]. The dynamic properties of MTs are thought to be modulated by tubulin posttranslational modifications [28, 29], as well as MT-associated proteins (MAPs) [30], including MAP2 and tau [30, 31]. MAP2 forms cross-bridges structures between MTs [32, 33], participates in organelle transport within this neuronal compartment [34, 35] and is a critical stabilizer of MTs in mature dendrites. Conversely, reducing MAP2 expression may lead to the inhibition of neuritogenesis [36]. Consistent with this notion, the severity of dendritic damage in the cortex of persons living with HIV (PLH) correlates with loss of MAP2 immunoreactivity [37, 38]. Considering that HIV does not infect neurons, the central question is whether HIV directly reduces MAP2 positive dendrites or does so indirectly via viral proteins or other biological mechanisms.
The main goals of this study were to evaluate whether MAP2 could be used as a biomarker for HAND, and whether HIV and gp120 share the same intracellular mechanism of neurotoxicity that could be targeting MAP2 and the dendritic compartment. We report that both HIV and gp120 change the levels of MAP2 through the binding of gp120 to MTs. Learning more about the intracellular mechanisms of gp120 neurotoxicity could lead to a better understanding of HIV neuropathogenesis.
Materials and Methods
Reagents.
HIVADA and HIVIIIB were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: from Dr. R. Gallo [39, 40] and were used at a concentration of 3 ng/ml of p24. Gp120ADA (cat# 1081) and gp120IIIB (cat#1001) were purchased from ImmunoDX (Woburn, WA). Helix-A peptide was synthesized by and purchased from Genscript, Piscataway, NJ. All other chemicals were commercially obtained, reagent grade. Human MAP2 enzyme-linked immunosorbent assay (ELISA) kit was purchased from LifeSpan BioScience (Seattle, WA) and run according to manufacturer’s instructions.
Lipids including 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine (DiynePC), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-dibenzocyclooctyl (DBCO lipid), were purchased from Avanti Polar Lipids (Alabaster, AL). Anhydrous dimethylformamide (DMF) was purchased from SigmaMillipore. Detergent adsorbing BioBeads were obtained from BioRad (Hercules, CA).
Human Cerebrospinal Fluid Samples.
Cerebrospinal fluid (CSF) specimens from 30 PLH were analyzed for MAP2 by ELISA. All participants were assessed in the National Institute of Mental Health-funded HIV Neurobehavioral Research Center cohort at the University of California, San Diego. As previously described [41], comprehensive assessments included neuromedical and neurobehavioral evaluation, venipuncture, and lumbar puncture. All study procedures were approved by the Human Research Protections Program at the University of California, San Diego (irb.ucsd.edu) and all participants provided written informed consent, including consent for use of their specimens and data in future research.
After being selected, three participants were subsequently excluded from analyses: One participant had insufficient CSF volume to measure MAP2, one participant had evidence of active hepatitis (e.g., serum alanine transaminase 497 mg/dl), and one participant had renal failure (serum creatinine 3.8 mg/dl). The analyzed sample size was 27. Assay scientists were blinded to neuromedical and neurobehavioral data until the assays were completed. The standardized neuropsychological testing battery assessed motor functioning, executive functioning, processing speed, learning, recall, working memory/attention, and verbal fluency [42]. Scores from each test were transformed into demographically-adjusted T-scores after adjusting for effects of age, education, sex, and ethnicity [43]. Global deficit scores (GDS) were calculated and ranged from 0 to 5 with higher scores indicating poorer cognition. We evaluated GDS as a continuous measure reflecting severity or dichotomized it to define neurocognitive impairment (NCI, GDS ≥ 0.5). HAND diagnoses were assigned according to published criteria [44]. This pilot analysis was purposely designed to include participants who were either using (n=21, 77.8%) or not using (n=6, 22.2%) ART and who had HIV RNA either below or above 200 copies/ml in blood (10 of 21 of those taking ART had HIV RNA ≤ 200 copies/ml) or CSF (18 of 21 of those taking ART had HIV RNA ≤ 200 copies/ml). Table 1 describes additional characteristics of participants.
Table 1.
Characteristics of participants
| Characteristic | ||
|---|---|---|
| Age (years) | Median 38 | Interquartile range (IQR): 35–40 |
| Sex: 24 men (89%) | 24 (89%) | |
| Ethnicity (non-Black) | 20 (74%) | |
| AIDS (present) | 26 (96%) | |
| Nadir CD4+ T-cell count (cells/μl) | Median 13 | IQR 6–58 |
| Current CD4+ T-cell count (cells/μl) | Median 138 | IQR 91–349 |
| Current ART use | 21 (78%) | |
| Plasma HIV RNA among those off ART (log10 c/ml) | Median 4.7 | IQR 4.6–5.0 |
| CSF HIV RNA among those off ART (log10 c/ml) | Median 3.1 | IQR 1.8–4.0 |
| Global Deficit Score | Median 0.64 | IQR 0.14–2.5 |
| Neurocognitive Impairment | 16 (59%) | |
| HAND Diagnosis | 16 (59%) |
21 of 27 participants were using ART. 10 of 21 participants taking ART had plasma HIV RNA ≤ 200 copies/ml. 18 of 21 participants taking ART had CSF HIV RNA ≤ 200 copies/ml. 14 (67%) of the 27 participants had CSF HIV RNA ≤ 50 copies/ml.
Rat cortical neurons.
Animals studies were done in strict accordance with the Laboratory Animal Welfare Act, with National Institutes of Health Guide for the Care and Use of Laboratory Animals, and after approval from the Georgetown University Animal Care and Use Committee.
Primary rat cortical neurons were prepared from the cortex of embryonic day 17–18 Sprague–Dawley rats (Charles River, MA, USA) following an established protocol [45]. Cells were seeded (0.5 ×106/ml) onto poly-L-lysine pre-coated plates or glass coverslips in Neurobasal Medium containing 2% B27 supplement, 25 nM glutamate, 0.5 mM L-glutamine, and 1% antibiotic-antimycotic solution (ThermoFisher Scientific, Waltham, MA). Cultures were grown at 37°C in 5% CO2/95% air for 14 days prior to the experiments.
Immunocytochemistry.
Cultures were grown at 37°C in 5% CO2/95% air for 7 days on glass coverslips. Cultures contained ~5% non-neuronal cells. To determine the length of neuronal processes, neurons were fixed in 4% paraformaldehyde/phosphate buffer with 4% sucrose for 20 min at room temperature. Fixed cells were blocked and permeabilized in 5% non-fat milk in TBS-T (150nM NaCl, 20mM Tris-base, pH 7.5, 0.1% Triton X100) for 1 hr at 23°C. Cells were incubated overnight at 4°C with mouse anti-MAP2 antibody (1:10000; MilliporeSigma, St. Louis, MO). Coverslips were washed with TBS-T and corresponding fluorescence-conjugated secondary antibody (1:2000; ThermoFisher Scientific) were applied for 1 hr at room temperature. Coverslips were washed with TBS-T and mounted with Fluoro-Gel with TES buffer (Electron Microscopy Science, Hatfield, PA). Cells were imaged using a Nikon Eclipse (Nikon Inc, Los Angeles, CA). Image scale was calibrated and length of MAP2 positive processes was measured in three randomly selected fields (10 neurons per field) using ImageJ as described previously [22].
Cell viability.
The viability of primary cortical neurons was estimated by Hoechst 33258 and propidium iodide (Hoechst/PI; MilliporeSigma) co-staining and visualized using a fluorescence microscope Olympus IX71 as previously described [46, 47]. Hoechst/PI-positive cells were then counted using ImageJ and expressed as a percentage of the total number of neurons.
Helix-A and nanolipoprotein particle preparation.
Helix-A peptide was synthesized with an N-terminal tetramethylrhodamine (TMR) fluorophore and C-terminal azide functional group and (TMR-NDMVEQMHEDIISLWDQSL-azide) (Genscript, Piscataway, NJ), solubilized in anhydrous dimethyl sulfoxide (DMSO), and incubated with a 18:1 DBCO PE lipid (18:1 PDP PE) in anhydrous DMF for 16 hr at room temperature. A scramble peptide (NDMVEQMHEDPIPSLWPSLK) was used as a negative control. The Helix-A:lipid product was purified by High Performance Liquid Chromatography. To form nanolipoprotein particles (NLPs), DiynePC and DOPC (20:80 molar ratio, respectively) were incubated with apoA1 protein (40:1 lipid:protein ratio), and assembled according to a previously reported procedure [48]. Samples were purified by size exclusion chromatography (Superdex 200, 10/300 GL column, GE Healthcare, Piscataway, NJ) and crosslinked [48]. Subsequently, Helix-A:lipid in dimethyl sulfoxide (DMSO) was added to crosslinked NLPs (10:1), dialyzed in a 3.5k molecular weight-cutoff dialysis cassette to remove any un-incorporated peptide, filter sterilized, and lyophilized with 200mM trehalose.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) or JMP Pro version 14.3 (JMP, Cary, NC, USA). Data were evaluated for homogeneity of variance prior to analysis. MAP2 concentrations were normally distributed and did not require transformation for parametric statistical tests. Statistical significance was set at p<0.05. While the sample size was small, multivariate regression of MAP2 concentrations in CSF was performed using backward stepwise Akaike Information Criterion-guided selection and included variables that had significant or trend level p values. Recursive partitioning [49] was performed comparing MAP2 concentrations in CSF to NCI. All data were analyzed by Student’s t-test or one-way ANOVA followed by multiple comparisons by Tukey’s test.
Results
MAP2 concentrations in CSF are elevated in PLH.
Synaptodendritic injury can explain cognitive abnormalities found in PLH [37]. However, data from postmortem brains may not reveal whether loss of synapses is the cause or consequence of HAND. Because neuronal proteins from the brain may be elevated in CSF following neuronal injury, we measured MAP2 by ELISA in the CSF of PLH.
Higher MAP2 concentrations in CSF were associated with non-black ethnicity (β=1.06, p=0.019) and fewer leukocytes in CSF (β=−0.42, p=0.018) with trends toward HIV RNA in CSF ≤ 50 copies/ml (β=1.47, p=0.072) and lower serum total protein (β=−0.92, p=0.09). In this pilot study, p values were >0.10 for age (0.54), sex (0.13), nadir CD4+ T-cell count (0.78), current CD4+ T-cell count (0.98), ART use (0.50), HIV RNA in plasma (0.22), serum creatinine (0.22), and serum albumin (0.41). On bivariate analysis, higher MAP2 concentrations in CSF were associated with lower serum total protein (β=−1.20, p=0.021) and HIV RNA in CSF ≤ 50 copies/ml (β=1.88, p=0.018) (model R2=0.30, p=0.014, see Fig. 1A). These variables were included as covariates in multivariate analyses of GDS values.
Figure 1. Association between MAP2, HIV and NCI.
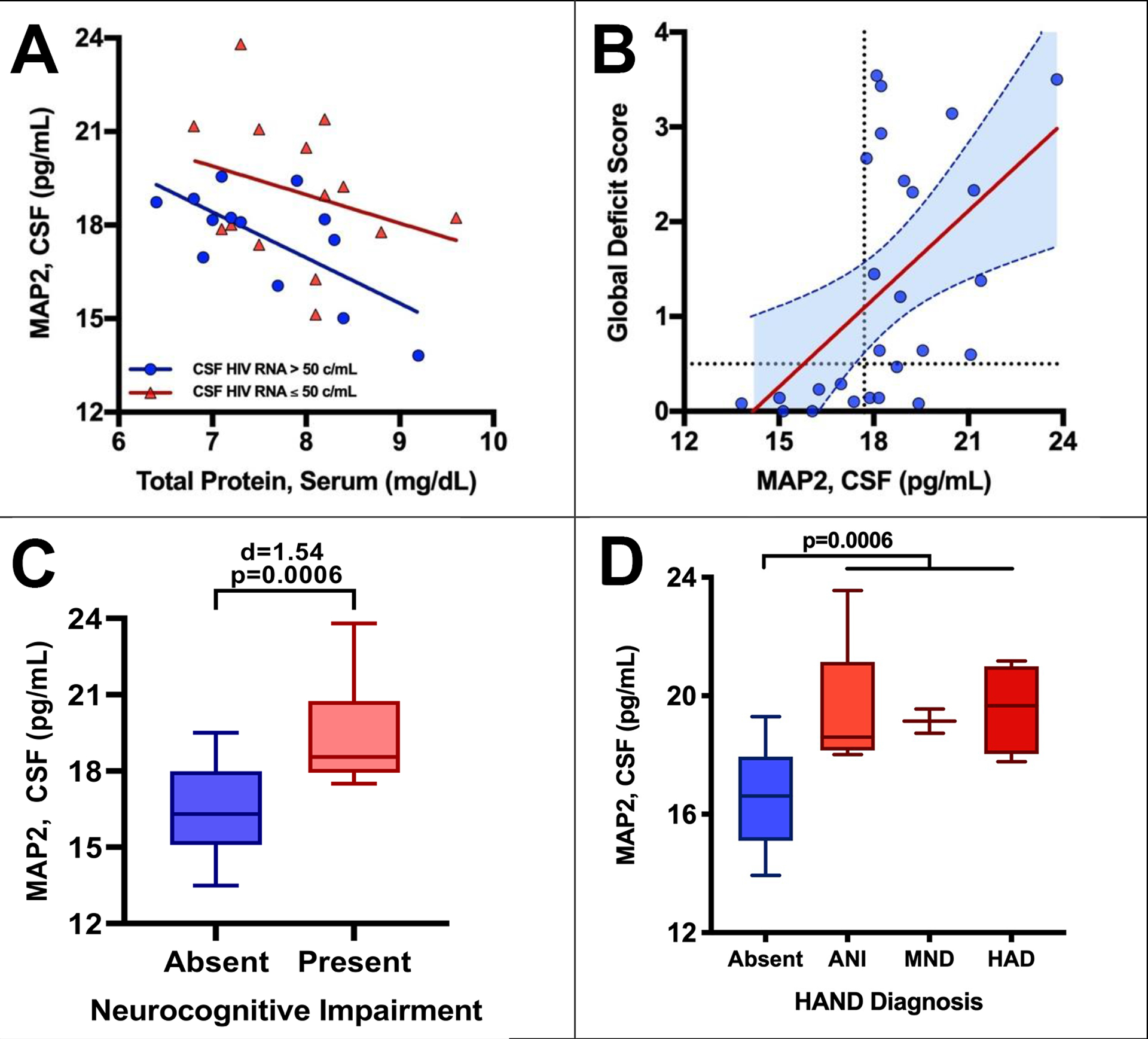
Higher MAP2 concentrations in CSF were associated with (A) lower serum total protein and HIV RNA in CSF, (B) worse GDS values, (C) NCI, and (D) HAND diagnosis. The dotted lines in graph B denote the threshold value for NCI (GDS ≥0.5) and the threshold value for MAP2 levels (MAP2=17.7 pg/ml), identified by recursive partitioning.
Worse GDS values were associated with higher MAP2 in CSF (β=0.31, p=0.0062), ART use (β=0.55, p=0.0052), higher serum glucose (β=0.01, p=0.053), and fewer leukocytes in CSF (β=−0.22, p=0.031). On bivariate analysis, worse GDS values were associated with ART use (β=0.66, p=0.043) and higher MAP2 (β=0.41, p=0.0017) (model R2=0.47, p=0.007, see Fig. 1B). Recursive partitioning [49] was performed comparing MAP2 concentrations in CSF to GDS to identify a potentially informative threshold value for MAP2. It revealed a cut-point value for MAP2 levels at 17.7 pg/ml (Fig. 1B, vertical dotted line). No participants with MAP2 levels below this value had a GDS value in the impaired range.
Similar results were seen when comparing MAP2 to the binary variable, NCI, or the multilevel categorical variable, HAND diagnosis. For NCI, the difference between the means of MAP2 levels and the presence or absence of NCI was large with a Cohen’s d of 1.54 (Fig. 1C). MAP2 levels were higher in HAND groups than cognitive normal and were similar in all HAND subgroups, asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD) (Fig. 1D).
HIVADA is neurotoxic to rat neurons.
MAP2 is a structural protein that has been implicated in the maintenance and elongation of dendrites [51, 52]. Thus, an increase in MAP2 levels in the CSF may indicate a loss of dendritic synapses in the CNS. To confirm this hypothesis, we exposed primary rat cortical neurons to HIVADA (3ng/ml of p24) and dendritic degeneration was examined by measuring the length of MAP2 positive neurites for up to 24 hr. As a control for the experiments, neurons were also exposed to gp120ADA (5 nM), which we have previously shown promotes dendritic pruning [22]. We found that HIV or gp120 exhibited a time-dependent effect in shortening MAP2-positive processes when compared to neurons exposed to heat-inactivated HIV or gp120 (Figs. 2A–B), suggesting that HIV, similarly to gp120, reduces the length of dendrites.
Figure 2. HIVADA has a neurotoxic profile similar to gp120.
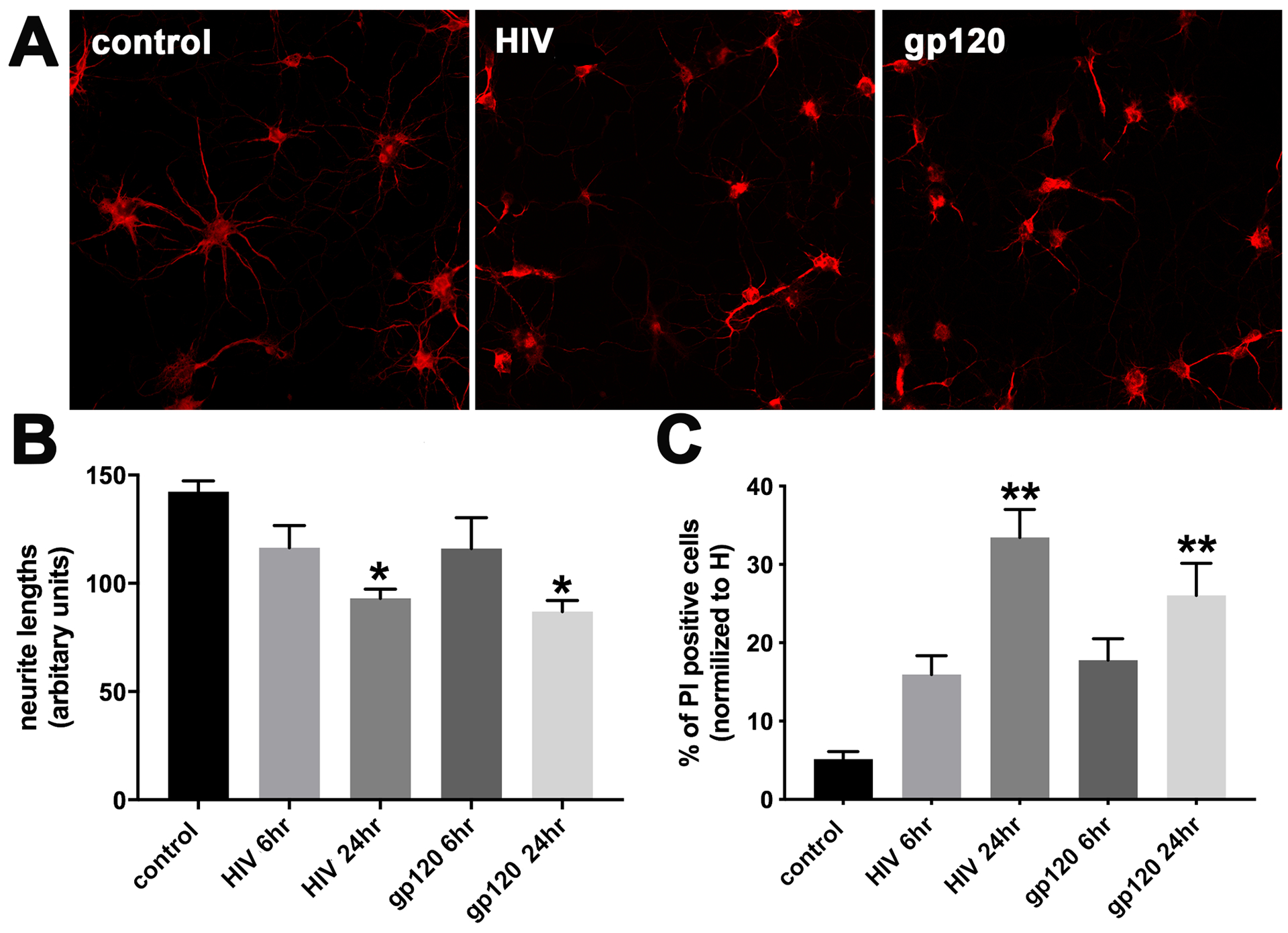
Cortical neurons were exposed to boiled HIVADA (control), HIVADA or gp120ADA for 6 and 24 hr. A. Representative images of neurons exposed for 24 hr to the indicated stimuli and stained for MAP2. B. Neurite length was measured by Image J as described in Materials and Methods. Data are the mean ± SD of three separate experiments. C. Neuronal viability was determined by counting the number of neurons positive for PI. Data are the mean ± SD of three separate experiments. *p<0.01 and **p<0.001 vs control (ANOVA and Tukey’s test).
To determine whether HIV-mediated morphological changes of neuronal processes associate with cell loss, sister cultures were analyzed for Hoechst/PI for various time points. Neurons exposed to HIV or gp120 for 6 hr showed few PI positive nuclei (Fig. 2C); however, neurons exposed to HIV or gp120 for 24 hr exhibited significantly more PI positive nuclei than control cells, suggesting that HIV reduces neuronal viability by 24 hr. The changes on both MAP2 immunoreactivity as well as Hoechst/PI positive nuclei were also reproducible by using an equimolar concentration of the T-tropic strain HIVIIIB (data not shown), supporting previous data that there is no strain-specific neurotoxic effect [22, 53].
The neurotoxic effect of HIV is chemokine receptor mediated.
HIV does not infect neurons. However, we have previously shown that HIV can shed gp120, which, in turn, is internalized into neurons by a chemokine receptor-mediated mechanism, and promotes dendritic simplification [54]. To determine whether the neurotoxic effect of HIV is mediated by gp120 binding to CCR5, neurons were exposed to HIVADA alone or in combination with d-Alapeptide T-amide (DAPTA), a CCR5 receptor antagonist [55], which we have previously used to block the neurotoxic effect of M-tropic gp120 [56]. DAPTA prevented the HIV- mediated decrease of MAP2 positive processes (Fig. 3A) as well as the loss of neurons (Fig. 3B). Similar results were obtained using gp120 and DAPTA (not shown). Thus, it appears that HIV neurotoxicity in vitro is mediated by gp120.
Figure 3. The CCR5 antagonist DAPTA prevents HIV-mediated neurotoxicity.
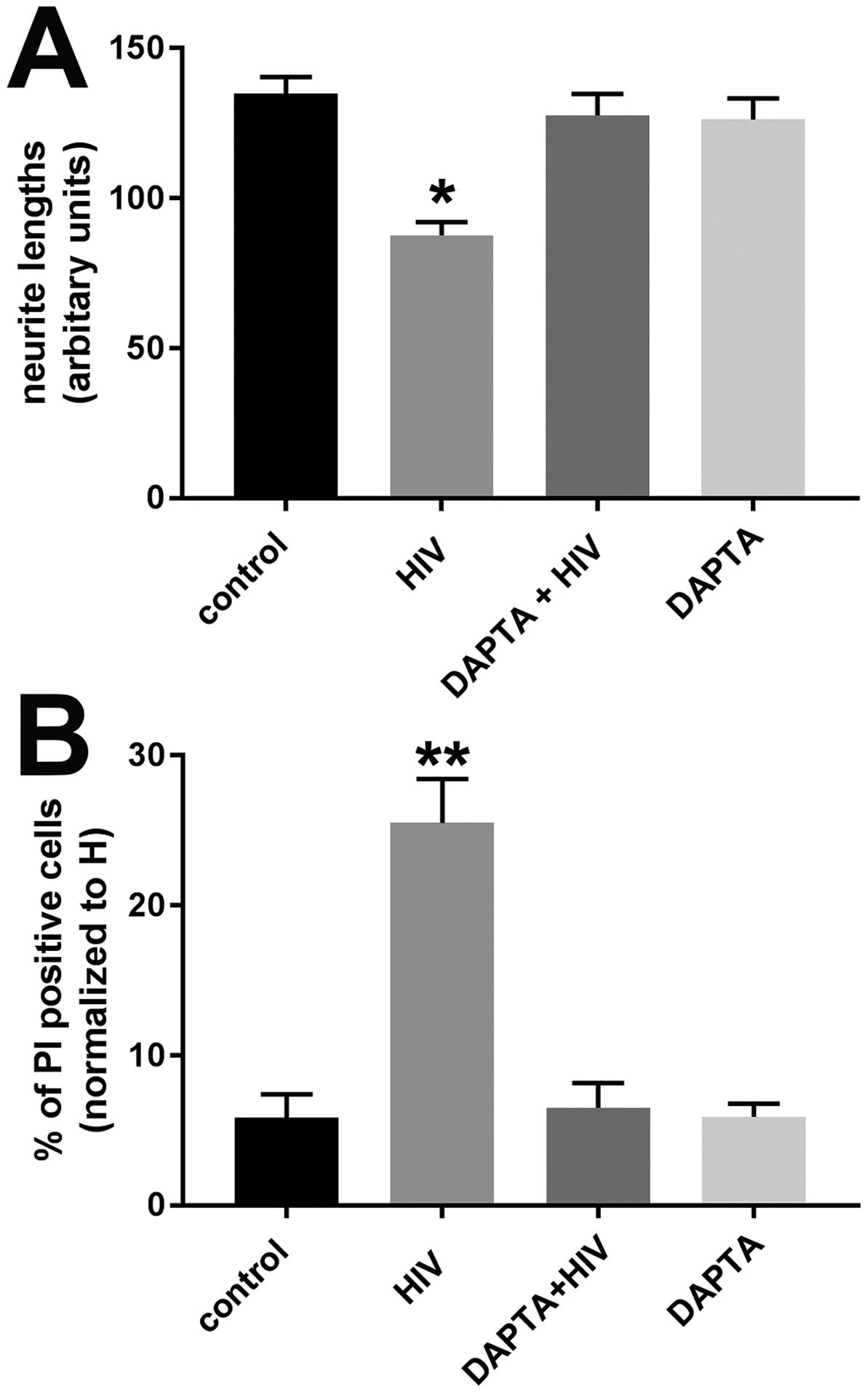
Cortical neurons were exposed to boiled HIVADA (control), HIVADA alone or in combination with DAPTA for 24 hr. A. Neurite length was measured by Image J. Data are the mean ± SD of three separate experiments. B. Neuronal viability was determined by counting the number of neurons positive for PI. Data are the mean ± SD of three separate experiments. *p<0.01 and **p<0.001 vs control (ANOVA and Tukey’s test).
HIV neurotoxicity is prevented by Helix-A NLPs.
Next, we investigated how HIV, through gp120, promotes neurite pruning. One of the mechanisms proposed to explain the neurotoxic effect of gp120 is the ability of the envelope protein to bind directly to TUBB3 by a α-helical domain. Indeed, Helix-A peptide, which displaces gp120 from binding to TUBB3, is neuroprotective against gp120 [22]. Therefore, we used Helix-A peptide as an additional tool to test whether HIV-mediated neurotoxic effect could occur through a gp120-mediated intracellular mechanism. Because Helix-A peptide does not cross cell membranes [22], we used NLPs (see Materials and Methods) as a carrier vehicle to facilitate cellular uptake. NLPs are discoidal high-density lipoprotein mimetics (also known as nanodiscs) that have been used extensively for in vitro and in vivo delivery applications [48, 57, 58]. NLPs with crosslinked bilayers have demonstrated significantly enhanced stability in biological milieu [48], suggesting their utility for delivering peptide-based therapeutics, such as Helix-A. The Helix-A peptide was covalently conjugated to an 18:1 DBCO PE lipid and subsequently incorporated into the NLPs to form the Helix-A NLPs for efficacy evaluation. Primary neurons were then exposed to HIVADA and Helix-A NLPs alone or in combination, for 24 hr. Neurons were also exposed to NLPs conjugated with a scramble peptide (see Materials and Methods) that we routinely use as a negative control [22, 53]. Helix-A NLPs blocked the HIVADA-mediated loss of dendrites (Fig. 4A) as well as the increase in PI positive neurons (Fig. 4B). The scramble peptide did not prevent HIV-induced neurotoxicity (data not shown). Thus, it appears that HIV is neurotoxic through a gp120-dependent mechanism that encompasses the ability of gp120 to bind to MTs.
Figure 4. Helix-A NLPs block HIV-mediated neurotoxicity.
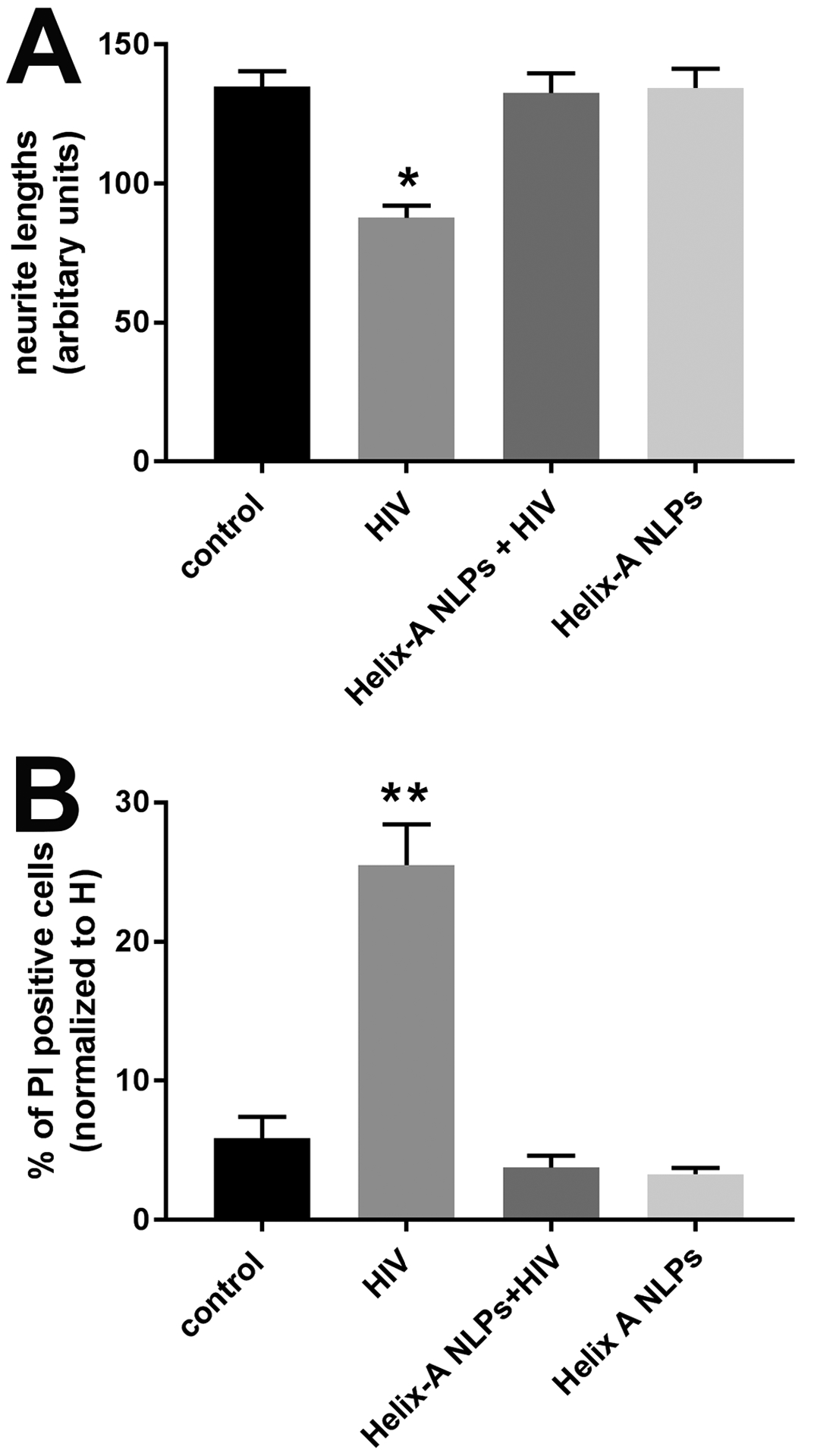
Cortical neurons were exposed to boiled HIVADA (control), HIVADA alone or in combination with Helix-A NLPs for 24 hr. Neurons were then either fixed and stained for MAP2 or imaged for Hoechst/PI. A. Quantitative analysis of MAP2 positive processes after various conditions. Data are the mean ± SEM of three independent experiments (n=60 neurons per group per experiment). B. Neuronal viability was determined by counting the number of neurons positive for PI. *p<0.01 and **p<0.001 vs control (ANOVA and Tukey’s test).
Discussion
Although HIV does not infect neurons, neurons can degenerate in PLH. Pathological signs include reduced synaptodendric complexity [37, 59], loss of neurotrophic factors [13, 60], immune activation and neuroinflammation [61]. Yet, the precise mechanisms linking HIV, neurodegeneration, and HAND are not entirely known. Much attention has been given to direct neurotoxicity associated with viral proteins. In this study we show that HIV induces loss of dendrites in rat neurons through a gp120-related mechanism supporting the hypothesis that HIV impairs synaptic integrity even in the absence of neuroinflammation.
As a result of ART, the incidence of HAD, the most severe form of HAND, has dramatically declined and the milder forms, ANI and MND, persist [61]. Nevertheless, no conclusive biomarkers to distinguish HAND from other nervous system conditions and this is a key gap in the development of new interventions. CSF studies have previously identified potential biomarkers to diagnose HAND and establish links between neuropathologic processes and cognitive impairment. For example, dopamine levels are decreased in the CSF of PLH and correlate with loss of nigrostriatal neurons even before extrapyramidal symptoms are observed [62]. Likewise, phosphorylated Tau, which is decreased in the CSF in HAND [63], could be used to assess axonal integrity [64]. Other studies have shown that markers of axonal and dendritic damage are elevated in PLH. These include neurofilament light chain (NFL) proteins in the CSF [65] and the postsynaptic protein neurogranin in postmortem cerebral cortex [66]. Considering the neuropathology of HAND, these markers could correlate with the neurodegeneration process observed in HAND. Indeed, increased NFL levels in the CSF appear to predict HAND [67] several months before the onset of the disease [68]. However, NFL proteins are cytoskeletal components of neurons that are particularly abundant in axons. Thus, they may not be suitable to detect dendritic injury. MAP2 is highly enriched in dendrites and plays a key role in MT polymerization [69] and formation of dendrites [51]. MAP2 levels in the CSF or blood can detect neuronal injury after stroke [70], or contusive injury[71]. Here we show that HIV increases MAP2 immunoreactivity in the CSF of patients exhibiting ANI, suggesting that MAP2 could be used to as an early biomarker for dendritic injury and could provide a sensitive measurement of brain damage in virally suppressed patients before progression to more severe forms of HAND.
HAND neuropathology is associated with alteration in neuronal function and connections. Given that ART may also impair neuronal function, identification of biomarkers that can distinguish the neuronal effects of HIV from those of ART is important. To this end, we have used rat cortical neurons to characterize the molecular mechanisms whereby HIV promotes dendritic injury. Our data show that the HIV has a neurotoxic profile in neuronal cells that lack CD4 expression and even in the absence of comorbidity. In addition, the neurotoxic effect of HIV was prevented by DAPTA, a well-known CCR5 receptor antagonist. Ligands for CCR5 include CCL5 [72] and the M-tropic strain of gp120 [73] as well as commercially available drugs, such as maraviroc [74]. Previous studies have shown that CCL5 is neuroprotective against gp120 [7] whereas gp120 activation of CCR5 or CXCR4 chemokine receptors is directly involved in HIV-associated neuronal damage [6, 75, 76]. HIV sheds gp120 in vitro, which, in turn, is endocytosed into neurons [54]. Thus, the idea that gp120 endocytosis into neurons mediates the neuronal effects of HIV is plausible. In support to this, our data show that the neurotoxic effect of HIV is prevented by Helix-A, a peptide that prevents gp120-mediated neurotoxicity by displacing gp120 from binding to MTs [22]. Such binding impairs MT assembly and function [47]. Thus, we propose that HIV neurotoxicity is mediated by gp120 binding to neuronal MTs. These considerations could explain why activation of CCR5 by gp120 results in neuronal injury whereas activation of the same receptor by CCL5 is neuroprotective [75, 77].
Changes in dendritic morphology have been reported in HAND and are reproduced experimentally by gp120. These include short-segment dendrite branching and loss of spines [16, 78, 79]. How binding to MTs by gp120 leads to dendritic loss is still under investigation. MTs are very dynamic structures and undergo cycles of polymerization and depolymerization, which alter their functionality and length. Dynamic stability is crucial for MT function and is regulated by several intracellular factors, including MAP2. Gp120 shares tubulin binding site with MAP2 [22, 80]. MAP2 stabilizes MTs in dendrites by binding to both tubulin and F-actin [81]. In addition, it participates in organelle transport and scaffold function within this neuronal compartment [34, 35]. Thus, altered MAP2 binding to MTs could impair the basic function of MTs, which could lead to decrease branching. More studies are needed to prove this hypothesis.
In this study we have used pharmacological tools to reveal potential mechanisms whereby HIV decreases neuronal viability. Our data show similarities between the neurotoxic effects of HIV and those of gp120 which point at the hypothesis that HIV sheds gp120. However, an important limitation of our project is that we used an in vitro model to test our hypothesis. Moreover, although Helix-A blocked the effect of HIV, we cannot exclude that in vivo other viral proteins could participate in promoting the degeneration of synapses that is seen in HAND. Lastly, we have shown that MAP2 levels are increased in the CSF of PLH and that this increase is associated with worse neurocognitive function. However, further studies with a larger size of PLH subgroups are required to confirm these findings.
In conclusion, our findings support the hypothesis that HIV utilizes gp120 to impair the function of the neuronal cytoskeleton. Although preliminary, our data provide evidence for a new therapeutic target that could be used to prevent or limit HIV-induced neurocognitive impairment.
Acknowledgments.
VA, MM, EDW and IM performed the studies, analyzed data, and helped with the writing of the manuscript; SFG and NOF prepared and purified nanolipoprotein particles. AA, and SLL analyzed the human data and helped with the writing of the manuscript. TI prepared and purified HIV. All authors reviewed the results and approved the final version of the manuscript.
Supported by HHS grants 1R01 NS079172 and 1R21NS1040001 to IM, F31 NS107106 to EDW, K24MH097673 and P30MH062512 to S.S.L., 1R01 AG062387 and 1R21MH118092 to A.A.
References
- 1.Le LT, Spudich SS. HIV-Associated Neurologic Disorders and Central Nervous System Opportunistic Infections in HIV. Semin Neurol 2016; 36(4):373–381. [DOI] [PubMed] [Google Scholar]
- 2.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 2015; 45:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol 2010; 67(6):699–714. [DOI] [PubMed] [Google Scholar]
- 4.Smail RC, Brew BJ. HIV-associated neurocognitive disorder. Handb Clin Neurol 2018; 152:75–97. [DOI] [PubMed] [Google Scholar]
- 5.Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS 2005; 19 Suppl 3:S72–78. [DOI] [PubMed] [Google Scholar]
- 6.Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A 1998; 95(24):14500–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ 2007; 14(2):296–305. [DOI] [PubMed] [Google Scholar]
- 8.Nath A, Steiner J. Synaptodendritic injury with HIV-Tat protein: What is the therapeutic target? Exp Neurol 2014; 251:112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atluri VS, Kanthikeel SP, Reddy PV, Yndart A, Nair MP. Human synaptic plasticity gene expression profile and dendritic spine density changes in HIV-infected human CNS cells: role in HIV-associated neurocognitive disorders (HAND). PLoS One 2013; 8(4):e61399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant AK, Ellis RJ, Umlauf A, Gouaux B, Soontornniyomkij V, Letendre SL, et al. Antiretroviral therapy reduces neurodegeneration in HIV infection. AIDS 2015; 29(3):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols MJ, Gates TM, Soares JR, Moffat KJ, Rae CD, Brew BJ, et al. Atrophic brain signatures of mild forms of neurocognitive impairment in virally suppressed HIV infection. AIDS 2019; 33(1):55–66. [DOI] [PubMed] [Google Scholar]
- 12.Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation 2004; 1(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci 2012; 32(28):9477–9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Bae M, Tovar-y-Romo LB, Patel N, Bandaru VV, Pomerantz D, et al. The human immunodeficiency virus coat protein gp120 promotes forward trafficking and surface clustering of NMDA receptors in membrane microdomains. J Neurosci 2011; 31(47):17074–17090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, et al. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci 2002; 21(3):493–501. [DOI] [PubMed] [Google Scholar]
- 16.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 1994; 367(6459):188–193. [DOI] [PubMed] [Google Scholar]
- 17.Berth S, Caicedo HH, Sarma T, Morfini G, Brady ST. Internalization and axonal transport of the HIV glycoprotein gp120. ASN neuro 2015; 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci 2003; 23(13):5715–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzel ED, Bachis A, Avdoshina V, Taraballi F, Tasciotti E, Mocchetti I. Endocytic Trafficking of HIV gp120 is Mediated by Dynamin and Plays a Role in gp120 Neurotoxicity. J Neuroimmune Pharmacol 2017; 12(3):492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teodorof C, Divakar S, Soontornniyomkij B, Achim CL, Kaul M, Singh KK. Intracellular mannose binding lectin mediates subcellular trafficking of HIV-1 gp120 in neurons. Neurobiol Dis 2014; 69:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci 2006; 26(25):6771–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avdoshina V, Taraballi F, Dedoni S, Corbo C, Paige M, Saygideger Kont Y, et al. Identification of a binding site of the human immunodeficiency virus envelope protein gp120 to neuronal-specific tubulin. J Neurochem 2016; 137(2):287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avdoshina V, Fields JA, Castellano P, Dedoni S, Palchik G, Trejo M, et al. The HIV protein gp120 alters mitochondrial dynamics in neurons Neurotoxicity research 2016; 29(4):583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baird FJ, Bennett CL. Microtubule defects & Neurodegeneration. Journal of genetic syndromes & gene therapy 2013; 4:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMurray CT. Neurodegeneration: diseases of the cytoskeleton? Cell Death Differ 2000; 7(10):861–865. [DOI] [PubMed] [Google Scholar]
- 26.Brunden KR, Trojanowski JQ, Smith AB 3rd, Lee VM, Ballatore C. Microtubule-stabilizing agents as potential therapeutics for neurodegenerative disease. Bioorg Med Chem 2014; 22(18):5040–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartelli D, Ronchi C, Maggioni MG, Rodighiero S, Giavini E, Cappelletti G. Microtubule dysfunction precedes transport impairment and mitochondria damage in MPP+ -induced neurodegeneration. J Neurochem 2010; 115(1):247–258. [DOI] [PubMed] [Google Scholar]
- 28.Schulze E, Asai DJ, Bulinski JC, Kirschner M. Posttranslational modification and microtubule stability. J Cell Biol 1987; 105(5):2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janke C, Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci 2010; 33(8):362–372. [DOI] [PubMed] [Google Scholar]
- 30.Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci 1998; 23(8):307–311. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein LS, Gunawardena S. Flying through the drosophila cytoskeletal genome. J Cell Biol 2000; 150(2):F63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiomura Y, Hirokawa N. Colocalization of microtubule-associated protein 1A and microtubule-associated protein 2 on neuronal microtubules in situ revealed with double-label immunoelectron microscopy. J Cell Biol 1987; 104(6):1575–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirokawa N, Hisanaga S, Shiomura Y. MAP2 is a component of crossbridges between microtubules and neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etch immunoelectron microscopy and reconstitution studies. J Neurosci 1988; 8(8):2769–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol 2005; 6(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol 2000; 61(2):133–168. [DOI] [PubMed] [Google Scholar]
- 36.Caceres A, Mautino J, Kosik KS. Suppression of MAP2 in cultured cerebellar macroneurons inhibits minor neurite formation. Neuron 1992; 9(4):607–618. [DOI] [PubMed] [Google Scholar]
- 37.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 2007; 8(1):33–44. [DOI] [PubMed] [Google Scholar]
- 38.Aprea S, Del Valle L, Mameli G, Sawaya BE, Khalili K, Peruzzi F. Tubulin-mediated binding of human immunodeficiency virus-1 Tat to the cytoskeleton causes proteasomal-dependent degradation of microtubule-associated protein 2 and neuronal damage. J Neurosci 2006; 26(15):4054–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 1984; 224(4648):497–500. [DOI] [PubMed] [Google Scholar]
- 40.Ratner L, Haseltine W, Patarca R, Livak KJ, Starcich B, Josephs SF, et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 1985; 313(6000):277–284. [DOI] [PubMed] [Google Scholar]
- 41.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cysique LA, Franklin D Jr., Abramson I, Ellis RJ, Letendre S, Collier A, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. Journal of clinical and experimental neuropsychology 2011; 33(5):505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol 2012; 26(6):894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia 2010; 58(13):1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozzi SJ, Borelli G, Ryan K, Steiner JP, Reglodi D, Mocchetti I, et al. PACAP27 is protective against tat-induced neurotoxicity. J Mol Neurosci 2014; 54(3):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avdoshina V, Caragher SP, Wenzel ED, Taraballi F, Mocchetti I, Harry GJ. The viral protein gp120 decreases the acetylation of neuronal tubulin: potential mechanism of neurotoxicity. J Neurochem 2017; 141(4):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilmore SF, Blanchette CD, Scharadin TM, Hura GL, Rasley A, Corzett M, et al. Lipid Cross-Linking of Nanolipoprotein Particles Substantially Enhances Serum Stability and Cellular Uptake. ACS Appl Mater Interfaces 2016; 8(32):20549–20557. [DOI] [PubMed] [Google Scholar]
- 49.Athey S, Imbens G. Recursive partitioning for heterogeneous causal effects. Proc Natl Acad Sci U S A 2016; 113(27):7353–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods 2009; 14(4):323–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol 2002; 158(3):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khuchua Z, Wozniak DF, Bardgett ME, Yue Z, McDonald M, Boero J, et al. Deletion of the N-terminus of murine map2 by gene targeting disrupts hippocampal ca1 neuron architecture and alters contextual memory. Neuroscience 2003; 119(1):101–111. [DOI] [PubMed] [Google Scholar]
- 53.Avdoshina V, Taraballi F, Tasciotti E, Uren A, Mocchetti I. Helix-A peptide prevents gp120-mediated neuronal loss. Mol Brain 2019; 12(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mocchetti I, Bachis A, Esposito G, Turner SR, Taraballi F, Tasciotti E, et al. Human immunodeficiency virus-associated dementia: a link between accumulation of viral proteins and neuronal degeneration. Current trends in neurology 2014; 8:71–85. [PMC free article] [PubMed] [Google Scholar]
- 55.Polianova MT, Ruscetti FW, Pert CB, Ruff MR. Chemokine receptor-5 (CCR5) is a receptor for the HIV entry inhibitor peptide T (DAPTA). Antiviral Res 2005; 67(2):83–92. [DOI] [PubMed] [Google Scholar]
- 56.Bachis A, Biggio F, Major EO, Mocchetti I. M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol 2009; 4(1):150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilmore SF, He W, Rasley A, Fischer NO. Strategies for Functionalizing Lipoprotein-Based Nanoparticles. . Control of Amphiphile Self-Assembling at the Molecular Level: Supra-Molecular Assemblies with Tuned Physicochemical Properties for Delivery Applications 2017; American Chemical Society:131–150. [Google Scholar]
- 58.Fischer NO, Weilhammer DR, Dunkle A, Thomas C, Hwang M, Corzett M, et al. Evaluation of nanolipoprotein particles (NLPs) as an in vivo delivery platform. PLoS One 2014; 9(3):e93342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol 2004; 107(2):97–110. [DOI] [PubMed] [Google Scholar]
- 60.Bharti AR, Woods SP, Ellis RJ, Cherner M, Rosario D, Potter M, et al. Fibroblast growth factors 1 and 2 in cerebrospinal fluid are associated with HIV disease, methamphetamine use, and neurocognitive functioning. HIV AIDS (Auckl) 2016; 8:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12(4):234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Obermann M, Küper M, Kastrup O, Yaldizli O, Esser S, Thiermann J, et al. Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. Journal of Neurology 2009; 256(6):948–953. [DOI] [PubMed] [Google Scholar]
- 63.Ozturk T, Kollhoff A, Anderson AM, Howell CJ, Loring DW, Waldrop-Valverde D, et al. inked CSF reduction of phosphorylated tau and IL-8 in HIV associated neurocognitive disorder. Scientific reports 2019; 9:8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 2006; 129(Pt 11):3035–3041. [DOI] [PubMed] [Google Scholar]
- 65.Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 2014; 9(12):e116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guha D, Wagner MCE, Ayyavoo V. Human immunodeficiency virus type 1 (HIV-1)-mediated neuroinflammation dysregulates neurogranin and induces synaptodendritic injury. J Neuroinflammation 2018; 15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis 2013; 207(11):1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis 2007; 195(12):1774–1778. [DOI] [PubMed] [Google Scholar]
- 69.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 2009; 10(5):319–332. [DOI] [PubMed] [Google Scholar]
- 70.Park D, Joo SS, Lee HJ, Choi KC, Kim SU, Kim YB. Microtubule-associated protein 2, an early blood marker of ischemic brain injury. J Neurosci Res 2012; 90(2):461–467. [DOI] [PubMed] [Google Scholar]
- 71.Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma 2006; 23(8):1241–1253. [DOI] [PubMed] [Google Scholar]
- 72.Vangelista L, Secchi M, Lusso P. Rational design of novel HIV-1 entry inhibitors by RANTES engineering. Vaccine 2008; 26(24):3008–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, et al. Structure of a V3-containing HIV-1 gp120 core. Science 2005; 310(5750):1025–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS 2016; 30(4):591–600. [DOI] [PubMed] [Google Scholar]
- 75.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A 1999; 96(14):8212–8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, et al. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol 1998; 8(10):595–598. [DOI] [PubMed] [Google Scholar]
- 77.Bachis A, Mocchetti I. Brain-derived neurotrophic factor is neuroprotective against human immunodeficiency virus-1 envelope proteins. Ann NY Acad Sci 2005; 1053(1):247–257. [DOI] [PubMed] [Google Scholar]
- 78.Bachis A, Wenzel E, Boelk A, Becker J, Mocchetti I. The neurotrophin receptor p75 mediates gp120-induced loss of synaptic spines in aging mice. Neurobiol Aging 2016; 46:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, et al. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol 2013; 19(5):418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uchimura S, Oguchi Y, Katsuki M, Usui T, Osada H, Nikawa J, et al. Identification of a strong binding site for kinesin on the microtubule using mutant analysis of tubulin. EMBO J 2006; 25(24):5932–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roger B, Al-Bassam J, Dehmelt L, Milligan RA, Halpain S. MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr Biol 2004; 14(5):363–371. [DOI] [PubMed] [Google Scholar]


