Abstract
Objective:
Coronary microvascular dysfunction (CMD) is a predictor of cardiac death in diabetes mellitus (DM), independent of traditional cardiovascular (CV) risk factors. Rheumatoid arthritis (RA) is a chronic inflammatory condition with excess CV risk compared to the general population, where CMD is hypothesized to play a role. However, there are limited data on CMD in RA and association with clinical outcomes. The objective of this study was to compare the prevalence of CMD in RA to DM, and test association with all-cause mortality.
Methods:
We performed a retrospective cohort study using data from a registry of all patients undergoing stress myocardial perfusion positron emission tomography (PET) as part of routine clinical care from 2006–2017. Inclusion criteria was a normal perfusion scan. RA or DM patients were classified using previously published approaches. Coronary flow reserve (CFR) was calculated on all patients in the registry and linked with mortality data. CMD was defined as CFR<2.0.
Results:
We studied n=73 patients with RA, and n=441 with DM. Among patients with a normal perfusion scan, the prevalence of CMD in RA was similar to DM, p=0.2. CMD was associated with increased risk for all-cause mortality in RA (HR 2.4, 95% CI 1.4, 4.2), as well as increased risk for cardiac related death at rates similar to DM.
Conclusions:
These findings suggest an important role for CMD as a potential contributor to excess CV risk and mortality in RA, as previously observed in DM, as well as evidence for a mechanistic link between inflammation and CVD.
Rheumatoid arthritis (RA) is a chronic inflammatory condition where patients are at 1.5-fold risk of cardiovascular (CV) risk compared to individuals in the general population of a similar age, gender, and traditional CVD risk factors(1, 2). CV risk calculators developed for use in the general population underestimate future CV risk in RA(3–6). This excess risk has been attributed to inflammation, however the mechanism linking inflammation to CV risk have not been elucidated. One potential mechanism may involve the pro-inflammatory state of RA on atherosclerosis and coronary vasomotor function, thereby increasing the risk of atherothrombotic and ischemic complications(7, 8). Indeed, previous studies observed a higher prevalence of coronary microvascular dysfunction (CMD) in RA compared to non-RA patients, present even in early RA(9). While atherosclerosis and coronary vasomotor dysfunction are hypothesized to play a role in the increased CV risk in RA(9, 10), data linking CMD with clinical outcomes in RA are limited(11).
Coronary flow reserve (CFR) is used to detect CMD. Calculated as the ratio of peak hyperemic myocardial blood flow over that at rest, CFR is emerging as a powerful quantitative prognostic imaging marker of clinical cardiovascular risk.(12, 13) CFR can be accurately measured non-invasively by positron emission tomography (PET) and in the absence of obstructive CAD, it provides a robust and reproducible clinical measure of the integrated hemodynamic effects of diffuse atherosclerosis and CMD on myocardial tissue perfusion across the entire coronary circulation. A reduced CFR consistently identifies patients at increased risk of adverse cardiac events, including MI and death(14, 15). For example, diabetic patients with impaired CFR without overt obstructive CAD, here reflecting microvascular dysfunction, show a risk of cardiac death comparable to, and possibly higher than, that for nondiabetic patients with known CAD(16, 17). We designed this study to test the hypotheses that RA patients, considered a human model of inflammation, will have a similar burden of CMD compared to patients with DM, and that a reduced CFR reflecting CMD will be associated with higher risk of death.
MATERIALS AND METHODS
Study population
We performed a retrospective cohort study of all patients undergoing rest/stress cardiac positron emission tomography (PET) assessing for obstructive CAD at Brigham and Women’s Hospital between January 1, 2006 and October 28, 2017; all patients were entered into a registry study as previously described(17). Patients with known CAD, history of myocardial infarction, coronary revascularization, heart transplantation or moderate or severe valvular disease were excluded, as were those with abnormal myocardial perfusion PET scans reflecting obstructive CAD. Patients with RA were identified using a validated algorithm for RA using electronic medical record (EMR) data with a PPV of 94%(18). Subjects classified by the algorithm as having RA were further chart reviewed to confirm that the RA diagnosis was present at the time of the cardiac PET scan. Information on DM was available through review of medical history and interviews performed on the day of the scan as described previously(17).
PET Imaging
The rest/stress cardiac positron emission tomography (PET) was performed using a standard PET-computed tomography scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI). Details of the protocol are described in Gupta, et al(12). Briefly, patients followed a standardized protocol including abstaining from caffeine and methylxanthine-containing substances and drugs for 24 hours prior to the scan. Myocardial blood flow (MBF) at rest and at maximal hyperemia were measured with 82Rubidium (1480–2200 MBq) or 13N-ammonia (700–900 MBq) as the flow tracer. The stress agents used included dipyridamole, adenosine, regadenoson, or dobutamine; the choice of stress agent was selected based on the agent preferred by the lab as well as the patient comorbidities. The CFR was calculated as the ratio of maximal myocardial blood flow at peak hyperemia over that at rest for the left ventricle and corrected for differences in baseline heart rate-pressure product as previously described. Measurements of CFR and MBF were not available in the clinical reports during the study period and, therefore, did not impact clinical care.
Clinical data assessment
Information regarding both biologic and non-biologic disease modifying anti-rheumatic drugs (DMARDs) to treat RA were obtained through medical record review. Data on diabetes treatments, other traditional CV risk factors such as hypertension and smoking history, as well as medications for primary and secondary prevention of CVD were obtained at the time of the study through patient interview and review of the medical history as previously described(17).
Outcome data
The primary outcome was corrected global CFR, reflecting coronary vascular health of the entire left ventricle. MBF was quantified by 4 operators with an intraclass correlation of 0.94 (95% CI 0.88–0.98)(17). Mortality data was obtained through integrating data from the Partners Healthcare Research Patient Data Repository(19), the Social Security Index, the National Death Index, death certificates, and telephone calls. Ascertainment of cause of death was performed using blinded adjudication of these same records(20).
Statistical analysis
Univariate analyses were performed to compare demographics of the RA population compared to DM. The primary analysis was performed comparing patients with RA compared to DM. If a patient had a concurrent diagnosis of RA and DM, they were included in the RA cohort for the primary analysis. The distribution of CFR was then compared between the two populations. Kaplan-Meier survival curves were constructed to compare the rate of all-cause mortality in RA compared to DM, and was further stratified by CFR<2.0, a cut-off correlated with increased cardiac event rates(21–23). A CFR<2.0 in a patient with a normal perfusion scan is generally considered a sign of CMD.
Using Cox proportional hazards regression models, we tested the association between CFR and all-cause mortality adjusted by age, gender, RA or DM status, traditional CV risk factors including hypertension, hyperlipidemia, smoking status (y/n) at baseline (model 1). Baseline covariates were assessed in the one-year period prior to the scan. A potential confounder for CFR, rest left ventricular ejection fraction (LVEF) obtained at the time of the scan was also included in the model 1. Additionally, we constructed a second model (model 2), including only covariates that resulted in an HR change of >5% for the predictor, CFR, with mortality, in a base model of age and gender. Finally, a third model (model 3) included covariates in model 1 with the addition of other potential confounders including statins, steroid use (prednisone equivalent 7.5mg or higher), and BMI. A sensitivity analysis was performed excluding subjects with a concurrent diagnosis of RA and DM.
In subgroup analyses tested the association between CFR<2 and CVD related deaths only among patients who died during the study period. We first constructed a 2×2 table of CVD adjudicated death vs other causes of death, and CFR<2 vs CFR≥2. Differences between the groups were tested using the chi-square test. The association between CFR<2 and CVD related deaths was further adjusting for age, gender, and RA/DM status in a logistic regression model.
All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC). All aspects of this study were approved by the Partners Healthcare Institutional Review Board.
RESULTS
Over the study period, 73 subjects with RA and 441 subjects with DM met the inclusion or exclusion criteria (Table 1). The mean age of both were similar at 63 years. The proportion of women was higher in RA than in DM group (73% vs 56%, respectively). Patients with DM had a higher prevalence of hypertension and dyslipidemia. Eighteen percent of subjects had concurrent RA and DM.
Table 1.
Comparison of CV risk factors and CFR between RA and DM subjects with a normal stress myocardial perfusion scan.
| Clinical characteristics | RA, n=73 | DM=441 | p-value |
|---|---|---|---|
| Age, mean (SD) | 63.3 (12.2) | 62.8 (11.7) | 0.72 |
| Female, n (%) | 55 (75.3%) | 248 (56.2%) | 0.0021 |
| Race | |||
| White, n (%) | 42 (57.5) | 210 (47.6) | 0.12 |
| Black, n (%) | 20 (27.4) | 108 (24.5) | 0.60 |
| Other, n (%) | 11 (15.1) | 123 (27.9) | 0.02 |
| RA clinical factors | |||
| RF or anti-CCP positive, n (%) | 33 (64.7)* | n/a | n/a |
| MTX use, n (%) | 17 (23.3) | 2 (0.4) | n/a |
| Other non-biologic DMARD use, n (%) | 14 (19.2) | 23 (5.2) | <0.0001 |
| Biologic DMARD use, n (%) | 17 (23.3) | 2 (0.4) | n/a |
| Prednisone >7.5mg, n (%) | 14 (19.2) | 24 (5.4) | <0.001 |
| DM clinical factors | |||
| Hba1c %, mean (SD) | n/a | 7.5 (1.7)** | n/a |
| Oral hypoglycemic agent, n (%) | 2 (2.7) | 133 (30.2%) | <0.0001 |
| Insulin, n (%) | 13 (17.8) | 213 (48.3) | <0.0001 |
| CV risk factors | |||
| BMI kg/m2, mean (SD) | 33.3 (9.0) | 34.7 (8.5) | 0.10 |
| BMI ≥30 kg/m, n (%) | 43 (58.9) | 305 (69.3) | 0.08 |
| Hypertension, n (%) | 58 (79.5) | 393 (89.1) | 0.02 |
| Dyslipidemia, n (%) | 43 (58.9) | 337 (76.4) | 0.002 |
| Smoking, n (%) | 2 (2.7) | 39 (8.8) | 0.10 |
| Statin, n (%) | 36 (50.0) | 283 (64.0) | 0.02 |
| Imaging findings | |||
| Rest LVEF (%) | 61.6 (8.7) | 57.4 (11.8) | 0.008 |
| Stress LVEF reserve >0 | 66.7 (9.5) | 61.5 (12.6) | 0.004 |
| Stress MBF, ml/g/min | 2.2 (0.8) | 1.9 (0.8) | 0.007 |
| Rest MBF, mL/g/min | 1.1 (0.4) | 1.1 (0.4) | 0.24 |
| CFR | 2.0 (0.7) | 1.9 (0.7) | 0.23 |
Abbreviations: anti-CCP= antibodies to cyclic citrullinated peptide, BMI= body mass index, CFR= coronary flow reserve, DMARD= disease modifying anti-rheumatic drugs, LVEF= left ventricular ejection fraction, MBF= myocardial blood flow
available in n=51 from the electronic medical record
available in n=284 from the electronic medical record
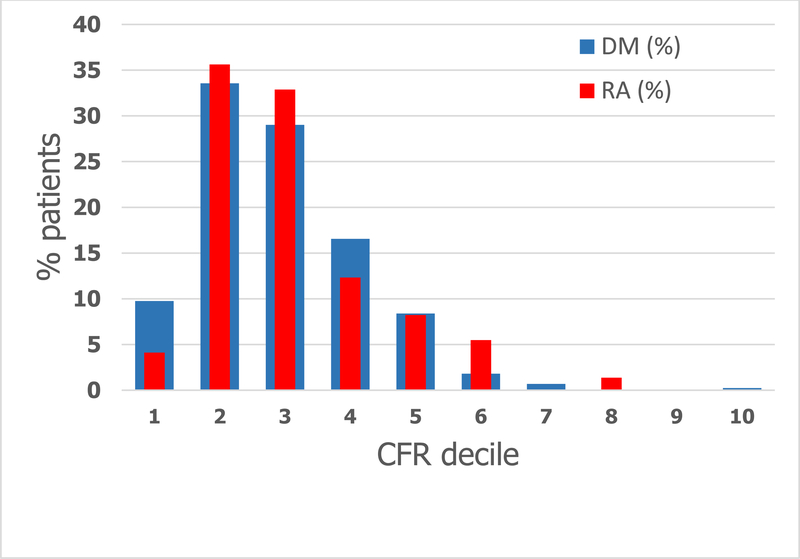
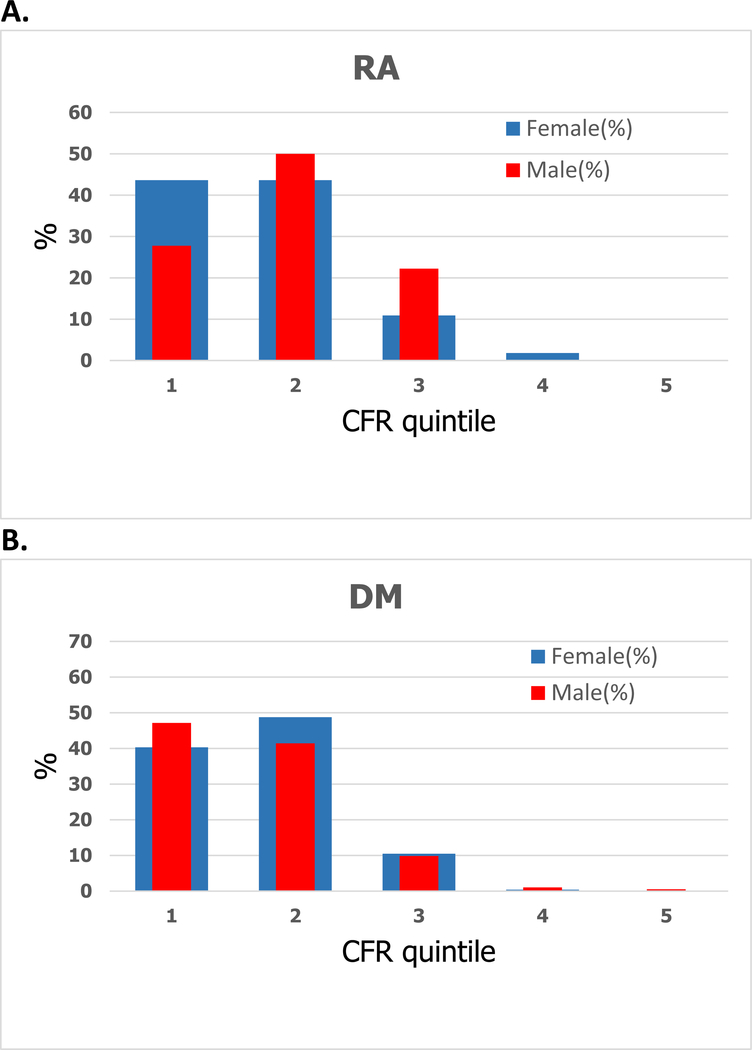
The distribution of CFR was similar between patients with RA and DM, with no significant difference between the mean CFRs of the two groups (Figure 1, p=0.17). In both populations, over 50% of subjects had a CFR <2, 54% in RA and 64% in DM (Figure 1). Among RA patients, the distribution of CFR was similar when stratified by sex, p=0.31 (Figure 2A). Similarly, among patients with DM, there was no difference in distribution between females and males, p=0.73 (Figure 2B).
Figure 1.
Distribution of coronary flow reserve in RA (red) compared to DM (blue), categorized by deciles of CFR (normal CFR≥2).
| Decile | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| CFR range | ≥0.6, ≤1.2 | >1.2, ≤1.7 | >1.7, ≤2. 2 | >2.2, ≤2.8 | >2.8, ≤3.3 | >3.3, ≤3.9 | >3.9, ≤4.4 | >4.4, ≤4.9 | >4.9, ≤5.5 | >5.5, ≤6.0 |
Figure 2.
Distribution of coronary flow reserve categorized into quintiles and stratified by sex among (A) RA and (B) DM (normal CFR≥2).
| Quintile | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| CFR range | ≥0.6, ≤1.7 | >1.7, ≤2.8 | >2.8, ≤3.8 | >3.8, ≤4.9 | >4.9, ≤6.0 |
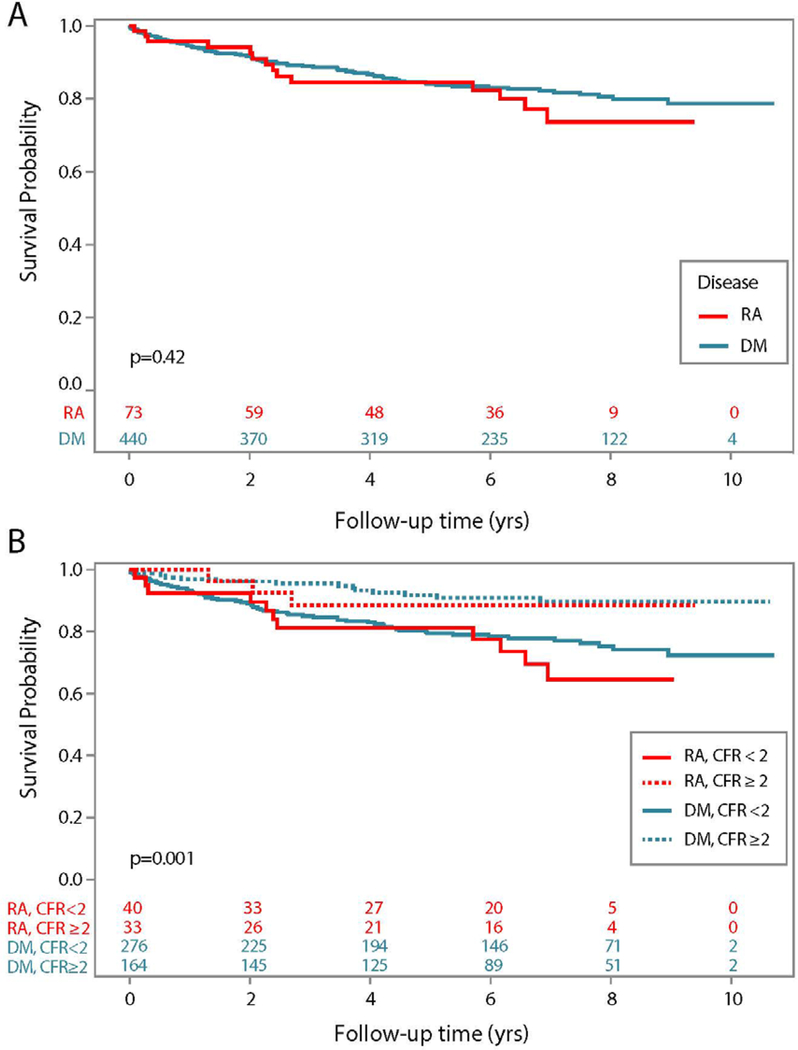
We observed a total of 88 deaths during a mean follow-up period of 2.7 (standard deviation (SD) 2.3) years, n=14 in RA and n=74 in DM (cause of death was adjudicated in n=12 RA, n=67 DM). Among patients with normal perfusion scans, there were no significant differences in survival among RA compared to DM, p=0.41 (Figure 3A). However, significant differences were observed when the cohort was stratified by CFR<2 or CFR≥2. In each cohort, RA or DM, patients with evidence of CMD (CFR<2) had a higher mortality rate, p<0.0001 (Figure 3B). Among RA and DM, the increased risk of mortality for a patient with normal perfusion, but CFR<2 were similar.
Figure 3.
Survival curves for all-cause mortality in (A) the overall RA cohort compared to DM, and (B) RA compared to DM further stratified by CFR <2.0 (impaired) or ≥2 (normal) within each group.
In RA and DM, CFR<2 was associated with an increased risk for all-cause mortality (HR 2.4, 95% CI 1.4, 4.2) (Table 2), adjusting for age, gender, and CV risk factors. None of the measured covariates changed the HR between CFR and mortality by >5% when tested individually in a base model. Additionally, we observed no significant change in the association between CFR and mortality when the model was further adjusted for statins, BMI, and prednisone use at baseline (HR 2.33, 95% CI 1.36 3.98). Results from the sensitivity analysis excluding patients with a concurrent diagnosis of RA and DM (n=18), and adjusting for the same covariates in model 1, yielded a similar relationship between CFR<2 and mortality (HR 2.57, 95% CI 1.48, 4.47).
Table 2.
Cox proportional hazard model for association between CFR and all-cause mortality in RA and DM.
| Clinical characteristics | HR (95% CI) |
|---|---|
| Age | 1.05 (1.03–1.07) |
| Female gender | 0.71 (0.45–1.12) |
| RA vs DM | 1.60 (0.87–2.93) |
| Hyperlipidemia | 0.78 (0.48–1.28) |
| Hypertension | 0.82 (0.39–1.73) |
| Smoking | 2.23 (1.15–4.33) |
| LV ejection fraction | 0.98 (0.96–0.99) |
| CFR<2 | 2.43 (1.40–4.22) |
Abbreviations: RA=rheumatoid arthritis, DM=diabetes mellitus, LV=left ventricular, CFR=coronary flow reserve
Of the total deaths (n=88), we observed n=35 deaths related to cardiac causes; in RA n=6 (43%) deaths were due to cardiac causes compared to n=29 (40%) among patients with DM. Patients with CFR<2 were more likely to suffer from a CVD related death (p=0.04, Table 3). This association remained after adjusting for age, female gender, and RA/DM status, OR 3.9 (95% CI 1.1, 18.4).
Table 3.
CVD related deaths stratified by CFR<2 among all deaths (n=88).
| CFR<2 | ||||
|---|---|---|---|---|
| Yes | No | Total | ||
| CVD-related death | Yes | 32 | 3 | 35 |
| No | 39 | 14 | 53 | |
| Total | 71 | 17 | 88 | |
DISCUSSION
Prior studies observed a higher prevalence of CMD in RA compared to non-RA patients(9), suggesting that CMD may contribute to the excess CV risk observed in RA(2, 24, 25). However, there are limited data demonstrating associations between CMD with clinical outcomes in RA(11). To provide context, we directly compared the prevalence of CMD in RA to DM, a condition where the impact on CVD and mortality is better understood. Patients with DM without a history of CVD have similar risk for a future myocardial infarction (MI) as a patient with a prior MI(26); thus, DM is considered a CVD risk equivalent. In patients with DM, CMD is associated with increased cardiac mortality after adjusting for traditional CV risk factors, as well as additional imaging parameters associated with increased poor cardiac outcomes such as the extent of ischemia or scar(16, 17). In the present study, we observed that the prevalence of CMD was similar in RA and DM among patients undergoing a cardiac stress test who had no evidence of obstructive CAD. As well, we observed similarly poor outcomes for patients with CMD regardless of whether they had RA or DM.
Similar to findings from published studies in DM, all-cause mortality was higher in RA patients with evidence of CMD (CFR<2) compared to those who did not, despite no evidence of obstructive CAD. While we anticipated an elevated risk of mortality among RA patients with CMD, we did not anticipate levels similar to DM. Despite the relatively small number of CVD related deaths (n=35), we observed that patients with CMD with either RA or DM were more likely to die from a CVD-related cause compared to all other causes, again recalling that these patients did not have obstructive CAD on imaging. These data highlight a potentially important role for more studies using CFR to improve identification of RA patients at increased CV risk.
Since RA can be considered a human model of inflammation, this study provides data to support a potential mechanistic link between inflammation and CMD. In RA, inflammation is considered the main driver of CMD(27–29). Higher levels of RA disease activity are associated with measurements of endothelial dysfunction such as flow mediated dilatation(30, 31). Treatment of RA patients with anakinra, an interleukin 1 antagonist, was associated with improved flow-mediated dilation, as well as CFR, further supporting the hypothesis linking inflammation with CMD(32). Inflammation of the myocardium can also be detected on imaging, which may impact endothelial function of the microvascular bed(28, 33, 34). Persistent or recurrent flares over time may therefore lead to endothelial dysfunction and CMD, subclinical myocardial injury, predisposing to increased risk of MI and CHF even in the absence of obstructive CAD(15, 35). In patients with DM, the impaired vasomotor function is attributed to changes in the vascular endothelium due to hyperglycemia and insulin resistance, with some contributions from inflammation(36, 37).
Consistent with the female predominance of RA, 75% of the RA cohort was female compared to 56% in the DM cohort. This female predominance however, was unlikely to have contributed to the equivalent prevalence of CMD in RA compared to DM. Prior studies have found that both groups are equally likely to have CMD in the preclinical stage of CAD(13, 20). We observed no significant difference in the distribution of CFR values among females and males in this study, corroborating with prior data.
Limitations of this study include the use of observational data from a single large academic center. Detailed information on RA disease activity was not collected as part of routine medical care and thus were not available for analysis. RA disease duration was not available in all subjects and therefore its potential effects were not examined in this study. However, information regarding treatments and timing of RA diagnoses were verified using medical record review. The study population comprised of patients who underwent a cardiac stress test ordered as part of routine care. This allowed for similar selection criteria for subjects with RA and DM but cannot provide information for subjects not considered to be at risk for coronary artery disease. A few of the baseline clinical characteristics of RA and DM patients differed and adjusting in models may not fully account for confounding. However, the data were reassuring since none of the potential covariates resulted in a change >5% in the HR between CFR and mortality.
While this is the largest study with CFR and clinical outcomes in RA, the subgroup analysis of patients with CVD related deaths was relatively small and need to be re-examined in future studies. Lastly, we observed 4 subjects in the DM cohort with a prescription for MTX or a biologic DMARD. Those subjects had the following diagnoses on medical record review: inflammatory myositis, psoriatic arthritis, idiopathic thrombocytopenia, and sarcoidosis. The impact of including subjects with other inflammatory conditions in the DM cohort would more likely have resulted in DM patients having more severe impairment of CFR than RA.
In conclusion, our study found that RA patients had a similar distribution of CMD compared to patients with DM, a condition where CMD is considered an independent predictor for CVD and cardiac mortality. Among a population undergoing cardiac stress test as part of routine care with normal perfusion scans, CMD demonstrated by a reduced CFR, was associated with an increased risk for all-cause mortality rate, as well as CVD-related death. These data support CFR as a promising imaging biomarker to assist in improving CV risk stratification among patients with RA, in addition to its importance in DM. Our findings also support a mechanistic link between inflammation, CMD, and CV risk in RA. The CFR data are obtained from clinically available stress myocardial perfusion PET scans, performed as part of clinical care in several institutions. Thus, this approach can be easily translated into clinical practice. Studies are underway to determine whether CFR is modifiable by controlling inflammation in RA, and the potential overall impact on CV risk.
SIGNIFICANCE AND INNOVATIONS.
Prior studies suggest coronary microvascular dysfunction (CMD) is present even in early RA; CMD is an independent predictor of cardiac mortality in the general population, particularly in patients with DM
This study adds new data on the association between CMD and clinical outcomes in RA, demonstrating an association with all-cause mortality and cardiac related death in RA
We provide context for the mortality rates associated with CMD by comparing mortality in RA with a DM cohort; all-cause mortality in RA with CMD were similar to those with DM and CMD
CMD is a potentially important risk factor for CV risk in RA that can be measured using clinically available methods for cardiac stress testing
Acknowledgements
This study was funded by NIH R01 HL127118, NIH P30 AR072577, and the Harold and DuVal Bowen Fund.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis and rheumatism. 2008;59(12):1690–7. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Curhan GC, Rimm EB, Cannuscio CC, Karlson EW. Cardiovascular risk factors in women with and without rheumatoid arthritis. Arthritis and rheumatism. 2004;50(11):3444–9. [DOI] [PubMed] [Google Scholar]
- 3.Arts EE, Popa C, Den Broeder AA, Semb AG, Toms T, Kitas GD, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Annals of the rheumatic diseases. 2013;74(4):668–74. [DOI] [PubMed] [Google Scholar]
- 4.Arts EE, Popa CD, Den Broeder AA, Donders R, Sandoo A, Toms T, et al. Prediction of cardiovascular risk in rheumatoid arthritis: performance of original and adapted SCORE algorithms. Annals of the rheumatic diseases. 2016;75(4):674–80. [DOI] [PubMed] [Google Scholar]
- 5.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Z, Yang N, Everett BM, Frits M, Iannaccone C, Coblyn J, et al. Impact of Changes in Inflammation on Estimated Ten-Year Cardiovascular Risk in Rheumatoid Arthritis . Arthritis & rheumatology (Hoboken, NJ. 2018;70(9):1392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American College of Cardiology. 2009;54(23):2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340(2):115–26. [DOI] [PubMed] [Google Scholar]
- 9.Atzeni F, Sarzi-Puttini P, De Blasio G, Delfino L, Tomasoni L, Turiel M. Preclinical impairment of coronary flow reserve in patients with rheumatoid arthritis. Ann N Y Acad Sci 2007;1108:392–7. [DOI] [PubMed] [Google Scholar]
- 10.Atzeni F, Turiel M, Hollan I, Meroni P, Sitia S, Tomasoni L, et al. Usefulness of cardiovascular biomarkers and cardiac imaging in systemic rheumatic diseases. Autoimmunity reviews. 2010;9(12):845–8. [DOI] [PubMed] [Google Scholar]
- 11.Fent GJ, Greenwood JP, Plein S, Buch MH. The role of non-invasive cardiovascular imaging in the assessment of cardiovascular risk in rheumatoid arthritis: where we are and where we need to be. Annals of the rheumatic diseases. 2017;76(7):1169–75. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, et al. Integrated Noninvasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients With Stable Coronary Artery Disease. Circulation. 2017;136(24):2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, et al. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation. 2017;135(6):566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safdar B, Ong P, Camici PG. Identifying Myocardial Ischemia due to Coronary Microvascular Dysfunction in the Emergency Department: Introducing a New Paradigm in Acute Chest Pain Evaluation. Clin Ther 2018;40(11):1920–30. [DOI] [PubMed] [Google Scholar]
- 15.Camici PG, Crea F. Coronary microvascular dysfunction. The New England journal of medicine. 2007;356(8):830–40. [DOI] [PubMed] [Google Scholar]
- 16.Murthy VL, Di Carli MF. Non-invasive quantification of coronary vascular dysfunction for diagnosis and management of coronary artery disease. J Nucl Cardiol 2012;19(5):1060–72; quiz 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126(15):1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao KP, Cai T, Gainer V, Goryachev S, Zeng-treitler Q, Raychaudhuri S, et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2010;62(8):1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy S, Churchill S, Bry L, Chueh H, Weiss S, Lazarus R, et al. Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res 2009;19(9):1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DD, Donohue TJ, Younis LT, Bach RG, Aguirre FV, Wittry MD, et al. Correlation of pharmacological 99mTc-sestamibi myocardial perfusion imaging with poststenotic coronary flow reserve in patients with angiographically intermediate coronary artery stenoses. Circulation. 1994;89(5):2150–60. [DOI] [PubMed] [Google Scholar]
- 22.Schulman DS, Lasorda D, Farah T, Soukas P, Reichek N, Joye JD. Correlations between coronary flow reserve measured with a Doppler guide wire and treadmill exercise testing. American heart journal. 1997;134(1):99–104. [DOI] [PubMed] [Google Scholar]
- 23.Chamuleau SA, Meuwissen M, Koch KT, van Eck-Smit BL, Tio RA, Tijssen JG, et al. Usefulness of fractional flow reserve for risk stratification of patients with multivessel coronary artery disease and an intermediate stenosis. Am J Cardiol 2002;89(4):377–80. [DOI] [PubMed] [Google Scholar]
- 24.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Annals of the rheumatic diseases. 2012;71(9):1524–9. [DOI] [PubMed] [Google Scholar]
- 25.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis and rheumatism. 2001;44(12):2737–45. [DOI] [PubMed] [Google Scholar]
- 26.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. The New England journal of medicine. 1998;339(4):229–34. [DOI] [PubMed] [Google Scholar]
- 27.Erre GL, Buscetta G, Paliogiannis P, Mangoni AA, Carru C, Passiu G, et al. Coronary flow reserve in systemic rheumatic diseases: a systematic review and meta-analysis. Rheumatol Int 2018;38(7):1179–90. [DOI] [PubMed] [Google Scholar]
- 28.Raza K, Banks M, Kitas GD. Reversing myocardial microvascular disease in a patient with rheumatoid arthritis. The Journal of rheumatology. 2005;32(4):754–6. [PubMed] [Google Scholar]
- 29.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30(15):1837–43. [DOI] [PubMed] [Google Scholar]
- 30.Ormseth MJ, Oeser AM, Cunningham A, Bian A, Shintani A, Solus J, et al. Reversing vascular dysfunction in rheumatoid arthritis: improved augmentation index but not endothelial function with peroxisome proliferator-activated receptor gamma agonist therapy. Arthritis & rheumatology (Hoboken, NJ. 2014;66(9):2331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turiel M, Tomasoni L, Sitia S, Cicala S, Gianturco L, Ricci C, et al. Effects of long-term disease-modifying antirheumatic drugs on endothelial function in patients with early rheumatoid arthritis. Cardiovasc Ther 2010;28(5):e53–64. [DOI] [PubMed] [Google Scholar]
- 32.Ikonomidis I, Tzortzis S, Andreadou I, Paraskevaidis I, Katseli C, Katsimbri P, et al. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7(4):619–28. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi Y, Giles JT, Hirano M, Yokoe I, Nakajima Y, Bathon JM, et al. Assessment of myocardial abnormalities in rheumatoid arthritis using a comprehensive cardiac magnetic resonance approach: a pilot study. Arthritis Res Ther 2010;12(5):R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amigues I, Tugcu A, Russo C, Giles JT, Morgenstein R, Zartoshti A, et al. Myocardial Inflammation, Measured Using 18-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography (FDG PET-CT) is Associated with Disease Activity in Rheumatoid Arthritis. Arthritis & rheumatology (Hoboken, NJ. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J. 2016;37(23):1799–806. [DOI] [PubMed] [Google Scholar]
- 36.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. Journal of the American College of Cardiology. 2003;41(8):1387–93. [DOI] [PubMed] [Google Scholar]
- 37.Quinones MJ, Hernandez-Pampaloni M, Schelbert H, Bulnes-Enriquez I, Jimenez X, Hernandez G, et al. Coronary vasomotor abnormalities in insulin-resistant individuals. Annals of internal medicine. 2004;140(9):700–8. [DOI] [PubMed] [Google Scholar]