Abstract
Novel coronavirus (2019-nCov) infection (COVID-19) rapidly spread across China and 25 countries in the worldwide, which infected not only adults but also children, even neonates. Each year, about 15 million newborns are delivered in China. Newborn screening (NBS) helps effectively prevent some mental retardation, premature death, and adverse outcomes in the early stage of baby, which could detect some inherited metabolic disorders (IMDs). During this COVID-19 epidemic, how to balance the risk of infected 2019-nCov and the risk of disability and teratogenesis of IMDs. Expert members of NBS extra quality assessment in National Clinical Center of Laboratory (NCCL) give a brief consensus for NBS of IMDs in the COVID-2019 epidemic, hoping that the brief consensus could be reference for NBS of IMDs in the other epidemic areas or periods all over the world.
Keywords: Novel coronavirus (2019-nCov), newborn screening (NBS), inherited metabolic disorders (IMDs)
Since December 2019, a sustained increasing number of cases with novel coronavirus (2019-nCov) infection (COVID-19) originated in Wuhan has been identified, rapidly spread across China and 25 countries in the worldwide. By Feb 25, more than 77,000 cases had been diagnosed as COVID-19 in China, and the mortality was about 3.42%. Only adults are susceptible to 2019-nCov infection in early reports (1,2). But, more children (3) with COVID-19 were diagnosed recently, the youngest being 30 h after birth (4). The children’s health and medical care is raised great attention during the epidemic of COVID-2019.
Neonates are thought to be susceptible to the virus because their immune system is not well developed. The newborn screening (NBS) for inherited metabolic disorders (IMDs) helps effectively prevent some mental retardation, premature death, and adverse outcomes in the early stage of a baby, which could detect some treatable IMDs, including congenital hypothyroidism (CH), hyperphenylalaninemia (HPA), congenital adrenocortical hyperplasia (CAH), glucose-6-phosphatase (G6PD) deficiency, methylmalonic academia (MMA), maple syrup urine disease (MSUD), primary carnitine deficiency (PCD), and so on. NBS is one of the effect measures to prevent birth defects, which can screen and diagnose early, treat timely and effectively, to avoid or reduce disability, to improve the quality of children life, and ultimately improve the quality of population. NBS is a highly systematized program with multiple procedures, including informed consent, blood collection, laboratory testing, and complicated post-testing management in the different medical organizations (5), with the whole process of screening, confirmation, diagnosis, treatment, follow-up and management.
Each year, about 15 million newborns are delivered. During this epidemic, there still some newborns are produced and have potential harmful for NBS. The members belonging to the subdivision of NBS extra quality assessment in National Clinical Center of Laboratory (NCCL) have contributed to great efforts for NBS in China. We aim to elicit a contingency consensus for NBS of IMDs in the COVID-2019 epidemic, mainly focused on the whole strategy for NBS.
For screening of NBS for IMDs
Health education and informed consent
The medical staff of delivery hospitals, regardless of the 2019-nCoV epidemic area or not, still need to give routine health education for the guardians of neonates about NBS for IMDs, including the disease type, purpose, significance, cost, method, importance of early screening diagnosis/treatment, and sign the informed consent.
Besides, the medical staff must explain the harmful of COVID-19 in detail to the neonates’ guardians. If the parturients and newborns are infected with the 2019-nCoV, they are easy to have various complications, such as myocarditis and meningitis, which will cause serious consequences and even endanger life if not treated in time. Meanwhile, the medical staff must detailed enquire neonates’ guardians whether their family members closely contacted the diagnosed or suspected COVID-19 cases, any influenza-like symptoms or normal temperature recently, and record them correctly.
Sample collecting and testing
The sample collection and detection are performed as the routine process of NBS for IMDs during this period. But for the neonates whose mothers are suspected or diagnosed COVID-19 would not be performed routine NBS, until these neonates are released from quarantine after 14-day isolation and clinical observation. And the results of 2019-nCoV’s RNA detection from these neonates are negative in twice, they are permitted to collect dried blood spots (DBS) for routine NBS. Only when the neonates have clear family history of IMDs, exhibit clinical manifestation of IMDs with life crisis at the early stage, the NBS for IMDs should be performed and transferred to the superior hospitals immediately. And the subsequent procedure will strictly follow the prevention and control law of infectious diseases of China (6). The medical staff shall fully perform personal biosafety protection (wearing medical protective clothing and gloves, masks, goggles, etc.) (7-9), collect specimen from heel blood (or using other tests’ venous blood for DBS), put it in a special closed and isolated area (e.g., biosafety cabinet) for drying them naturally, seal and keep them with “one sample one storage bag” with biosafety hazard signs, and inform NBS center with enough biosafety protection conditions to test the single specimen independently. After the tests, the storage area for sample must be thoroughly disinfected by ultraviolet irradiation or disinfectants later (7-9).
For the newborns suspected or diagnosed COVID-19 should be postpone the sample collection time till they are excluded from the 2019-nCoV infection, and perform the routine NBS. The medical staff of laboratory should protect themselves in the corresponding biosafety level and test the 2019-nCoV RNA according to SOP recommended by the National Health Committee (7-9).
After test samples from suspected or diagnosed COVID-19 neonates, the specimens were still sealed in accordance with the “one sample one bag” or directly discarded as medical waste referred to “The biological safety guidelines for 2019-nCoV laboratory” (2nd Edition) (8). After finishing the test, the NBS laboratory should be disinfected thoroughly in the air, floor, table and ground according to the requirements (7-9).
Reexamination of neonates with positive results in the first screening
For the newborns with positive IMDs results in the first screening, the medical staff should inform the family (by phone, e-mail, or WeChat public) to collect sample for reexamination in their nearest delivery hospital with full biosafety self-protection.
For the newborns with positive results in the first screening of suspected or diagnosed COVID-19 parturient, we suggest that they should not go to delivery hospital for sample collecting reexamination until the neonates are excluded 2019-nCoV infection. But if the newborns are suspected for G6PD deficiency in the first screening, the family should strictly obey the rules for the G6PD deficiency patients from then, including avoiding some fava beans, medicine and serious infection, lasting the end of the epidemic stage. However, if the neonates have the urgent manifestation of acute hemolysis, they should be taken into the hospital promptly.
For diagnosis and treatment of NBS for IMDs
The newborns with IMDs positive results of recalling reexamination need further differential diagnosis and confirmation. The IMDs newborns should be treated by IMDs specialist followed the related guidelines (10). During this COVID-19 epidemic, we suggest different treatment for different IMDs as in Table 1.
Table 1. Diagnosis and treatment suggestion for common IMDs during COVID-19 epidemic.
| IMD name | Major abnormal tests | Suggestion |
|---|---|---|
| CH | TSH ↑ | Check the thyroid function in local conditional hospitals, report the test results, consult treatment from specialist in NBS center telemedicine; back to NBS center for full examination and therapy instruction face to face when the end of the epidemic; if the local hospital is not able to diagnose CH, those should go to NBS center for confirmation and treatment instruction with full self-protection and private transportation |
| HPA | Phe ↑ | If Phe value is 2–6 mg/dL, collect DBS monthly in local hospital for re-examination to monitor Phe concentration; go to NBS center for confirm and diagnosis when the epidemic alleviated. But once Phe value exceeds 6 mg/dL, go to the NBS immediately to finish the diagnosis and start treatment |
| CAH | 17-OHP ↑ | Without abnormal symptoms and 17-OHP value <100 nmol/L, put off the visiting time of NBS for confirmation. But with manifestations, e.g., poor response, feeding difficulties, malignant vomiting, diarrhea., go to the hospital for diagnosis. If 17-OHP value >100 nmol/L or with obvious symptoms, go to NBS center immediately and start treatment asap |
| Multiple IMDs | TMS, abnormal multiple IMDs indicators | Relevant indicators slightly increased without abnormal manifestation or family history of IMDs, delayed diagnosis appropriately; If the relevant indicators significantly increased, with a family history and obvious abnormal manifestations, diagnosis and examination immediately |
| Non-confirmed IMDs | Screened positive results but not IMDs diagnosis | Contact the specialist in NBS center in time with abnormal manifestations. Confirmed IMDs, immediately start individualized therapy and plans (the local cases be given therapeutic guidance by telemedicine |
↑, adding. NBS, newborn screening; DBS, dried bloodspots; CH, congenital hypothyroidism; HPA, hyperphenylalaninemia; CAH, congenital adrenocortical hyperplasia; IMDs, inherited metabolic disorders; Phe, phenylalanine; TSH, thyroid stimulating hormone; 17-OHP, 17α-hydroxyprogesterone; TMS, tandem mass spectrometry; e.g., for example; asap, as soon as possible.
For follow-up and management of NBS for IMDs
Most IMDs are chronic diseases, which require long-term medication and regular review. To better control the epidemic, government has adopted closed management to reduce cross-infection caused by personnel flow. For IMDs children, under the condition of guaranteeing medication, the reexamination should be postponed during the epidemic, suggested to keep contact with the medical staff of the NBS center through the telecommunication at any time, to minimize the impact on disease control. For those confirmed CH or other IMDs, MMA and propionic acidemia, as well as amino acid metabolic diseases such as HPA requiring long-term special food control, they are suggested not stop taking medication or special food.
If those IMDs children have the manifestation of fever, stress, or overeating during the epidemic, they are easy to induce acute exacerbation of the disease, and suggested to consult NBS specialist online or remotely guidance for medicine or dietary adjustment. If those IMDs children have poor appetite, poor complexion, frequent vomiting and diarrhea, they are suggested to see a doctor in time with personal biosafety protection.
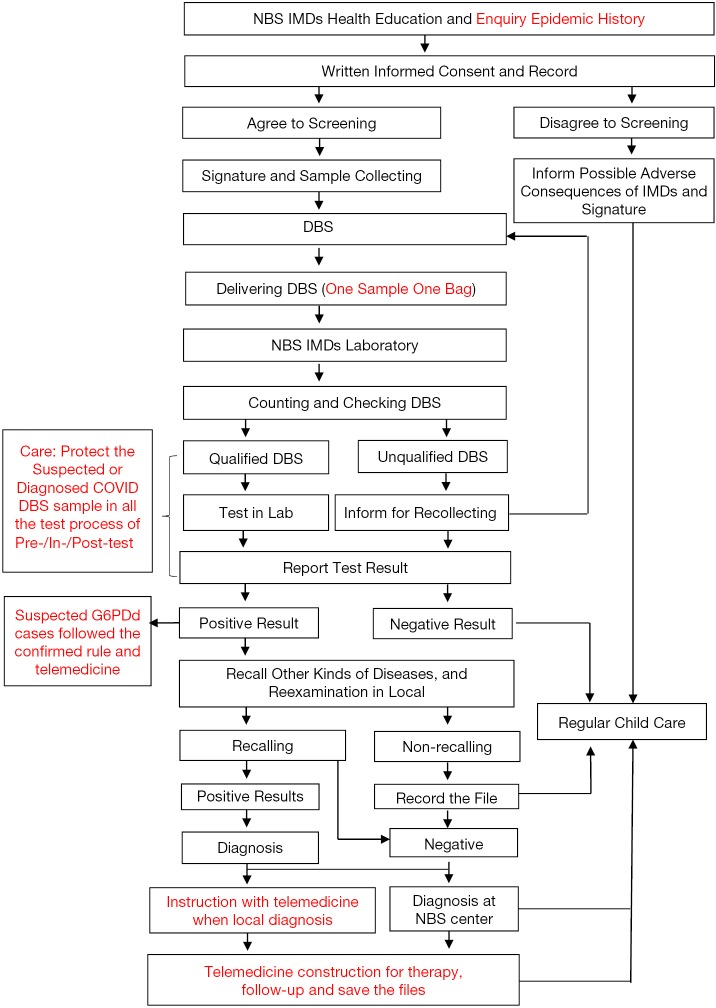
The recommended flow of NBS for IMDs during the COVID-19 period is shown in Figure 1.
Figure 1.
The flow chart for newborn screening of IMDs during the COVID-19 period. The figure shows the flow chart for newborn screening of IMDs. And the difference during COVID-19 is marked in red. NBS, newborn screening; IMDs, inherited metabolic disorders; DBS, dried bloodspots; COVID, novel coronavirus infection disease; Lab, laboratory.
Suggestion for protection of IMDs children with during epidemic of COVID-19 (11,12)
Self-monitoring
Considering that infants and young children are unable to effectively express discomfort, the guardians should pay attention to monitoring body temperature, mental appetite, whether with some clinical symptoms such as cough, diarrhea, etc. If neonates have fever, respiratory symptoms, cough, runny nose, vomiting, diarrhea and other gastrointestinal symptoms, they are suggested to consult the specialist of NBS center by telemedicine or see a doctor nearby.
Self-protection
Considering that health habit is good for prevention infection diseases, the guardians should practice hand hygiene and personal hygiene, maintain respiratory hygiene, wear masks correctly.
Suggestions for diet and home
Considering that children with non-special diet need eat more balanced diet, the guardians should give them high-quality protein food, fresh vegetables, fruits, enough water, cooked thoroughly, ventilation and cleaning at home, to avoid intimate contact, ensure adequate sleep and improve immunoresistance.
Suggestions for psychological counseling
Considering that IMDs children stay at home for a long time, with small space for activities, they may have long-term depressed mood, poor appetite and other manifestations, the guardians should use a variety of parent-child activities for interaction and regulation, such as poetry reading, painting, calligraphy, radio gymnastics, shuttle kicking, rope skipping and other sports.
Therefore, family members need to weigh the pros and cons fully, evaluate rationally, and well protected. We emphasize that although most short-term delayed reviews may not have a significant impact on children’s health, this does not mean that no review is needed. However, review should be conducted immediately after the end of COVID-2019 epidemic, to improve disease control and reduce the risk of disability and teratogenesis of IMDs. Most of our recommendations are based on Chinese current status of medical system. The contingency suggestion will be continuously modified, which should be updated based on the ability of diagnosis and treatment in district hospital. Medical service providers should also continually update their knowledge and skills on prevention and control of the COVID-2019.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was partly financial supported by Chongqing Science and Technology Bureau (cstc2019jscx-msxm0189). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The fund was supported by Lin Zou.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.60). The authors have no conflicts of interest to declare.
References
- 1.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med 2020;382:1199-207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr 2020. [Epub ahead of print]. 10.1007/s12519-020-00345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Qi H, Bao L, et al. A contingency plan for the management of the 2019 novel coronavirus outbreak in neonatal intensive care units. Lancet Child Adolesc Health 2020;4:258-9. 10.1016/S2352-4642(20)30040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Zou L, Wang Z. Newborn screening and prenatal diagnosis laboratory management, 1st ed.; People's Health Publishing House; Beijing, China, 2018:1-3. [Google Scholar]
- 6.The prevention and control law of infectious diseases of the People's Republic of China. Available online: https://baike.so.com/doc/5412518-5650655.html
- 7.Tong Y, Wang M, Xu W, et al. Proposal for detection of 2019-nCoV nucleic acid in clinical laboratories. Chn J Lab Med 2020;43:E003. [Google Scholar]
- 8.Wang C. Biosafety Consensus for 2019 Novel Coronavirus Pneumonia in clinical laboratory. Chn J Lab Med 2020;43:E001. [Google Scholar]
- 9.WHO. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Available online: https://www.renrendoc.com/p-49426389.html
- 10.van Spronsen FJ, van Wegberg AM, Ahring K, et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol 2017;5:743-56. 10.1016/S2213-8587(16)30320-5 [DOI] [PubMed] [Google Scholar]
- 11.National Health Commission of the People’s Republic of China. Management plan for the persons suspicious exposing or close contacting the patients with Novel coronavirus pneumonia. Available online: http://www.nhc.gov.cn/xcs/zhengcwj/202001/c67cfe29ecf1470e8c7fc47d3b751e88.shtml
- 12.National Health Commission of the People’s Republic of China. Novel coronavirus transmission routes and prevention guidelines. Available online: http://www.nhc.gov.cn/jkj/s3578/202001/9e73060017d744aeafff8834fc0389f4.shtml
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as