Abstract
Background
OTU domain-containing protein 3 (OTUD3), as a deubiquitinase (DUB) belonging to the ovarian tumor protease (OTU) family, has been reported to suppress tumor via OTUD3-PTEN signaling axis. Glioma is the most common primary intracranial tumor with high invasiveness and poor prognosis. Although less than half of the patients have phosphatase and tension homologue deleted in chromosome 10 (PTEN) mutations or homozygous deletions, two-thirds of glioma possess diminished PTEN expression. Hence, it is conceivable that other obscure mechanisms may cause the decreased expression of the PTEN protein.
Methods
OTUD3 expression was assessed in human normal and glioma tissues at The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/) and Genotype-Tissue Expression (GTEx) database (https://commonfund.nih.gov/GTex). The mRNA levels of OTUD3 in C6 cells and primary astrocytes were detected using real-time fluorescence quantitative PCR. Western blot was performed to assay PTEN and OTUD3 protein expression in C6 cells and primary astrocytes. By generating Kaplan-Meier curves, we predicted the association between OTUD3 expression and prognosis in glioma patients.
Results
(I) OTUD3 transcription was markedly downregulated in glioma based on microarray data for gene expression between human gliomas and normal brain samples. (II) The mRNA levels of OTUD3 in C6 cells was significantly lower than that of in primary astrocytes. (III) The expressions of protein PTEN and OTUD3 in C6 cells were significantly decreased when compared with primary astrocytes. (IV) Glioma patients with high expression of OTUD3 had a longer survival time than patients with low expression.
Conclusions
Our present findings demonstrated that low expression of OTUD3 in glioma may be involved in PTEN related glioma and may contribute to patient survival.
Keywords: OTU domain-containing protein 3 (OTUD3), phosphatase and tension homologue deleted in chromosome 10 (PTEN), glioma, survival
Introduction
Glioma is the most prevalent primary intracranial tumor generated from the neuroepithelial cells of the central nervous system (CNS) with high invasiveness and poor prognosis. Depending on the histological classification of the World Health Organization (WHO) graded criteria, the most commonly occurring types of gliomas included astrocytoma, oligodendroglioma, and oligoastrocytomas (1,2). Currently, treatments for glioma mainly include neurosurgery, radiotherapy, and chemotherapy. However, on account of the tumor’s rapid cell proliferation, highly invasive, robust migration, increased neovascularization and lack of specific treatment so that the recurrence rate of glioma is extremely high and the median survival time is only 12–15 months (3). Therefore, it is critical to develop more effective therapeutic strategies for glioma.
Maintenance of protein homeostasis is the crucial process that comprises an integrated network of proteins in the normal functioning of the cell. Deregulation of protein stability plays a key role in the pathogenesis and progression of numerous cancer types (4,5). Ubiquitination and deubiquitination mediated by ubiquitin ligase (E3) and deubiquitinase (DUB) are able to precisely regulate the balance of protein content in cells. Deubiquitination mediated by DUBs (deubiquitylating enzymes) as a reverse process of ubiquitination is capable of regulating oncogenes and tumor suppressors. Abnormal DUBs activity including overexpression and loss of function can promote cancer. However, due to the diversity of the substrate of DUBs, it is difficult to determine whether the role of DUBs in a tumorigenesis is absolutely promoted or inhibited (6). The importance of DUBs in cellular functions and carcinogenesis has been recently reported (7-10). OTU domain-containing protein 3 (OTUD3) as one of the deubiquitinating enzymes from the OTU family has been reported to suppress the breast tumor via OTUD3-PTEN signaling axis (11). Alternatively, evidence suggests that OTUD3 may influence the stability of the glucose-regulated protein 78-kDa (GRP78) and promotes the tumorigenesis in lung cancer (12). However, the role of OTUD3 in other tumors including glioma remains unclear.
In this study, we examined OTUD3 mRNA levels in glioma based on microarray data for gene expression between human brain gliomas and normal brain samples. Moreover, the mRNA levels of OTUD3 in C6 cells were significantly decreased compared with primary astrocytes, which consistent with that microarray data. In addition, protein expression levels of PTEN and OTUD3 were down-regulated in C6 cells. Finally, we predict that high expression of OTUD3 improves patient outcomes. In conclusion, low expression of OTUD3 in glioma cells may be involved in the pathogenesis of glioma.
Methods
Regent
The OTUD3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were designed and synthesized by TAKARA (Qingdao, China). Fetal bovine serum (FBS) and TRIzol reagent were purchased from Invitrogen (CA, USA). Dulbecco’s modified Eagle’s medium (DMEM) and other chemical drugs were purchased from Sigma (St. Louis, MO, USA).
Database
The expression level of OTUD3 for glioblastoma (GBM) and low-grade glioma (LGG) patients and clinical data were downloaded from The Cancer Genome Atlas (TCGA) data portal (https://www.cancer.gov/). Clinical data and OTUD3 expression information of normal control cases were obtained from Genotype-Tissue Expression (GTEx) data portal (https://commonfund.nih.gov/GTEx/). All the data were performed differential analysis.
Primary astrocyte and C6 cells culture
C6 cells were grown in DMEM containing 10% fetal bovine serum (FBS) and 1% mixture of streptomycin and maintained in 5% CO2/95% humidified air at 37 °C. C6 cells were seeded in six-well plates and allowed to cultivate confluently prior to testing.
Primary rat astrocyte cultures were obtained from the midbrain of newborn Sprague-Dawley rats (1 to 2 days) by mechanical dissociation and all cells were cultured in DMEM/F12 (Ham’s F 12 nutrient medium) supplemented by a 10% fetal bovine serum (FBS), 1% mixture of streptomycin and penicillin under the stable atmosphere of 5% CO2/95% humidified air at 37 °C. After 7–9 days of cultivation, the culture was shaken for 16–18 hours at 220 rpm to remove the oligodendrocytes and microglia. Determination of the primary astrocytes purity was greater than 95% by immunostaining with anti-glial fibrillary acid protein antibody (GFAP). The primary astrocytes were removed with 0.25% trypsin for 3–5 min and replanted onto 6-well plates for the next experiments. Our work was approved by the local Ethics Commission.
Total RNA extraction and real-time quantitative PCR
One mL of TRIzol reagent was added to each well of six-well plates and the total RNA of the cells in each well was collected. Reverse transcription of total RNA into cDNA using a First Strand cDNA Synthetic Kit. The mRNA levels of OTUD3 (forward: 5'-AGAACCTGGAAGCTGAGAGTCACAC-3'; reverse: 5'-TTCACTCTGTCACCAGGCTCAAG-3') were determined using quantitative PCR with TB Green reagents. GAPDH (forward: 5'-GGCACAGTCAAGGCTGAGAATG-3'; reverse: 5'-ATGGTGGTGAAGACGCCAGTA-3') served as the endogenous control. Amplification of cDNA was carried out as the following steps: 95 °C for 30 s, followed by 95 °C for 5 s and 60 °C for 34 s for total 40 cycles. The relative mRNA levels were calculated and estimated by the 2−ΔΔCt method.
Western blotting
At the designated time, total cell lysates obtained from cells and equal amount of protein lysates (20 µg) were separated by 10% SDS-PAGE, and then transferred to a 0.45 µm pore size PVDF membrane. Membranes were blocked with 5% nonfat milk and incubated with primary antibodies for PTEN (ab32199, Abcam) at 1:10,000 dilution, at 4 °C overnight. After washing 3 times with 1× TBS containing 0.05% Tween 20 (TBST), membranes were incubated with HRP-conjugated secondary antibodies at room temperature for 1–2 hrs. Bound antibodies were detected by the ECL chemiluminescence reagent and signals were quantified by Image J software. The experimental results were expressed as the ratio of the target protein to the gray average of the GAPDH bands.
Overall survival curve
The overall survival analysis of OTUD3 differential expression was generated by Kaplan-Meier curves and tested by Log-rank test.
Statistical analysis
All experiments were repeated at least three times to ensure the results were representative, and data were presented as mean ± SEM. Analysis was performed using SPSS 17 and Graphpad 5.0. Student’s t test was performed to compare between two groups and P value less than 0.05 was indicated statistical significance.
Result
Depressed OTUD3 mRNA levels in glioma samples and C6 cells
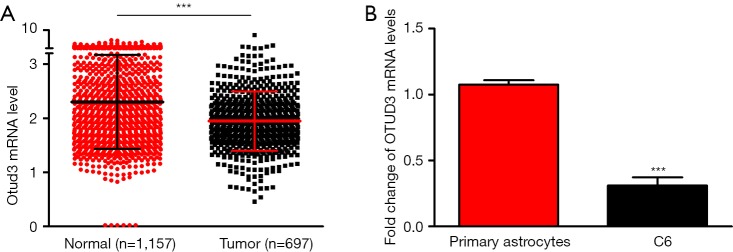
We analyzed the expression of OTUD3 in the TCGA and GTEx datasets and found that the mRNA levels of OTUD3 in glioblastoma were lower than normal brain tissues (Figure 1A). For further verification, we examined mRNA levels of OTUD3 in primary astrocytes and C6 cells. Analogous to the data from bioinformatic analysis, the downregulated OTUD3 was also found in C6 cells (Figure 1B).
Figure 1.
The mRNA expression levels were down-regulated in clinical brain glioblastoma samples and C6 cells. (A) Comparison of OTUD3 mRNA expression in normal brain tissues and glioma from GTEx and TCGA (normal: 1,157 cases from GTEx; tumor: 697 cases from TCGA, including GBM and LGG). Compared with the normal group, ***P<0.0001. (B) Detection of OTUD3 mRNA level in primary astrocytes and C6 cells by real-time quantitative PCR. Quantification data represent 3 independent experiments ± SEM. n=6, t-test; compared with the primary astrocytes group, ***P<0.001.
Depressed PTEN and OTUD3 protein levels in C6 cells and glioma samples
To explore the change of OTUD3-PTEN signaling axis, we detected the PTEN and OTUD3 protein levels in C6 cells and primary astrocytes (Figure 2A). Western blot assays showed that PTEN protein level in C6 cells was lower than in primary astrocytes (Figure 2B). Consistent with PTEN, the protein level of OTUD3 in C6 cells was also decreased (Figure 2C).
Figure 2.
PTEN and OTUD3 protein level in C6 cells was lower than in primary astrocytes. (A) Identification of primary astrocytes by GFAP. Cultures were immunostained by GFAP antibody (green) to confirm the purity of the primary astrocytes. In terms of total DAPI counts, 95% of the cells were astrocytes. Scale bars: 100 μm. (B) Indicated PTEN protein levels were detected in C6 cells and primary astrocytes. Quantification data represent 3 independent experiments ± SEM. n=3, t-test, **P<0.01. (C) OTUD3 protein levels were detected in C6 cells and primary astrocytes. Quantification data represent 3 independent experiments ± SEM. n=3, t-test, *P<0.05.
Correlation of OTUD3 expression with overall survival
In order to explore the potential impact of OTUD3 on overall survival, we first obtained 697 tumor data including glioblastoma (GBM) and low-grade glioma (LGG) in the TCGA database, simultaneously obtained 1,157 normal brain tissue information in the GTEx database. Then, we generated Kaplan-Meier survival curves from the acquired clinical and expression data. After log-rank test, we showed that high expression of OTUD3 predicts better overall survival (Figure 3).
Figure 3.
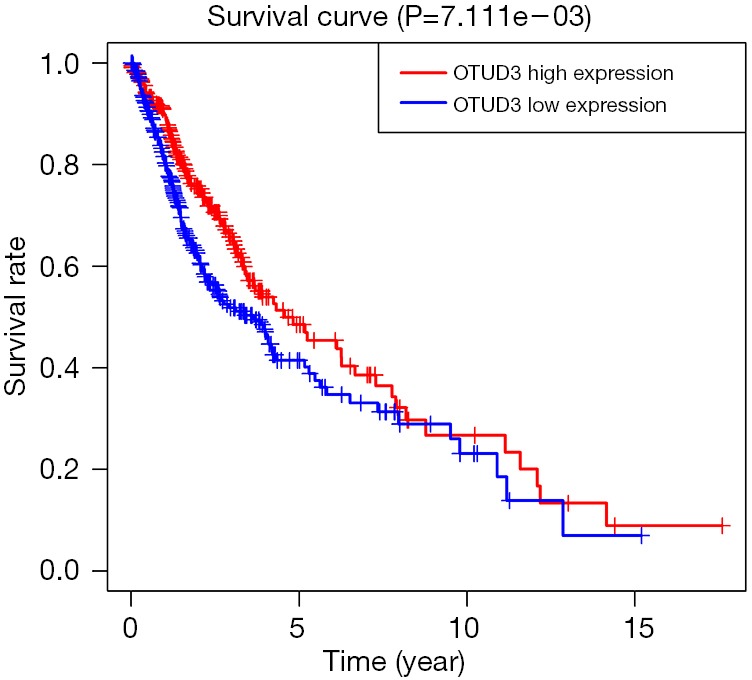
The prognostic value of OTUD3 expression. Survival curves for patients with GBM and LGG were plotted by the Kaplan-Meier plotter (n=697). The red line indicates patients with expression above the median and the blue line indicates patients with expressions below the median.
Discussion
Glioma is the most common aggressive brain tumor characterized by high invasiveness and considerably poor prognosis (13,14). But an understanding of the molecular mechanism of glioma is not clear. At present, targeting inhibition of proteostasis factors to trigger endoplasmic reticulum stress-induced tumor apoptosis is one of the current cancer therapies (9,15). There is another tumor treatment that targets DUBs and thereby mediates tumor promoters or suppressors (16-18). However, it is difficult to judge the role of DUBs in tumorigenesis because the specificity of DUBs may depend on tissue types and stage of malignancy (17,19-21). Evidence suggested that the deubiquitylase OTUD3 inhibited the occurrence of breast cancer through stabilizing PTEN protein to suppress PI3K/AKT signaling. The positive correlation between OTUD3 and PTEN also existed in hepatocellular cancer, colon cancer and cervical cancer (11). In contrast, OTUD3 plays a positive role in lung tumorigenesis by stabilizing GRP78 levels (12). Therefore, OTUD3 has diversiform pathological effects on tumorigenesis in different tissues. However, the role and expression of OTUD3 in glioma remain mysterious.
PTEN, as a major tumor suppressor gene, was originally identified in 1997 on human chromosome 10q23, which could convert PIP3 into PIP2 to antagonize the PIP3/AKT signaling pathway (22-24). PTEN is among the most commonly mutated tumor suppressor genes in human cancers. Loss of PTEN function is associated with multiple human cancers like endometrial cancer, glioblastoma, melanoma, lung and breast cancer. Therefore, PTEN plays a pivotal role in tumorigenesis and tumor progression (25-27). Here, we extracted biological omics data from the TCGA and GTEx databases and detected the expression of OTUD3 on profiles of gliomas. Resultantly, we determined the apparent up-regulation of OTUD3 levels in gliomas. Previous studies have reported that the microarray data-based predicted outcome has higher superiority than traditional histological criteria (28). In the present study, qRT-PCR and Western blotting methods are used to further explore the parallel expression of OTUD3 in C6 glioma cells. The results show that the PTEN and OTUD3 levels in C6 cells were striking down-regulated. Finally, bioinformatics data showed that high expression of OTUD3 predicts better overall survival.
Conclusions
Collectively, our present findings illuminated that low expression of OTUD3 in glioma cells may be associated with the pathogenesis of glioma. Moreover, high expression of OTUD3 may improve survival time in patients with glioma. In the further study, we will verify the effect of OTUD3 on the development of glioma and the underlying mechanism.
Acknowledgments
Funding: This work was supported by the NSFC (31771110, 31701020), National Key Research and Development Program of China (2016YFC1306505), Qingdao Municipal Science and Technology Project (16-6-2-2-nsh), Open project program of the State Key Laboratory of Neuroscience (SKLN-201607), Taishan Scholars Construction Project and the Key Research and Development Program of Shandong Province (2018GSF118042).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our work was approved by the local Ethics Commission.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Barnholtz-Sloan JS, Ostrom QT, Cote D. Epidemiology of Brain Tumors. Neurol Clin 2018;36:395-419. 10.1016/j.ncl.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol 2017;19:v1-v88. 10.1093/neuonc/nox158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghotme KA, Barreto GE, Echeverria V, et al. Gliomas: New Perspectives in Diagnosis, Treatment and Prognosis. Curr Top Med Chem 2017;17:1438-47. 10.2174/1568026617666170103162639 [DOI] [PubMed] [Google Scholar]
- 4.Mofers A, Pellegrini P, Linder S, et al. Proteasome-associated deubiquitinases and cancer. Cancer Metastasis Rev 2017;36:635-53. 10.1007/s10555-017-9697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastola P, Oien DB, Cooley M, et al. Emerging Cancer Therapeutic Targets in Protein Homeostasis. Aaps j 2018;20:94. 10.1208/s12248-018-0254-1 [DOI] [PubMed] [Google Scholar]
- 6.Carvalho AS, Rodriguez MS, Matthiesen R. Review and Literature Mining on Proteostasis Factors and Cancer. Methods Mol Biol 2016;1449:71-84. 10.1007/978-1-4939-3756-1_2 [DOI] [PubMed] [Google Scholar]
- 7.Cheng J, Guo J, North BJ, et al. Functional analysis of deubiquitylating enzymes in tumorigenesis and development. Biochim Biophys Acta Rev Cancer 2019;1872:188312. [DOI] [PubMed]
- 8.Gilberto S, Peter M. Dynamic ubiquitin signaling in cell cycle regulation. J Cell Biol 2017;216:2259-71. 10.1083/jcb.201703170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer 2018;18:69-88. 10.1038/nrc.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mevissen TET, Komander D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu Rev Biochem 2017;86:159-92. 10.1146/annurev-biochem-061516-044916 [DOI] [PubMed] [Google Scholar]
- 11.Yuan L, Lv Y, Li H, et al. Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat Cell Biol 2015;17:1169-81. 10.1038/ncb3218 [DOI] [PubMed] [Google Scholar]
- 12.Du T, Li H, Fan Y, et al. The deubiquitylase OTUD3 stabilizes GRP78 and promotes lung tumorigenesis. Nat Commun 2019;10:2914. 10.1038/s41467-019-10824-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman F. Glioblastoma update: molecular biology, diagnosis, treatment, response assessment, and translational clinical trials. F1000Res 2017;6:1892. 10.12688/f1000research.11493.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 2017;8:91779-94. 10.18632/oncotarget.21586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed E, Cao Y, Rodriguez PC. Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy. Cancer Immunol Immunother 2017;66:1069-78. 10.1007/s00262-017-2019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansour MA. Ubiquitination: Friend and foe in cancer. Int J Biochem Cell Biol 2018;101:80-93. 10.1016/j.biocel.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 17.Pfoh R, Lacdao IK, Saridakis V. Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocr Relat Cancer 2015;22:T35-54. 10.1530/ERC-14-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrigan JA, Jacq X, Martin NM, et al. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov 2018;17:57-78. 10.1038/nrd.2017.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sack LM, Davoli T, Li MZ, et al. Profound Tissue Specificity in Proliferation Control Underlies Cancer Drivers and Aneuploidy Patterns. Cell 2018;173:499-514.e23. 10.1016/j.cell.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Arcy P, Wang X, Linder S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther 2015;147:32-54. 10.1016/j.pharmthera.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 21.He M, Zhou Z, Wu G, et al. Emerging role of DUBs in tumor metastasis and apoptosis: Therapeutic implication. Pharmacol Ther 2017;177:96-107. 10.1016/j.pharmthera.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997;275:1943-7. 10.1126/science.275.5308.1943 [DOI] [PubMed] [Google Scholar]
- 23.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997;15:356-62. 10.1038/ng0497-356 [DOI] [PubMed] [Google Scholar]
- 24.Chen CY, Chen J, He L, et al. PTEN: Tumor Suppressor and Metabolic Regulator. Front Endocrinol (Lausanne) 2018;9:338. 10.3389/fendo.2018.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol 2018;19:547-62. 10.1038/s41580-018-0015-0 [DOI] [PubMed] [Google Scholar]
- 26.Haddadi N, Lin Y, Travis G, et al. PTEN/PTENP1: 'Regulating the regulator of RTK-dependent PI3K/Akt signalling', new targets for cancer therapy. Mol Cancer 2018;17:37. 10.1186/s12943-018-0803-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez-Garcia V, Tawil Y, Wise HM, et al. Mechanisms of PTEN loss in cancer: It's all about diversity. Semin Cancer Biol 2019;59:66-79. 10.1016/j.semcancer.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 28.Tomczak K, Czerwinska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn) 2015;19:A68-77. 10.5114/wo.2014.47136 [DOI] [PMC free article] [PubMed] [Google Scholar]