Abstract
Background
Secondhand smoke (SHS) may be related to worse outcomes in chronic obstructive pulmonary disease (COPD), but the reported SHS prevalence in different studies varied from 27% to 65% and the effects of SHS are still questionable among these patients. The study aims were to estimate the objective SHS prevalence and explore the SHS impact on outcomes among COPD patients without active smoking.
Methods
A cross-sectional design combined with longitudinal death outcome. We selected COPD patients over 40 years old based on the spirometry from National Health and Nutrition Examination Survey (2007–2012), and used the tobacco-specific biomarkers [cotinine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanonol] to determine exposure statuses (active smoking, SHS exposure, or no smoke exposure). Then we estimated the short-term (past 2–4 days) and medium-term (past 6–12 weeks) SHS prevalence among 334 patients without active smoking. Weighted multiple regressions were performed to assess the associations between medium-term SHS exposure and outcomes (symptoms, health status, medical institution visits, and death).
Results
Among the patients without active smoking, the objective prevalence rates of short-term and medium-term SHS were 66.65% [95% confidence interval (CI), 59.63–73.67%] and 34.91% (95% CI, 28.86%–40.96%), respectively. Medium-term SHS exposure showed a significant effect (odds ratio, 3.57; 95% CI, 1.22–10.40) on more chronic coughing after adjusting for the covariates and indicated a trend of unadjusted increasing death risk (log-rank test, P=0.01).
Conclusions
Among COPD patients without active smoking, both short-term and medium-term SHS exposure are prevalent. Chronic cough may be the most susceptible patient-centred outcome related to medium-term SHS exposure. The crude longitudinal trend of elevated death risk associated with medium-term SHS exposure deserves further study.
Keywords: Chronic obstructive pulmonary disease (COPD), prevalence, outcome, secondhand smoke (SHS), tobacco biomarker
Introduction
Chronic obstructive pulmonary disease (COPD) remains a primary cause of mortality worldwide (1), and both its global prevalence and disability-adjusted life-years have increased during recent decades (2,3). Exposure to tobacco smoke, including exposure to secondhand smoke (SHS), is the most common risk factor for COPD (4,5). While the relationship between SHS and the incidence of COPD is established, knowledge regarding the mechanism by which SHS affects the outcomes of COPD patients is relatively limited. Several studies have suggested that SHS exposure may be related to a higher frequency of COPD-related death (6-8), higher exacerbation or readmission rates (9-11), and a poorer health status (10-13). However, the verification of these results remains warranted because these studies had one or more flaws which included the lack of spirometry measurements, only self-reported assessments of SHS exposure, a non-COPD cohort, or a limited covariate adjustment. Moreover, the reported SHS prevalence among COPD patients varied from 27% to 65% in previous studies (10-12), thus, the fundamental question that whether objective SHS exposure is indeed prevalent is still uncertain. Due to active smokers always cause SHS exposure to themselves and smoking cessation should be their priority, we focused on those COPD patients without active smoking (former smokers and never smokers) in this study.
Between 2007 and 2012, the U.S. National Health and Nutrition Examination Survey (NHANES) conducted spirometry measurements for obstructive lung diseases, and also contained measurements of tobacco-specific biomarkers. Using these valuable national data, we constructed a representative COPD sample and established suitable objective criteria for distinguishing three exposure statuses: active smoking, passive smoking (SHS), or no smoke exposure. Our study aims were as follow: (I) to estimate the objectively measured SHS prevalence and explore exposure-related risk factors among COPD patients without active smoking; and (II) to explore the relationships between objective SHS exposure and patient-centered outcomes (respiratory symptoms, health status indicators) and disease-centred outcomes (medical institution visits, death statuses during follow-up).
Methods
Study population
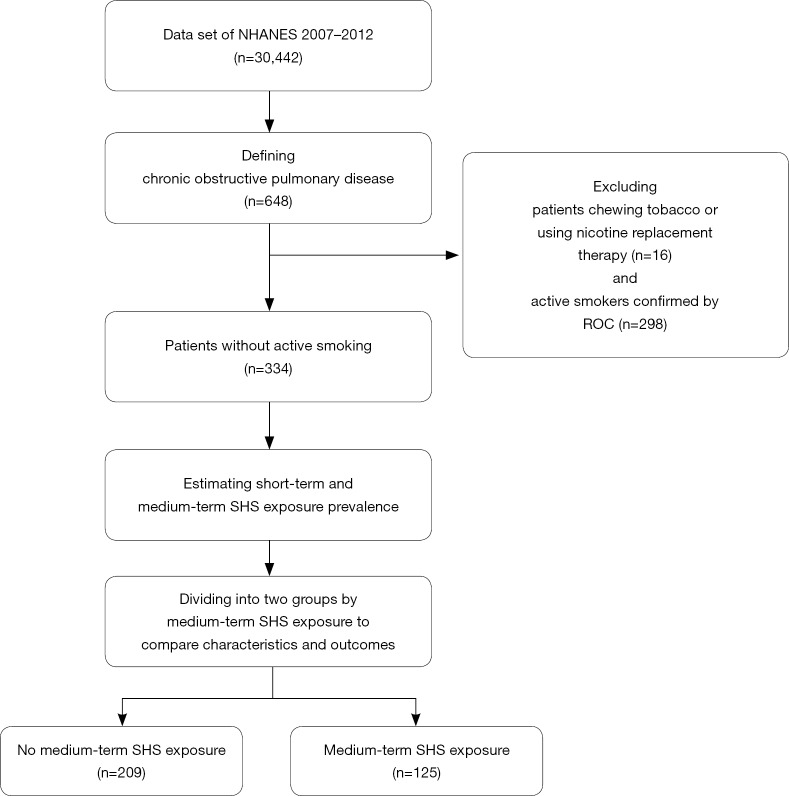
The public NHANES database contains data from a cross-sectional, complex, multistage survey using probability sampling. The survey combines interviews with mobile examination center (MEC) examinations. Each subject was assigned an interview weight and a MEC weight which were calculated by the NHANES team. Informed consent was obtained from all participants, and the National Center for Health Statistics Research Ethics Review Board approved the protocol. The 2-year survey cycles from 2007 to 2012 have 30,442 participants with all ages. More detailed information regarding the NHANES is provided on the website (14). The flow chart of this study is presented in Figure 1.
Figure 1.
Flow chart of this study. Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. NHANES, National Health and Nutrition Examination Survey; ROC, receiver operating characteristic; SHS, secondhand smoke.
Spirometry and definition of COPD
The spirometry procedures followed the recommendations of the American Thoracic Society (15). If the first forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio below the lower limit of normal (LLN) or below 70%, eligible participants were asked to repeat the spirometry measurement after inhaling a β2-adrenergic bronchodilator medication. Each complete spirometry measurement was seen as available if both the FEV1 and FVC had a qualified grade A, B, or C.
Based on the definition of COPD in a previous study which estimated the COPD prevalence in NHANES (16), we defined the COPD as patients who were older than 40 years of age and met one of the following criteria: (I) available results of the post-bronchodilator spirometry measurement with a FEV1/FVC <70%; (II) only available results of the pre-bronchodilator spirometry measurement with a FEV1/FVC <70% and COPD history; or (III) no available results of both spirometry measurements, but COPD history and daily supplementary oxygen use. COPD history was defined as previous or current emphysema or current chronic bronchitis. We included asthma and never-smoking patients because asthma has been regarded as a risk factor of COPD (17), and never-smoking patients constitutes a significant part of all COPD subjects (18,19). In total, 648 participants met this definition.
We established the following three categories of obstructive limitation based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) grading: GOLD 1 (FEV1 ≥80% predicted), GOLD 2 (50%≤ FEV1 <80% predicted), and GOLD 3 or 4 (FEV1 <50% predicted). Equations predictive of FEV1 among different ethnicities were derived from the NHANES III data (20). Since the “other” category of ethnicity was mainly Asian, the predictive equation for non-Hispanic white was used with a multiplied factor of 0.88 for this classification (21).
Tobacco biomarkers
Serum cotinine and urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanonol (NNAL) were the available tobacco-specific biomarkers measured by liquid chromatography tandem mass spectrometry in NHANES. Urinary creatinine was also provided to calculate the urine NNAL/creatinine, which is a ratio indicator adjusted for the renal function and has the same meaning as NNAL. Patients chewing tobacco or using nicotine replacement therapy were excluded (n=16), because these behaviors could disturb the quantification of the biomarkers.
Active smoking behavior
Based on the answers to the questions “Have you smoked at least 100 cigarettes during your life?” and “Do you now smoke cigarettes?”, the self-reported active, former, or never smoking behavior was confirmed. By using the self-reported active smoking behavior as the binary outcome and the biomarkers as the independent variables, we performed three receiver operating characteristic curve (ROC) analyses and objectively identified and excluded 298 active smokers. The remaining 334 patients without active smoking were included in the final analysis.
SHS exposure
The self-reported SHS exposure was regarded as present if the patient reported exposure to SHS at home or at the workplace. Based on different elimination half-life ranges of the biomarkers, serum cotinine was used for estimating the prevalence of short-term SHS exposure (past 2–4 days) (22), while urine NNAL was used for estimating the prevalence of medium-term SHS exposure (past 6–12 weeks) (23). Due to any concentration of a tobacco-specific biomarker indicates an increased risk (24), the limit of detections of serum cotinine and urine NNAL, which were 0.015 and 0.001 ng/mL, were used as the cut-off points to identify patients with short-term or medium-term SHS exposure, respectively. Because longer SHS exposure is more harmful (12,25,26), we chose urine NNAL as the optimal indicator of exposure for subsequent analysis. Finally, 125 patients were identified as having medium-term SHS exposure, and the remaining 209 patients were assigned to the no medium-term SHS exposure group.
Outcome variables and covariates
The following four categories of outcomes were included: (I) respiratory symptoms, including chronic cough, chronic sputum, wheezing, and shortness of breath; (II) health status indicators, including fair or poor general health, worse health in the recent 1 year; (III) self-reported medical institution visits, including outpatient visits ≥3 times per year and hospitalization ≥1 time per year; (IV) death statuses during follow-up (through December 31, 2015), which were obtained from the linkage data of the National Death Index. The former two categories were patient-centred outcomes (27), and the latter two categories were regarded as disease-centred outcomes in this study. The detailed definitions of the binary outcomes in the former three categories are listed in Table S1.
Table S1. Definitions of the binary outcomes.
| Outcome variables | Definitions |
|---|---|
| Chronic cough | Coughing on most days for 3 consecutive months or more during the year |
| Chronic sputum | Coughing phlegm on most days for 3 consecutive months or more during the year |
| Wheezing | Wheezing or whistling in the chest during the year |
| Shortness of breath | Shortness of breath either when hurrying on the level ground or walking up a slight hill |
| Fair or poor general health | Self-reported fair or poor health in general |
| Worse health in the recent 1 year | Compared with the previous 12 months, general health is worse |
| Outpatient visits ≥3 times per year | During the previous 12 months, more than 3 visits to a doctor or other health care professional without being hospitalized overnight |
| Hospitalization ≥1 time per year | Having been a patient in a hospital overnight during the previous 12 months |
The covariates that had been considered were age, gender, ethnicity, education, marital status, family income-to-poverty ratio grading, body mass index, GOLD grading, smoking history, self-reported SHS exposure, asthma, cancer, stroke, hypertension, coronary heart disease, heart failure, diabetes, recent acute infectious disease, sedentary activity minutes per day, sleeping time, and commonly used inhaler medications (inhaled bronchodilators and inhaled combination of bronchodilators and corticosteroids). Definitions of some comorbidities are listed in Table S2.
Table S2. Definitions of part of comorbidities.
| Comorbidities | Definitions |
|---|---|
| Coronary heart disease | Coronary heart disease was combined with angina and heart attack |
| Hypertension | Systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or the use of antihypertensive medications |
| Diabetes | Having diabetes or borderline diabetes |
| Recent acute infectious disease | Having had a head cold, chest cold, diarrhea, flu, pneumonia, or ear infection during the most recent 30 days |
Statistical analysis
The best Youden indexes were chosen to develop the optimal cut-off points in the ROC analyses. The prevalence estimates of SHS exposure were based on the 334 patients without active smoking. The prevalence rates were not adjusted because the NHANES had used data from the U.S. Census Bureau to adjust for demographic factors during poststratification (28). When estimating the strength of the agreement between the self-reported and objective measure exposure statuses, the objective exposure statuses were used as the gold standards. Comparisons of the characteristics between the group with medium-term SHS exposure and the group without tobacco exposure were performed by using Rao-Scott modified χ2 tests for the categorical variables and t-tests for the continuous variables. Both crude and adjusted (age groups and gender) stratified prevalence rates were estimated. In order to clarify the effect of potential risk factors on medium-term SHS exposure, we established a weighted multiple logistic model by using the stepwise strategy. Weighted multiple logistic or Cox regressions were performed to assess the associations between short-term or medium-term SHS exposure and the binary or time-to-event outcomes. Each tobacco biomarker in a regression model was first used as a binary variable and then as a continuous variable. The basic covariates used for adjustments were sociodemographic factors, GOLD grading, smoking history, and inhaled medicines. In order to account for the complex study design, sampling, nonresponses and poststratification, all analyses incorporated the MEC weights and were performed by using specific survey procedures in the SAS 9.4 (SAS Institute Inc, Cary, NC, USA). The level of statistical significance was assumed at P<0.05.
Sensitivity analyses
Because the standard of FEV1/FVC <0.7 may lead to overdiagnosis among older subjects (29-31), we also used the criterion of FEV1/FVC < LLN to define COPD and performed our analyses again.
Results
Biomarker efficiency for distinguishing active smoking
By comparing the AUCs, we found that all three tobacco exposure indicators shown in Figure 2 had adequate similar differentiation ability. In addition, the urine NNAL/creatinine has the same half-life range as NNAL and some missing values. Thus, we only chose urine NNAL and serum cotinine for subsequent analyses. After determining the cut-off points for NNAL and cotinine by the best Youden indexes, we defined the objective active smoking behavior as urine NNAL ≥0.020 ng/mL or serum cotinine ≥17.423 ng/mL. The strength of the agreement between the self-reported and the objective active smoking behavior was high [kappa =0.86; 95% confidential interval (CI), 0.80–0.91]. Among all the COPD patients, the objective active smoking rate was 45.95% (95% CI, 41.16–50.75%).
Figure 2.
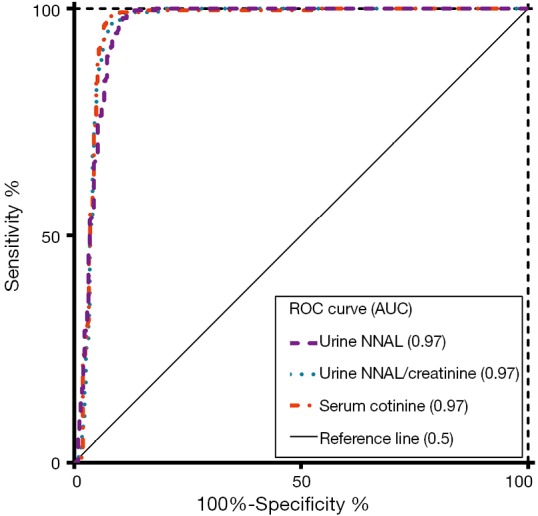
Comparisons of the ROC curves of the tobacco-specific biomarkers used to distinguish the active smoking behavior. Urine creatinine can be used to calculate the urine NNAL/creatinine ratio, which is an adjusted indicator of NNAL. The sensitivity and specificity values at the optimal cut-off points were as follows: urine NNAL, 99.60% and 86.70%, respectively; urine NNAL/creatinine, 97.50% and 90.20%, respectively; and serum cotinine, 98.30% and 91.60%, respectively. Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. AUC, area under the curve; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanonol; ROC, receiver operating characteristic.
Objective SHS exposure prevalence
The agreement between the self-reported SHS exposure and the objective short-term (kappa =0.04; 95% CI, −0.03 to 0.11) or medium-term (kappa =0.08; 95% CI, −0.04 to 0.21) SHS exposure was not significant. By using the biomarkers to measure the SHS exposure among the patients without active smoking, the prevalence rates of short-term and medium-term SHS exposure were 66.65% (95% CI, 59.63–73.67%) and 34.91% (95% CI, 28.86–40.96%), respectively.
Risk factors of medium-term SHS exposure
According to the comparison of the characteristics between two groups in Table 1, medium-term SHS exposure was significantly related to ethnicity, education, family income-to-poverty ratio grading, smoking history, self-reported SHS at home, and only inhaling bronchodilators among COPD patients without active smoking. No significant differences were observed for the other variables included in Table 1 and Table S3, such as gender or seven comorbidities. According to the more intuitive results from a multivariate perspective in Table 2, patients who were former smokers [odds ratio (OR), 3.77; 95% CI, 1.29–10.99] and reporters of SHS exposure at home (OR, 23.30; 95% CI, 4.90–110.94) had higher probabilities of medium-term SHS exposure, on the other hand, patients who were older than 60 years of age (OR, 0.34; 95% CI, 0.13–0.87), were Mexican and other ethnicities (vs. white, OR, 0.30; 95% CI, 0.11–0.82), and had an high family income-to-poverty ratio grading (vs. middle, OR, 0.31; 95% CI, 0.15–0.66) were less likely exposed to medium-term SHS. If education or only inhaling bronchodilators was added in the model, neither of them was significant. We chose the significant factors in Table 2 (except age groups) to calculate crude and adjusted stratified medium-term SHS prevalence rates in Table S4, and we found that the adjusted stratified rates were very similar with the crude stratified rates.
Table 1. Characteristics of COPD patients without active smoking by medium-term SHS exposure.
| Characteristic variables† | Medium-term SHS exposure | P value | |
|---|---|---|---|
| No | Yes | ||
| Sample size | 209 | 125 | – |
| Estimated population (weighted)‡ | 3,063,227 | 1,642,858 | – |
| Age groups, % | 0.15 | ||
| 40–59 years | 34.96 | 46.73 | |
| ≥60 years | 65.04 | 53.27 | |
| Male, % | 63.26 | 62.02 | 0.88 |
| Ethnicity, % | 0.03* | ||
| Non-Hispanic white | 86.40 | 87.20 | |
| Non-Hispanic black | 4.20 | 8.44 | |
| Mexican-American and other | 9.40 | 4.36 | |
| Education, % | 0.04* | ||
| College or above | 70.14 | 56.89 | |
| High school or below | 29.86 | 43.11 | |
| Family income-to-poverty ratio grading, % | <0.01* | ||
| High (>3.50) | 61.77 | 38.52 | |
| Middle (>1.30–3.50) | 22.42 | 37.01 | |
| Low (0.00–1.30) | 6.33 | 15.92 | |
| Missing | 9.48 | 8.55 | |
| Smoking history, % | <0.01* | ||
| Former | 53.30 | 78.30 | |
| Never | 46.70 | 21.70 | |
| Self-reported SHS at home, % | <0.01* | ||
| Yes | 0.57 | 9.91 | |
| No | 98.71 | 89.84 | |
| Missing | 0.72 | 0.25 | |
| GOLD grading, % | 0.08 | ||
| 1 | 62.84 | 44.25 | |
| 2 | 23.29 | 38.00 | |
| 3 or 4 | 5.83 | 10.38 | |
| Missing | 8.04 | 7.37 | |
| Only inhaling bronchodilator | 0.03* | ||
| Yes | 3.22 | 14.35 | |
| No | 96.78 | 85.65 | |
Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. †, except for age group, gender, and GOLD grading, other non-significant characteristics are listed in Table S3. Because the sample weights led to inconsistencies with the proportions in cells, the patient counts are not shown; ‡, estimated population (weighted) means the population size represented by the sample size; *, statistical significance at the P<0.05 level when comparing between two groups. COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SHS, secondhand smoke.
Table S3. Other characteristics of COPD patients without active smoking by medium-term SHS exposure based on FEV1/FVC <70%.
| Characteristic variables† | Medium-term SHS exposure | P value | |
|---|---|---|---|
| No | Yes | ||
| Sample size | 209 | 125 | – |
| Estimated population (weighted)‡ | 3,063,227 | 1,642,858 | – |
| Age, mean (SE), years | 63.00 (0.98) | 62.13 (1.15) | 0.57 |
| Marital status, % | 0.33 | ||
| Married or living with partner | 79.96 | 75.00 | |
| Other | 20.04 | 25.00 | |
| BMI grading, % | 0.09 | ||
| Obese (≥30 kg/m2) | 26.06 | 40.73 | |
| Overweight (25–29.9 kg/m2) | 43.65 | 38.17 | |
| Normal or underweight (<25 kg/m2) | 28.51 | 19.81 | |
| Missing | 1.78 | 1.29 | |
| Pack-years among former smokers, mean (SE) | 31.90 (3.30) | 37.37 (4.85) | 0.38 |
| Self-reported SHS at the workplace, % | 0.62 | ||
| Yes | 5.96 | 3.67 | |
| No | 50.75 | 47.87 | |
| Unemployed | 43.29 | 48.46 | |
| FEV1, mean (SE), L/s | 2.67 (0.11) | 2.45 (0.11) | 0.18 |
| Comorbidities | |||
| Asthma, % | 28.58 | 23.57 | 0.49 |
| Cancer, % | 25.67 | 21.18 | 0.54 |
| Stroke, % | 5.82 | 4.06 | 0.57 |
| Hypertension, % | 56.06 | 56.05 | 0.99 |
| Coronary heart disease, % | 14.54 | 12.89 | 0.72 |
| Heart failure, % | 4.11 | 5.35 | 0.63 |
| Diabetes, % | 14.18 | 17.84 | 0.42 |
| Sedentary time per day, mean (SE), minutes | 386.92 (17.06) | 388.84 (22.22) | 0.95 |
| Inhaling bronchodilator and corticosteroid, % | 13.16 | 14.96 | 0.72 |
Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. †, because the sample weights led to inconsistencies with the proportions in cells, the patient counts are not shown; ‡, estimated population (weighted) means the population size represented by the sample size. BMI, body mass index; COPD, chronic obstructive lung disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SHS, secondhand smoke; SE, standard error.
Table 2. Significant risk factors for medium-term SHS exposure in weighted multiple logistic regression.
| Characteristic variables | Adjusted OR (95% CI) | P value |
|---|---|---|
| Age group: ≥60 vs. 40–59 years | 0.34 (0.13–0.87) | 0.03* |
| Ethnicity: black vs. white | 1.66 (0.70–3.94) | 0.24 |
| Ethnicity: Mexican-American and other vs. white | 0.30 (0.11–0.82) | 0.02* |
| Family income-to-poverty ratio grading: high vs. middle | 0.31 (0.15–0.66) | <0.01* |
| Family income-to-poverty ratio grading: low vs. middle | 1.69 (0.68–4.23) | 0.25 |
| Smoking history: former vs. never | 3.77 (1.29–10.99) | 0.02* |
| Self-reported SHS at home: yes vs. no | 23.30 (4.90–110.94) | <0.01* |
Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. *, statistical significance of the coefficients at the P<0.05 level in the model. CI, confidence interval; OR, odds ratio; SHS, secondhand smoke
Table S4. Crude and adjusted stratified medium-term SHS exposure prevalences in COPD patients without active smoking.
| Characteristic variables | Crude rates (95% CI) | Adjusted rates (95% CI) |
|---|---|---|
| Ethnicity, % | ||
| Non-Hispanic black | 51.87 (36.01–67.47) | 53.09 (38.81–67.37) |
| Non-Hispanic white | 35.12 (28.10–42.65) | 35.18 (27.97–42.40) |
| Mexican-American and other | 19.91 (9.72–34.08) | 22.92 (13.21–32.62) |
| Family income-to-poverty ratio grading, % | ||
| High (>3.50) | 25.06 (17.69–33.68) | 22.37 (15.89–28.85) |
| Middle (>1.30–3.50) | 46.96 (35.77–58.37) | 47.29 (34.95–59.63) |
| Low (0.00–1.30) | 57.43 (43.38–70.66) | 58.51 (46.16–70.85) |
| Smoking history, % | ||
| Former | 44.07 (35.93–52.45) | 46.49 (37.62–55.37) |
| Never | 19.95 (10.81–32.15) | 18.37 (9.04–27.58) |
| Self-reported SHS at home, % | ||
| Yes | 90.33 (61.42–99.53) | 91.78 (80.56–100.00) |
| No | 32.80 (26.95–39.07) | 32.74 (26.52–38.97) |
Adjusted stratified prevalences were standardized by age groups and gender. Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. COPD, chronic obstructive lung disease; CI, confidential interval; SHS, secondhand smoke.
Association between SHS exposure and outcomes
According to the results of bivariate analyses in Table 3, medium-term SHS exposure was significantly associated with a higher possibility of chronic coughing (OR, 2.55; 95% CI, 1.17–5.57), wheezing (OR, 1.99; 95% CI, 1.07–3.73), and fair or poor general health (OR, 2.24; 95% CI, 1.19–4.21) among patient-centred outcomes. All patients in the final study sample had eligible follow-up statuses of mortality. The median survival times of the two groups were undefined. The median follow-up times (and interquartile ranges) of the exposure group and the control group were 67 [46–92] months and 76 [60–94] months, respectively. The log-rank test was significant (P=0.01) for comparison between groups in Figure 3, however, the bivariate analysis using the complex-survey-specific weighted Cox model showed a non-significant effect of SHS on all-cause death [hazard ratio (HR), 1.21; 95% CI, 0.62–2.36; Table 3].
Table 3. Effect of medium-term SHS exposure (yes vs. no) on outcomes in bivariate and multiple (adjusted) models.
| Outcome variables | Effect type† | Bivariate models | Multiple models‡ | |||
|---|---|---|---|---|---|---|
| Effect (95% CI) | P value | Effect (95% CI) | P value | |||
| Patient-centred outcomes | ||||||
| Chronic cough | OR | 2.55 (1.17–5.57) | 0.02* | 3.57 (1.22–10.40) | 0.02* | |
| Chronic sputum | OR | 1.56 (0.69–3.52) | 0.28 | 1.78 (0.54–5.85) | 0.34 | |
| Wheezing | OR | 1.99 (1.07–3.73) | 0.03* | 1.78 (0.72–4.40) | 0.20 | |
| Shortness of breath | OR | 1.94 (0.94–3.98) | 0.07 | 1.56 (0.63–3.85) | 0.33 | |
| Fair or poor general health | OR | 2.24 (1.19–4.21) | 0.01* | 1.53 (0.61–3.85) | 0.36 | |
| Worse health in the past year | OR | 1.35 (0.60–3.08) | 0.46 | 0.77 (0.28–2.06) | 0.59 | |
| Disease-centred outcomes | ||||||
| Outpatient visits ≥3 times per year | OR | 1.01 (0.54–1.88) | 0.98 | 0.63 (0.31–1.29) | 0.20 | |
| Hospitalization ≥1 time per year | OR | 1.15 (0.40–3.35) | 0.79 | 0.39 (0.09–1.72) | 0.21 | |
| Death during follow-up | HR | 1.21 (0.62–2.36) | 0.56 | 0.72 (0.34–1.54) | 0.39 | |
Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. †, the reference category was the group without medium-term SHS exposure; ‡, multiple models were adjusted by age, gender, ethnicity, family income-to-poverty ratio grading, GOLD grading, smoking history, inhaled bronchodilators, and inhaled combination of bronchodilators and corticosteroids; *, statistical significance at the P<0.05 level. CI, confidential interval; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; OR, odds ratio; SHS, secondhand smoke.
Figure 3.
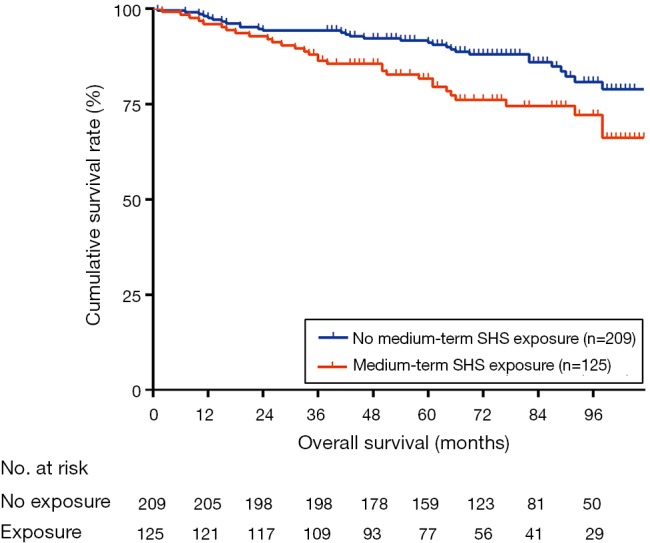
Survival curves of chronic-obstructive-pulmonary-disease patients without active smoking by medium-term SHS exposure. Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. SHS, secondhand smoke.
After adjusting for the covariates in Table 3, medium-term SHS exposure remained a significant effect (OR, 3.57; 95% CI, 1.22–10.40) on more chronic coughing, other patient-centred outcomes and all disease-centred outcomes (including death) were non-significant. When NNAL was used as a continuous variable, the dose-response relationship between medium-term SHS exposure and other outcomes were negative except for the chronic cough (OR, 1.19; 95% CI, 1.04–1.37; Table S5).
Table S5. Effect of medium-term SHS exposure (as a continuous variable) on outcomes in bivariate and multiple (adjusted) models.
| Outcome variables | Effect type† | Bivariate models | Multiple models‡ | |||
|---|---|---|---|---|---|---|
| Effect (95% CI) | P value | Effect (95% CI) | P value | |||
| Patient-centred outcomes | ||||||
| Chronic cough | OR | 1.16 (1.04–1.29) | <0.01* | 1.19 (1.04–1.37) | 0.01* | |
| Chronic sputum | OR | 1.06 (0.96–1.16) | 0.28 | 1.04 (0.91–1.19) | 0.53 | |
| Wheezing | OR | 1.07 (0.96–1.19) | 0.24 | 1.10 (0.92–1.32) | 0.27 | |
| Shortness of breath | OR | 1.04 (0.94–1.15) | 0.41 | 0.98 (0.85–1.12) | 0.72 | |
| Fair or poor general health | OR | 1.05 (0.96–1.14) | 0.29 | 0.93 (0.79–1.10) | 0.38 | |
| Worse health in the past year | OR | 1.01 (0.89–1.15) | 0.83 | 0.91 (0.73–1.13) | 0.36 | |
| Disease-centred outcomes | ||||||
| Outpatient visits ≥3 times per year | OR | 0.94 (0.86–1.04) | 0.21 | 0.92 (0.84–1.01) | 0.08 | |
| Hospitalization ≥1 time per year | OR | 1.00 (0.91–1.11) | 0.96 | 0.92 (0.72–1.16) | 0.46 | |
| Death during follow-up | HR | 1.06 (0.97–1.16) | 0.19 | 1.05 (0.93–1.20) | 0.43 | |
Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. †, the effects were derived from the β values corresponding to an increment of 0.001 ng/mL of NNAL in the models; ‡, multiple models were adjusted by age, gender, ethnicity, family income-to-poverty ratio grading, GOLD grading, smoking history, inhaled bronchodilators, and inhaled combination of bronchodilators and corticosteroids; *, statistical significance at the P<0.05 level. SHS, secondhand smoke; OR, odds ratio; HR, hazard ratio; CI, confidential interval; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanonol; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
The short-term SHS exposure, whether as a binary or continuous variable, had non-significant associations with all the outcomes mentioned above in both bivariate and multiple models. These non-significant data are not shown in this paper.
Sensitivity analysis results
Under the criterion of FEV1/FVC < LLN, the cut-off points of urine NNAL and serum cotinine were 0.027 and 26.486 ng/mL, respectively, but the new definition of the objective active smoking behavior had no substantial effect on subsequent results. The prevalence rates of short-term and the medium-term SHS exposure were 69.19% (95% CI, 61.52–76.87%) and 37.82% (95% CI, 30.08–45.56%), respectively. The results of the between-group comparison in Table S6 showed that education level, family income-to-poverty ratio, smoking history, and self-reported SHS at home remained significant, but ethnicity maintained a nonsignificant difference trend. Similarly, the log-rank test of survival curves was significant (P=0.03), but the bivariate weighted Cox model showed a non-significant trend (HR, 1.25; 95% CI, 0.53–2.92). The outcome-related results in Table S7 indicated that the effect of medium-term SHS exposure (as a binary variable) on more chronic coughing was still significant (OR, 4.66; 95% CI, 1.39–15.58) after adjusting for the same covariates. The continuous medium-term SHS exposure had a critical significant effect (OR, 1.14; 95% CI, 1.00–1.30; P=0.06) on more chronic coughing. The short-term SHS exposure, whether as a binary or continuous variable, still had non-significant associations with all the outcomes in the sensitivity analyses (data are also not shown).
Table S6. Characteristics of COPD patients without active smoking by medium-term SHS exposure based on FEV1/FVC < LLN.
| Characteristic variables | Medium-term SHS exposure† | P value | |
|---|---|---|---|
| No | Yes | ||
| Sample size | 123 | 93 | – |
| Estimated population (weighted)‡ | 1,798,094 | 1,093,623 | – |
| Age, mean (SE), years | 61.80 (1.32) | 61.42 (1.48) | 0.85 |
| Male, % | 64.64 | 49.16 | 0.08 |
| Ethnicity, % | 0.35 | ||
| Non-Hispanic white | 87.46 | 83.42 | |
| Non-Hispanic black | 6.11 | 10.83 | |
| Mexican-American and other | 6.43 | 5.75 | |
| Marital status, % | 0.01* | ||
| Married or living with partner | 86.30 | 68.98 | |
| Other | 13.70 | 31.02 | |
| Education of college or above, % | 63.65 | 47.96 | 0.04* |
| Family income-to-poverty ratio grading, % | <0.01* | ||
| High (>3.50) | 65.92 | 33.25 | |
| Middle (>1.30–3.50) | 19.90 | 38.83 | |
| Low (0.00–1.30) | 6.27 | 18.01 | |
| Missing | 7.91 | 9.91 | |
| BMI grading, % | 0.62 | ||
| Obese (≥30 kg/m2) | 23.20 | 30.96 | |
| Overweight (25–29.9 kg/m2) | 44.37 | 42.88 | |
| Normal or underweight (<25 kg/m2) | 29.39 | 25.07 | |
| Missing | 3.04 | 1.09 | |
| Smoking history, % | 0.04* | ||
| Former | 60.96 | 78.82 | |
| Never | 39.04 | 21.18 | |
| Pack-years among former smokers, mean (SE) | 33.01 (4.43) | 40.85 (5.09) | 0.25 |
| Self-report SHS at home, % | 0.03* | ||
| Yes | 0.00 | 9.50 | |
| No | 100.00 | 90.12 | |
| Missing | 0.00 | 0.38 | |
| Self-reported SHS at the workplace, % | 0.39 | ||
| Yes | 7.11 | 2.76 | |
| No | 49.63 | 46.18 | |
| Unemployed | 43.26 | 51.06 | |
| FEV1, mean (SE), L/s | 2.65 (0.12) | 2.29 (0.16) | 0.07 |
| GOLD grading, % | 0.33 | ||
| 1 | 51.08 | 37.98 | |
| 2 | 26.95 | 37.42 | |
| 3 or 4 | 8.27 | 13.26 | |
| Missing | 13.70 | 11.34 | |
| Comorbidities | |||
| Asthma, % | 28.91 | 23.57 | 0.49 |
| Cancer, % | 30.06 | 25.08 | 0.58 |
| Stroke, % | 5.06 | 4.70 | 0.93 |
| Hypertension, % | 60.42 | 45.64 | 0.07 |
| Coronary heart disease, % | 15.17 | 14.23 | 0.83 |
| Heart failure, % | 6.41 | 7.18 | 0.84 |
| Diabetes, % | 13.13 | 16.49 | 0.54 |
| Sedentary time per day, mean (SE), minutes | 382.39 (22.92) | 359.99 (20.98) | 0.48 |
| Only inhaling bronchodilator, % | 5.48 | 21.15 | 0.03* |
| Inhaling bronchodilator and corticosteroid, % | 18.68 | 10.94 | 0.20 |
Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. †, because the sample weights led to inconsistencies with the proportions in cells, the patient counts are not shown; ‡, estimated population (weighted) means the population size represented by the sample size; *, statistical significance at the P<0.05 level when comparing between two groups. COPD, chronic obstructive lung disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LLN, lower limit of normal; SHS, secondhand smoke; SE, standard error; BMI, body mass index; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table S7. Effect of medium-term SHS exposure (yes vs. no) on outcomes in bivariate and multiple (adjusted) models based on FEV1/FVC < LLN.
| Outcome variables | Effect type† | Bivariate models | Multiple models‡ | |||
|---|---|---|---|---|---|---|
| Effect (95% CI) | P value | Effect (95% CI) | P value | |||
| Patient-centred outcomes | ||||||
| Chronic cough | OR | 2.20 (1.17–4.14) | 0.02* | 4.66 (1.39–15.58) | 0.01* | |
| Chronic sputum | OR | 1.71 (0.75–3.90) | 0.20 | 3.43 (0.84–13.98) | 0.08 | |
| Wheezing | OR | 1.93 (0.70–5.34) | 0.20 | 3.36 (0.83–13.64) | 0.09 | |
| Shortness of breath | OR | 1.78 (0.77–4.08) | 0.17 | 1.64 (0.50–5.41) | 0.41 | |
| Fair or poor general health | OR | 1.86 (0.83–4.20) | 0.13 | 0.85 (0.15–4.70) | 0.85 | |
| Worse health in the past year | OR | 1.17 (0.34–4.01) | 0.80 | 0.37 (0.12–1.13) | 0.08 | |
| Disease-centred outcomes | ||||||
| Outpatient visits ≥3 times per year | OR | 0.91 (0.36–2.30) | 0.83 | 0.46 (0.18–1.22) | 0.12 | |
| Hospitalization ≥1 time per year | OR | 1.85 (0.64–5.35) | 0.25 | 0.56 (0.12–2.56) | 0.45 | |
| Death during follow-up | HR | 1.25 (0.53–2.92) | 0.60 | 0.66 (0.27–1.65) | 0.37 | |
Data obtained from the National Health and Nutrition Examination Survey (2007–2012), National Center for Health Statistics. †, the reference category was the group without medium-term SHS exposure; ‡, multiple models were adjusted by age, gender, ethnicity, family income-to-poverty ratio grading, GOLD grading, smoking history, inhaled bronchodilators, and inhaled combination of bronchodilators and corticosteroids; *, statistical significance at the P<0.05 level. SHS, secondhand smoke; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LLN, lower limit of normal; CI, confidential interval; OR, odds ratio; HR, Hazard Ratio; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Discussion
Based on the representativeness of the NHANES and the objectivity of the SHS and spirometry measures, our study provides a fundamental answer and suggests that even after removing the threat of active smoking, up to 66.65% of patients experience short-term exposure and 34.91% of patients experience medium-term exposure to SHS. Comparing to the SHS exposure prevalence of 17.86% based on the cotinine data of NHANES (2011–2012) among adult nonsmokers who were older than 40 years of age (32), we can find that the COPD patients without active smoking, a population that have worse health outcomes, suffer from 3.73 times higher short-term SHS exposure than the general population without active smoking in the same age group. In addition, patients with more SHS exposure are younger, are non-Hispanic black or white, have a lower socioeconomic status, are former smokers, or report SHS at home. These risk factor results are consistent with the findings of Putcha N and colleagues (11) and instructive to target the key subgroups for prevention.
Prior studies from Eisner MD and colleagues suggest that higher SHS measures are associated with worse patient-centred outcomes which are mainly dyspnea and disease-specific quality of life (10,12,13). The results reported by Putcha N and colleagues also show that self-reported SHS exposures are related to worse respiratory symptoms and worse scores of disease-specific questionnaires or scales (11). Moreover, the association between SHS and greater airway wall thickness in COPD has recently been highlighted (11), and airway wall thickness is related to respiratory symptoms (33). Therefore, a possible causality frame from components of SHS to worse patient-centred outcomes has been preliminarily established. But these studies failed to adjust for the potential confounding effect of the daily usage of inhaler drugs which can benefit the symptoms, quality of life, and exacerbation among COPD patients (17). After adjusting for the covariates including inhaler medicines, our study indicates that chronic cough may be the most susceptible patient-centred outcome related to medium-term SHS exposure. Due to cough is an important determinant of health-related quality of life among stable COPD patients (34), chronic cough may play a considerable role to worsen the quality of life among the patients with SHS exposure.
On the other hand, the effects of SHS exposure on disease-centred outcomes are relatively uncertain. Two longitudinal studies did not show a significant relationship between SHS and hospitalization of COPD patients using an adjusted model (9,10), but other studies suggest that SHS or PM 2.5 may increase severe exacerbations in certain subgroups, such as current smokers, obese patients (11,35). At a level of society, the legislated bans on smoking seem to be associated with the reduced rates of exacerbation or admission to hospital in COPD population in some developed countries (36-38) and this advance may be mainly ascribed to the decrease of SHS. Regarding the final outcome of death, the results showing that SHS elevated the COPD-related death risk can be only found from non-COPD cohorts (6-8), which could not exclude the effect of SHS exposure on increasing the morbidity of COPD. In this study, medium-term SHS exposure had no apparent association with disease-related outcomes which included nonspecific medical institution visits. According to our knowledge, our study is the first to present the survival curves in a COPD cohort by medium-term SHS exposure (Figure 3). The non-significant results of weighted Cox models (both adjusted and unadjusted) could be limited by the sample size, but the significant log-rank test might be interpreted prudently as a clue that medium-term SHS exposure might associate with the increased risk of death. However, more longitudinal studies are needed to clarify the dose-effect relationship between SHS and disease-centred outcomes.
Based on the higher prevalence, evidently worse impacts on patient-centred outcomes, and probably harmful effects on disease-centred outcomes, the SHS exposure deserves more general interventions among COPD patients without active smoking. In addition to individual smoking cessation, more general preventions, like health educations for patient’s whole families and public smoking bans, are necessary to be strengthened in order to eliminate the exposure of all tobacco smoke during individual and social disease management. Moreover, according to our knowledge, the mechanism by which SHS cause the onset of COPD is still limited. Woodruff and his colleagues established a biological link between SHS exposure and the development of inflammatory processes refer to COPD in mice due to alveolar macrophage recruitment and activation (39). Hartney and colleagues found that four-week exposure to second hand smoke can lead to airspace leukocyte infiltration and decreased lung elastance (40). John G and colleagues observed that after sidestream exposure there was an inflammatory reaction consisting of macrophages and diminished granulocyte-macrophage colony-stimulating factor (GM-CSF) levels due to elevated CO concentrations in COPD mouse models (41). More research, like COPD animal model induced by SHS or study at human level, is needed in this area.
Our study has several limitations. First, because some patients of GOLD 3 or 4 might not receive spirometry, the final sample was more representative of GOLD 1 and 2 patients. However, this restriction likely does not affect the reliability of our conclusion because the medium-term SHS exposure rate would likely to be higher if more severe patients were included, furthermore, Putcha N and colleagues found that SHS might be more harmful among GOLD 1 and 2 patients (11). Second, the NHANES lacks detailed SHS questionnaires which can help to assess the duration or pattern of SHS exposure, thus by only using the biomarkers, we cannot separate the effect of SHS from total smoke exposure among active smoking patients. Third, the NHANES also lacks COPD-specific questionnaires or scales and COPD-specific medical institution visits, thus, the related outcomes deserve further study. Fourth, subject to the limited final sample size, we acknowledge that the negative results of outcomes lack power and deserve further investigation. Fifth, the data is a little out of date, however, we believe that our prevalence estimations, which can be regarded as a baseline reference for future research, were more referable than previous studies in this area and the harmful biological effect of SHS on COPD patients should not change over time. Finally, although cross-sectional data is suitable for the purpose of estimating the prevalence, the cross-sectional association analyses of most outcomes does not support a high level of causality.
Conclusions
In summary, given the representativeness of the sample and the objectivity of the SHS and spirometry measurements, up to 66.65% of COPD patients without active smoking have experienced short-term exposure of SHS, and 34.91% of them have experienced medium-term exposure of SHS. Chronic cough may be the most susceptible patient-centred outcome related to medium-term SHS exposure. More longitudinal studies based on prospective COPD population are needed to further clarify the relationship between SHS and outcomes in the future.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors would like to acknowledge the patients and staff of the NHANES.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81573262) and the Fundamental Research Funds for the Central Universities, HUST (No. 2016YXZD042). Funds supported data analysis but had no role in the study design, the interpretation of results, or writing of the paper.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study used the data of the public NHANES database and waived the need for approval by our local ethics committee.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.145). The authors have no conflicts of interest to declare.
References
- 1.GBD 2016 Mortality Collaborators. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1084-150. 10.1016/S0140-6736(17)31833-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1260-344. 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017;5:691-706. 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer F, Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public Health 2015;15:1202. 10.1186/s12889-015-2489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin P, Jiang CQ, Cheng KK, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet 2007;370:751-7. 10.1016/S0140-6736(07)61378-6 [DOI] [PubMed] [Google Scholar]
- 6.Hirayama T. Non-smoking wives of heavy smokers have a higher risk of lung cancer: a study from Japan. Br Med J (Clin Res Ed) 1981;282:183-5. 10.1136/bmj.282.6259.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandler DP, Comstock GW, Helsing KJ, et al. Deaths from all causes in non-smokers who lived with smokers. Am J Public Health 1989;79:163-7. 10.2105/AJPH.79.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ukawa S, Tamakoshi A, Yatsuya H, et al. Passive smoking and chronic obstructive pulmonary disease mortality: findings from the Japan collaborative cohort study. Int J Public Health 2017;62:489-94. 10.1007/s00038-016-0938-1 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Aymerich J, Farrero E, Felez MA, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003;58:100-5. 10.1136/thorax.58.2.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisner MD, Iribarren C, Yelin EH, et al. The impact of SHS exposure on health status and exacerbations among patients with COPD. Int J Chron Obstruct Pulmon Dis 2009;4:169-76. 10.2147/COPD.S4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putcha N, Barr RG, Han MK, et al. Understanding the impact of second-hand smoke exposure on clinical outcomes in participants with COPD in the SPIROMICS cohort. Thorax 2016;71:411-20. 10.1136/thoraxjnl-2015-207487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisner MD, Jacob P, 3rd, Benowitz NL, et al. Longer term exposure to secondhand smoke and health outcomes in COPD: impact of urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Nicotine Tob Res 2009;11:945-53. 10.1093/ntr/ntp091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisner MD, Balmes J, Yelin EH, et al. Directly measured secondhand smoke exposure and COPD health outcomes. BMC Pulm Med 2006;6:12. 10.1186/1471-2466-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Survey Methods and Analytic Guidelines. National Health and Nutrition Examination Survey. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (Accessed October 1, 2019).
- 15.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 16.Tilert T, Dillon C, Paulose-Ram R, et al. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007-2010. Respir Res 2013;14:103. 10.1186/1465-9921-14-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GOLD Board and Science Committee. Global Strategy for The Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2019 Report. Available online: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf (Accessed November 7, 2019).
- 18.Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax 2015;70:822-9. 10.1136/thoraxjnl-2015-206938 [DOI] [PubMed] [Google Scholar]
- 19.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest 2011;139:752-63. 10.1378/chest.10-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179-87. 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Kawut SM, Shahar E, et al. Performance of American Thoracic Society-Recommended Spirometry Reference Values in a Multiethnic Sample of Adults The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest 2010;137:138-45. 10.1378/chest.09-0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benowitz NL, Jacob P, 3rd. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther 1994;56:483-93. 10.1038/clpt.1994.169 [DOI] [PubMed] [Google Scholar]
- 23.Goniewicz ML, Havel CM, Peng MW, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev 2009;18:3421-5. 10.1158/1055-9965.EPI-09-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avila-Tang E, Al-Delaimy WK, Ashley DL, et al. Assessing secondhand smoke using biological markers. Tob Control 2013;22:164-71. 10.1136/tobaccocontrol-2011-050298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan JM, Knezevich AD, Wang RW, et al. Urinary levels of the tobacco-specific carcinogen N'-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 2011;32:1366-71. 10.1093/carcin/bgr125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan JM, Gao YT, Murphy SE, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res 2011;71:6749-57. 10.1158/0008-5472.CAN-11-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008;31:416-69. 10.1183/09031936.00099306 [DOI] [PubMed] [Google Scholar]
- 28.Mirel LB, Mohadjer LK, Dohrmann SM, et al. National Health and Nutrition Examination Survey: estimation procedures, 2007-2010. Vital Health Stat 2 2013;(159):1-17. [PubMed] [Google Scholar]
- 29.Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction - Use percentage of FEV1/FVC ratio below the fifth percentile, not < 70%. Chest 2007;131:349-55. 10.1378/chest.06-1349 [DOI] [PubMed] [Google Scholar]
- 30.Brazzale DJ, Upward AL, Pretto JJ. Effects of changing reference values and definition of the normal range on interpretation of spirometry. Respirology 2010;15:1098-103. 10.1111/j.1440-1843.2010.01830.x [DOI] [PubMed] [Google Scholar]
- 31.Quanjer PH, Brazzale DJ, Boros PW, et al. Implications of adopting the Global Lungs Initiative 2012 all-age reference equations for spirometry. Eur Respir J 2013;42:1046-54. 10.1183/09031936.00195512 [DOI] [PubMed] [Google Scholar]
- 32.Homa DM, Neff LJ, King BA, et al. Vital signs: disparities in nonsmokers' exposure to secondhand smoke--United States, 1999-2012. MMWR Morb Mortal Wkly Rep 2015;64:103-8. [PMC free article] [PubMed] [Google Scholar]
- 33.Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med 2010;181:353-9. 10.1164/rccm.200907-1008OC [DOI] [PubMed] [Google Scholar]
- 34.Deslee G, Burgel PR, Escamilla R, et al. Impact of current cough on health-related quality of life in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016;11:2091-7. 10.2147/COPD.S106883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormack MC, Belli AJ, Kaji DA, et al. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. Eur Respir J 2015;45:1248-57. 10.1183/09031936.00081414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naiman A, Glazier RH, Moineddin R. Association of anti-smoking legislation with rates of hospital admission for cardiovascular and respiratory conditions. CMAJ 2010;182:761-7. 10.1503/cmaj.091130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dusemund F, Baty F, Brutsche MH. Significant reduction of AECOPD hospitalisations after implementation of a public smoking ban in Graubunden, Switzerland. Tob Control 2015;24:404-7. 10.1136/tobaccocontrol-2013-051290 [DOI] [PubMed] [Google Scholar]
- 38.Galán I, Simón L, Boldo E, et al. Changes in hospitalizations for chronic respiratory diseases after two successive smoking bans in Spain. PLoS One 2017;12:e0177979. 10.1371/journal.pone.0177979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodruff PG, Ellwanger A, Solon M, et al. Alveolar macrophage recruitment and activation by chronic second hand smoke exposure in mice. COPD 2009;6:86-94. 10.1080/15412550902751738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartney JM, Chu H, Pelanda R, et al. Sub-chronic exposure to second hand smoke induces airspace leukocyte infiltration and decreased lung elastance. Front Physiol 2012;3:300. 10.3389/fphys.2012.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John G, Kohse K, Orasche J, et al. The composition of cigarette smoke determines inflammatory cell recruitment to the lung in COPD mouse models. Clin Sci (Lond) 2014;126:207-21. 10.1042/CS20130117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as