Abstract
Background
Due to the “ceiling effect” of respiratory depression and the non-addictiveness, the consumption of dezocine is increasing quickly in the cancer surgery perioperative period for security and comfort reasons in China. Former studies find dezocine inhibits the norepinephrine transporters (NET) and serotonin transporters (SERT) and sigma-1opioid receptors. Given the complexity of the molecular mechanism, the effect of dezocine on tumor cells need to be studied. In this study, we investigated the effect of dezocine on HepG2 and Hep 3B liver cancer cell lines growth and glycolysis, and the molecular mechanisms behind.
Methods
HepG2 and Hep 3B cells viability and migration were measured by CCK8, Wound healing and transwell assay, Extracellular acidification rate (ECAR) was used to index the aerobic glycolysis of liver cancer cells and western blot analysis showed protein expression levels in the cells. SC79, an agonist of Akt, and the siRNA silence of Akt1 aimed to regulate Akt1 activity and expression in the reverse experiments.
Results
Dezocine played opposite roles in HepG2 and Hep 3B cells viability and migration in a concentration-dependent manner (P<0.01). Dezocine has diverse effects on aerobic glycolysis and adjusts the serine/threonine kinase 1 (Akt1)-glycogen synthase kinase-3β (GSK-3β) pathway. The effects of SC79 and the siRNA silence of Akt1 could reverse the effects of dezocine on HepG2 and Hep 3B cells.
Conclusions
As an analgesic drug widely used in clinical practice, dezocine play reversed roles on HepG2 and Hep 3B cells viability and migration targeting Akt1/GSK-3β pathway then the glycolysis in a concentration-dependent manner.
Keywords: Dezocine, glycolysis, liver cancer
Introduction
Dezocine is an opioid receptor partial agonist/antagonist developed by the American Home Products Corporation in 1970’s (1), which is increasingly popular in China as an alternative medication for perioperative pain management (2). As it exhibiting a “ceiling effect” for respiratory depression (3,4) and alleviating morphine-induced dependence without addiction (5), dezocine is used widely including cancer surgery.
As necessary analgesic drugs in the perioperative period, the influence of opioids on tumors has attracted the attention of researchers (6). Opioids can affect tumors by activating a series of pathways in the cell via opioid receptors or other receptors (7,8). Dezocine, a mixed partial MOR agonist and KOR antagonist, is not categorized as a controlled substance and has been used for postoperative analgesia for more than a decade. Dezocine is also discovered as an inhibitor of the norepinephrine (NET) and serotonin transporters (SERT) and sigma-1receptor (9). Dezocine acts on a variety of receptors with different affinities in cells. Moreover, these receptors have different effects on tumor cells, we wonder what effects of dezocine exactly has on tumor cells in different concentrations.
The long-term prognosis of cancer patients is affected by many aspects, and the perioperative period is recognized as an important window affecting tumor outcomes increasingly (6,10). Perioperative stress and perioperative intervention are considered as two mutually balanced factors (11). For Anesthesiologists, only a detailed understanding of the effects of perioperative drugs on the tumor can make the tumor disease tend in a good direction. While widely used in the perioperative period, the effect of dezocine on tumors is shorted of study. The study aims to find the effect of dezocine on liver cancer.
Methods
Cell cultures and reagents
Human liver cancer cells HepG2 (ATCC@ HB-8065™) and Hep 3B (ATCC® HB-8064™) were cultured in DMEM cell culture medium (Hyclone, Australia) with 10% fetal bovine serum (FBS) (Gibico, Australia). Cells were cultured in an incubator containing 5% CO2 at 37 °C. Cell medium was changed every other day, and cells were passaged when reaching 90% confluence. Cells at the passage of 4−10 were used in the present study.
SC79, an Akt activator, was purchased from MCE, USA (Cat. No. HY-18749). Dezocine was obtained from Young’s River pharmaceutical group (Taizhou, Jiangsu, China) with 99.9% purity. The concentrations of dezocine range from 1 to 8 µg/mL according to the former studies (5,9). The reagents used in the present study were all dissolved in DMSO (Sigma, St. Louis, MO, USA, SKU: PHR1309). The final concentration of DMSO was adjusted to 0.01% to avoid potential nonspecific effects. After being treated by dezocine or SC79 for 6 hours in a concentration of 10 µg/mL, the experiment acted as follows.
Cell viability analysis
Cell suspensions (4×104 cells/mL) were added in wells of the 96-multiwell culture plate and cultured in the incubator. After corresponding treatments, 10 µL CCK-8 was added in wells and the cells were further cultured for 24 or 48 h. Finally, the optical density at 450 nm was detected using an immunoplate reader. The cell viability curve was determined by accounting mean ± SD of optical density for every six wells.
Wound healing assay
Cells were seeded onto six-well culture plates (2×106 cells/well) in DMEM containing 10% FBS and cultured overnight till to an 80% confluency. After washed with PBS, the cell monolayer was scraped with a sterile 200 µL pipette tip to create a wound. Then, cells were cultured with medium containing dezocine in different concentrations or vehicle DMSO (0.01%) for another 24 h, respectively. Then, cell migration was observed and the images were captured at 0 and 24 h with an inverted microscope. All experiments were carried out in 3 times.
Cell transwell assay
Modified Boyden chamber assays were conducted using 24-well Transwell polyester membrane filter inserts with 8 µm pores and 0.33 cm2 surface area (Corning Inc., Corning, NY, USA) at a density of 500,000 cells/ml per transwell (upper chamber). DMEM medium without FBS in the upper chamber and total DMEM medium with 10% FBS in the bottom chambers of the transwells. After culturing for 24 h, the cells from the upper chambers were removed, and the migrated cells on the undersides of the membranes were stained with crystal violet (Beyotime, Haimen, China). Migratory cells were imaged and counted in high power microscope micro-photographs (field area: 0.98 mm2) taken under bright light (Olympus Tokyo, Japan) using Image Pro Plus 6.0 software (Rockville, MD).
Measurement of extracellular acidification rate of cells
The extracellular flux changes of oxygen and protons in the media caused by cells were measured by the XF96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA). Real-time measurements of cellular bioenergetics including oxygen consumption rates, glycolysis, ATP production and respiratory capacity performed in a noninvasive way (12). HepG2 and Hep 3B cells were seeded in the microplate at a density of 8×104 cells/well in each 80 µL of DMEM. After 24 hours of incubation at 37 °C in 5% CO2, baseline cellular respiration was measured. This device measures extracellular acidification rate (ECAR) in 96-well plates after sequentially adding to each well 20 µL of Glucose, 22 µL of an oligomycin, 25 µL of 2DG to reach working concentrations of 1.26, 1, 0.5, and 0.5 µM, respectively.
Western Blotting
The target proteins in cells were detected by western blot analysis. Equal amounts of proteins achieved from cells were separated by 10% SDS-polyacrylamide gels and immobilized on polyvinylidene fluoride membranes (Millipore, Billeruca, MA) using a full-wet electroblotting system. The membranes were blocked with 5% fat-free milk in PBST for 1 hour, then hybridized with 1,000-1 dilution of specific primary antibodies overnight at 4 °C. The membranes were washed with PBST, and then incubated with a 1,500-1 dilution of Goat anti-rabbit IgG, HRP conjugate (Proteintech, Wuhan, China) for 1 hour at room temperature. After washing, the signals were visible after development with a chemiluminescence (ECL) reagent (Millipore Corporation, USA) and detected using a LAS-4000 mini CCD camera (GE Healthcare).
siRNA treatment of Akt1
Human AKT1-specific siRNA (siAKT1) targeting the AKT gene (NM-001014431.2) and control siRNA (siNC) were supplied by Shanghai GenePharma Co, Ltd (Shanghai, People’s Republic of China) as follows:
Akt1 No. 1 sense, 5'-UGCCCUUCUACAACCAGGATT-3'; antisense, 5'-UCCUGGUUGUAGAAGGGCATT-3';
Akt1 No. 2 sense, 5'-GGCCACGATGACTTCCTTC-3'; antisense, 5'- GAAGGGAGTCGTCGTGGCC-3';
Control sense, 5'-UCCGUUUCGGUCCACAUUCTT-3'; and antisense, 5'-GAAUGUGGACCGAAACGGATT-3'.
Cells were seeded at 5×105 cells per well in 6-well plates in DMEM containing 10% FBS without penicillin and streptomycin overnight. Transfection experiments were performed with OPTI-MEM serum-free medium and Lipofectamine 3000 reagent with a final siRNA concentration of 50 or 100 nM. The cells were collected for CCK8 assay and protein extraction after 24 h of siRNA transfection.
Statistical analysis
Data were obtained from at least 3 independent experiments and expressed as the mean ± SD. Groups were compared using a one-way analysis of variance followed by pairwise comparisons between groups with Dunnett’s t-test. SPSS software version 25 (IBM), Image J and GraphPad Prism software version 7.0 (GraphPad Software, Inc.) were used for statistical analyses, and differences were considered significant at P<0.05.
Results
Dezocine played different roles on cell viability and migration in HepG2 and Hep 3B cells concentration-related
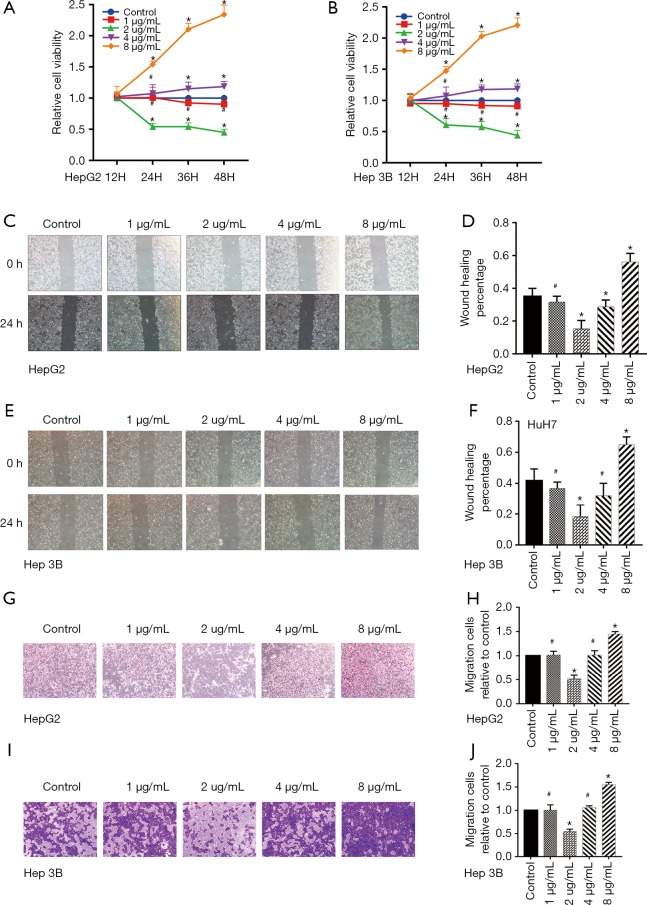
CCK8 assay was used to value the effect of dezocine (0, 1, 2, 4, 8 µg/mL) on cell viability in HepG2 and Hep 3B cells. As shown in Figure 1A,B, cell viability was decreased in the concentration of 2 µg/mL, while increased in 8 µg/mL. To evaluate the effects of dezocine on liver cancer cell migration wound healing assays and transwell experiments were performed. As shown in Figure 1C,D,E,F,G,H,I,J, dezocine treatment effectively decreased wound closure rate and the number of cells in transwell experiments 2 µg/mL compared with the control group while increased in 8 µg/mL in HepG2 and Hep 3B cells. The above results revealed that dezocine informed different effects on cell growth and migration in liver cancer. Thus, 2 and 8 µg/mL dezocine were selected in the following in vitro experiments to found the mechanism behind it.
Figure 1.
Dezocine played concentration-related opposite roles on cell viability and migration in HepG2 and Hep 3B cells. Liver cancer Cells were incubated with Dezocine (0, 1, 2,4, 8 μg/mL) for 24 h. The cell viability was measured by CCK8 assay, cell migrate ability were measured by Wound healing and transwell assay. (A,B) Cell viability of HepG2 and Hep 3B cells in different concentrations of dezocine. (C,D,E,F) Cell migrate ability of HepG2 and Hep 3B cells in different concentrations of dezocine indicated by wound healing assay (scale bar: 200 µm). (G,H,I,J) Cell migrate ability of HepG2 and Hep 3B cells in different concentrations of dezocine indicated by transwell assay (cells were stained with crystal violet, scale bar: 200 µm). Data are represented as means ± SEM, n=3. *, P<0.05 vs. cells treated without dezocine; #P>0.1 vs. cells treated without dezocine.
Dezocine has diverse effects on aerobic glycolysis of liver cancer cells at different concentrations
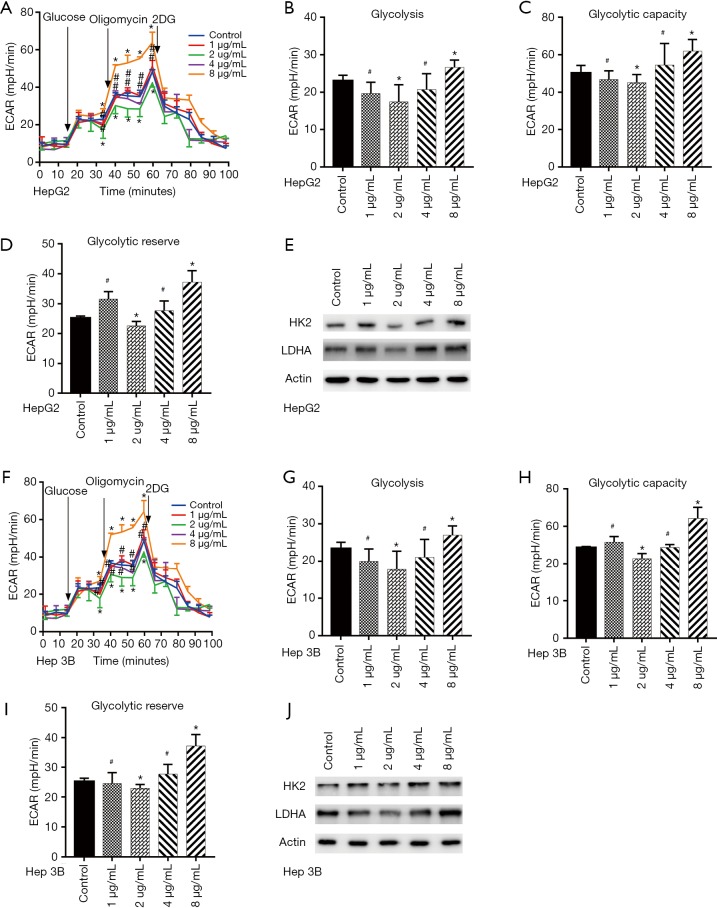
Having learned that glycolysis is the main energy provider for tumor metastasis, we then set to explore whether dezocine effected on cell mobility by modulating glycolysis. As shown in Figure 2A,B,C,D, dezocine treatment effectively modified Extracellular Acidification Rate (ECAR) (Figure 2A), cells glycolysis (Figure 2B), glycolysis capacity (Figure 2C) and glycolysis reserve of HepG2 cells. Further investigation exhibited that the expression of crucial glycolytic enzymes HK2 and LDHA in HepG2 cells harmony with the same trends as cell glycolysis which also regulated by dezocine (Figure 2E). Meanwhile, dezocine showed the same effects on Hep 3B cells exactly (Figure 2F,G,H,I,J) There is above results suggested that dezocine may effect on cell mobility by modifying glycolysis in liver cancer cells.
Figure 2.
Dezocine has diverse effects on aerobic glycolysis of liver cancer cells at different concentrations. (A) Dezocine treatment modified extracellular acidification rate (ECAR) of HepG2. (B) Cells glycolysis of HepG2 cells treated by dezocine. (C) Glycolysis capacity of HepG2 cells treated by dezocine. (D) Glycolysis reserve of HepG2 cells treated by dezocine. (E) Expression of HK2 and LDHA in HepG2 cells treated by dezocine. (F) Dezocine treatment modified ECAR of Hep 3B cells. (G) Cells glycolysis of Hep 3B cell treated by dezocine. (H) Glycolysis capacity of Hep 3B cell treated by dezocine. (I) Glycolysis reserve of Hep 3B cells treated by dezocine. (J) Expression of HK2 and LDHA in Hep 3B cells treated by dezocine. Data are represented as means ± SEM, n=3. *, P<0.05 vs. cells treated without dezocine; #P>0.1 vs. cells treated without dezocine.
Akt1/GSK 3β pathway share the same trend of cell mobility and glycolysis while dezocine-treated
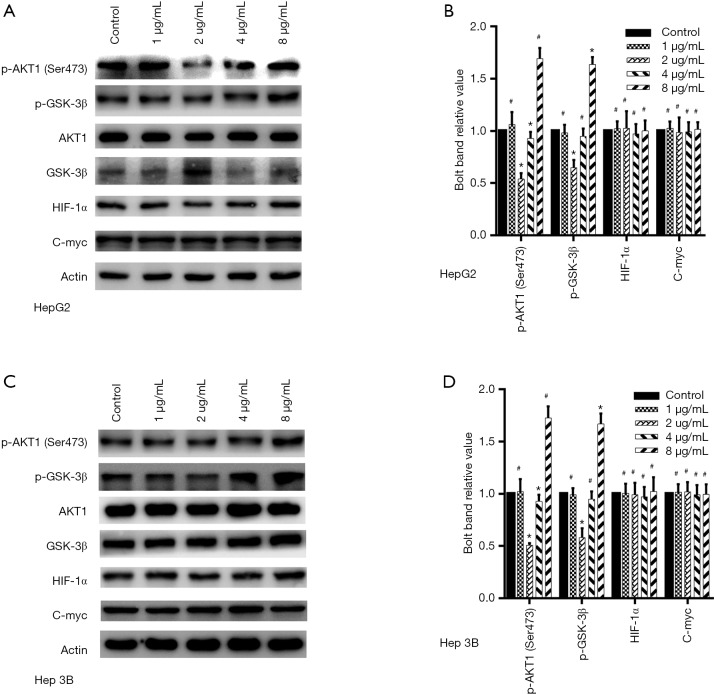
According to previous studies, Akt1, HIF-1α, C-myc proteins associate with induced glycolysis and reduced gluconeogenesis in cancer cells, so we detected which of above proteins involved in the effect of dezocine on liver cancer cells. The activation of Akt1was changed while exposed by dezocine while HIF-1α and C-myc show no difference (Figure 3). Serine/threonine kinase 1 (Akt1)-glycogen synthase kinase-3β (GSK-3β) pathway regulates the hepatic gene transcription for glucose metabolism, such as GLUT1 and HK2 (13,14). The Akt1/GSK-3β pathway activation has been associated with induced glycolysis. We examed the procession and activation of Akt1/GSK-3β and found the activation of Akt1/GSK-3β changed in the same way as liver cancer glycolysis. The phosphorylation of Akt1 and GSK-3β inhibited in 2 µg/mL and activated in 8 µg/mL (Figure 3).
Figure 3.
Changes of Akt1/GSK-3β pathway treated by dezocine at different concentrations in protein level. (A,B) GSK-3β and Akt1 phosphorylation in HePG2 cells shared diverse changes after being treated by dezocine in 2 and 8 µg/mL, while HIF-1α and C-myc didn’t have statistically significant difference. (C,D) GSK-3β and Akt1 phosphorylation in Hep 3B cells shared diverse changes after being treated by dezocine in 2 and 8 µg/mL, while HIF-1α and C-myc didn’t have statistically significant difference. Data are represented as means ± SEM, n=3. *, P<0.05 vs. cells treated without dezocine; #P>0.1 vs. cells treated without dezocine.
Dezocine regulated HepG2 and Hep 3B cells glycolysis targeting Akt1/GSK 3β pathway
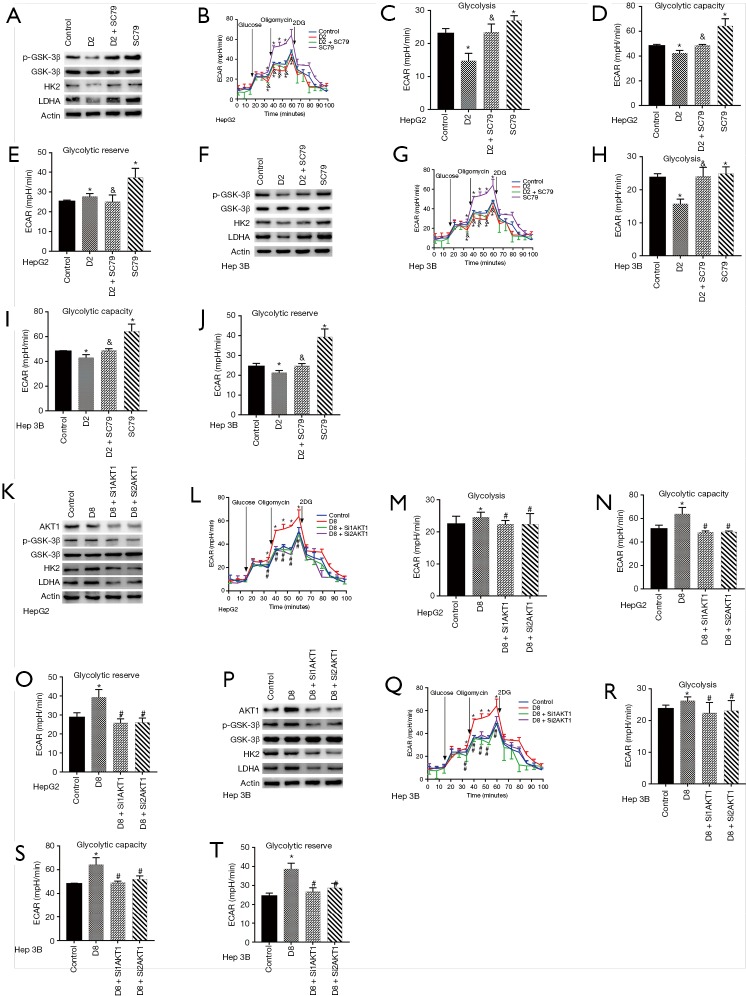
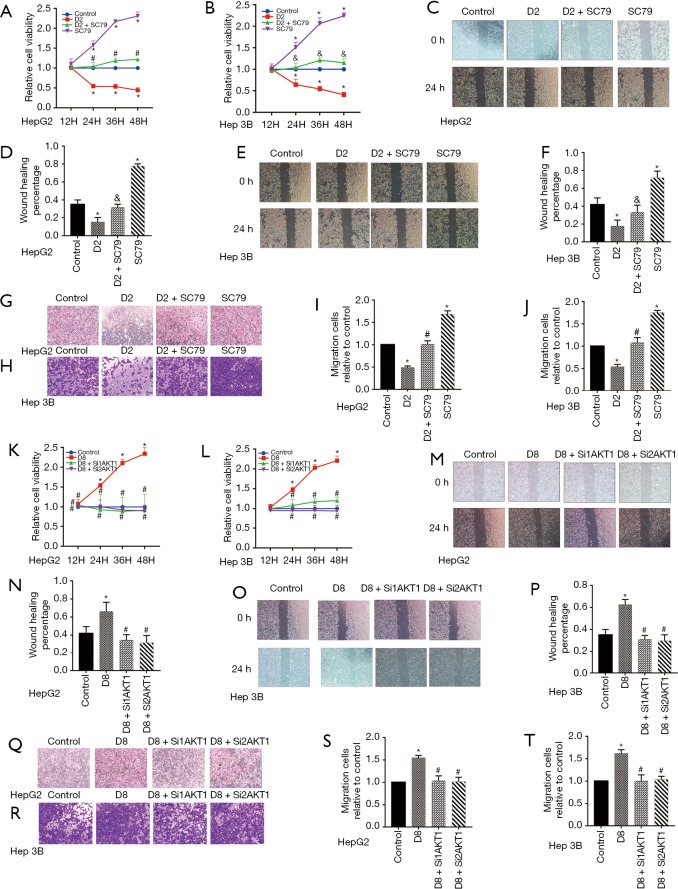
We altered the activity of the Akt1/GSK-3β signaling pathway to investigate whether it involves dezocine modulation of the activity of liver tumor cells glycolysis. SC79, Agonist of Akt, reversed the inhibition of dezocine on GSK-3β phosphorylation also the HK2 and LDHA expression in HepG2 and Hep 3B cells (Figure 4). Moreover, SC79 reversed the downgraded glycolysis of HepG2 and Hep 3B cells induced by dezocine (Figure 4B,C,D,E,G,H,I,J). On the other hand, the activated GSK and overexpression of HK2, LDHA did no longer reappear in the concentration of 8 µg/mL dezocine, when Akt1 was silenced by siRNA (Figure 4K,P). There was also no intense glycolysis in the concentration of 8 µg/mL dezocine as Akt1 was silenced (Figure 4L,M,N,O,Q,R,S,T).
Figure 4.
Dezocine regulated HepG2 and Hep 3B cells glycolysis targeting Akt1/GSK-3β pathway. (A) The activation of GSK-3β and expression of HK2, LDHA in HepG2 cells were inhibited in 2 µg/mL, and be reversed by Akt agonist SC79. (B) The ECAR of HepG2 cells was reduced in 2 µg/mL, then recovered after treated by SC79. (C) Cells glycolysis of HepG2 cells treated by dezocine in 2 µg/mL and by SC79. (D) Glycolysis capacity of HepG2 cells treated by dezocine in 2 µg/mL and by SC79. (E) Glycolysis reserve of HepG2 and Hep 3B cells treated by dezocine in 2 µg/mL and by SC79. (F,G,H,I,J) The activation of GSK-3β and expression of HK2, LDHA, ECAR, cells glycolysis, glycolysis capacity and glycolysis reserve in Hep 3B cells were inhibited in 2 µg/mL, and be reversed by Akt agonist SC79. (K) The Akt1/GSK-3β pathway and the expression of HK2 and LDHA in HepG2 cells was upregulated in 8 µg/mL and was attenuated after SiRNA treating. (L) The ECAR of HepG2 cells was increased in 8 µg/mL, then recovered after treated by SiRNA. (M) Cells glycolysis of HepG2 cells treated by dezocine in 8 µg/mL and by SiRNA. (N) Glycolysis capacity of HepG2 cells treated by dezocine in 8 µg/mL and by SiRNA. (O) Glycolysis capacity of HepG2 and Hep 3B cells treated by dezocine in 8 µg/mL and by SiRNA. (P,Q,R,S,T) The activation of GSK-3β and expression of HK2, LDHA, ECAR, cells glycolysis, glycolysis capacity and glycolysis reserve in Hep 3B cells treated by dezocine in 8 µg/mL and by SiRNA. Data are represented as means ± SEM, n=3. *, P<0.05 vs. cells treated without dezocine; #, P>0.1 vs. cells treated without dezocine; &, P<0.05 vs. cells treated by SC79.
Dezocine regulated HepG2 and Hep 3B cells viability and migration targeting Akt1/GSK 3β pathway
Not only glycolysis, but cancer cell biological characteristics were also reversed by raise or silence Akt1 protein. Cancer cell activity could not be suppressed by dezocine in the concentration of 2 µg/mL (Figure 5A,B), as well as the wound closure rate and trans cell numbers showing a consistent trend (Figure 5C,D,E,F,G,H,I,J). The AKT silence made dezocine in the concentration of 8 µg/mL can not to upregulate cell activity (Figure 5K,L) or promote the migration of tumor cells (Figure 5M,N,O,P,Q,R,S,T).
Figure 5.
Dezocine regulated HepG2 and Hep 3B cells viability and migration targeting Akt1/GSK 3β pathway. (A,B) Cell viability of HepG2 and Hep 3B cells in Medium containing dezocine of 2 µg/mL and Akt agonist SC79. (C,D,E,F) Cell migrate ability of HepG2 and Hep 3B cells treated by dezocine of 2 µg/mL and Akt agonist SC79 by wound healing assay (scale bar: 200 µm). (G,H,I,J) Cell migrate ability of HepG2 and Hep 3B cells treated by dezocine of 2 µg/mL and Akt agonist SC79 by transwell (cells were stained with crystal violet, scale bar: 200 µm). (K,L) Cell viability of HepG2 and Hep 3B cells treated with dezocine of 8 µg/mL and SiRNA. (M,N,O,P) Cell migrate ability of HepG2 and Hep 3B cells treated with dezocine of 8 µg/mL and SiRNA by wound healing assay (scale bar: 200 µm). (Q,R,S,T) Cell migrate ability of HepG2 and Hep 3B cells treated with dezocine of 8 µg/mL and SiRNA by transwell (cells were stained with crystal violet, scale bar: 200 µm). Data are represented as means ± SEM, n=3; *, P<0.05 vs. cells treated without dezocine; #, P>0.1 vs. cells treated without dezocine; &, P<0.05 vs. cells treated by SC79.
Conclusions
In the present study, we found that dezocine Shows opposed roles on cell viability and migration in liver cancer cell line HepG2 and Hep 3B in a concentration-dependent manner. The contrary effects of dezocine may be achieved by targeting the Akt1/GSK-3β pathway, then modifying the glycolysis.
Cancer cell metabolism is performed as an incremental uptake and transform of glucose to lactate via glycolysis. Changes in metabolism play an important role in cancer cell proliferation and growth (15,16). It was reported that GSK-3β was involved in aerobic glycolysis (17,18). Moreover, GSK-3β activity is modulated by the phosphorylation status of Ser9. Akt1 is a key protein kinase that regulates GSK-3β phosphorylation at Ser9. Akt1 attenuates GSK-3β activity via the upregulation of GSK-3β phosphorylation at Ser9. The present study indicated that dezocine could regulate the Akt1/GSK-3β pathway, modify the expressions of glycolytic enzymes such as HK2 and LDHA (19,20), change HepG2 and Hep 3B cells glycolysis, thus adjust HepG2 and Hep 3B cells viability and migration.
Due to the “ceiling effect” of respiratory depression and the non-addictiveness (3,4,21), the consumption of dezocine is increasing quickly in the perioperative period in China for security and comfort reasons (2). It is recognized that dezocine has the same analgesic effect as morphine, while less effect on inhibiting respiration (9,21). So dezocine is safer in the perioperative period, especially intraoperative analgesia. Clinical studies found that dezocine can prevent etomidate-induced myoclonus (22), prevention of postoperative catheter-related bladder discomfort (3,23). More and more new features of dezocine have been discovered in clinical. Recent studies have found that dezocine inhibits the norepinephrine (NET) and serotonin transporters (SERT) and opioid sigma-1receptor. Given the complexity of the molecular mechanism, the effect of dezocine on tumor cells need to be studied.
However, some limitations should be noted in this study. First, the present study was carried out only in vitro, and the effect of dezocine should be further confirmed by studies using in vivo systems. Second, we only used a single Human liver cancer cell line, more kinds of cancer cells should be employed in future studies. Third, the exact mechanism by which dezocine playing different roles on Akt1/GSK-3β single pathway should be further explored.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors want to thank Yangzi River Pharmaceuticals Group (Taizhou, Jiangsu, China) for providing dezocine.
Funding: This work was supported by the National Key Research and Development Program of China (No: 2018YFC2001904 CHM), the National Natural Science Foundation of China (No: 81873948 CHM, No: 81871591 WKC) and by the Youth Program of National Natural Foundation of China (No: 81901999 QCW).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the animal welfare and ethics group of the Department of Laboratory Animal Science of Fudan University (No. 201902027S).
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.28). The authors have no conflicts of interest to declare.
References
- 1.Fragen RJ, Caldwell N. Comparison of dezocine (WY 16, 225) and meperidine as postoperative analgesics. Anesth Analg 1978;57:563-6. 10.1213/00000539-197857050-00010 [DOI] [PubMed] [Google Scholar]
- 2.Sun Q, Zhou W, Wu B, et al. Dezocine: a novel drug to prevent fentanyl-induced cough during general anesthesia induction? J Anesth 2012;26:470. 10.1007/s00540-011-1318-x [DOI] [PubMed] [Google Scholar]
- 3.Gal TJ, DiFazio CA. Ventilatory and Analgesic Effects of Dezocine in Humans. Anesthesiology 1984;61:716-22. 10.1097/00000542-198412000-00015 [DOI] [PubMed] [Google Scholar]
- 4.Romagnoli A, Keats AS. Ceiling respiratory depression by dezocine. Clin Pharmacol Ther 1984;35:367-73. 10.1038/clpt.1984.45 [DOI] [PubMed] [Google Scholar]
- 5.Wu FX, Babazada H, Gao H, et al. Dezocine Alleviates Morphine-Induced Dependence in Rats. Anesth Analg 2019;128:1328-35. 10.1213/ANE.0000000000003365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 2019;394:1807-15. 10.1016/S0140-6736(19)32313-X [DOI] [PubMed] [Google Scholar]
- 7.Maher DP, Walia D, Heller NM. Morphine decreases the function of primary human natural killer cells by both TLR4 and opioid receptor signaling. Brain Behav Immun 2020;83:298-302. 10.1016/j.bbi.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 8.Singleton PA, Moss J, Karp DD, et al. The mu opioid receptor: A new target for cancer therapy? Cancer 2015;121:2681-8. 10.1002/cncr.29460 [DOI] [PubMed] [Google Scholar]
- 9.Liu R, Huang XP, Yeliseev A, et al. Novel molecular targets of dezocine and their clinical implications. Anesthesiology 2014;120:714-23. 10.1097/ALN.0000000000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wall T, Sherwin A, Ma D, et al. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth 2019;123:135-50. 10.1016/j.bja.2019.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis 2018;35:347-58. 10.1007/s10585-017-9862-x [DOI] [PubMed] [Google Scholar]
- 12.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today 2008;13:268-74. 10.1016/j.drudis.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 13.Luo W, Ai L, Wang BF, et al. High glucose inhibits myogenesis and induces insulin resistance by down-regulating AKT signaling. Biomed Pharmacother 2019;120:109498. 10.1016/j.biopha.2019.109498 [DOI] [PubMed] [Google Scholar]
- 14.Gao J, He X, Ma Y, et al. Chlorogenic Acid Targeting of the AKT PH Domain Activates AKT/GSK3β/FOXO1 Signaling and Improves Glucose Metabolism. Nutrients 2018. doi: . 10.3390/nu10101366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bechmann LP, Hannivoort RA, Gerken G, et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 2012;56:952-64. 10.1016/j.jhep.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Qiao Y, Wu Q, et al. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun 2017;8:15280. 10.1038/ncomms15280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh S, Kim H, Nam K, et al. Silencing of Glut1 induces chemoresistance via modulation of Akt/GSK-3β/β-catenin/survivin signaling pathway in breast cancer cells. Arch Biochem Biophys 2017;636:110-22. 10.1016/j.abb.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Lien EC, Lyssiotis CA, Juvekar A, et al. Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat Cell Biol 2016;18:572-8. 10.1038/ncb3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis J, Shaw LM. Insulin Receptor Substrate 2-mediated Phosphatidylinositol 3-kinase Signaling Selectively Inhibits Glycogen Synthase Kinase 3β to Regulate Aerobic Glycolysis. J Biol Chem 2014;289:18603-13. 10.1074/jbc.M114.564070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An J, Zhang Y, He J, et al. Lactate dehydrogenase A promotes the invasion and proliferation of pituitary adenoma. Sci Rep 2017;7:4734. 10.1038/s41598-017-04366-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YH, Chai JR, Xu XJ, et al. Pharmacological Characterization of Dezocine, a Potent Analgesic Acting as a κ Partial Agonist and µ Partial Agonist. Sci Rep 2018;8:14087. 10.1038/s41598-018-32568-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Yang Y, Zhou C, et al. Using dezocine to prevent etomidate-induced myoclonus: a meta-analysis of randomized trials. Drug Des Devel Ther 2017;11:2163-70. 10.2147/DDDT.S137464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang GF, Guo J, Qiu LL, et al. Effects of dezocine for the prevention of postoperative catheter-related bladder discomfort: a prospective randomized trial. Drug Des Devel Ther. 2019;13:1281-8. 10.2147/DDDT.S199897 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as