Abstract
Background
Comorbidity among cancer patients is prevalent and influential to prognosis after operation. Limited data are available on comorbidity evaluations in patients with intrahepatic cholangiocarcinoma (ICC). This study aimed to assess the comorbidity distribution in ICC patients and to adapt the Charlson Comorbidity Index (CCI) or the age-adjusted CCI (ACCI) for survival prediction.
Methods
The study cohort included 268 ICC patients treated with curative surgery from January 2000 to December 2007 at the Department of Liver Surgery, Zhongshan Hospital. The association between the comorbidity index and overall survival (OS) or disease-free survival (DFS). was analyzed by the Kaplan-Meier method. Multivariable analysis was established to select the determinant parameters.
Results
Major comorbid conditions of ICC patients included liver disease, hypertension, diabetes and ulcer. The median follow-up time was 25.5 months in the whole data set. Among the entire cohort, the 1-, 3- and 5-year OS rates were 55.3%, 26.0% and 15.6%, respectively. In multivariate analysis, the ACCI correlated with OS, and higher scores were associated with poorer prognosis (hazard ratio =1.134, 95% confidence interval: 1.015–1.267 and P value =0.026). CCI was not an independent predictive factor for OS or DFS.
Conclusions
In contrast to CCI, ACCI was a more promising model to accurately predict OS in ICC patients who underwent liver resection. Further research should be focused on the impact of comorbidity therapies.
Keywords: Intrahepatic cholangiocarcinoma (ICC), comorbidity, age-adjusted Charlson Comorbidity Index (ACCI), survival
Introduction
Intrahepatic cholangiocarcinoma (ICC), arising from the epithelial cells of the secondary bile duct and its branch in the liver, is the second most common primary liver malignancy in humans and accounts for up to 15% of primary liver cancer cases, next to hepatocellular carcinoma (HCC) (1-3). Over the past few decades, there has been a rapid uptrend of the incidence of ICC worldwide (4,5). Due to its high mortality and the swift progression of the tumor, therapies for ICC remain deficient (6). Surgical resection is still the mainstay for treatment and provides curative opportunity (3,5). In 2011, our institution proposed an outcome study based on a massive cohort of ICC patients who underwent resection. Specifically, the median survival was only 17.6 months (7). Such poor survival may be contributed by multiple factors.
ICC comprises different morphological features and molecular subsets. Determinant factors, including C-reactive protein (CRP), immune infiltrating condition and pathological characteristics, such as multiple lesions, tumor budding and vascular invasion, have been proven to be highly associated with outcomes after resection (8-11).
Comorbidities are chronic conditions that impact patients’ life quality, especially in long-term postoperative recovery. The management of comorbidities in cancer treatment is crucial to physicians. Recent studies have demonstrated the strong influence of comorbidities on survival after surgery in different kinds of solid neoplasms, including vulvar cancer, colorectal cancer and breast cancer (12-14). The Charlson Comorbidity Index (CCI), first proposed in 1984 by reviewing hospital charts, managed to account for the influence of a patients’ comorbidity condition in longitudinal studies (15). Since age has been subsequently determined to be correlated with prognosis, Charlson et al. modified the scoring system with the addition of patients’ age in 1994. The age-adjusted CCI (ACCI) incorporates the age as a correction variable of the final score by adding 1 point for every decade over 40 years old (16). Both CCI and ACCI have been widely validated in surgical and nonsurgical settings (17-19). Better understanding of comorbidity can promote the recognition of prognostic implications to malignancies. Nevertheless, the role of comorbidities in ICC has not yet been evaluated. Moreover, whether CCI or ACCI shows predictive performance in ICC patients needs further verification.
In the present study, we sought to assess the diversity and incidence of comorbidities in ICC patients through detailed history. We performed a cohort study to evaluate the prognostic capacities of comorbidities, and CCI and ACCI were calculated and stratified to predict the survival of patients after curative resections.
Methods
Data set
A total of 283 ICC patients who underwent liver resection between January 2000 and December 2007 at the Department of Liver Surgery, Zhongshan Hospital, Fudan University, were prospectively collected. The inclusion criteria were as follows: no preoperative anticancer therapy; without other malignancies; and diagnosed with ICC histopathologically. Patients diagnosed as HCC or combined hepatocellular-cholangiocarcinoma or hilar or extrahepatic cholangiocarcinoma, those with hepatic encephalopathy at the time of surgery, those with perioperative mortality and those with deficient follow-up information or surgery records were excluded from our study cohort. Ultimately, our data set selected 268 specimens. The study was approved by the institutional review board of Zhongshan Hospital and complied with the standards of the Declaration of Helsinki and current ethical guidelines.
Diagnosis and follow-up
Detailed history, complete physical examination and accessory tests were used for preoperative diagnosis. Blood was drawn from the patients for determination of the levels of platelets, hepatitis B surface antigen (HBsAg), anti-hepatitis C virus (HCV) antibody, serum albumin (ALB), total bilirubin (TB), alanine aminotransferase (ALT), aspartate transaminase (AST), γ-glutamyl transferase (GGT), α-fetoprotein (AFP), carbohydrate 19-9 (CA19-9), and carcinoembryonic antigen (CEA). Imagining examinations included abdominal ultrasound, contrast-enhanced computed tomography (CT), magnetic resonance imaging, and positron emission tomography (PET), if needed. Confirmative diagnosis relied on pathological results of the resected tissue, assessed by authoritative experts.
The study was censored on April 2012. A standardized follow-up protocol was adopted for all patients. Patients had follow-up visits with blood tests for liver function and tumor markers every 3 months. Radiological examinations included computed tomography or abdominal magnetic resonance imaging scans every 6 months for the first 2 years. The end-points of the study were overall survival (OS) and disease-free survival (DFS). OS was defined as the interval between the date of surgery and the date of patient death or the last follow-up. DFS was defined as the time from the date of resection to the date of recurrence, metastasis, or last follow-up.
Comorbidities and ACCI
Patients’ comorbidities based on detailed history were assessed rigorously on the basis of the disease definition (15). CCI incorporated different medical conditions, with each weighted from 1 to 6 points. Patients’ age was sorted and counted according to ACCI. Both the CCI and ACCI scores were then calculated by the appropriate formula (Table 1).
Table 1. Different weights assigned for specific conditions in the age adjusted Charlson Comorbidity Index and patients’ distribution.
| Scores | Conditions | N=268, n (%) |
|---|---|---|
| Assigned weights for disease | ||
| 1 | Myocardial infarction | 0 (0.0) |
| Congestive heart failure | 1 (0.4) | |
| Peripheral vascular disease | 0 (0.0) | |
| Dementia | 2 (0.7) | |
| Cerebrovascular disease | 3 (1.1) | |
| Chronic pulmonary disease | 4 (1.5) | |
| Ulcer disease | 12 (4.5) | |
| Diabetes | 13 (4.9) | |
| Hypertension | 36 (13.4) | |
| Mild liver disease | 83 (31.0) | |
| 2 | Moderate or severe renal disease | 0 (0.0) |
| Hemiplegia | 1 (0.4) | |
| Malignant lymphoma | 2 (0.7) | |
| Any tumor | 10 (3.7) | |
| 3 | Moderate or severe liver disease | 41 (15.3) |
| 6 | Metastatic solid tumor | 0 (0.0) |
| Acquired immune deficiency syndrome | 0 (0.0) | |
| Assigned weights for age | ||
| 1 | For each decade over age 40 years (up to 4 points) |
Statistical methods
Demographic, clinical and tumor characteristics are described as summary statistics and are presented as percentages for categorical variables and medians (ranges) for continuous variables. We evaluated categorical variables using the Pearson Chi-square test. The Mann-Whitney U test was employed to compare continuous variables. The Kaplan-Meier method and log-rank test were used to estimate OS and DFS. Univariable and multivariable analysis were established based on a logistic regression model.
Statistical inferences were two-sided, and a P value <0.05 was considered statistically significant. All the statistical tests were performed using SPSS 22.0 (SPSS, Chicago, IL)
Selection of most predictive comorbidities in ACCI
Data were analyzed using the Statistical Analysis System (SAS, Version 9.4). The models were limited to the 6 most predictive comorbidities in ICC patients after adjusting for age. Partial R-square values were calculated to appraise the independent proportion of explained variance within ACCI scores by each comorbidity included in the model. The 6 most predictive comorbidities accounted for the variance of ACCI scores.
Results
Comorbidity distributions in the data set
The distributions of different comorbidities are summarized in Table 1. Among 268 ICC patients, 83 were diagnosed with mild liver disease, which was the most common comorbidity (31%). In the group of 1-point comorbidities, hypertension, diabetes and ulcer disease ranked next to mild liver disease, with ratios of 13.4%, 4.9% and 4.5%, respectively. Among the comorbidities that scored 2 points, solid tumor history was found in 10 patients (3.7%). Meanwhile, 2 patients (0.7%) displayed cooccurrence of both malignant lymphoma and ICC. One patient (0.4%) had hemiplegia. Among the comorbidities that accounted for more than 2 points, moderate and severe liver diseases were declared in only 41 patients (15.3%).
Clinicopathological characteristics of patients
The demographic and clinicopathological data are shown in Table 2. The proportions of males and females were 60.4% and 39.6%, respectively. The median age of the data set was 55 years (range, 27–89 years). Among 268 patients, 85 patients were positive for HBsAg (31.7%). The median max-diameter of the tumor was 6.0 cm (range, 1.0–18.0). Most patients (n=150) had stage I ICC. The population ratios for stage II, III, and IVa were 16.8%, 3.0%, and 24.3%, respectively. Most nodules of ICC patients did not have complete capsules (87.1%). The median score of ACCI was 3 (range, 0–6). The distributions of ACCI were low (≤2), medium [3] and high (≥4) groups in 36.6%, 22.8%, and 40.7% of patients, respectively. Similar results for CCI are shown in Table 2.
Table 2. Demographic, clinical, and tumor characteristics of patients with intrahepatic cholangiocarcinoma.
| Patient demographics | Variables | N (%) |
|---|---|---|
| Gender | Female | 106 (39.6) |
| Male | 162 (60.4) | |
| Age (y) | Median (R) | 55 (27, 89) |
| HBsAg | − | 183 (68.3) |
| + | 85 (31.7) | |
| Anti-HCV | − | 257 (97.0) |
| + | 8 (3.0) | |
| TB (mg/dL) | <17 | 192 (71.6) |
| ≥17 | 76 (28.4) | |
| AST (U/L) | Median (R) | 28 (10, 246) |
| ALB (g/dL) | Median (R) | 44 (26, 57) |
| ALT (U/L) | <35 | 161 (60.3) |
| ≥35 | 106 (39.7) | |
| PT (s) | <13 | 244 (91.0) |
| ≥13 | 24 (9.0) | |
| AFP (ng/mL) | <20 | 227 (88.3) |
| ≥20 | 30 (11.7) | |
| CEA (μg/mL) | <5 | 191 (77.0) |
| ≥5 | 57 (23.0) | |
| CA19-9 (U/mL) | <37 | 88 (36.1) |
| ≥37 | 156 (63.9) | |
| CCI Score | Median (R) | 1 (0, 6) |
| 0 | 103 (38.4) | |
| 1 | 87 (32.5) | |
| ≥2 | 78 (29.1) | |
| ACCI Score | Median (R) | 3 (0, 6) |
| ≤2 | 98 (36.6) | |
| 3 | 61 (22.8) | |
| ≥4 | 109 (40.7) | |
| Max-diameter(cm) | Median (R) | 6.0 (1.0, 18.0) |
| Tumor number | Median (R) | 1 (1, 20) |
| Lymphoid metastasis | None | 206 (76.9) |
| Yes | 62 (23.1) | |
| Tumor capsule | None & partial | 229 (87.1) |
| Complete | 34 (12.9) | |
| Differentiation | I | 2 (1.0) |
| I–II, II | 118 (59.0) | |
| II–III, III | 79 (39.5) | |
| III–IV, IV | 1 (0.5) | |
| TNM | I | 150 (56.0) |
| II | 45 (16.8) | |
| III | 8 (3.0) | |
| IVa | 65 (24.3) | |
| MVI | None | 234 (87.3) |
| Yes | 34 (12.7) | |
| VI | None | 234 (87.3) |
| Yes | 34 (12.7) |
Values are presented as No. (%) or median (minimum, maximum). Median (R), median (range); HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; TB, total bilirubin; PT, prothrombin time; AST, aspartate transaminase; ALB, albumin; AFP, α-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate 19-9; ALT, alanine aminotransferase; CCI, Charlson Comorbidities Index; ACCI, age-adjusted Charlson Comorbidities Index; TNM, tumor node metastasis stage; VI, vascular invasion; MVI, micro vascular invasion.
The median follow-up time was 25.5 months (range, 1 to 134) in the whole data set. Among the entire cohort, 88.1% of the patients (236/268) developed a recurrence, and 81.0% of the patients (217/268) died during follow-up. The 1-, 3- and 5-year OS rates were 55.3%, 26.0% and 15.6%, respectively; the 1-, 3- and 5-year DFS rates were 43.5%, 18.7% and 10.8%, respectively.
CCI and survival
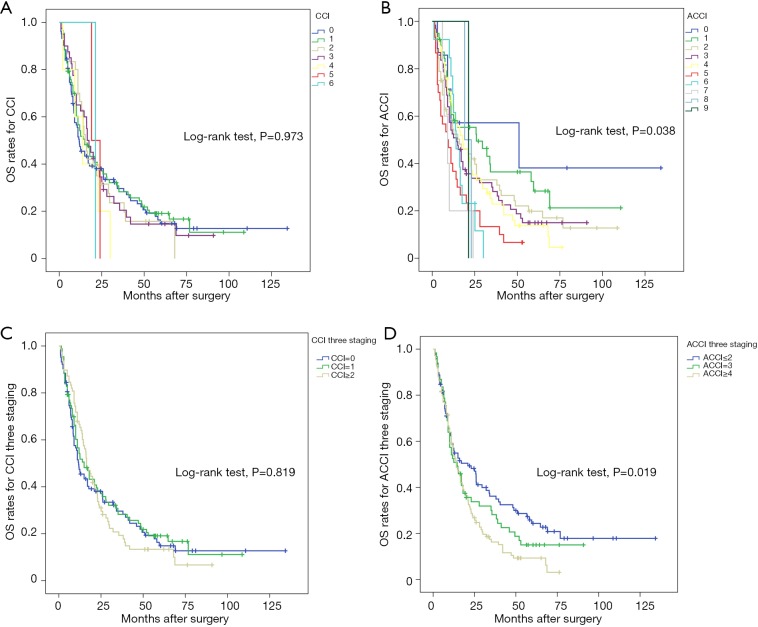
To investigate the capacities of the CCI and ACCI to predict prognosis, the 5-year OS and DFS rates according to the comorbidity index were estimated using Kaplan-Meier curves. In Figure 1, we evaluated the relationship between comorbidity index and OS with both continuous and categorical variables. When using continuous variables (Figure 1B), patients with higher ACCI scores showed increasing risk of worse OS (P=0.038). The median survival times in ACCI =0, 1, 2, 3 and 5 stratification were 50.9, 25.9, 16.0, 14.5 and 9.0 months, respectively. In contrast, there was no significant difference between CCI scores and OS times (P=0.973). From the prospective of categorical variables, three stages of ACCI also revealed efficacious prognostic performance, with 3-year OS rates =34.9%, 26.2%, 15.1% and 5-year OS rates =22.7%, 15.0%, 9.3% in the low, moderate and high groups (P=0.019).
Figure 1.
Kaplan-Meier curve estimates of overall survival according to (A) CCI, (B) ACCI, (C) CCI three staging and (D) ACCI three staging. Survival probability is plotted on the Y-axis against postoperative time on the X-axis. Different color stands for different index scores. ACCI, age-adjusted Charlson Comorbidity Index; CCI, Charlson Comorbidity Index.
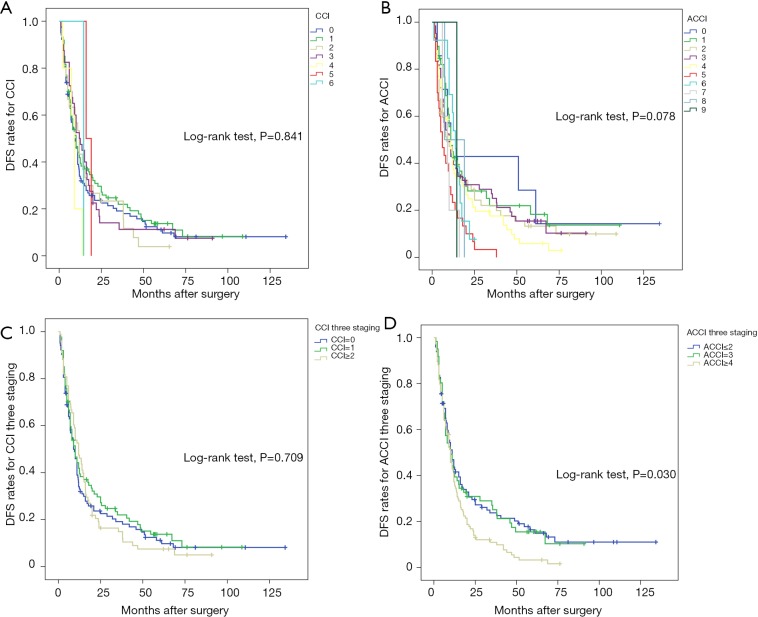
In Figure 2, we assessed DFS according to CCI or ACCI. No statistical significance was found in CCI or ACCI when considering continuous variables (P=0.841 and 0.078, respectively). Unlike CCI stratification, ACCI stratification displayed a strong influence on the DFS (P=0.030).
Figure 2.
Kaplan-Meier curve estimates of disease-free survival according to (A) CCI, (B) ACCI, (C) CCI three staging and (D) ACCI three staging. Survival probability is plotted on the Y-axis against postoperative time on the X-axis. Different color stands for different index scores. ACCI, age-adjusted Charlson Comorbidity Index; CCI, Charlson Comorbidity Index.
Predictive factors for OS and DFS
Utilizing a Cox proportional hazard model, we performed a multivariable analysis to define the predictive determinants (Table 3). For OS, CEA [hazard ratio (HR): 1.804, 95% CI: 1.133–2.874, P=0.013], CA19-9 (HR: 1.838, 95% CI: 1.219–2.771, P=0.004), max-diameter (HR: 1.078, 95% CI: 1.004–1.158, P=0.040), tumor differentiation (P=0.007), ACCI (HR: 1.134, 95% CI: 1.015–1.267, P=0.026) and ACCI classification (P=0.040) were determined to be independent prognostic factors. For DFS, the prognostic determinants were AFP (HR: 1.840, 95% CI: 1.042–3.249, P=0.035), max diameter (HR: 1.088, 95% CI: 1.019–1.163, P=0.012), micro vascular invasion (MVI) (HR: 7.374, 95% CI: 1.812–30.005, P=0.005), CEA (HR: 1.809, 95% CI: 1.161–2.819, P=0.009) and vascular invasion (VI) (HR: 0.632, 95% CI: 0.424–0.941, P=0.024). The multivariable analysis on DFS is shown in the Table S1.
Table 3. Cox proportional hazards regression model showing the multivariate analysis of variables with OS and DFS according to ACCI.
| Variable | OS | DFS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex (female/male) | 1.122 | 0.724–1.740 | 0.606 | 1.281 | 0.848–1.935 | 0.239 | |
| HBsAg (yes/no) | 0.651 | 0.426–0.994 | 0.047 | 0.691 | 0.469–1.017 | 0.061 | |
| Anti-HCV (yes/no) | 0.388 | 0.090–1.675 | 0.205 | 0.354 | 0.083–1.507 | 0.160 | |
| AFP (≥20/<20, ng/mL) | 1.710 | 0.932–3.139 | 0.083 | 1.840 | 1.042–3.249 | 0.035 | |
| CEA (≥5/<5, ng/mL) | 1.804 | 1.133–2.874 | 0.013 | 1.809 | 1.161–2.819 | 0.009 | |
| CA19-9 (≥37/<37, U/mL) | 1.838 | 1.219–2.771 | 0.004 | 1.155 | 0.791–1.686 | 0.456 | |
| ALT (≥35/<35, U/L) | 0.932 | 0.558–1.556 | 0.786 | 0.732 | 0.450–1.191 | 0.209 | |
| AST, U/L | 1.004 | 0.998–1.010 | 0.202 | 1.004 | 0.998–1.010 | 0.190 | |
| PT (≥13/<13, s) | 0.635 | 0.304–1.327 | 0.227 | 0.630 | 0.320–1.243 | 0.183 | |
| TB (≥17/<17, μmol/L) | 1.456 | 0.924–2.295 | 0.106 | 1.365 | 0.889–2.096 | 0.155 | |
| Lymphoid metastasis (yes/no) | 1.536 | 0.983–2.400 | 0.059 | 1.439 | 0.942–2.199 | 0.093 | |
| Tumor numbers | 0.984 | 0.892–1.086 | 0.751 | 0.973 | 0.874–1.084 | 0.620 | |
| Max diameter, cm | 1.078 | 1.004–1.158 | 0.040 | 1.088 | 1.019–1.163 | 0.012 | |
| VI | 1.038 | 0.692–1.556 | 0.857 | 0.632 | 0.424–0.941 | 0.024 | |
| MVI | 1.107 | 0.269–4.548 | 0.888 | 7.374 | 1.812–30.005 | 0.005 | |
| Capsule | 0.731 | 0.400–1.338 | 0.310 | 0.617 | 0.344–1.105 | 0.104 | |
| Tumor differentiation | 0.007 | 0.063 | |||||
| I vs. I–II, II | 1.816 | 0.119–27.656 | 0.668 | 0.691 | 0.047–10.081 | 0.787 | |
| I–II, II vs. II–III, III | 1.195 | 0.140–10.213 | 0.871 | 0.423 | 0.050–3.547 | 0.427 | |
| II–III, III vs. III–IV, IV | 2.308 | 0.261–20.386 | 0.452 | 0.669 | 0.078–5.753 | 0.715 | |
| ACCI | 1.134 | 1.015–1.267 | 0.026 | 1.069 | 0.964–1.186 | 0.205 | |
| ACCI 3 staging | 0.040 | 0.253 | |||||
| ≤2 vs. 3 | 0.706 | 0.462–1.078 | 0.107 | 0.793 | 0.535–1.175 | 0.247 | |
| 3 vs. ≥4 | 1.254 | 1.077–1.398 | 0.045 | 0.679 | 0.417–1.105 | 0.119 | |
OS, overall survival; DFS, disease free survival; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; TB, total bilirubin; PT, prothrombin time; AST, aspartate transaminase; ALB, albumin; AFP, α-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate 19-9; ALT, alanine aminotransferase; CCI, Charlson Comorbidities Index; ACCI, age-adjusted Charlson Comorbidities Index; TNM, tumor node metastasis stage; VI, vascular invasion; MVI, micro vascular invasion.
Table S1. Cox proportional hazards regression model showing the multivariate analysis of variables with OS and DFS according to CCI.
| Variable | OS | DFS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex (female/male) | 1.173 | 0.751–1.832 | 0.482 | 1.353 | 0.889–2.059 | 0.158 | |
| Age, years | 1.030 | 1.011–1.050 | 0.002 | 1.028 | 1.010–1.046 | 0.003 | |
| HBsAg (yes/no) | 0.927 | 0.554–1.549 | 0.771 | 1.009 | 0.631–1.614 | 0.971 | |
| Anti-HCV (yes/no) | 0.350 | 0.081–1.517 | 0.160 | 0.306 | 0.072–1.303 | 0.109 | |
| AFP (≥20/<20, ng/mL) | 1.674 | 0.913–3.072 | 0.096 | 1.793 | 1.015–3.166 | 0.044 | |
| CEA (≥5/<5, ng/mL) | 1.710 | 1.071–2.731 | 0.025 | 1.709 | 1.093–2.672 | 0.019 | |
| CA19-9 (≥37/<37, U/mL) | 1.820 | 1.202–2.757 | 0.005 | 1.150 | 0.782–1.690 | 0.477 | |
| ALT (≥35/<35, U/L) | 0.940 | 0.561–1.574 | 0.815 | 0.744 | 0.455–1.215 | 0.237 | |
| AST, U/L | 1.006 | 0.999–1.012 | 0.082 | 1.006 | 1.000–1.012 | 0.050 | |
| PT (≥13/<13, s) | 0.711 | 0.338–1.495 | 0.369 | 0.690 | 0.350–1.363 | 0.285 | |
| TB (≥17/<17, μmol/L) | 1.420 | 0.895–2.253 | 0.137 | 1.334 | 0.866–2.057 | 0.191 | |
| Lymphoid metastasis (yes/no) | 1.612 | 1.031–2.520 | 0.036 | 1.487 | 0.973–2.274 | 0.067 | |
| Tumor numbers | 0.980 | 0.890–1.080 | 0.686 | 0.971 | 0.874–1.079 | 0.589 | |
| Max diameter, cm | 1.092 | 1.015–1.175 | 0.018 | 1.106 | 1.033–1.185 | 0.004 | |
| VI | 0.980 | 0.645–1.488 | 0.924 | 0.592 | 0.395–0.889 | 0.011 | |
| MVI | 1.233 | 0.289–5.258 | 0.777 | 8.608 | 2.081–35.612 | 0.003 | |
| Capsule | 0.709 | 0.384–1.306 | 0.270 | 0.589 | 0.326–1.063 | 0.079 | |
| Tumor differentiation | 0.015 | 0.109 | |||||
| I vs. I–II, II | 0.996 | 0.062–15.894 | 0.997 | 0.361 | 0.024–5.517 | 0.464 | |
| I–II, II vs. II–III, III | 1.038 | 0.121–8.920 | 0.973 | 0.367 | 0.043–3.097 | 0.357 | |
| II–III, III vs. III–IV, IV | 1.909 | 0.215–16.956 | 0.562 | 0.553 | 0.064–4.784 | 0.590 | |
| CCI | 0.972 | 0.820–1.153 | 0.747 | 0.901 | 0.764–1.063 | 0.217 | |
| CCI 3 staging | 0.980 | 0.503 | |||||
| ≤2 vs. 3 | 1.002 | 0.594–1.692 | 0.993 | 1.304 | 0.788–2.159 | 0.302 | |
| 3 vs. ≥4 | 0.956 | 0.572–1.599 | 0.864 | 1.006 | 0.620–1.630 | 0.982 | |
OS, overall survival; DFS, disease free survival; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; TB, total bilirubin; PT, prothrombin time; AST, aspartate transaminase; ALB, albumin; AFP, α-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate 19-9; ALT, alanine aminotransferase; CCI, Charlson Comorbidities Index; TNM, tumor node metastasis stage; VI, vascular invasion; MVI, micro vascular invasion.
The 6 most predictive comorbidities in ACCI
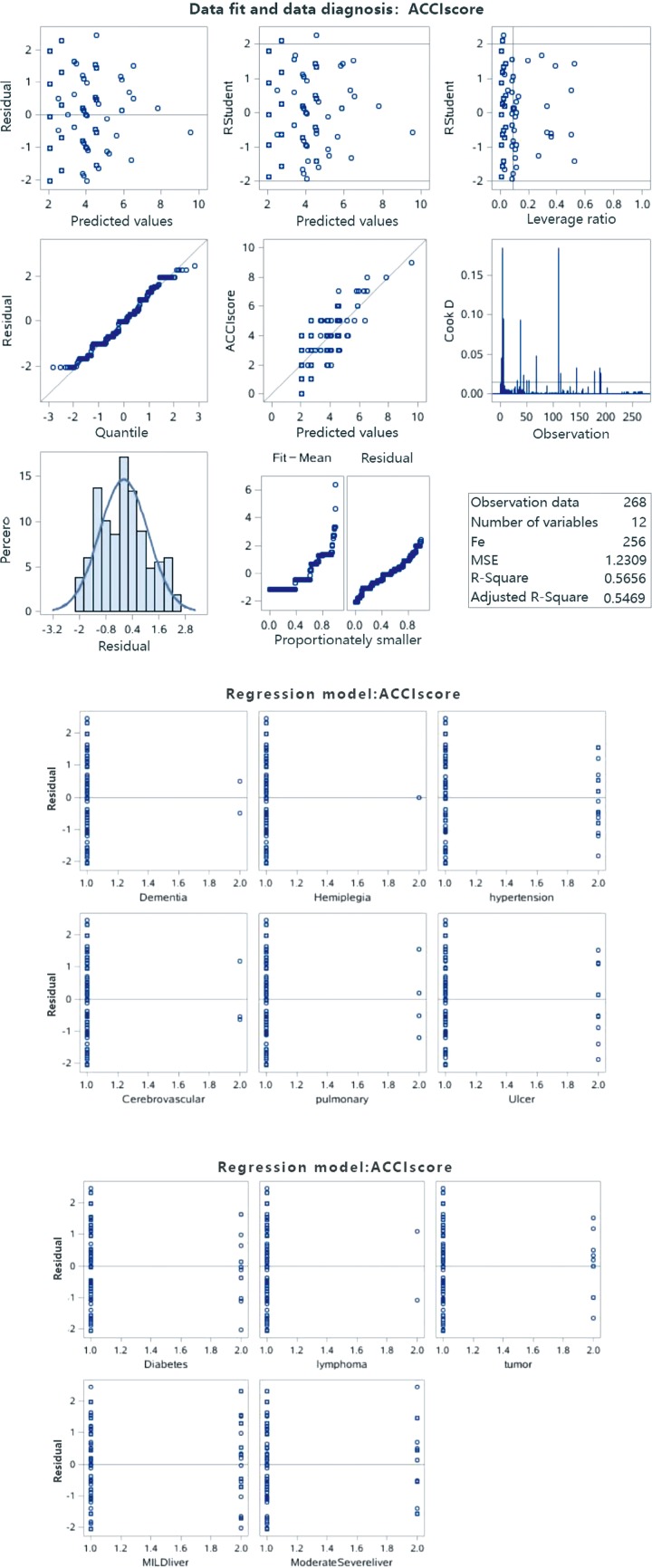
Table 4 revealed the results of the multivariate linear regression analysis and explained the variance (R2) of ACCI scores by the 6 most predictive comorbidities (explaining approximately 56.56%). The candidates were as follows: moderate or severe liver disease, ulcer, tumor, diabetes, mild liver disease and lymphoma. The calculation process is shown in the Figure S1.
Table 4. Explained variance (R2) of ACCI by the 6 most predictive factors.
| Order | Comorbidity | Partial R2 | R2 | C(p) | F | Pr > F |
|---|---|---|---|---|---|---|
| 1 | Moderate and severe liver disease | 0.2294 | 0.3941 | 104.474 | 98.82 | <0.001 |
| 2 | Ulcer | 0.0559 | 0.4499 | 73.4218 | 26.41 | <0.001 |
| 3 | Tumor | 0.0473 | 0.4973 | 47.4101 | 24.39 | <0.001 |
| 4 | Diabetes | 0.0288 | 0.5260 | 32.3989 | 15.65 | <0.001 |
| 5 | Mild liver disease | 0.0287 | 0.5548 | 17.4072 | 16.58 | <0.001 |
| 6 | lymphoma | 0.0108 | 0.5656 | 12.9937 | 6.39 | 0.0121 |
All models are adjusted for age. ACCI, age-adjusted Charlson Comorbidities Index.
Figure S1.
The calculation process for the multivariate liner regression analysis. The data results satisfy the condition of multiple linear regression: the homogeneity of independent normal variance. The 6 most predictive comorbidities explain approximately 56.56% of the variance (R2) of ACCI scores.
Discussion
Despite the development in surgical techniques, management after curative resection is a priority in cancer therapy, in which long-term comorbidity contributes greatly to survival. In our present study, we originally investigated the distribution of comorbidities in ICC patients treated with surgery and assessed the prognostic performance of ACCI. From Table 1, we clearly found that liver disease was the most common comorbidity, regardless of severity. In the CCI, the definition of liver disease mainly depended on cirrhosis, portal hypertension and a history of variceal bleeding (15). Therefore, the pathophysiological changes of the primary tumor could elucidate the high incidence of liver disease (20,21). Hypertension, diabetes and ulcer occupied the 2nd–4th major proportions, which was broadly consistent with recent epidemiological studies (22). In particular, gastrointestinal ulcer occurred more frequently in ICC patients than in other solid tumors. This phenomenon was mainly attributed to the derivative effects of portal hypertension and coagulation disorder (23).
CCI was initially developed to evaluate one-year mortality rates in medical care. However, along with the improvements of surgical techniques and postoperative management, the indication of CCI or ACCI expanded to appraise patients’ long-term survival after surgery (18,24,25). In our study, for the first time, CCI and ACCI were utilized to determine the influence of comorbidity in ICC. It turned out that ACCI performed well in predicting outcomes after ICC resection. The 5-year OS rates in the ACCI low, moderate and high groups were 22.7%, 15.0% and 9.3%, with P values =0.019 (Figure 1D). Interestingly, the Kaplan-Meier curve for continuous variables depicted a meticulous survival comparison (Figure 1B). In the ACCI =7, 8, 9 groups, OS rates decreased to 0% after one year of recovery. The low populations in these classifications, which deteriorated the diversity, may be the explanation for these results. However, the other stratifications had similar prognostic trends, in which survival time declined incrementally with higher ACCI scores. All these results highlighted the decisive role of comorbidity management for ICC patients. Timely and considerate treatments for preoperative comorbidities may bring about decent survival after resection.
Growing evidence has suggested that age carries a considerable weight in predicting the prognosis of patients with different cancers (26-28). Researchers obtained corresponding results in ICC patients. There was a prominent survival difference between the high ACCI score group and the low group. One positive finding was that patients without any comorbidities and who were under 40 years old had a 3-year OS of 57.1% and a 5-year OS of 38.1% (Figure 1D). The data visually underlined the potentially strong link between younger patients and better prognosis. Careful treatment of elderly individuals, more comprehensive screening and rapid surgical decision would be of great advantage in improving outcomes. In contrast to ACCI, CCI failed to show statistical significance in both OS and DFS (Figures 1C,2C). To some extent, adjusting age to CCI was a relatively profound method to evaluate the role of comorbidities.
In view of ACCI’s outstanding performance in the survival curve, we selected ACCI rather than CCI as one of the candidates in the multivariable analysis. As a result, CEA, CA19-9, max-diameter, tumor differentiation, ACCI and ACCI classification were indicative of poorer OS (Table 3). Consistently, preoperative CA19-9 and tumor size were also identified as independent predictive parameters in our previous study (10). Of note, a recent study from He et al. demonstrated that supplementing CEA to CA19-9 had a better effect for survival prediction (29). For DFS, the prognostic determinants were AFP, CEA, Max diameter, MVI and VI. Vascular invasion, known as the potential evidence of tumor cell metastasis, has recently been proven to be related to ICC recurrence (30). Based on the manifestation of serum markers, tumor morphology and ACCI, we achieved further recognition of ICC from the clinical and hematological prospective.
The SAS analysis results revealed that moderate or severe liver disease played the most decisive role among all the comorbidities. This was possibly caused by the potential relationship between primary malignancy and its pathophysiological changes in the liver. The subsequent candidates included serious diseases, such as solid tumor or lymphoma, and widely known conditions, such as ulcer or diabetes. Further studies should explore more specific comorbidity therapies, such as drug intake, or habit changes and patients’ compliance to the treatments.
Although our study covered a large sample of an ICC cohort, it had limitations. First, it was a retrospective study, which inevitably brought about selection bias. Second, our data were derived from a single center; further validation with additional data sets is needed. Third, the diversity of multiple comorbidities was insufficient. Fourth, further studies should be proposed to pursue the association between specific comorbidities and treatment benefits.
Conclusions
Compared with the CCI, the ACCI is a more promising model to accurately predict OS in ICC patients treated with curative resection. The results in our study highlight the importance of comorbidities in ICC patients, including liver disease, hypertension, diabetes and ulcer. Future research should focus on the impact of comorbidity therapy.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank the study participants and the research staff for their contribution to this project.
Funding: This study was founded by the Grants from the National Natural Science Foundation of China (Grant No. 81472674, 81773067), Shu Guang project of Shanghai Municipal Education Commission and Shanghai Education Development Foundation (Grant No. 13SG04).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board of Zhongshan Hospital (No. Y2017-279) and complied with the standards of the declaration of Helsinki and current ethical guidelines.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.23). The authors have no conflicts of interest to declare.
References
- 1.Njei B. Changing pattern of epidemiology in intrahepatic cholangiocarcinoma. Hepatology 2014;60:1107-8. 10.1002/hep.26958 [DOI] [PubMed] [Google Scholar]
- 2.Sia D, Villanueva A, Friedman SL, et al. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017;152:745-61. 10.1053/j.gastro.2016.11.048 [DOI] [PubMed] [Google Scholar]
- 3.Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95-111. 10.1038/nrclinonc.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Yang T, Wu M, et al. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett 2016;379:198-205. 10.1016/j.canlet.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 5.Sirica AE, Gores GJ, Groopman JD, et al. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology 2019;69:1803-15. 10.1002/hep.30289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moeini A, Sia D, Bardeesy N, et al. Molecular Pathogenesis and Targeted Therapies for Intrahepatic Cholangiocarcinoma. Clin Cancer Res 2016;22:291-300. 10.1158/1078-0432.CCR-14-3296 [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Zeng ZC, Tang ZY, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol 2011;22:1644-52. 10.1093/annonc/mdq650 [DOI] [PubMed] [Google Scholar]
- 8.Yeh YC, Lei HJ, Chen MH, et al. C-Reactive Protein (CRP) is a Promising Diagnostic Immunohistochemical Marker for Intrahepatic Cholangiocarcinoma and is Associated With Better Prognosis. Am J Surg Pathol 2017;41:1630-41. 10.1097/PAS.0000000000000957 [DOI] [PubMed] [Google Scholar]
- 9.Chan KM, Tsai CY, Yeh CN, et al. Characterization of intrahepatic cholangiocarcinoma after curative resection: outcome, prognostic factor, and recurrence. BMC Gastroenterol 2018;18:180. 10.1186/s12876-018-0912-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian MX, Zhou YF, Qu WF, et al. Histopathology-based immunoscore predicts recurrence for intrahepatic cholangiocarcinoma after hepatectomy. Cancer Immunol Immunother 2019;68:1369-78. 10.1007/s00262-019-02371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M, Yamauchi N, Ushiku T, et al. Tumor Budding in Intrahepatic Cholangiocarcinoma: A Predictor of Postsurgery Outcomes. Am J Surg Pathol 2019;43:1180-90. 10.1097/PAS.0000000000001332 [DOI] [PubMed] [Google Scholar]
- 12.Boakye D, Rillmann B, Walter V, et al. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: A systematic review and meta-analysis. Cancer Treat Rev 2018;64:30-9. 10.1016/j.ctrv.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Minicozzi P, Van Eycken L, Molinie F, et al. Comorbidities, age and period of diagnosis influence treatment and outcomes in early breast cancer. Int J Cancer 2019;144:2118-27. 10.1002/ijc.31974 [DOI] [PubMed] [Google Scholar]
- 14.Di Donato V, Page Z, Bracchi C, et al. The age-adjusted Charlson comorbidity index as a predictor of survival in surgically treated vulvar cancer patients. J Gynecol Oncol 2019;30:e6. 10.3802/jgo.2019.30.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 16.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 17.Takemura K, Takenaka Y, Ashida N, et al. Age-adjusted Charlson Comorbidity Index predicts prognosis of laryngopharyngeal cancer treated with radiation therapy. Acta Otolaryngol 2017;137:1307-12. 10.1080/00016489.2017.1362112 [DOI] [PubMed] [Google Scholar]
- 18.Boakye D, Walter V, Jansen L, et al. Magnitude of the Age-Advancement Effect of Comorbidities in Colorectal Cancer Prognosis. J Natl Compr Canc Netw 2020;18:59-68. 10.6004/jnccn.2019.7346 [DOI] [PubMed] [Google Scholar]
- 19.Bagni K, Chen IM, Johansen AZ, et al. Prognostic impact of Charlson's Age-Comorbidity Index and other risk factors in patients with pancreatic cancer. Eur J Cancer Care (Engl) 2020;111:e13219. [DOI] [PubMed] [Google Scholar]
- 20.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69-76. 10.1016/j.jhep.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. 10.1016/S0140-6736(13)61903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta HB, Sura SD, Adhikari D, et al. Adapting the Elixhauser comorbidity index for cancer patients. Cancer 2018;124:2018-25. 10.1002/cncr.31269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang CS, Baik GH, Kim JH, et al. Peptic ulcer disease in liver cirrhosis and chronic hepatitis: impact of portal hypertension. Scand J Gastroenterol 2014;49:1051-7. 10.3109/00365521.2014.923501 [DOI] [PubMed] [Google Scholar]
- 24.Yang CC, Fong Y, Lin LC, et al. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg 2018;53:235-40. 10.1093/ejcts/ezx215 [DOI] [PubMed] [Google Scholar]
- 25.Kahl A, du Bois A, Harter P, et al. Prognostic Value of the Age-Adjusted Charlson Comorbidity Index (ACCI) on Short- and Long-Term Outcome in Patients with Advanced Primary Epithelial Ovarian Cancer. Ann Surg Oncol 2017;24:3692-9. 10.1245/s10434-017-6079-9 [DOI] [PubMed] [Google Scholar]
- 26.Shah S, Boucai L. Effect of Age on Response to Therapy and Mortality in Patients With Thyroid Cancer at High Risk of Recurrence. J Clin Endocrinol Metab 2018;103:689-97. 10.1210/jc.2017-02255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aquina CT, Mohile SG, Tejani MA, et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. Br J Cancer 2017;116:389-97. 10.1038/bjc.2016.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner H, Castro FA, Eberle A, et al. Death certificate only proportions should be age adjusted in studies comparing cancer survival across populations and over time. Eur J Cancer 2016;52:102-8. 10.1016/j.ejca.2015.10.059 [DOI] [PubMed] [Google Scholar]
- 29.He C, Zhang Y, Song Y, et al. Preoperative CEA levels are supplementary to CA19-9 levels in predicting prognosis in patients with resectable intrahepatic cholangiocarcinoma. J Cancer 2018;9:3117-28. 10.7150/jca.25339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song P, Midorikawa Y, Nakayama H, et al. Patients' prognosis of intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma after resection. Cancer Med 2019;8:5862-71. 10.1002/cam4.2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as