Abstract
Background
This study was conducted retrospectively to investigate the survival of patients undergoing gastric cancer surgery with epidural combined with general anesthesia (EGA) and general anesthesia alone (GA).
Methods
We retrospectively analyzed 596 patients with gastric cancer who were scheduled for radical resection. Propensity score matching was performed at a 1:1 ratio between GA (n=97) and EGA (n=97) to reduce selection bias. Univariate and multivariate analyses were used to identify factors significantly correlated with recurrence and/or metastasis and prognosis. The 5-year overall survival rates of patients receiving EGA and GA alone were compared.
Results
After the propensity scores were matched, there were 97 patients who underwent EGA and 97 patients who underwent GA. For the entire population, reconstruction type, pN stage, and complications were significantly correlated with prognosis based on multivariate analyses. For patients with a recurrence and/or metastasis, lymphadenectomy and pN stage were shown to be independent prognostic factors by multivariate analysis.
Conclusions
In summary, patients might benefit from EGA as a result of better analgesic and anti-inflammatory effects, fewer postoperative complications, higher safety, and a lower rate of metastasis and recurrence is conducive to postoperative recovery in patients with gastric cancer.
Keywords: Epidural anesthesia, general anesthesia, gastric cancer, metastasis, recurrence
Introduction
Gastric cancer is a major global public health problem, and the incidence continues to rise, thus posing a serious threat to human health (1,2). Remarkably, the incidence of gastric cancer in China is highest worldwide (3,4). Radical surgery remains the primary curative modality for resected gastric cancer (5). Surgical incisions may interfere with the synthesis and secretion of various inflammatory cytokines, leading to inflammatory reactions. The severity of the inflammatory response has a great impact on the treatment effects, causing dismal survival and prognosis among the patients (6). Previous study has shown that various perioperative elements may impair cellular immunity, thereby increasing cellular immunosuppressive effects, further inducing tumor recurrence and metastasis, and reducing the survival time of patients (7). Therefore, protecting immune function and the stress response during the perioperative period is very important to improve the prognosis of tumor patients.
Anesthesia is an inescapable application during the perioperative period, and various anesthesia methods may have different influences on postoperative recovery, short-term adverse reactions, and even tumor metastasis and recurrence (8). One possible reason may be that anesthesia can regulate the recurrence or metastasis of cancer by directly affecting the biological behavior of tumor cells or improving the tumor microenvironment (9). General anesthesia (GA) and epidural anesthesia are commonly used for patients undergoing gastric cancer surgery. Moreover, epidural anesthesia has the potential to reduce the incidence of side effects, cancer recurrence, and metastasis (10,11).
Recently, the potential survival benefits of anesthesia techniques for different cancer types has received increasing attention (10-17); however, the roles of anesthesia techniques in improving survival and reducing complications after cancer surgery are conflicting rather than conclusive. In a retrospective study, Christopherson et al. (18) concluded that the type of anesthesia did not appear to affect long-term survival after colon cancer surgery. However, another retrospective study by Zhong et al. (19) suggested that epidural combined with general anesthesia (EGA) can improve the prognosis of ovarian cancer after surgery. In a randomized controlled trial, Tsui et al. (20) found no difference in disease-free survival between combined general/epidural anesthesia and general anesthesia alone in prostate cancer. This study was conducted to compare the effects of EGA versus GA in patients undergoing gastric cancer surgery.
Methods
Patients
Between February 1984 and February 2010 patients with gastric cancer without metastases who underwent gastrectomy were entered into a retrospectively maintained database. A total of 596 patients with locally advanced gastric cancer underwent total or subtotal gastrectomy with D2 or >D2 lymphadenectomies. All patients achieved a potentially curative resection for histologically-proven gastric adenocarcinoma. This study has been approved by the Ethics Committee of the Fourth Affiliated Hospital, China Medical University [Approval Number: EC-2018-KS-085(YJ)]. All patient records and information were anonymized and de-identified prior to analysis.
Included and excluded standards
The inclusion criteria were as follows: patients with EGA or GA; histologically-proven adenocarcinoma; negative resection margins (R0); potentially curable and curative operation was performed; complete medical records; patients <70 years of age; and standard D2 (D2) or extended D2 (D2+) lymphadenectomy. The exclusion criteria were as follows: preoperative adjuvant therapy; laparoscopic-assisted gastric cancer surgery; stage IV cancer; previous or concomitant cancer; and emergency surgery.
Follow-up
The follow-up of the entire population was complete until death or the cutoff date (February 2015). All patients had physical examinations with histories and had serum carcinoembryonic antigen (CEA) levels assessed every 1–3 months for the first post-operative year, and every 6–12 months thereafter. Twelve patients were lost to follow-up and were excluded. The follow-up rate was 98.0%. Overall, a total of 596 patients with locally advanced gastric cancer were included in this study.
Propensity score matching
Because this study is a retrospective study, this study was inevitably affected by selection bias due to baseline feature imbalance. Therefore, we used propensity score matching studies to reduce selection bias. The propensity score for all cases was calculated by a logistic regression model in which the type of anesthesia was considered to be a dependent variable for all clinicopathologic covariates shown in Table 1. Patients who underwent GA were matched to the patients who underwent EGA according to the propensity score and matched by a 1:1 fixed ratio between GA (n=97) and EGA (n=97). After matching, the normalized difference of each covariate was applied to compare the balance of the matching group. After the propensity scores were matched, there were 97 patients who underwent GA and 97 patients who underwent EGA.
Table 1. Comparison of demographic and clinicopathological variables between the two groups according to the types of anesthesia before and after propensity score matching.
| Variables | Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Anesthesia | All | P value‡ | Anesthesia | All | P value‡ | ||||
| EGA (n=97) | GA (n=499) | EGA (n=97) | GA (n=97) | ||||||
| Sex | 0.008 | 0.208 | |||||||
| Female | 16 (16.5) | 140 (28.1) | 156 (26.2) | 16 (16.5) | 10 (10.3) | 26 (13.4) | |||
| Male | 81 (83.5) | 359 (71.9) | 440 (73.8) | 81 (83.5) | 87 (89.7) | 168 (86.6) | |||
| Age, years | 0.224 | 0.110 | |||||||
| <65 | 65 (67.0) | 366 (73.3) | 431 (72.3) | 65 (67.0) | 75(77.3) | 140 (72.2) | |||
| ≥65 | 32 (33.0) | 133 (26.7) | 165 (27.7) | 32 (33.0) | 22 (22.7) | 54 (27.8) | |||
| Tumor size, cm | 0.622 | 0.349 | |||||||
| ≤5 | 65 (67.0) | 347 (69.5) | 412 (69.1) | 65 (67.0) | 71 (73.2) | 136 (70.1) | |||
| >5 | 32 (33.0) | 152 (30.5) | 184 (30.9) | 32 (33.0) | 26 (26.8) | 58 (29.9) | |||
| Histologic grade | 0.686 | 0.832 | |||||||
| Grade I | 5 (5.2) | 40 (8.0) | 45 (7.6) | 5 (5.2) | 8 (8.2) | 13 (6.7) | |||
| Grade II | 35 (36.1) | 153 (30.7) | 188 (31.5) | 35 (36.1) | 28 (28.9) | 63 (32.5) | |||
| Grade III | 54 (55.7) | 273 (54.7) | 327 (54.9) | 54 (55.7) | 57 (58.8) | 111 (57.2) | |||
| Grade IV | 3 (3.1) | 33 (6.6) | 36 (6.0) | 3 (3.1) | 4 (4.1) | 7 (3.6) | |||
| pT stage | 0.201 | 0.203 | |||||||
| pT1 | 15 (15.5) | 75 (15.0) | 90 (15.1) | 15 (15.5) | 26 (26.8) | 41 (21.1) | |||
| pT2 | 32 (33.0) | 111 (22.2) | 143 (24.0) | 32 (33.0) | 26 (26.8) | 58 (29.9) | |||
| pT3 | 26 (26.8) | 175 (35.1) | 201 (33.7) | 26 (26.8) | 24 (24.7) | 50 (25.8) | |||
| pT4 | 24 (24.7) | 138 (27.7) | 162 (27.2) | 24 (24.7) | 21 (21.6) | 45 (23.2) | |||
| pN stage | 0.471 | 0.103 | |||||||
| pN0 | 40 (41.2) | 233 (46.7) | 273 (45.8) | 40 (41.2) | 35 (36.1) | 75 (38.7) | |||
| pN1 | 24 (24.7) | 105 (21.0) | 129 (21.6) | 24 (24.7) | 18 (18.6) | 42 (21.6) | |||
| pN2 | 20 (20.6) | 101 (20.2) | 121 (20.3) | 20 (20.6) | 21 (21.6) | 41(21.1) | |||
| pN3 | 13 (13.4) | 60 (12.0) | 73 (12.2) | 13 (13.4) | 23 (23.7) | 36 (18.6) | |||
| Gastrectomy | 0.102 | 0.409 | |||||||
| Total | 4 (4.1) | 40 (8.0) | 44 (7.4) | 4 (4.1) | 2 (2.1) | 6 (3.1) | |||
| Subtotal | 93 (95.9) | 459 (92.0) | 552 (92.6) | 93 (95.9) | 95 (97.8) | 188 (96.9) | |||
| Lymphadenectomy | 0.843 | 0.507 | |||||||
| D2 | 51 (52.6) | 262 (52.5) | 313 (52.5) | 51 (52.6) | 49 (50.5) | 100 (51.5) | |||
| D2+ | 25 (25.8) | 138 (27.7) | 163 (27.3) | 25 (25.8) | 36 (37.1) | 61 (31.4) | |||
| D3 | 21 (21.6) | 99 (19.8) | 120 (20.1) | 21 (21.6) | 12 (12.4) | 33 (17.0) | |||
| Number of LNs retrieved | 0.998 | 0.775 | |||||||
| Adequate, n≥16 | 49 (50.5) | 252 (50.5) | 301 (50.5) | 49 (50.5) | 47 (48.5) | 96 (49.5) | |||
| Inadequate, n<16 | 48 (49.5) | 247 (49.5) | 295 (49.5) | 48 (49.5) | 50 (51.5) | 98(50.5) | |||
| Reconstruction type | 0.169 | 0.170 | |||||||
| Billroth I | 83 (85.6) | 403 (80.8) | 486 (81.5) | 83 (85.6) | 88 (90.7) | 171 (88.1) | |||
| Billroth II | 12 (12.4) | 76 (15.2) | 88 (14.8) | 12 (12.4) | 9 (9.3) | 21 (10.8) | |||
| Roux-Y | 2 (2.1) | 20 (4.0) | 22 (3.7) | 2 (2.1) | 0 (0) | 2 (1.0) | |||
| Chemotherapy | 0.709 | 0.269 | |||||||
| No | 83 (85.6) | 434 (87.0) | 517 (86.7) | 83 (85.6) | 88 (90.7) | 171 (88.1) | |||
| Yes | 14 (14.4) | 65 (13.0) | 79 (13.3) | 14 (14.4) | 9 (9.3) | 23 (11.9) | |||
| Complications† | <0.001 | <0.001 | |||||||
| Absent | 96 (99.0) | 456 (91.4) | 552 (92.6) | 96 (99.0) | 61 (62.9) | 157 (80.9) | |||
| Present | 1 (1.0) | 43 (8.6) | 44 (7.4) | 1 (1.0) | 36 (37.1) | 37 (19.1) | |||
| Blood loss, mL | 0.005 | 0.058 | |||||||
| ≤200 | 63 (64.9) | 247 (49.5) | 310 (52.0) | 63 (34.9) | 75 (77.3) | 138 (71.1) | |||
| >200 | 34 (35.1) | 252 (50.5) | 286 (48.0) | 34 (35.1) | 22 (22.7) | 56 (28.9) | |||
| Lymphatic vessel invasion | 0.415 | 0.108 | |||||||
| Negative | 82 (84.5) | 437 (87.6) | 519 (87.1) | 82 (84.5) | 73 (75.3) | 155 (79.9) | |||
| Positive | 15 (15.5) | 62 (12.4) | 77 (12.9) | 15 (15.5) | 24 (24.7) | 39 (20.1) | |||
| Locoregional recurrence | 0.514 | 0.701 | |||||||
| Absent | 82 (84.5) | 408 (81.8) | 490 (82.2) | 82 (84.5) | 80 (82.5) | 162 (83.5) | |||
| Present | 15 (15.5) | 91 (18.2) | 106 (17.8) | 15 (15.5) | 17 (17.5) | 32 (16.5) | |||
| Distant metastasis | 0.683 | 0.433 | |||||||
| Absent | 71 (73.2) | 355 (71.1) | 426 (71.5) | 71 (73.2) | 66 (68.0) | 137 (70.6) | |||
| Present | 26 (26.8) | 144 (28.9) | 170 (28.5) | 26 (26.8) | 31 (32.0) | 57 (29.4) | |||
†, including urinary infection/retention, delayed gastric emptying, intestinal obstruction/ileus, pneumonia, abdominal infection/abscess, wound infection/dehiscence, subphrenic infection/abscess, dumping syndrome, anastomotic fistula, postoperative hemorrhage, pancreatic fistula, and cholecystitis. ‡, P values were calculated by the χ2-test. The P value for significance was <0.05. EGA, epidural plus general anesthesia; GA, general anesthesia; LNs, lymph nodes.
Clinicopathologic characteristics
The clinicopathological features that were investigated for prognosis included sex, age, blood loss, complications, gastrectomy, reconstruction type, tumor size, histologic grade, lymphatic vessel invasion (LVI), number of lymph nodes (LNs) retrieved, depth of invasion (pT stage), number of regional LN metastases (pN stage), inadequate or adequate LNs retrieved, chemotherapy, locoregional recurrence, and distant metastasis (Table 1).
Pathology
Two pathologists independently examined the sections and differences of opinion were resolved by discussion to reach a consensus and establish the final diagnosis. The carcinoma lesions together with the surrounding gastric wall were fixed in formalin and cut into multiple 5-mm slices that were parallel to the lesser curvature (5). As many LNs as possible were retrieved for adequate staging. According to the current guidelines for gastric cancer, examining at least 16 LNs is strongly recommended for adequate staging (2,21-24). The 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging classification for carcinoma of the stomach was applied to re-stage all patients in this study. The pathology report mainly included tumor size, pT, pN, status of margin, LVI, status of lymph nodes, number of LNs retrieved, and histologic grade.
Statistical analysis
The 5-year overall survival (OS) rates were calculated using Kaplan-Meier survival analysis. The number at risk was also shown in all Kaplan-Meier curves (Figure 1). Two sided χ2 tests or two-tailed t-tests were performed for comparison of clinicopathologic features between patients who underwent a gastrectomy with EGA and GA. Univariate and multivariate analyses were applied to identify the prognostic factors. A P value <0.05 was defined as statistically significant. The SPSS statistical software (version 22.0) was used for all statistical analyses (SPSS, Inc., Chicago, IL, USA). The work has been reported in line with the STROCSS criteria (25).
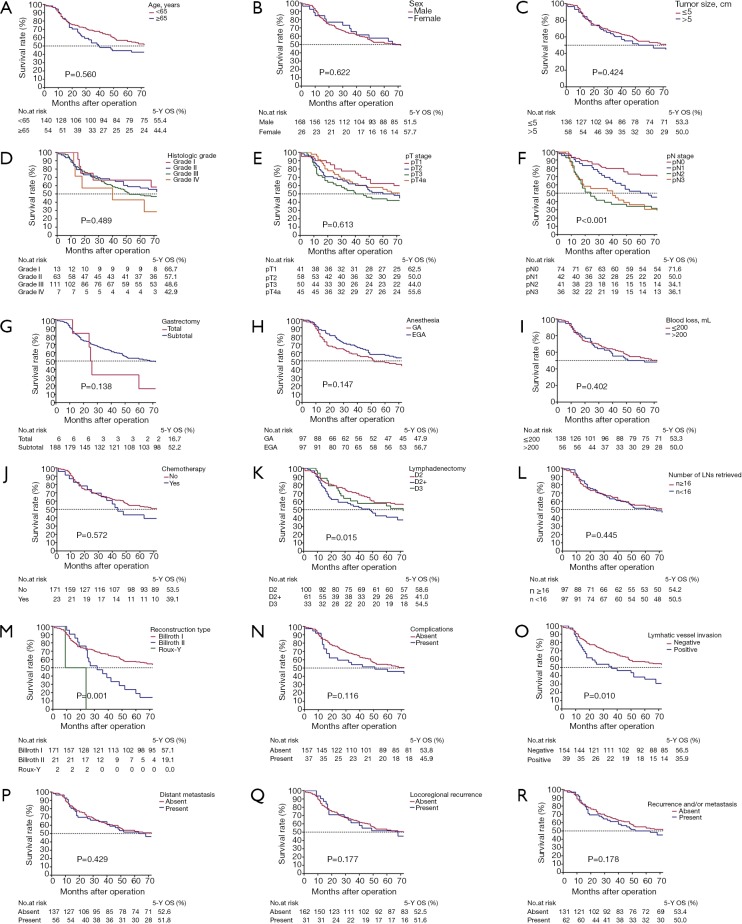
Figure 1.
Kaplan–Meier survival curves for 5-year OS stratified by different prognostic factors with statistical significance based on the (A) age, (B) sex, (C) tumor size, (D) histologic grade, (E) pT stage, (F) pN sage, (G) gastrectomy, (H) anesthesia, (I) blood loss, (J) chemotherapy, (K) lymphadenectomy, (L) number of LNs retrieved, (M) reconstruction type, (N) complications, (O) lymphatic vessel invasion, (P) distant metastasis, (Q) locoregional recurrence, (R) locoregional recurrence and/or distant metastasis. LNs, lymph nodes; EGA, epidural plus general anesthesia; GA, general anesthesia; 5-Y OS, 5-year overall survival rate.
Results
Patient characteristics before propensity score matching
The clinicopathological characteristics are summarized in Table 1. Five hundred and ninety-six gastric cancer patients who underwent a gastrectomy were assessed for eligibility in this study. Of the 596 patients, 97 patients underwent a gastrectomy with EGA (median age, 55.8 years) and 499 patients underwent a gastrectomy with GA (median age, 62.3 years). Of patients who underwent a gastrectomy with EGA, 16 patients (16.5%) were females and 81 patients (83.5%) were males. Of patients who underwent a gastrectomy with GA, 140 patients (28.1%) were females and 359 patients (71.9%) were males. Among the confounding factors unrelated to anesthesia, the two groups were well-balanced with respect to age (P=0.224), gastrectomy (P=0.102), reconstruction type (P=0.169), tumor size (P=0.622), histologic grade (P=0.686), LVI (P=0.415), number of LNs retrieved (P=0.998), pT stage (P=0.201), pN stage (P=0.471), and chemotherapy (P=0.709). There was a significant difference in sex (P=0.008) between the two groups. Importantly, among the post-operative prognostic factors associated with anesthesia, no significant difference was demonstrated in locoregional recurrence (P=0.514) and distant metastasis (P=0.683). There are significant differences in blood loss (P=0.005) and complications (P<0.001).
Patient characteristics after propensity score matching
Based on propensity score matching analysis, the clinicopathologic features are listed in Table 1. The clinicopathologic characteristics of the propensity score-matched cohort (194 patients) are summarized in Table 1. Ninety-seven patients underwent a gastrectomy with EGA (median age, 55.8 years) and 97 patients underwent a gastrectomy with GA (median age, 58.0 years). Of the patients who underwent a gastrectomy with EGA, 16 patients (16.5%) were females and 81 patients (83.5%) were males. Of the patients who underwent a gastrectomy with GA, 10 patients (10.3%) were females and 87 patients (89.7%) were males. Among the confounding factors unrelated to anesthesia, the two groups were well-balanced with respect to age, gastrectomy, reconstruction type, tumor size, histologic grade, LVI, number of LNs retrieved, pT stage, pN stage, chemotherapy, and sex (all, P>0.05) between the two groups. Among the postoperative prognostic factors associated with anesthesia, there were no significant differences between locoregional recurrence (P=0.701), blood loss (P=0.058), and distant metastasis (P=0.433). There were significant differences in the complications between the two groups (P<0.001).
Outcomes
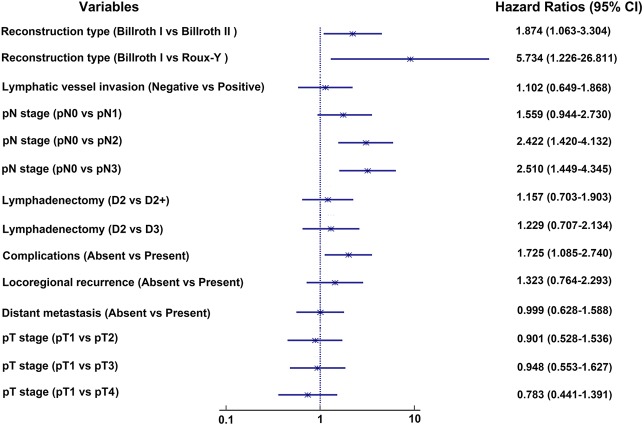
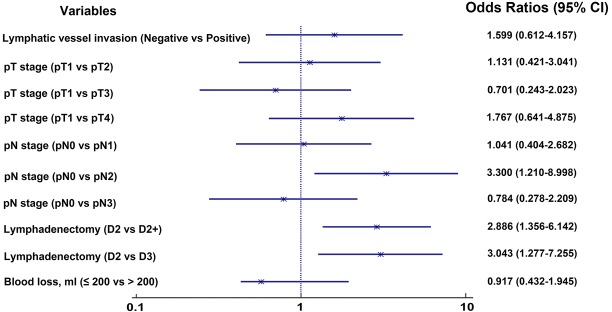
Univariate analysis demonstrated that reconstruction type (P=0.001), pN stage (P<0.001), lymphatic vessel invasion (P=0.015) and lymphadenectomy (P=0.010) were significantly associated with prognosis for the entire population (Table 2). We plotted survival curves for various clinical and pathologic factors (Figure 1). EGA had similar survival rates for patients who underwent a gastrectomy compared with GA (56.7% for EGA and 47.9% for GA, log-rank test, P=0.147, Figure 1H). We included the factors that were significantly associated with prognosis and those that were generally considered to be associated with prognosis in clinical work, such as pT stage, complications, locoregional recurrence and distant metastasis, in the multivariate analysis. The multivariate analysis showed that pN stage (P=0.003), reconstruction type (P=0.011), and complications (P=0.021) were independent prognostic factors associated with prognosis (Table 3, Figure 2). For patients with recurrence and/or metastases, univariate analysis showed lymphadenectomy (P=0.020) to be an independent factor correlated with prognosis (Table 4). We included lymphadenectomy and factors commonly associated with recurrence and/or metastasis (pT stage, pN stage, blood loss, and LVI) into the multivariate analysis. The multivariate analysis showed that pN stage (P=0.012) and lymphadenectomy (P=0.006) were independent prognostic factors for patients with recurrence and/or metastases (Table 4, Figure 3).
Table 2. Univariate analyses of prognostic factors for the entire study population.
| Variables | N (%) | 5-year OS (%) | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Sex | 0.622 | ||||
| Female | 26 (13.4) | 57.7 | 1.000 | – | – |
| Male | 168 (86.6) | 51.5 | 1.136 | 0.683–1.890 | 0.622 |
| Age, years | 0.560 | ||||
| <65 | 140 (72.2) | 55.4 | 1.000 | – | – |
| ≥65 | 54 (27.8) | 44.4 | 1.117 | 0.771–1.619 | 0.560 |
| Tumor size, cm | 0.424 | ||||
| ≤5 | 136 (70.1) | 53.3 | 1.000 | – | – |
| >5 | 58 (29.9) | 50.0 | 1.159 | 0.807–1.665 | 0.424 |
| Histologic grade | 0.489 | ||||
| Grade I | 13 (6.7) | 66.7 | 1.000 | – | – |
| Grade II | 63 (32.5) | 57.1 | 0.912 | 0.444–1.875 | 0.803 |
| Grade III | 111 (57.2) | 48.6 | 1.002 | 0.502–1.999 | 0.996 |
| Grade IV | 7 (3.6) | 42.9 | 1.726 | 0.641–4.649 | 0.280 |
| pT stage | 0.613 | ||||
| pT1 | 41 (21.1) | 62.5 | 1.000 | – | – |
| pT2 | 58 (29.9) | 50.0 | 1.245 | 0.755–2.051 | 0.391 |
| pT3 | 50 (25.8) | 44.0 | 1.403 | 0.849–2.318 | 0.186 |
| pT4 | 45 (23.2) | 55.6 | 1.286 | 0.772–2.145 | 0.334 |
| pN stage | <0.001 | ||||
| pN0 | 75 (38.7) | 71.6 | 1.000 | – | – |
| pN1 | 42 (21.6) | 50.0 | 1.533 | 0.976–2.473 | 0.063 |
| pN2 | 41 (21.1) | 34.1 | 2.426 | 1.526–3.857 | <0.001 |
| pN3 | 36 (18.6) | 36.1 | 2.419 | 1.509–3.877 | <0.001 |
| Gastrectomy | 0.138 | ||||
| Total | 6 (3.1) | 16.7 | 1.000 | – | – |
| Subtotal | 188 (96.9) | 52.3 | 0.506 | 0.205–1.245 | 0.138 |
| Lymphadenectomy | 0.015 | ||||
| D2 | 100 (51.5) | 58.6 | 1.000 | – | – |
| D2+ | 61 (31.4) | 41.0 | 1.740 | 1.189–2.545 | 0.004 |
| D3 | 33 (17.0) | 54.5 | 1.444 | 0.904–2.306 | 0.124 |
| Number of LNs retrieved | 0.445 | ||||
| Adequate, n≥16 | 96 (49.5) | 54.2 | 1.000 | – | – |
| Inadequate, n<16 | 98 (50.5) | 50.5 | 1.141 | 0.813–1.601 | 0.445 |
| Reconstruction type | 0.001 | ||||
| Billroth I | 171 (88.1) | 57.1 | 1.000 | – | – |
| Billroth II | 21 (10.8) | 19.0 | 2.202 | 1.355–3.578 | 0.001 |
| Roux-Y | 2 (1.0) | 0.0 | 5.739 | 1.390–23.692 | 0.016 |
| Chemotherapy | 0.572 | ||||
| No | 171 (88.1) | 53.5 | 1.000 | – | – |
| Yes | 23 (11.9) | 39.1 | 1.163 | 0.689–1.962 | 0.572 |
| Complications† | 0.116 | ||||
| Absent | 157 (80.9) | 53.8 | 1.000 | – | – |
| Present | 37 (19.1) | 45.9 | 1.394 | 0.921–2.108 | 0.116 |
| Blood loss, mL | 0.402 | ||||
| ≤200 | 138 (71.1) | 53.3 | 1.000 | – | – |
| >200 | 56 (28.9) | 50.0 | 0.850 | 0.582–1.243 | 0.402 |
| Lymphatic vessel invasion | 0.010 | ||||
| Negative | 82 (84.5) | 56.5 | 1.000 | – | – |
| Positive | 15 (15.5) | 35.9 | 1.690 | 1.134–2.520 | 0.010 |
| Locoregional recurrence | 0.177 | ||||
| Absent | 162 (83.5) | 52.5 | 1.000 | – | – |
| Present | 32 (16.5) | 51.6 | 1.351 | 0.873–2.091 | 0.177 |
| Distant metastasis | 0.429 | ||||
| Absent | 137 (70.6) | 52.6 | 1.000 | – | – |
| Present | 57 (29.4) | 51.8 | 1.162 | 0.801–1.684 | 0.429 |
| Locoregional recurrence or distant metastasis | 0.178 | ||||
| Absent | 131 (67.5) | 53.4 | 1.000 | – | – |
| Present | 63 (32.5) | 50.0 | 1.279 | 0.894–1.831 | 0.178 |
| Anesthesia | 0.147 | ||||
| GA | 97 (50.0) | 47.9 | 1.000 | – | – |
| EGA | 97 (50.0) | 56.7 | 0.778 | 0.555–1.092 | 0.147 |
†, including urinary infection/retention, delayed gastric emptying, intestinal obstruction/ileus, pneumonia, abdominal infection/abscess, wound infection/dehiscence, subphrenic infection/abscess, dumping syndrome, anastomotic fistula, postoperative hemorrhage, pancreatic fistula, and cholecystitis. LNs, lymph nodes; EGA, epidural plus general anesthesia; GA, general anesthesia; HR, hazard ratio; CI, confidence intervals; OS, overall survival.
Table 3. Multivariable analyses of prognostic factors for the entire study population.
| Variables | N (%) | 5-year OS (%) | HR | 95%CI | P value |
|---|---|---|---|---|---|
| pT stage | 0.841 | ||||
| pT1 | 41 (21.1) | 62.5 | 1.000 | – | – |
| pT2 | 58 (29.9) | 50 | 0.901 | 0.528–1.536 | 0.701 |
| pT3 | 50 (25.8) | 44 | 0.948 | 0.553–1.627 | 0.847 |
| pT4 | 45 (23.2) | 55.6 | 0.783 | 0.441–1.391 | 0.404 |
| pN stage | 0.003 | ||||
| pN0 | 75 (38.7) | 71.6 | 1.000 | – | – |
| pN1 | 42 (21.6) | 50 | 1.559 | 0.944–2.730 | 0.083 |
| pN2 | 41 (21.1) | 34.1 | 2.422 | 1.420–4.132 | 0.001 |
| pN3 | 36 (18.6) | 36.1 | 2.510 | 1.449–4.345 | 0.001 |
| Lymphadenectomy | 0.746 | ||||
| D2 | 100 (51.5) | 58.6 | 1.000 | – | – |
| D2+ | 61 (31.4) | 41 | 1.157 | 0.703–1.903 | 0.566 |
| D3 | 33 (17.0) | 54.5 | 1.229 | 0.707–2.134 | 0.465 |
| Reconstruction type | 0.011 | ||||
| Billroth I | 171 (88.1) | 57.1 | 1.000 | – | – |
| Billroth II | 21 (10.8) | 19 | 1.874 | 1.063–3.304 | 0.300 |
| Roux-Y | 2 (1.0) | 0 | 5.734 | 1.226–26.811 | 0.260 |
| Complications† | 0.021 | ||||
| Absent | 157 (80.9) | 53.8 | 1.000 | – | – |
| Present | 37 (19.1) | 45.9 | 1.725 | 1.085–2.740 | 0.021 |
| Lymphatic vessel invasion | 0.720 | ||||
| Negative | 82 (84.5) | 56.5 | 1.000 | – | – |
| Positive | 15 (15.5) | 35.9 | 1.102 | 0.649–1.868 | 0.720 |
| Locoregional recurrence | 0.318 | ||||
| Absent | 162 (83.5) | 52.5 | 1.000 | – | – |
| Present | 32 (16.5) | 51.6 | 1.323 | 0.764–2.293 | 0.318 |
| Distant metastasis | 0.996 | ||||
| Absent | 137 (70.6) | 52.6 | 1.000 | – | – |
| Present | 57 (29.4) | 51.8 | 0.999 | 0.628–1.588 | 0.996 |
†, including urinary infection/retention, delayed gastric emptying, intestinal obstruction/ileus, pneumonia, abdominal infection/abscess, wound infection/dehiscence, subphrenic infection/abscess, dumping syndrome, anastomotic fistula, postoperative hemorrhage, pancreatic fistula, and cholecystitis. EGA, epidural plus general anesthesia; GA, general anesthesia; HR, hazard ratio; CI, confidence intervals; OS, overall survival.
Figure 2.
The forest plot shows multivariable analyses of prognostic factors for the entire study population. LNs, lymph nodes; EGA, epidural plus general anesthesia; GA, general anesthesia; CI, confidence intervals.
Table 4. Univariate and multivariate analysis of factors for patients with recurrence and/or metastasis.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| N (%) | 5-year OS (%) | P value | OR | 95% CI | P value | ||
| Sex | 0.870 | ||||||
| Female | 26 (13.4) | 57.7 | |||||
| Male | 168 (86.6) | 51.5 | |||||
| Age, years | 0.215 | ||||||
| <65 | 140 (72.2) | 55.4 | |||||
| ≥65 | 54 (27.8) | 44.4 | |||||
| Tumor size, cm | 0.509 | ||||||
| ≤5 | 136 (70.1) | 53.3 | |||||
| >5 | 58 (29.9) | 50 | |||||
| Histologic grade | 0.579 | ||||||
| Grade I | 13 (6.7) | 66.7 | |||||
| Grade II | 63 (32.5) | 57.1 | |||||
| Grade III | 111 (57.2) | 48.6 | |||||
| Grade IV | 7 (3.6) | 42.9 | |||||
| pT stage | 0.780 | 0.281 | |||||
| pT1 | 41 (21.1) | 62.5 | 1.000 | – | – | ||
| pT2 | 58 (29.9) | 50 | 1.131 | 0.421–3.041 | 0.807 | ||
| pT3 | 50 (25.8) | 44 | 0.701 | 0.243–2.023 | 0.511 | ||
| pT4 | 45 (23.2) | 55.6 | 1.767 | 0.641–4.875 | 0.271 | ||
| pN stage | 0.563 | 0.012 | |||||
| pN0 | 75 (38.7) | 71.6 | 1.000 | – | – | ||
| pN1 | 42 (21.6) | 50 | 1.041 | 0.404–2.682 | 0.934 | ||
| pN2 | 41 (21.1) | 34.1 | 3.300 | 1.210–8.998 | 0.020 | ||
| pN3 | 36 (18.6) | 36.1 | 0.784 | 0.278–2.209 | 0.645 | ||
| Gastrectomy | 0.991 | ||||||
| Total | 6 (3.1) | 16.7 | |||||
| Subtotal | 188 (96.9) | 52.3 | |||||
| Lymphadenectomy | 0.020 | 0.006 | |||||
| D2 | 100 (51.5) | 58.6 | 1.000 | – | – | ||
| D2+ | 61 (31.4) | 41 | 2.886 | 1.356–6.142 | 0.006 | ||
| D3 | 33 (17.0) | 54.5 | 3.043 | 1.277–7.255 | 0.012 | ||
| Number of LNs retrieved | 0.479 | ||||||
| Adequate, n≥16 | 96 (49.5) | 54.2 | |||||
| Inadequate, n<16 | 98 (50.5) | 50.5 | |||||
| Reconstruction type | 0.953 | ||||||
| Billroth I | 171 (88.1) | 57.1 | |||||
| Billroth II | 21 (10.8) | 19 | |||||
| Roux-Y | 2 (1.0) | 0 | |||||
| Chemotherapy | 0.473 | ||||||
| No | 171 (88.1) | 53.5 | |||||
| Yes | 23 (11.9) | 39.1 | |||||
| Complications† | 0.960 | ||||||
| Absent | 157 (80.9) | 53.8 | |||||
| Present | 37 (19.1) | 45.9 | |||||
| Blood loss, ml | 0.598 | 0.821 | |||||
| ≤200 | 138 (71.1) | 53.3 | 1.000 | – | – | ||
| >200 | 56 (28.9) | 50 | 0.917 | 0.432–1.945 | 0.821 | ||
| Lymphatic vessel invasion | 0.784 | 0.338 | |||||
| Negative | 82 (84.5) | 56.5 | 1.000 | – | – | ||
| Positive | 15 (15.5) | 35.9 | 1.599 | 0.612–4.157 | 0.338 | ||
| Anesthesia | 0.699 | ||||||
| GA | 97 (50.0) | 47.9 | |||||
| EGA | 97 (50.0) | 56.7 | |||||
†, including urinary infection/retention, delayed gastric emptying, intestinal obstruction/ileus, pneumonia, abdominal infection/abscess, wound infection/dehiscence, subphrenic infection/abscess, dumping syndrome, anastomotic fistula, postoperative hemorrhage, pancreatic fistula, and cholecystitis. LNs, lymph nodes; EGA, epidural plus general anesthesia; GA, general anesthesia; OR, odds ratio; CI, confidence intervals; OS, overall survival.
Figure 3.
The forest plot shows multivariate analysis of factors for patients with recurrence and/or metastasis. LNs, lymph nodes; EGA, epidural plus general anesthesia; GA, general anesthesia; CI, confidence intervals.
Discussion
Recently, increasing evidence has suggested that the anesthesia technique may affect cancer recurrence and survival outcomes; however, no studies comparing EGA and GA on survival in patients undergoing gastric cancer surgery have been conducted. In addition, complete resection with negative margins has been recommended as the standard goal for patients with resected gastric cancer (2,23,24). Thus, only patients with resected gastric cancer who underwent curable complete resection were included in this study. Considering that significantly fewer LNs may be retrieved during D1 compared with D2 or D2+ lymphadenectomy and may thus lead to a significantly higher incidence of recurrences and poorer prognosis, the results would not be reliable if D1 lymphadenectomies were included in this study. Therefore, only patients who underwent radical resection with D2 or >D2 lymphadenectomy were enrolled in this study. The average lifespan of men and women in China is 74 and 77 years, respectively. Therefore, the long-term effect of curative gastrectomy for gastric cancer may not be evaluable in patients >70 years of age; thus, we only included patients <70 years of age.
It is worth noting that the roles of epidural anesthesia in improving survival outcomes for prostatectomies are conflicting. Biki et al. (26) demonstrated that patients who had GA as a substitute for epidural anesthesia for postoperative opioids following open prostatectomy surgery was associated with substantially less risk of biochemical cancer recurrence. In another retrospective study, however, no significant difference existed between GA plus postoperative ketorolac-morphine anesthesia and GA plus intraoperative and postoperative thoracic epidural anesthesia with respect to the biochemical recurrence free survival, cancer specific survival, or OS for patients undergoing open retropubic radical prostatectomies (17). Furthermore, EGA significantly decreased the incidence of recurrence following radical prostatectomy compared with GA plus opioid infusion (20).
Similarly, the effects of epidural anesthesia on survival for patients who underwent colorectal cancer surgery are also conflicting rather than conclusive. A large cohort study conducted by Cummings et al. (27) confirmed that patients with non-metastatic colorectal cancer benefit from epidural anesthesia with respect to survival, rather than cancer recurrence, compared with non-epidural anesthesia. Gupta et al. (11) found a reduction in all-cause mortality after rectal, but not colon cancer, in patients with epidural anesthesia, as compared with patient-controlled anesthesia. EGA was confirmed to be associated with enhanced survival among patients without metastases before 1.46 years (18); however, no significant decrease in cancer recurrence was demonstrated for patients with epidural anesthesia undergoing colorectal cancer surgery compared to patients without epidural anesthesia (28).
The pathogenesis of tumor metastases has been studied previously (15,29). Tumor metastases may be associated with the balance between tumor potential metastases and anti-metastatic host defenses (15). Epidural anesthesia may decrease the neuroendocrine stress and prevent the immunosuppression induced by surgery and GA, thus decreasing the incidence of post-operative infectious complications and tumor metastases (29). Our study suggested that patients who underwent gastric cancer surgery had similar survival rates between EGA and GA. No significant differences in the 5-year OS rates were shown for the entire population (56.7% for EGA, 47.9% for GA, log-rank test, P=0.147). Moreover, similar results could be found in terms of locoregional recurrence, distant metastasis, and overall survival. Notably, patients who had EGA had less intraoperative blood loss and postoperative complications compared to patients who received GA; however, this retrospective study was based on a long-term period that varied from operation-to-operation. Therefore, the results of our study must be interpreted with caution and should be clarified in further prospective, randomized controlled studies.
In this study postoperative complications were defined as new morbid conditions requiring therapeutic intervention. Studies have shown a beneficial effect of epidural anesthesia on postoperative complications. The use of epidural anesthesia can reduce the incidence of respiratory complications compared with general anesthesia (30). Pöpping et al. (31) showed that the use of epidural analgesia can reduce lung complications after abdominal and thoracic surgery (possibly due to early mobilization), reduce opioid consumption, and improved cough. For patients undergoing colorectal surgery, combined use of thoracic epidural anesthesia promotes early mobilization and recovery of bowel function, thereby reducing gastrointestinal complications (32,33). A study by Mohamad et al. (34) demonstrated that epidural anesthesia significantly reduced postoperative major adverse cardiac events in patients with coronary artery disease undergoing surgery for large abdominal tumors. In our patients, post-operative complications were significantly reduced in the EGA group compared with the GA group (P<0.001).
Tumor resection can cause severe trauma and severe post-operative pain, significantly increasing the levels of cortisol (COR) and C-reactive protein (CRP), and stimulating the body’s perioperative stress response (35). Zhong et al. (19) compared the effects of EGA and GA on the prognosis of patients with ovarian cancer. Compared with GA, EGA had a better analgesic effect, better improvement in the propensity score-matched cohort (194 patients) perioperative stress response, and a reduction in the inflammatory response (19). Moslemi et al. (36) and Han et al. (37) showed that EGA can improve the analgesic effect of refractory pain compared with GA, has less of an impact on patient immune function, a better analgesic effect, and higher safety.
This study also had some limitations. First, the data obtained in this study were from February 1984 to February 2010. During these 26 years, surgical technology has developed rapidly, so we may have to interpret the conclusions of this study with caution. In addition, although we used the method of propensity score matching analysis to reduce the selection bias, the number of patients included was relatively small, so a large sample data study is needed to further verify the results.
Conclusions
In conclusion, this study failed to show a significant reduction in the incidence of cancer recurrence and/or metastasis in patients with EGA compared with patients with GA. In addition, EGA had a similar 5-year survival rate compared to patients with GA; however, in combination with published studies, patients might benefit from EGA due to better analgesic and anti-inflammatory effects, fewer postoperative complications, higher safety, and a lower rate of metastasis and/or recurrence, which are conducive to post-operative recovery in patients with gastric cancer.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study has been approved by the Ethics Committee of the Fourth Affiliated Hospital, China Medical University [Approval Number: EC-2018-KS-085(YJ)]. All patient records and information were anonymized and deidentified prior to analysis.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.127). CDZ reports grants from China Scholarship Council (201908050148) and Japan China Sasakawa Medical Fellowship (2017816) during the conduct of the study. The other authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Amin MB, Edge SB, Ggreene FL, et al. AJCC cancer staging manual. New York: Springer; 2017. [Google Scholar]
- 3.Zheng R, Zeng H, Zhang S, et al. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 2017;36:66. 10.1186/s40880-017-0234-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 5.Zhang CD, Shen MY, Zhang JK, et al. Prognostic significance of distal subtotal gastrectomy with standard D2 and extended D2 lymphadenectomy for locally advanced gastric cancer. Sci Rep 2015;5:17273. 10.1038/srep17273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Wu L, Zhang M, et al. Effects of general anesthesia with combined epidural anesthesia on inflammatory response in patients with early-stage gastric cancer undergoing tumor resection. Exp Ther Med 2019;17:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SN, Vokali E, Lund AW, et al. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 2014;35:814-24. 10.1016/j.biomaterials.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 8.Choi WJ, Baek S, Joo EY, et al. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget 2017;8:87667. 10.18632/oncotarget.21034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou BJ, Du Y, Gu SX, et al. General anesthesia combined with epidural anesthesia maintaining appropriate anesthesia depth may protect excessive production of inflammatory cytokines and stress hormones in colon cancer patients during and after surgery. Medicine (Baltimore) 2019;98:e16610. 10.1097/MD.0000000000016610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmitti G, Soliz J, Aloia TA, et al. Positive impact of epidural analgesia on oncologic outcomes in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol 2016;23:1003-11. 10.1245/s10434-015-4933-1 [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Björnsson A, Fredriksson M, et al. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. Br J Anaesth 2011;107:164-70. 10.1093/bja/aer100 [DOI] [PubMed] [Google Scholar]
- 12.Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev 2017;36:159-77. 10.1007/s10555-016-9647-8 [DOI] [PubMed] [Google Scholar]
- 13.Gao H, Meng XY, Wang HQ, et al. Association between anaesthetic technique and oncological outcomes after colorectal carcinoma liver metastasis resection. Int J Med Sci 2019;16:337. 10.7150/ijms.28016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogelaar FJ, Lips DJ, van Dorsten FR, et al. Impact of anaesthetic technique on survival in colon cancer: a review of the literature. Gastroenterol Rep (Oxf) 2016;4:30-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis 2018;35:347-58. 10.1007/s10585-017-9862-x [DOI] [PubMed] [Google Scholar]
- 16.Hiller JG, Hacking M, Link E, et al. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiol Scand 2014;58:281-90. 10.1111/aas.12255 [DOI] [PubMed] [Google Scholar]
- 17.Wuethrich PY, Hsu Schmitz SF, Kessler TM, et al. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: a retrospective study. Anesthesiology 2010;113:570-6. [DOI] [PubMed] [Google Scholar]
- 18.Christopherson R, James KE, Tableman M, et al. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg 2008;107:325-32. 10.1213/ane.0b013e3181770f55 [DOI] [PubMed] [Google Scholar]
- 19.Zhong S, Zhong XX, Zhong XM, et al. Comparison between the effect of epidural anesthesia combined with epidural analgesia and general anesthesia combined with intravenous analgesia on prognosis of ovarian cancer patients. Oncol Lett 2019;17:5662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsui BC, Rashiq S, Schopflocher D, et al. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can J Anaesth 2010;57:107-12. 10.1007/s12630-009-9214-7 [DOI] [PubMed] [Google Scholar]
- 21.Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317. 10.1245/s10434-006-9218-2 [DOI] [PubMed] [Google Scholar]
- 22.Songun I, Putter H, Kranenbarg MK, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. 10.1016/S1470-2045(10)70070-X [DOI] [PubMed] [Google Scholar]
- 23.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajani JA, D'Amico TA, Almhanna K, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:1286-312. 10.6004/jnccn.2016.0137 [DOI] [PubMed] [Google Scholar]
- 25.Agha RA, Borrelli MR, Vella-Baldacchino M, et al. The STROCSS statement: strengthening the reporting of cohort studies in surgery. Int J Surg 2017;46:198-202. 10.1016/j.ijsu.2017.08.586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biki B, Mascha E, Moriarty DC, et al. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence. Anesthesiology 2008;109:180-7. 10.1097/ALN.0b013e31817f5b73 [DOI] [PubMed] [Google Scholar]
- 27.Cummings KC, Xu F, Cummings LC, et al. A Comparison of Epidural Analgesia and Traditional Pain Management Effects on Survival and Cancer Recurrence after ColectomyA Population-based Study. Anesthesiology 2012;116:797-806. 10.1097/ALN.0b013e31824674f6 [DOI] [PubMed] [Google Scholar]
- 28.Kim R. Anesthetic technique for cancer surgery: Harm or benefit for cancer recurrence? Eur J Surg Oncol 2018;44:557-8. 10.1016/j.ejso.2018.02.207 [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Xiao J, Shen S, et al. Emerging effect of anesthesia on post-operative tumor recurrence and metastasis. J Int Med Res 2019;47:3550-8. 10.1177/0300060519861455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausman MS, Jewell ES, Engoren M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: does avoiding general anesthesia reduce the risk of postoperative complications? Anesth Analg 2015;120:1405-12. 10.1213/ANE.0000000000000574 [DOI] [PubMed] [Google Scholar]
- 31.Pöpping DM, Wenk M, Van Aken HK. Neurologic Complications After Epidural Analgesia. Anasthesiol Intensivmed Notfallmed Schmerzther 2012;47:336-43. [DOI] [PubMed] [Google Scholar]
- 32.Chen WK, Ren L, Wei Y, et al. General anesthesia combined with epidural anesthesia ameliorates the effect of fast-track surgery by mitigating immunosuppression and facilitating intestinal functional recovery in colon cancer patients. Int J Colorectal Dis 2015;30:475-81. 10.1007/s00384-014-2098-1 [DOI] [PubMed] [Google Scholar]
- 33.Taqi A, Hong X, Mistraletti G, et al. Thoracic epidural analgesia facilitates the restoration of bowel function and dietary intake in patients undergoing laparoscopic colon resection using a traditional, nonaccelerated, perioperative care program. Surg Endosc 2007;21:247-52. 10.1007/s00464-006-0069-5 [DOI] [PubMed] [Google Scholar]
- 34.Mohamad MF, Mohammad MA, Hetta DF, et al. Thoracic epidural analgesia reduces myocardial injury in ischemic patients undergoing major abdominal cancer surgery. J Pain Res 2017;10:887. 10.2147/JPR.S122918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sendasgupta C, Makhija N, Kiran U, et al. Caudal epidural sufentanil and bupivacaine decreases stress response in paediatric cardiac surgery. Ann Card Anaesth 2009;12:27. 10.4103/0971-9784.45010 [DOI] [PubMed] [Google Scholar]
- 36.Moslemi F, Rasooli S, Baybordi A, et al. A comparison of patient controlled epidural analgesia with intravenous patient controlled analgesia for postoperative pain management after major gynecologic oncologic surgeries: A randomized controlled clinical trial. Anesth Pain Med 2015;5:e29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han XR, Wen X, Li YY, et al. Effect of different anesthetic methods on cellular immune functioning and the prognosis of patients with ovarian cancer undergoing oophorectomy. Biosci Rep 2017;37. doi: . 10.1042/BSR20170915 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as