Abstract
Background
Sam68, an RNA-binding protein, exerts oncogenic functions in several types of cancer. However, the specific functions and mechanisms of Sam68 in colorectal cancer (CRC) had not been previously clarified. Pyruvate kinase muscle (PKM)2 is the key rate-limiting enzyme in glycolysis, and PKM2 maintains the glycolysis-dominant energy metabolism in most cancer cells.
Methods
CCK8 assay was performed to show the effect of Sam68 on cell growth. Pyruvate kinase activity and lactate detection assays were performed to analyze the effects of Sam68 on aerobic glycolysis. RNA immunoprecipitation (RIP) was used to detect the binding of Sam68 to the PKM2 sequence. Western blot and real-time PCR were executed to analyze the regulation of PKM2 by Sam68.
Results
Gain-of-function and loss-of-function studies showed that ectopic expression of Sam68 promoted glycolysis and cell proliferation in CRC cells, whereas Sam68 knockdown inhibited glycolysis and cell proliferation. Mechanically, Sam68 modulated the expression profile of pyruvate kinase (PKM2 or PKM1) by regulating its alternative splicing. Overexpression of Sam68 was associated with decreased PKM1/PKM2 ratio, which positively contributed to the glycolysis procedure. Sam68 significantly promoted cell proliferation and caused a decrease of PKM1/PKM2 ratio, resulting in the metabolism of glucose switched from oxidative phosphorylation to glycolysis in CRC cells. Besides, Sam68 enhanced PKM2 mRNA transport from the nucleus to cytoplasm and increased the expression of PKM2 protein, resulting in elevated pyruvate kinase activity and lactate production.
Conclusions
These findings suggested that Sam68 affected cell growth and glycolysis pathway by regulating the alternative splicing and expression of PKM2 in CRC.
Keywords: Colorectal cancer (CRC), sam68, pyruvate kinase muscle 2 (PKM2), glycolysis, alternative splicing
Introduction
Colorectal cancer (CRC) is the most common carcinoma found worldwide and the leading cause of cancer-related death (1). Reprogramming energy metabolism is critical in tumor development and progression and has been suggested as one of the hallmarks of cancer (2). Normal cells, under enough oxygenation, breathe aerobically through oxidative phosphorylation in mitochondria, whereas oxygen-deprived cells switch their energy metabolism to anaerobic form, producing lactic acid as their products. Even if oxygen is available, the tumor cells anaerobically absorb the excess glucose and produce a large volume of lactic acid. The strategic shift of energy production mechanism from oxidative phosphorylation to glycolysis, even in well-oxygenated conditions, is considered a hallmark of cancer metabolism (3). This describes the famous “Warburg Effect’’ or aerobic glycolysis (4). Although the end result is well-known and widely accepted (5), the underlying mechanisms and possible targeted principles involved in glucose metabolism remain elusive.
It is well-acknowledged now that cancerous cells prefer pyruvate consumption and lactate production of glycolysis despite the availability of enough oxygen. Pyruvate kinase M (PKM) 2 is the isoform of pyruvate kinase, which functions as the essential rate-limiting catalysis enzyme of the last step in the glycolytic pathway and promotes phosphoenol-pyruvate (PEP) conversion to pyruvate (6). By enhancing pyruvate kinase activity, cells display to utilize the glucose switch from pentose phosphate glycolytic to the glycolytic pathway, thereby providing the energy and meeting the biosynthetic demands for rapid growth (7). PKM2, as an essential regulator of the aerobic glycolytic pathway, plays a crucial role in tumor progression (8-10). Tumor cells enhance its expression through variable shearing of PKM2, promoting glycolysis and tumor development, but its mechanism has not been fully understood.
Sam68 is a tyrosine-phosphorylated, SRC-associated substrate of 68kDa protein that plays a crucial role in the progress of cell differentiation and development (11). Sam68 is known as an RNA binding protein (RBP) and regulates the alternative splicing of some cancer-related genes with recognition sequences (12). Abnormal expression of Sam68 was observed in many types of tumors, including CRC (13,14). Our earlier study revealed that Sam68 could interact with CYTOR to promote CRC progression (15). Herein, we showed that Sam68 contributed to the expression of cytoplasm PKM protein by promoting PKM2 mRNA alternative splicing and transportation from nuclear to cytoplasm, and increased PKM2 activity and lactate production. Our findings altogether show that Sam68 promotes aerobic glycolysis in CRC by enhancing the expression and activity of PKM2.
Methods
Cell lines and clinical samples
The CRC cell lines RKO and HCT116 were cultured according to the instructions recommended by the ATCC. These two types of cells were named by Genewiz Inc. (China) using short tandem repeat (STR) markers and were confirmed to be free of mycoplasma. CRC cohorts, including 120 human primary CRC tissues and their matched adjacent noncancerous tissues (NCTs), were obtained from the Affiliated Hospital of Jiangnan University. All patient data were obtained with informed consent, and the Clinical Research Ethics Committee approved the project of the participating institutions. The study was conducted following the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS).
Vector constructs and siRNA
The wild-type or mutant Sam68 sequences were cloned into pcDNA3.1-HA and constructed as described previously (15). The siRNAs of Sam68 (siSam68) and negative control (siNC) were bought from Suzhou GenePharma Co. Ltd (China). The sequences of siNC were: forward, 5'-UUCUCCGAACGUGUCACGUTT-3', reverse, 5'-ACGUGACACGUUCGGAGAATT-3'; the sequences of siSam68 were: forward, 5'-GAGACUGGUGCAAAGAUCUCUGUAU-3', reverse, 5'-AUACAGAGAUCUUUGCACCAGUCUC-3'.
Cell proliferation assay
Cell viability was measured using the cell counting kit 8 (CCK8) (Dojindo, Japan) according to the manufacturer’s instructions. CRC cell was seeded into 96-well cell culture plates, and each well held 2×103 cells in 100 liters of the medium. Then, siSam68 or siNC were transfected into the cells using lipofectamine 2000 (Invitrogen, USA). After 24, 48, 72, or 96 hours, every well was replaced with 100 µL fresh medium containing 10% CCK8, and then the cells were incubated at 37 °C for another 2 h. The absorbance was measured under the wavelength of 450 nm.
Western blotting
According to the manufacturer’s instructions, the nuclear and cytoplasmic parts of CRC cells were isolated using the PARIS Kit (Thermo Fisher, USA). The extracted protein was separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. The relative expression of target proteins were measured using the primary antibodies for Sam68 (CST, 1:1,000), PKM2 (CST, 1:1,000), HA (CST, 1:5,000) and GAPDH (Thermo Fisher, 1:5,000).
Pyruvate kinase activity and lactate detection
Pyruvate kinase activity Assay Kit and lactate detection Kit were performed from Jiancheng Biological Company (China) according to the manufacturer’s instructions.
Immunohistochemistry (IHC)
IHC analysis holding 120 previously constructed examples of CRC tissue arrays was used to determine the expression levels of Sam68 protein (16). IHC staining was performed on 4-mm sections of paraffin-embedded tissue samples. In short, the slides were incubated with anti-Sam68 antibody (CST, 1:200) at 4 °C overnight. The following steps were performed using the GTVision III Detection System/Mo&Rb (Gene Tech, China).
Total, cytoplasmic, and nuclear RNA and Protein isolate
According to the manufacturer’s agreement, RNA and proteins of the total nucleus and cytoplasm are separated from freshly cultured CRC cells.
RNA-binding protein immunoprecipitation (RIP) assay
According to the manufacturer’s agreement, RIP analysis was performed using EZ-Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, USA). HCT116 cells were transfected with Sam68 expression plasmids or their control vectors. Three days after transfection, these cells were collected for RIP assay. Antibody against HA (CST, USA) was used for RIP. After RNA extraction, the relative levels of PKM2 mRNA were assessed by RT-PCR using PrimeScript RT Reagent Kit (Takara, Japan) and Platinum™ SuperFi™ DNA polymerase (Thermo Fisher). The PCR products were loaded onto 2% agarose gel and visualized by ethidium bromide staining.
Quantitative RT-PCR assay
The total RNA was reverse transcribed into cDNA using the PrimeScript II 1st Strand Synthesis Kit (TaKaRa). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was conducted on the ViiA7 real-time PCR system using the UltraSYBR Mixture (CWBIO, China). The PKM2 expression level was normalized to ACTB expression level and calculated by the 2ΔΔCt method. The total RNA was isolated from cells and was measured by RT–PCR followed by PstI digestion to assess the PKM1/PKM2 ratio (17,18). The primers of Sam68 were listed: forward, 5'-CTCATGGCCGAGAAGGACTC-3', reverse, 5'-TGTACCACGTACCAAAGCCC-3'; The primers of PKM were: forward, 5'-CTGAAGGCAGTGATGTGGCC-3', reverse, 5'-ACCCGGAGGTCCACGTCCTC-3'; The primers of PKM2 were: forward, 5'-ACTCGGGCTGAAGGCAGTGA-3', reverse, 5'-TGTGGGGTCGCTGGTAATGG-3'; The primers of GAPDH were: forward, 5'-ACCACAGTCCATGCCATCAC-3', reverse, 5'-TCCACCACCCTGTTGCTGTA-3'.
Statistical analysis
All data were presented as the mean ± standard deviation. Student t-test, the Mann-Whitney U test and the x2 test were used to evaluate for significant differences between groups. The differences in survival rates between the two groups were determined by the Kaplan-Meier method and the log-rank test. Cox univariate and multivariate proportional hazards regression models were used to determine the independent factors that influence survival. The statistical analyses were conducted using the SPSS 20.0 (SPSS), and P<0.05 (*) were considered statistically significant.
Results
Sam68 promotes proliferation and aerobic glycolysis in CRC cells
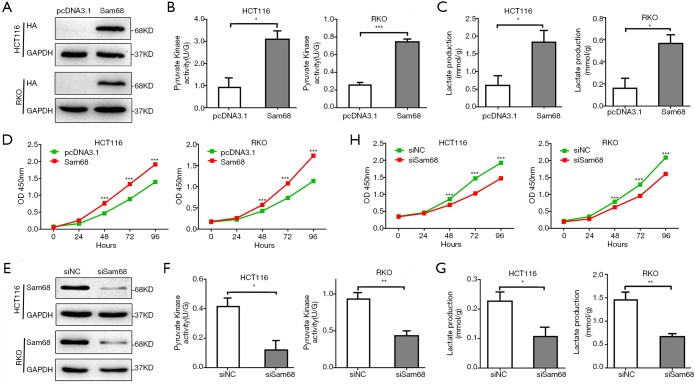
Aerobic glycolysis is an essential hallmark of tumor cells, characterized by elevated glucose uptake and lactate production. We assessed the effects of Sam68 on glycolysis and cell proliferation in CRC cells. Our data showed that Sam68 overexpression increased the activity of pyruvate kinase and promoted the production of lactate (Figure 1A,B,C), thereby induced cell proliferation in CRC cells (Figure 1D). In contrast, Sam68 knockdown significantly decreased pyruvate kinase activity and lactate levels (Figure 1E,F,G) and significantly inhibited cell growth in CRC cells (Figure 1H). Therefore, these data suggested that Sam68 regulated CRC proliferation by promoting glycolysis.
Figure 1.
Sam68 promotes CRC cells proliferation by regulating aerobic glycolysis. (A) The overexpression of Sam68 was determined by Western blotting. (B,C) The pyruvate kinase activity and lactate concentration were measured in CRC cells transfected with pcDNA3.1-Sam68 or pcDNA3.1. (D) Cell proliferation with Sam68-overexpressing HCT116 and RKO cells was measured by CCK8 assays. (E) The knockdown efficiency of Sam68 siRNA was determined by Western blotting. (F,G) The effect of Sam68 knockdown on the pyruvate kinase activity and lactate production in CRC cells. (H) Cells proliferation in the silence of Sam 68-HCT116 and RKO cells was measured using CCK8 assays. *P<0.05, **P<0.01, ***P<0.001. CRC, colorectal cancer; CCK8, cell counting Kit 8.
Identifying the function domains of Sam68 and Sam68 promotes aerobic glycolysis in CRC cells
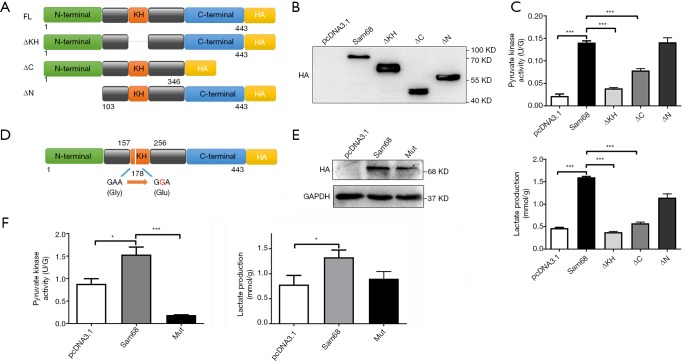
Sam68 protein was consists of three main domains, including the N-terminal domain, KH domain for RNA binding, and the C-terminal domain. To identify the functional domain of Sam68 regulating the glucose metabolism, we constructed four mutations, included HA-tagged of Sam68, KH-domain deleted (ΔKH), N-terminus domain deleted (ΔN) and C-terminus domain deleted (ΔC) (Figure 2A), and then we measured their effects on glycolysis in CRC cells. ΔN mutant of Sam68 retained the regulation functions of full-length Sam68 on pyruvate kinase activity and lactate production fairly, whereas ΔKH mutant lost the regulation effect of Sam68 on glycolysis (Figure 2B,C), suggesting that the KH domain was the critical function domain in the regulation of glycolysis. KH domain-mediated the sequence-specific RNA binding of Sam68, suggesting it may exert its function by regulating the RNA molecules. According to the structure of the Sam68 protein, the G178E amino acid of sam68 is vital for binding to its RNA targets (13). To identify the exact site of KH domain of Sam68 that regulate the process of glycolysis, we transfected HCT116 cells with either a HA-tagged Sam68 (HA-Sam68), a mutant with a point mutation in the KH domain (HA-Sam68 G178E) (13), or the control vector (pcDNA3.1-HA) (Figure 2D), and then detected the pyruvate kinase activity and lactate production. We found that both the pyruvate kinase activity and lactate production were increased with Sam68 overexpression and severely dampened with 178 sites of amino acid mutation in CRC cells (Figure 2E,F).
Figure 2.
The function domain of Sam68 mediates aerobic glycolysis. (A) Schematic diagram for Sam68-fusion protein (residues 1–443), full-length or designated mutants (ΔN lacks residues 1–102, ΔC lacks 347–443, and ΔKH lacks 162–228) fused with HA tag. (B,C) The pyruvate kinase activity and lactate production were measured in CRC cells transfected with different Sam68 mutants. (D) Schematic diagram of Sam68 mutation (G178E) in the KH domain. (E,F) The effects of Sam68 G178E mutation on the pyruvate kinase activity and lactate production in CRC cells. *P<0.05, ***P<0.001. CRC, colorectal cancer.
Sam68 is associated with PKM2 to affect glucose metabolism
To elucidate the detailed molecular mechanism of Sam68 in CRC glucose metabolism, we used several assays to show the potential Sam68-associated RNAs. Recent studies have shown that PKM2 plays a crucial role in tumorigenesis and progression through glycolysis and other pathways. PKM2 is the key rate-limiting enzyme regulating the cellular glycolytic activity and is highly expressed in a variety of malignant cells, including CRC (8). The result of RIP assays confirmed that Sam68 protein could directly bind to PKM2 mRNA (Figure 3A). To show the specific region of Sam68 binding to PKM2, we constructed a series of mutations with Sam68 region deletion based on the catRAPID omics analysis (http://s.tartaglialab.com/) (Figure 3B). A potential Sam68-binding region (aa.141–255) with PKM2 was predicted according to the catRAPID omics analysis. As Figure 3C showed that the KH domain of Sam68 was essential for the binding of Sam68 with PKM2. Then, we found that the G178E mutation in the KH domain (Figure 2D) is crucial to the binding of Sam68 to PKM2 (Figure 3D). There existed one potential Sam68-specific binding motif (ATAA, 1,692–1,695 nt) in PKM2 mRNA, and we constructed a PKM2 mutant with the motif deletion. HA-tagged Sam68 and PKM2 mutants were co-transfected into 293 T cells, and these cells were then subjected to RIP and PCR analyses. The results showed that Sam68 did not bind to PKM2 with the ATAA deletion mutation (Figure 3E).
Figure 3.
Sam68 is associated with PKM2 mRNA to affect glucose metabolism. (A) The binding of Sam68 to PKM2 mRNA in CRC cells transfected with Sam68-HA performed by RIP assays. (B) Predicted Sam68-binding region with PKM2 mRNA by CatRAPID omics (http://s.tartaglialab.com/). (C) Sam68-ΔKH mutant binding domain to PKM2 mRNA. (D) Sam68-G178E mutant binding domain to PKM2 mRNA. (E) Sam68 binding domain to PKM2 mRNA with the ATAA deletion. (F) The PKM2 mRNA levels analysis with overexpression of Sam68 in the cytoplasm. (G) The PKM2 protein levels in ectopic expression of Sam68 cells. **P<0.01. PKM2, pyruvate kinase muscle 2; CRC, colorectal cancer.
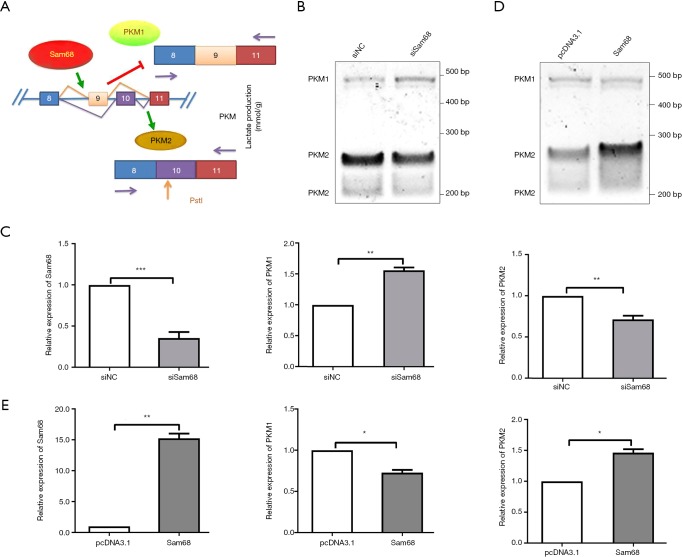
After splicing, PKM2 mRNA was transported to the cytoplasm to translate PKM2 protein and display its pyruvate kinase function. We demonstrated that ectopic Sam68 expression promoted the levels of PKM2 mRNA in cytoplasm, whereas Sam68 knockdown inhibited the cytoplasmic distribution of PKM2 mRNA, suggesting that Sam68 promoted the transport of PKM2 mRNA from the nucleolus to cytoplasm (Figure 3F). Meanwhile, the western blot results found that Sam68 overexpression enhanced the protein expression of PKM2 (Figure 3G).
Sam68 regulates PKM Splicing and increases PKM2 mRNA expression
We next speculated that whether Sam68 could regulate PKM2 splicing. Our data showed that Sam68 binds to EI9 of PKM (Figure 3A). The inclusion of PKM exon 9 (E9) promoted the formation of PKM2 mRNA (Figure 4A). The effects of the Sam68 silencing on PKM alternative splicing were further investigated by qRT-PCR (Figure 4B,C). In contrast, Sam68 overexpression resulted in the opposite phenomenon (Figure 4D,E). Sam68 knockdown resulted in an increase in PKM1/PKM2 mRNA, from 28% to 48%. Sam68 overexpression significantly decreased PKM1/PKM2 mRNA, from 69% to 27%.
Figure 4.
Sam68 promotes PKM2 splicing from PKM. (A) Diagrammatic sketch of PKM splicing assay with PstI digestion protocol. (B) PKM splicing assays in Sam68-depleted HCT116 cells. (C) The effects of the silence of sam68 on PKM splicing by qRT-PCR. (D) PKM splicing assays in Sam68-overexpressing HCT116 cells. (E) The effects of the Sam68 overexpression on the PKM splicing were investigated by qRT-PCR. *P<0.05, **P<0.01, ***P<0.001. PKM2, pyruvate kinase muscle 2.
Sam68 expression is positively correlated with PKM2 in CRC tissues
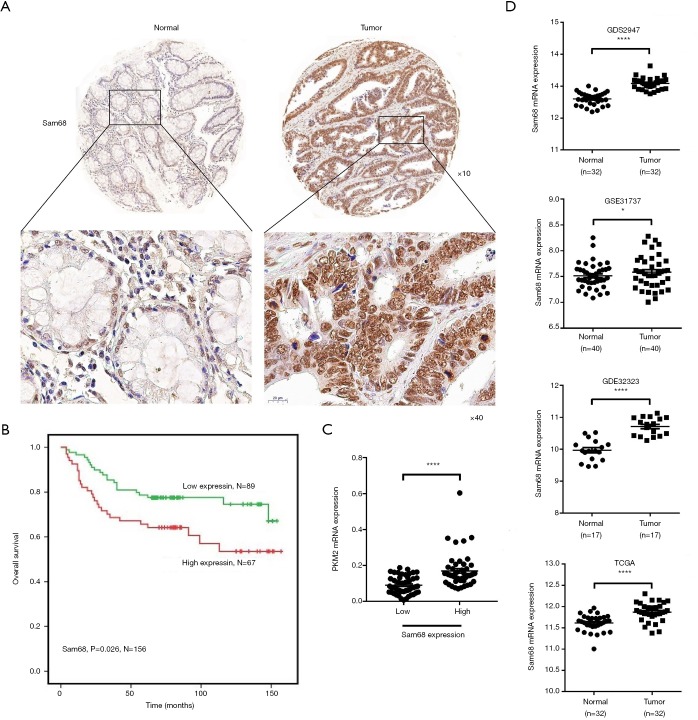
Although it was confirmed that Sam68 and PKM2 could promote CRC progression, their correlations in CRC were still unknown. IHC results confirmed that Sam68 protein expression was also upregulated in CRC tissues, and higher Sam68 protein expression levels were associated with more reduced survival rates (Figure 5A,B). Besides, the protein expression of Sam68 was positively correlated with the PKM2 mRNA expression in CRC tissues (Figure 5C). The analysis results from the TCGA RNA-seq database, GEO GDS2947 database, GEO GSE31737 database, GEO GSE32323 database further showed that Sam68 were also up-regulated at mRNA level in CRC samples compared with NCT samples (Figure 5D). Together, these data suggested that Sam68 could positively regulate PKM2 levels in CRC.
Figure 5.
Sam68 expression is positively correlated with PKM2 in CRC tissues. (A,B) Kaplan-Meier plots of overall survival versus Sam68 protein by IHC in CRC clinical samples from our tissue bank (×10, ×40). (C) The positive correlation between Sam68 protein and PKM2 mRNA expression in clinical CRC tissues. (D) Evaluation of the expression of Sam68 in CRC tissues and their paired normal tissues based on the data from the GDS2947 and TCGA databases. *P<0.05, ****P<0.0001. PKM2, pyruvate kinase muscle 2; CRC, colorectal cancer.
Discussion
CRC is the most frequently occurring form of high mortality cancer worldwide. The reasons causing CRC are numerous, such as genetic epigenetic alterations, damage to DNA, or other influences. Metabolic disturbance also could be regarded as a crucial factor in promoting CRC progress. The metabolism reprogramming of glucose inducing metabolic disturbance has been observed in many types of cancer, including CRC. Aerobic glycolysis, characterized by increased glucose consumption and lactate production, is the typical phenotype of glucose intake displayed in cancer cells. The main change of tumor cells in the glucose metabolism pathway converts to glycolysis, somewhat oxidative phosphorylation promotes cancer initiation and progression (5,19). The genes involved in glucose metabolic disturbance function as the oncogenes or tumor suppressors also played critical roles in that procedure. This study showed that Sam68 regulated glycolysis metabolism and displayed an oncogenic function in CRC cells.
The metabolism of glucose in CRC is involved in a series of proteins, such as glucose transporter 1, glucose transporter 4, hexokinase, 6-phosphofructokinase1, pyruvate kinase, and lactate dehydrogenase A (20). PKM1 and PKM2 were the isoforms of pyruvate kinase and encoded by the same gene, PKM, through alternative splicing. The active form of pyruvate kinases always displayed as tetramers, and PKM1 is showed functioning as a constitutively active tetramer. Various metabolites and functions could allosterically regulate PKM2 as a pyruvate kinase (tetramer) or a protein kinase (dimer). PKM2 expression and activity are transcriptionally or post-transcriptionally regulated by many different molecules (21). The abnormally expressed isoform of PKM2 could be observed in many types of cancer as the typical characteristic and presented high relevance with the tumor progress. For example, we revealed that the long non-coding RNA FEZF1-AS1 could bind to PKM2 and increased its stability by inhibiting its degradation in CRC cells, and the increased PKM2 protein expression is associated with metastasis and prognosis of CRC patients (8). Also, alternative splicing of PKM2 is a crucial mechanism mediating PKM2 upregulation in cancer cells (22-24). Switching from PKM1 to PKM2 subtype results in increased lactate production and decreased oxygen consumption, promoting the growth and metastasis of cancer cells (19). The transition of PKM2 contributes to the control of glycolysis and is essential for tumor cell proliferation and survival. Our study found that Sam68 functions as a PKM splicer and promoted PKM2 expression and activity, contributing to glycolysis in CRC cells.
Although Sam68 is already recognized as a tumorigenic factor (13,25), little was known about its detailed mechanisms in tumorigenesis and progression (12,15). It is previously reported that higher expression of Sam68 is associated with progression and poorer prognosis of several types of cancers, including CRC (26,27). We revealed that CYTOR drives CRC progression by interacting with Nucleolin and Sam68 (15). In this study, we revealed that Sam68 could enhance aerobic glycolysis by regulating PKM alternative splicing (22-25). Mechanistic investigations further found the exact binding sites in the Sam68 KH domain and its recognized RNA sequence in PKM2 mRNA. Through direct binding to the PKM and PKM2 RNA molecules, Sam68 promotes the splicing of PKM2 mRNA and helps it transported to the cytoplasm, resulting in increasing PKM2 protein levels and glycolysis activity in CRC cells. These data show that Sam68 is required for the essential metabolic switch, which tilts the metabolic program from oxidative phosphorylation to more acidic glycolysis, ensuring the growth of CRC cells. Despite the general reputation of Sam68 as an oncogenic factor, the metabolic features of Sam68 in CRC are a novel finding. We believe that Sam68 appears to be a potential target for future CRC treatment, and inhibiting PKM2 expression by mediating alternative splicing may be an available strategy for regulating cancer glycol-metabolism.
Conclusions
In this study, we found that Sam68 can enhance aerobic glycolysis by regulating PKM alternative splicing. Although sam68 is recognized as a carcinogenic factor, its metabolic characteristics in CRC are a discovery. Sam68 seems to be a potential target for CRC therapy in the future. Inhibition of PKM2 expression through the mediation of alternative splicing may be an effective strategy to regulate gluconeogenesis in cancer.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank Dr. Chen Chen for the critical reading of this article.
Funding: This work was supported by National Natural Science Foundation of China (81802462, 81972220 and 81672328), Natural Science Foundation of Jiangsu Province (BK20180618 and BE2019632), and Fundamental Research Funds for the Central Universities (NOJUSRP51619B), Medical Key Professionals Program of Jiangsu Province (AF052141), and Medical Innovation Team Program of Wuxi (CXTP003), the Fundamental Research Funds for the Central Universities (JUSRP11952).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Clinical Research Ethics Committees of the Affiliated Hospital of Jiangnan University, and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.108). The authors have no conflicts of interest to declare.
References
- 1.Wang X, Zhang L, Li H, et al. THBS2 is a Potential Prognostic Biomarker in Colorectal Cancer. Sci Rep 2016;6:33366. 10.1038/srep33366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rampal S, Yang MH, Sung J, et al. Association between markers of glucose metabolism and risk of colorectal adenoma. Gastroenterology 2014;147:78-87.e3. 10.1053/j.gastro.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Suzuki A, Puri S, Leland P, et al. Subcellular compartmentalization of PKM2 identifies anti-PKM2 therapy response in vitro and in vivo mouse model of human non-small-cell lung cancer. PLoS One 2019;14:e0217131. 10.1371/journal.pone.0217131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? (vol 41, pg 211, 2016). Trends Biochemical Sci 2016;41:287-7. 10.1016/j.tibs.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz L, Supuran CT, Alfarouk KO. The Warburg Effect and the Hallmarks of Cancer. Anticancer Agents Med Chem 2017;17:164-70. 10.2174/1871520616666161031143301 [DOI] [PubMed] [Google Scholar]
- 6.Filipp FV. Cancer metabolism meets systems biology: Pyruvate kinase isoform PKM2 is a metabolic master regulator. J Carcinog 2013;12:14. 10.4103/1477-3163.115423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vander Heiden MG, Christofk HR, Schuman E, et al. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol 2010;79:1118-24. 10.1016/j.bcp.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian Z, Zhang J, Li M, et al. LncRNA-FEZF1-AS1 Promotes Tumor Proliferation and Metastasis in Colorectal Cancer by Regulating PKM2 Signaling. Clin Cancer Res 2018;24:4808-19. 10.1158/1078-0432.CCR-17-2967 [DOI] [PubMed] [Google Scholar]
- 9.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metabolism 2016;23:27-47. 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YH, Li XF, Liu JT, et al. PKM2, a potential target for regulating cancer. Gene 2018;668:48-53. 10.1016/j.gene.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 11.Bielli P, Busa R, Paronetto MP, et al. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocrine-Related Cancer 2011;18:R91-102. 10.1530/ERC-11-0041 [DOI] [PubMed] [Google Scholar]
- 12.Frisone P, Pradella D, Di Matteo A, et al. SAM68: Signal Transduction and RNA Metabolism in Human Cancer. Biomed Res Int 2015;2015:528954. [DOI] [PMC free article] [PubMed]
- 13.Fu K, Su X, Wier EM, et al. Sam68/KHDRBS1 is critical for colon tumorigenesis by regulating genotoxic stress-induced NF-kappa B activation. Elife 2016. doi: . 10.7554/eLife.15018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caggiano C, Pieraccioli M, Panzeri V, et al. c-MYC empowers transcription and productive splicing of the oncogenic splicing factor Sam68 in cancer. Nucleic Acids Res 2019;47:6160-71. 10.1093/nar/gkz344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Yu H, Sun W, et al. The long non-coding RNA CYTOR drives colorectal cancer progression by interacting with NCL and Sam68. Mol Cancer 2018;17:110. 10.1186/s12943-018-0860-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bian Z, Jin L, Zhang J, et al. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep 2016;6:23892. 10.1038/srep23892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li N, Richard S. Sam68 functions as a transcriptional coactivator of the p53 tumor suppressor. Nucleic Acids Res 2016;44:8726-41. 10.1093/nar/gkw582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David CJ, Chen M, Assanah M, et al. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 2010;463:364-8. 10.1038/nature08697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto K, Sakamoto K, Kawai M, et al. Serum oxidative stress is an independent prognostic marker in colorectal cancer. Transl Cancer Res 2019;8:1699-708. 10.21037/tcr.2019.08.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graziano F, Ruzzo A, Giacomini E, et al. Glycolysis gene expression analysis and selective metabolic advantage in the clinical progression of colorectal cancer. Pharmacogenomics J 2017;17:258-64. 10.1038/tpj.2016.13 [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Zhang H, Hong H, et al. MiR-374b re-sensitizes hepatocellular carcinoma cells to sorafenib therapy by antagonizing PKM2-mediated glycolysis pathway. Am J Cancer Res 2019;9:765-78. [PMC free article] [PubMed] [Google Scholar]
- 22.Xie R, Chen X, Chen Z, et al. Polypyrimidine tract binding protein 1 promotes lymphatic metastasis and proliferation of bladder cancer via alternative splicing of MEIS2 and PKM. Cancer Lett 2019;449:31-44. 10.1016/j.canlet.2019.01.041 [DOI] [PubMed] [Google Scholar]
- 23.Calabretta S, Bielli P, Passacantilli I, et al. Modulation of PKM alternative splicing by PTBP1 promotes gemcitabine resistance in pancreatic cancer cells. Oncogene 2016;35:2031-9. 10.1038/onc.2015.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Z, Xia J, Xu H, et al. NEK2 Promotes Aerobic Glycolysis in Multiple Myeloma Through Regulating Splicing of Pyruvate Kinase. J Hematol Oncol 2017;10:17. 10.1186/s13045-017-0392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu K, Sun X, Xia X, et al. Sam68 is required for the growth and survival of nonmelanoma skin cancer. Cancer Med 2019;8:6106-13. 10.1002/cam4.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao WT, Liu JL, Wang ZG, et al. High expression level and nuclear localization of Sam68 are associated with progression and poor prognosis in colorectal cancer. BMC Gastroenterol 2013;13:126. 10.1186/1471-230X-13-126 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Sumithra B, Saxena U, Das AB. A comprehensive study on genome-wide coexpression network of KHDRBS1/Sam68 reveals its cancer and patient-specific association. Sci Rep 2019;9:11083. 10.1038/s41598-019-47558-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as