Abstract
Background
Portal vein embolization (PVE) is performed to induce hypertrophy of an insufficient future remnant liver (FRL) before major liver resection. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) aims to offer a more rapid and increased hypertrophy response. The first stage can be performed with complete or partial (laparoscopic) transection of the liver parenchyma. This study aimed to investigate the increase in FRL volume and function, as well as postoperative outcomes after PVE or complete- or partial-ALPPS1.
Methods
Patients with insufficient FRL undergoing either PVE or ALPPS underwent CT-volumetry and functional assessment using 99mTc-mebrofenin hepatobiliary scintigraphy (HBS). Severe complications and 90-day mortality were evaluated after liver resection.
Results
Seventy-two patients were included; 51 underwent PVE, 12 complete-ALPPS1 and 9 partial-ALPPS1 of which 7 laparoscopic. The median increase in FRL function was 1.5-, 1.7- and 1.3-fold higher, respectively, than the increase in volume; (P<0.01, P<0.01 and P=0.44). The target hypertrophy response did not differ between the groups, but was reached earlier in both ALPPS1 groups (8 and 10 days) compared to the PVE group (23 days). Of the resected patients, 18%, 30% and 17% had severe postoperative complications and the 90-day mortality was 2%, 25% and 0%, respectively.
Conclusions
Increase of FRL function exceeded increase of volume after both PVE and ALPPS1. The target hypertrophy response was reached earlier in ALPPS. Complete and partial-ALPPS1 showed comparable functional and volumetric hypertrophy responses. A (laparoscopic) partial-ALPPS1 is preferred considering lower morbidity and mortality rates after resection.
Keywords: Hepatectomy, liver regeneration, liver function tests, liver neoplasms, radionuclide imaging
Introduction
With associating liver partition and portal vein ligation (PVL) for staged hepatectomy (ALPPS), portal vein occlusion (PVO) is combined with parenchymal transection to induce more rapid and increased liver hypertrophy. Using this technique, introduced in 2012, larger liver resections can be performed in a shorter time frame compared to traditional PVO techniques, such as portal vein embolization (PVE) or conventional two-stage resection. Following the initially reported high morbidity and mortality rates of ALPPS (1), several modifications of this procedure have been introduced, such as partial ALPPS in which the extent of parenchymal transection at stage 1 is limited. The latter has also been proposed as a two-stage procedure in which stage 1 is undertaken laparoscopically with minimal parenchymal transection, in combination with sequential percutaneous PVE (2-4). The main advantage of this modification is decreased surgical impact and reduced congestion of the excluded segments leading to a decrease in interstage morbidity and mortality (5).
Using these two-stage, liver volume enhancing techniques, assessment of the future liver remnant is essential in order to monitor the hypertrophy rate and guide the timing of the second stage. Traditionally, this is performed with computed tomography (CT)-volumetry which uses volume as a surrogate measure of liver function. Alternatively, a true functional analysis using technetium-99m (99mTc) mebrofenin hepatobiliary scintigraphy (HBS) provides a quantitative dynamic test to assess liver function. Earlier reports have shown a discrepancy between future remnant liver (FRL) function and volume, especially after liver enhancing techniques (6,7), while functional analysis has shown to be superior to volumetry in the identification of patients with increased surgical risk (8).
Large experience with PVE has proven it to be a safe procedure in a wide range of patients. The long waiting time of several weeks until sufficient FRL growth increases the risk of tumor progression (9,10) leading to approximately 20–30% of patients not receiving final resection after PVE (7,11,12). The clear benefits of ALPPS over PVE alone are the accelerated and larger volumetric gain (13) after stage 1, and the increased resectability rate (14-16). Although modified techniques in ALPPS have shown the procedure to be safer (17), their influence on hypertrophy rate in terms of functional and volumetric increase of the FRL, needs further assessment.
The aim of this study was to compare the increase in FRL volume (FRLV) and function, as well as postoperative outcomes in patients undergoing PVE, complete- or partial-ALPPS1.
Methods
Patients
All consecutive patients that underwent PVE or any modification of the ALPPS procedure at the Amsterdam UMC, location AMC, between January 2012 and October 2018 were included. Patients underwent both CT-volumetry and functional HBS as standard practice during preoperative assessment and this was repeated after PVE or ALPPS stage 1.
PVE
Patients underwent embolization of the right portal system using a percutaneous transhepatic approach. Ultrasound examination was performed to determine whether an ipsilateral or a contralateral approach was best suitable for access to the portal veins. The portal branches were embolized using polyvinyl alcohol particles (300–500 nm, Cook, Bloomington, IN, USA) and coils (Tornado Embolization Microcoil; Cook).
ALPPS procedure
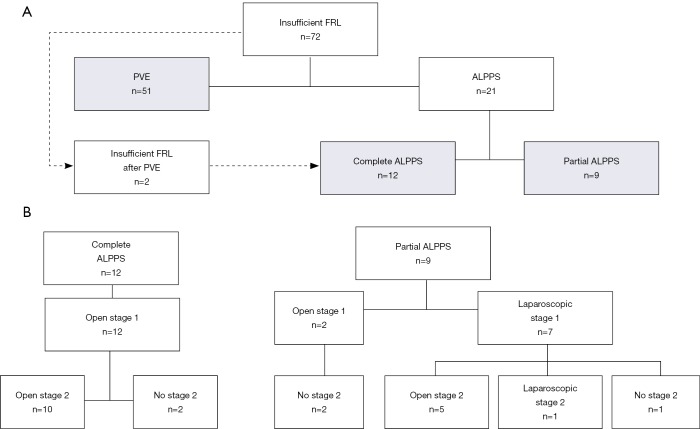
Patients with extensive bilateral disease (n=14), failed PVE (n=2) or unexpected tumor extent during exploration (n=5) were candidates for ALPPS. The surgical technique and its modifications have been described elsewhere (2,18). In case of bilobar disease, tumor deposits in the FRL were resected during the first stage. Subsequently, parenchymal transection was performed using the Cavitron Ultrasonic Surgical Aspirator (CUSA) (Valleylab, Boulder, CO, USA), either through the whole parenchyma with transection of the middle hepatic vein (in case of right trisectionectomy) up to the hepatic hilum (complete-ALPPS1, group B, n=12) or partially transecting the parenchyma (2–4 cm) while preserving the middle hepatic vein and staying well away from the hilar structures (partial-ALPPS1, group C, n=9). Partial-ALPPS1 was carried out laparoscopically using a 3–5 port technique (19), except in two cases in which the decision to perform partial-ALPPS1 was taken intraoperatively during open exploration due to unexpected tumor extension. The first stage was then followed by PVO. This was either conducted as simultaneous PVL in open procedures or as subsequent percutaneous PVE in the first days after stage 1. At stage 2, ten patients that had complete-ALPPS1 underwent open resection. In the partial-ALPPS1 group, six patients (all having undergone stage 1 laparoscopically) went on to stage 2 which was performed open except in one case in which the resection was completed laparoscopically. The technical aspects of the procedures are summarized in Table 1 and schematically shown in Figure 1. Patients were only subjected to stage 2 when liver function had increased sufficiently, and no disease progression, or extra-hepatic disease had been detected in the interstage interval.
Table 1. Technical details of PVE and ALPPS.
| Procedure details | PVE (N=51) | Complete ALPPS (N=12) | Partial ALPPS (N=9) |
|---|---|---|---|
| PVO technique, n (%) | |||
| PVE ipsilateral | 38 (74.5) | 4 (33.3) | 6 (66.7) |
| PVE contralateral | 13 (25.5) | 0 (0) | 1 (11.1) |
| PVL | 0 (0) | 8 (66.7) | 0 (0) |
| No PVO | 0 (0) | 0 (0) | 2 (22.2) |
| PVE after stage 1, n (%) | – | 2 (16.7) | 7 (77.8) |
| Days after stage 1 | 1 and 7 | 3 [2–4] | |
| PVL during stage 1, n (%) | – | 8 (66.7) | 0 |
| PVE before stage 1 (rescue ALPPS), n (%) | – | 2 (16.7) | 0 |
| Stage 1 and 2 open | – | 10 | 0 |
| Stage 1 and 2 laparoscopic | – | 0 | 1 |
| Stage 1 open, stage 2 laparoscopic | – | 0 | 0 |
| Stage 1 laparoscopic, stage 2 open | – | 0 | 5 |
| Stage 1 open, no stage 2 | – | 2 | 2 |
| Stage 1 laparoscopic, no stage 2 | – | 0 | 1 |
PVE, portal vein embolization; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; PVO, portal vein occlusion; PVL, portal vein ligation.
Figure 1.
Flowchart of patients included in the study (A) and surgical details of the ALPPS procedures (B). FRL, future remnant liver; PVE, portal vein embolization; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy.
CT-volumetry
Multiphase contrast-enhanced CT was carried out during preoperative assessment and after PVE or ALPPS stage 1 (MX-8000 or Brilliance; Philips Research Eindhoven, The Netherlands). The arterial phase images were acquired 35 seconds and porto-venous phase 70 seconds after injection of contrast. The portal-venous phase was used for volumetric assessment. The liver was outlined on an axial scan in a semi-automated fashion with manual adjustment to ensure that all extra-hepatic structures were excluded in order to calculate the total liver volume (TLV; cc). The tumor volume (TV; cc) and FRLV (cc) were calculated by manual delineation according to Couinaud’s functional segmentation of the liver (7,20). The volume share of the FRL (FRLV share; %) was calculated using the following formula:
| [1] |
A FRLV share of ≥30% was considered sufficient volume for undertaking safe resection (7).
Functional assessment using HBS
HBS was usually performed on the same day as or close to (range, 1–5 days) the time of volumetric assessment. The acquisition and processing were performed as described elsewhere (21). The dynamic early phase was used to calculate the mebrofenin uptake rate (MUR), representing total liver function (TLF; %/min). Subsequently, the single-photon emission computed tomography (SPECT) with low-dose CT, acquired in a period in which most of the activity is accumulated in the liver, was used to manually delineate the FRL and to calculate the functional share (i.e., functional volume) of the FRL (FRLF share; %). This is the percentage radioactivity counts in the FRL of the total hepatic activity. Consequently, FRL function (FRLF) was calculated as the product of the TLF and FRLF share, and divided by the body surface area (BSA) to compensate for individual metabolic requirements and is presented as %/min/m2.
A cut-off value of 2.7%/min/m2 was used to indicate sufficient FRLF as calculated previously (22).
Hypertrophy parameters
The increase of the FRLV, FRLV share, FRLF share and FRLF was calculated using the following formula:
| [2] |
To compare the hypertrophy responses, the kinetic growth rates (KGR) for FRLV share (KGRFRLV) and FRLF share (KGRFRLF) were calculated by dividing the point differences of the FRLV share and FRLF share between preoperative and interstage assessments by the number of days after PVE or ALPPS stage 1.
| [3] |
Clinical parameters
Baseline patients’ characteristics were recorded from a prospectively maintained, electronic database including tumor type, neo-adjuvant chemotherapy for patients with colorectal liver metastasis (CRLM) and preoperative biliary drainage for patients with biliary tumors. Outcome parameters included severe morbidity, post-hepatectomy liver failure (PHLF) and mortality after each stage or hepatectomy. Severe morbidity was defined according to Clavien-Dindo grade 3A or higher, PHLF was graded B or C according to the International Study Group of Liver Surgery (ISGLS) and mortality as death within 90 days after either stage of hepatectomy (23).
Statistical analysis
Continuous data was expressed as median with interquartile rage (IQR). Discrete variables were expressed as absolute numbers and relative frequencies. Differences in continuous variables between groups were tested using Mann-Whitney U test or Kruskal-Wallis test for unpaired data and Friedman test for paired data. Paired volumetric and functional data between baseline and at interstage were tested using Wilcoxon signed rank test. Differences in discrete variables were tested with a Fisher-Freeman-Halton Test. Statistical analysis were performed using IBM SPSS Statistics (version 24.0; IBM Corp., New York, USA).
Results
Patients
A total of 72 patients who underwent either PVE or ALPPS for insufficient FRL were included. Patients’ characteristics are summarized in Table 2. Overall, 51 patients underwent PVE, 12 patients underwent open complete-ALPPS1 and 9 patients underwent partial-ALPPS1, of which seven were performed laparoscopically. Two patients in the partial-ALPPS1 group underwent an open stage 1, this was because the decision to perform ALPPS was taken during intraoperative exploration. In the complete-ALPPS1 group, 2 patients had ALPPS after failed PVE (rescue-ALPPS), these patients were not included in the analysis in the PVE group (Figure 1). Furthermore, paired functional data was not available in two patients in the complete-ALPPS1 group because one patient did not undergo preoperative HBS and the other did not undergo interstage HBS; these patients were excluded from functional analysis. Figure 1 illustrates the procedures performed and Table 1 describes the technical aspects in detail.
Table 2. Patient characteristics.
| Patient characteristics | PVE (N=51) | Complete ALPPS (N=12) | Partial ALPPS (N=9) | P value |
|---|---|---|---|---|
| Age (years), median [IQR] | 64 [56–70] | 69 [61–72] | 61 [59–64] | 0.292 |
| Sex (male/female) | 32/19 | 7/5 | 4/5 | 0.645 |
| BMI (kg/m2), median [IQR] | 24.4 [22.8–26.6] | 25.8 [24.8–29.1] | 22.5 [22.3–25.5] | 0.034 |
| BSA (m2), median [IQR] | 1.94 [1.77–2.02] | 1.92 [1.72–2.24] | 1.80 [1.60–2.00] | 0.489 |
| Tumor type, n [%] | 0.001 | |||
| CRLM | 17 [33] | 11 [92] | 6 [67] | |
| HCC | 5 [10] | 0 [0] | 2 [22] | |
| Biliary | 26 [51] | 1 [8] | 0 [0] | |
| PHC | 20 [39] | 1 [8] | 0 [0] | |
| IHC | 6 [12] | 0 [0] | 0 [0] | |
| Benign | 2 [4] | 0 [0] | 1 [11] | |
| Other malignant | 1 [2] | 0 [0] | 0 [0] | |
| Neo-adjuvant chemotherapy, n [%] | 17 [33] | 9 [75] | 6 [67] | 0.015 |
| Cycles, median [IQR] | 4 [3–6] | 6 [4–11] | 7 [4–9] | 0.046 |
| Biliary drainage in patients with PHC (number/total PHC) | 17/20 | 1/1 | 0/0 | |
| ASA, n [%] | 0.035 | |||
| ≤2 | 46 [90] | 10 [83] | 5 [56] | |
| >2 | 5 [10] | 2 [17] | 4 [44] | |
| FRL, n [%] | 0.172 | |||
| Left (segments 1–4) | 28 [55] | 7 [58] | 8 [89] | |
| Left lateral (segments 1–3) | 23 [45] | 5 [42] | 1 [11] |
PVE, portal vein embolization; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; BMI, body mass index; BSA, body surface area; CRLM, colorectal liver metastasis; HCC, hepatocellular carcinoma; PHC, perihilar cholangiocarcinoma; IHC, intrahepatic cholangiocarcinoma; ASA, American Society of Anesthesiologists; FRL, future remnant liver; IQR, interquartile range.
The median time from PVE to CT-volumetry and HBS was significantly higher in the PVE group compared to complete-ALPPS1 and partial-ALPPS1 groups {23 [21–26] vs. 8 [5–13] and 10 [9–15] days, P<0.001 for HBS and 23 [22–26] vs. 9 [5–16] and 10 [8–14] days for CT, all P<0.01} (Table 3). The interstage time between both ALPPS groups did not differ significantly (P=0.067 for HBS and P=0.702 for CT).
Table 3. Volume and function parameters.
| Volume and function parameters | PVE (A) (N=51) | Complete ALPPS (B) (N=12) | Partial ALPPS (C) (N=9) | P value |
|---|---|---|---|---|
| Baseline, median [IQR] | ||||
| TLV (cc) | 1,853 [1,513–2,233] | 1,384 [1,198–1,869] | 1,424 [1,236–1,511] | 0.005 |
| FRLV (cc) | 409 [290–523] | 361 [280–480] | 369 [278–413] | 0.494 |
| FRLV share (%) | 23.4 [19.9–30.1] | 25 [19.8–30.1] | 25.6 [19.1–31.9] | 0.785 |
| TLF (%/min) | 14.2 [11.0–16.3] | 13.8 [12.0–16.1] | 14.8 [10.7–16.8] | 0.969 |
| FRLF share (%) | 25.2 [19.5–32.7] | 25.5 [15.8–36.0] | 29.0 [19.0–32.5] | 0.972 |
| FRLF (%/min/m2) | 1.88 [1.49–2.20] | 1.75 [1.08–2.44] | 2.07 [1.71–2.77] | 0.514 |
| Post-PVE or pre stage 2, median [IQR] | ||||
| Time (days) between stage 1 and HBS | 23 [21–26] | 8 [5–13] | 10 [9–15] | <0.001 |
| Time (days) between stage 1 and CT | 23 [22–26] | 9 [5–16] | 10 [8–14] | <0.001 |
| TLV (cc) | 1,830 [1,508–2,269] | 1,934 [1,406–2,679] | 1,639 [1,500–1,827] | 0.481 |
| FRLV (cc) | 553 [420–676] | 574 [450–739] | 554 [451–666] | 0.793 |
| FRLV share (%) | 32.4 [27.1–40.1] | 31.2 [26.5–36.3] | 32.7 [30.5–41.5] | 0.652 |
| TLF (%/min) | 13.8 [12.2–15.5] | 13.6 [11.9–14.5] | 15.0 [13.7–15.8] | 0.098 |
| FRLF share (%) | 44.7 [35.3–54.0] | 40.0 [35.5–52.5] | 43.0 [34.0–45.5] | 0.613 |
| FRLF (%/min/m2) | 3.15 [2.47–3.92] | 2.67 [2.50–3.41] | 3.36 [2.77–4.41] | 0.322 |
PVE, portal vein embolization; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; TLV, total liver volume; FRLV, future remnant liver volume; TLF, total liver function; FRLF, future remnant liver function; HBS, hepatobiliary scintigraphy; CT, computed tomography; IQR, interquartile range.
Volume increase
All included patients had undergone CT-volumetry before and after PVE or ALPPS stage 1. Volumetric data are summarized in Table 3.
The FRLV increased from 409 [290–523] to 553 [420–674] cc in the PVE group (P<0.001], from 361 [280–480] to 574 [450–739] cc in the complete-ALPPS1 group (P=0.002) and from 369 [278–413] to 554 [451–666] cc in the partial-ALPPS1 group (P=0.008).
The FRLV share increased from 23.4% (19.9–30.1%) to 32.4% (27.1–40.1%) in the PVE group (P<0.001), from 25.0% (19.8–30.1%) to 31.2% (26.5–36.3%) in the complete-ALPPS1 group (P=0.05) and from 25.6% (19.1–31.9%) to 33.0% (30.5–41.5%) in the partial-ALPPS1 group (P=0.008).
There were no significant differences in KGRFRLV between PVE and complete-ALPPS1 [2.6% (1.4–3.4%) vs. 5.9% (1.3–8.4%), P=0.077]. KGRFRLV was however significantly increased after partial-ALPPS1 as compared to PVE [7.2% (3.6–11.7%) vs. 2.6% (1.4–3.4%), P=0.001]. The KGRFRLV between complete- and partial-ALPPS1 did not differ significantly [5.9% (1.3–8.4%) vs. 7.2% (3.6–11.7%), P=0.477] (Figure 2).
Figure 2.
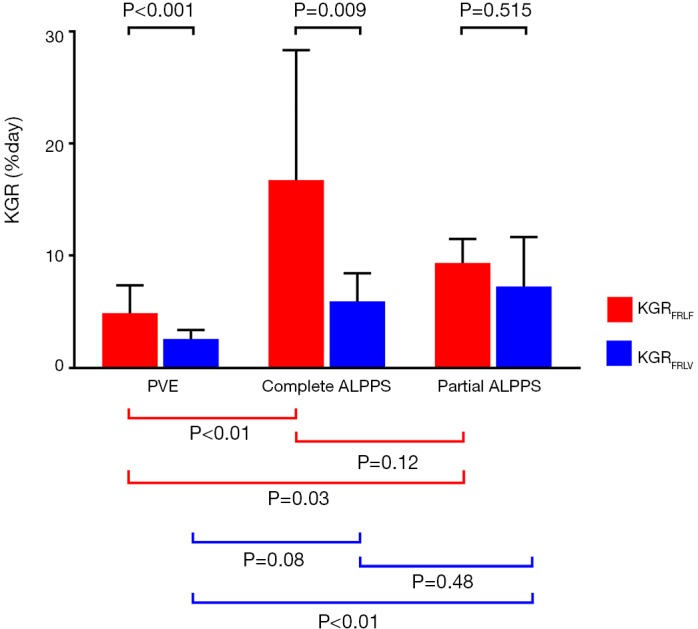
Median (IQR) KGR (%/day) FRLF share and FRLV share. PVE, portal vein embolization; IQR, interquartile range; KGR, kinetic growth rates; FRLF, FRL function; FRLV, FRL volume; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy.
Functional increase
There were no changes in TLF after PVE or ALPPS stage 1 in all groups (P=0.506 for PVE, P=0.508 for complete-ALPPS1 and P=0.477 for partial-ALPPS1), nor did TLF differ between the groups (Table 3).
The FRLF increased from 1.88 (1.49–2.20) to 3.15 (2.47–3.92) %/min/m2 in the PVE group (P<0.001), from 1.75 (1.08–2.44) to 2.67 (2.50–3.41) %/min/m2 in the complete-ALPPS1 group (P=0.009) and from 2.07 (1.71–2.77) to 3.36 (2.77–4.41) %/min/m2 in the partial-ALPPS1 group (P=0.008).
The FRLF share increased from 25.2% (19.5–32.7%) to 44.7% (35.3–54.0%) in the PVE group (P<0.001), from 25.5% (15.8–36.0%) to 40.0% (35.5–52.5%) in the complete-ALPPS1 group (P=0.008) and from 29.0% (19.0–32.5%) to 43.0% (34.0–45.5%) in the partial-ALPPS1 group (P=0.008).
The KGRFRLF was significantly higher in both the complete-ALPPS1 [16.7% (7.8–28.4%)] and partial-ALPPS1 [9.3% (6.4–11.5%)] compared to the PVE group [4.9% (3.2–7.4%)], P=0.002 and P=0.03 respectively. There was no significant difference in KGRFRLF between both ALPPS groups (P=0.102) (Figure 2).
Functional increase versus volume increase
The median increase in FRLF share exceeded the median increase in FRLV share in all groups and was 1.8-, 2.5- and 1.7-fold greater for PVE, complete- and partial-ALPPS1, respectively (P<0.001, P=0.007 and P=0.441).
Moreover, the median KGRFRLF exceeded the median KGRFRLV in all groups and was 1.8-, 2.8- and 1.3-fold greater for PVE, complete- and partial-ALPPS1, respectively (P<0.001, P<0.009 and P=0.515) (Figure 2).
Postoperative outcomes
In the PVE group, 14 patients did not undergo resection for reasons of tumor progression in 12 patients, which was detected either at repeat preoperative imaging or during laparotomy. In the other two patients, resection was cancelled because of locoregional metastasis or too small FRL, respectively.
In the patients who underwent complete-ALPPS1, two patients did not undergo stage 2. One patient had insufficient FRL hypertrophy and one patient (with liver cirrhosis) died after stage 1 due to fulminant liver failure.
Of the nine patients that underwent partial-ALPPS1, three did not undergo stage 2. In two patients, there were severe complications after stage 1 which delayed recovery; these patients later developed tumor progression making further resection not feasible. The third patient had developed severe adhesions at the liver hilum for which reason resection was aborted (Figure 1).
Surgical details and postoperative outcomes are presented in Table 4. In the complete ALPPS group, two patients had severe complications during the interstage period. One patient had refractory shock leading to liver ischemia and the other, obstructive ileus and fascial dehiscence requiring reoperation. In the partial ALPPS groups, three patients had severe complications. One patient had portal vein thrombosis and pulmonary embolism, and two patients had fluid collections that needed drainage. The median FRLF of patients without severe complications was higher than the patients with severe complications; this was however not significant; 3.4 (2.6–4.6) vs. 2.9 (2.6–3.2) %/min/m2, P=0.13. For FRLV share this was 33.8% (30.2–40.5%) vs. 29.1% (25.1–39.1%), P=0.16.
Table 4. Clinical outcomes.
| Clinical outcomes | PVE (A) (N=51) | Complete ALPPS (B) (N=12) | Partial ALPPS (C) (N=9) |
|---|---|---|---|
| Resected, n [%] | 37 [73] | 10 [83] | 6 [67] |
| Time (days) between PVE/stage 1 and resection | 44 [37–57] | 15 [10–19] | 17 [14–42] |
| Type resection, n [%] | |||
| Right | 22 [59] | 7 [70] | 6 [100] |
| Extended right | 15 [41] | 3 [30] | 0 [0] |
| Open | 37 [100] | 10 [100] | 0 [0] |
| Laparoscopic | 0 [0] | 0 [0] | 6 [100] |
| Severe complications*, n [%] | |||
| After stage 1 | – | 2/12 [17] | 3/9 [33] |
| After stage 2/resection | 9/37 [24] | 3/10 [30] | 1/6 [17] |
| Liver failure**, n [%] | |||
| After stage 1 | – | 1/12 [8] | 1/9 [11] |
| After stage 2/resection, n [%] | 1/37 [3] | 2/10 [20] | 0/6 [0] |
| 90-day mortality, n [%] | 1/37 [3] | 3/12 [25] | 0/9 [0] |
*, according to Clavien-Dindo grade 3a or higher; **, according to ISGLS grade B or C. PVE, portal vein embolization; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy.
Three patients after complete ALPPS died within 90 days, all had severe postoperative complications that ultimately led to PHLF. One patient died at interstage due to refractory shock and liver ischemia. The other two developed septic complications after resection leading to PHLF and ultimately multi-organ failure and death. The FRLF in these patients was borderline sufficient in two cases (2.57 and 2.68%/min/m2) but with a sufficient FRLV share (32.0% and 47.6%, respectively). The third patient had a sufficient FRLF of 3.17%/min/m2, but an insufficient FRLV share of 23.8%.
Two patients had undergone previous PVE that resulted in insufficient FRL hypertrophy after a waiting time of 2 and 12 months, respectively. ALPPS was decided after which in one patient, FRLF share increased from 18% to 28% (corresponding with an FRLF increase from 1.6 to 2.7%/min/m2) while the FRLV had marginally increased from 20.4% to 23.8%. This patient underwent resection but then developed abdominal sepsis and fascial dehiscence requiring ICU admission and reoperation, leading to PHLF and sepsis, ultimately leading to his demise, 68 days after liver resection. The other patient did not show any increase in function or volume after ALPPS1 and therefore, resection was declined.
Discussion
This single center study includes both functional and volumetric data of 72 patients who underwent PVE, complete-, or partial-ALPPS1 as liver enhancing techniques. Complete and partial-ALPPS1 showed comparable functional and volumetric hypertrophy responses, whereas the target hypertrophy response was reached earlier after ALPPS1 compared to PVE. Furthermore, the increase of FRLF exceeded the increase in volume after both PVE and ALPPS1. Lower morbidity and mortality rates after resection were observed in the partial-ALPPS1 group compared to the complete-ALPPS1 group, with no mortality after resection in the (modified, less invasive) partial-ALLPS1 group.
Clinical introduction of ALPPS was burdened by the high morbidity and mortality rates of the procedure as reported in the initial series (1,24,25). The early experience consisted mainly of open stage 1 in which complete parenchymal transection was undertaken. In these cases, the first stage was the challenging part and was followed by a less aggressive second stage. Initial poor outcomes were most likely associated with complications in the interstage course due to the extent of stage 1 (26). In the more recent series, technical modifications towards less invasive ALPPS procedures have been developed comprising partial parenchymal transection at stage 1 and a laparoscopic approach (27). By avoiding laparotomy with extensive parenchymal transection and/or hilar dissection during the first stage, the surgical impact is limited, thereby reducing the risk of potential major interstage complications. In addition, by reducing surgical trauma in the first stage, less inflammatory reaction and fewer adhesions will facilitate the second stage in patients with better preserved condition (3).
Technical modifications leading to less invasive ALPPS procedures have shown to be independently associated with decreased morbidity and mortality rates, that are now comparable with standard outcomes accepted for extended liver resections (17). Another reason for the decrease in morbidity in patients with limited parenchymal dissection could be the preservation of the middle hepatic vein. This reduces congestion to segment 4, thereby reducing the risk of biliary leakage and septic complications. Also, minimizing initial transection avoids impairment of arterial blood supply to segment 4 (28). Furthermore, during the course of this study, increased experience of the surgical team will also have had a positive impact on outcomes. Our experience reflects this evolution showing high mortality in the early cases of this series in which complete ALPPS1 was pursued, while outcomes improved with no mortality in the recent cases using partial (laparoscopic) ALPPS1.
Partial parenchymal transection up to 2–4 cm in this study led to a hypertrophy response which was comparable to that of the complete-ALPPS1 group. This finding suggests that limited parenchymal transection is sufficient to induce the accelerated and larger response, as also has been described in previous reports (3,29). The exact mechanisms of the accelerated hypertrophy response in ALPPS are not fully understood. One hypothesis is that because of the parenchymal partition, collateral portal vein perfusion of the deportalized liver is limited, this however does not seem of influence in partial ALPPS considering the comparable hypertrophy response (30). Other authors suggest that the inflammatory response associated with the hepatocyte damage due to parenchymal transection leads to a more pronounced regeneratory stimulus (31).
In this study the increase in liver function after ALPPS1 exceeded the volume increase, a finding that was not observed in other studies, including our own (32-34). Results of the latter studies were likely influenced by the drop in TLF after stage one, due to inclusion of patients with hyperbilirubinemia in whom competitive uptake of mebrofenin and bilirubin occurred at HBS (35). In our management protocol, HBS is not performed when bilirubin exceeds 30–50 µmol/L, which likely contributed to the overall larger functional increase in the present series; there was no case of hyperbilirubinemia after ALPPS stage 1 while median bilirubin was 7 [6–11] µmol/L. Adequate understanding of the HBS technique and its limitations are essential in order to prevent misinterpretations (21). On the other hand, animal and human studies did show that after ALPPS1, the proliferating hepatocytes were morphologically immature (36,37). This could explain the smaller increase in function compared to volume, but does not explain the drop in TLF. Another explanation is the slightly longer time interval between stage 1 and interstage assessment in the present series (a median of 8 days after complete-ALPPS1 and 10 days after partial-ALPPS1) providing more time for the hepatocytes to mature which translates into improved function at HBS.
It is important to rely on functional assessment rather than only volume of the FRL for the decision to perform resection, whether after PVE or the first stage of ALPPS. In this series, 8 patients underwent resection with insufficient FRLV (<30%) but sufficient FRLF (>2.7%/min/m2) with no PHLF or mortality, further emphasizing the additional value of functional assessment. HBS has been implemented in our center since 2012 in the preoperative work-up of patients considered for major liver resection (22). Since the introduction of functional assessment using HBS in our department, the rate of PHLF decreased significantly. Patients that developed PHLF or severe complications had lower FRLF compared to patients without PHLF. However, the number of events was insufficient to analyze whether lower FRLF was the primary risk factor for the increased complication rate. Hence, there is a clear benefit of measuring function rather than volume alone in the selection of patients that are borderline resectable due to insufficient FRL.
There are several limitations to this study, foremost the retrospective design. The small number of patients who underwent ALPPS and the heterogeneity of the groups limited the analysis and requires further assessment in larger cohorts. Furthermore, there were no strict criteria whether to perform complete or partial ALPPS in this study. The choice to perform minimally invasive, partial-ALPPS1 rather was a change of policy due to the initial high morbidity and mortality experienced with complete-ALPPS1 as elaborated above.
Conclusions
In conclusion, the increase of FRLF exceeded increase of volume after both PVE and ALPPS1. The target hypertrophy response was larger and was reached earlier in ALPPS. Complete (open) and partial (laparoscopic) ALPPS1 showed comparable hypertrophy responses. The discrepancy between function and volume increase further emphasizes the importance of functional assessment of liver function. A (laparoscopic) partial-ALPPS1 is preferred considering lower morbidity and mortality rates after resection.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Medical Research Involving Human Subjects Act (WMO) does not apply to this study and that an official approval of this study was granted by Medical Ethics Review Committee of the Academic Medical Center (No. W19_160 #19.196).
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.191). The authors have no conflicts of interest to declare.
References
- 1.Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg 2015;262:780-5; discussion 785-6. 10.1097/SLA.0000000000001450 [DOI] [PubMed] [Google Scholar]
- 2.Linecker M, Kron P, Lang H, et al. Too Many Languages in the ALPPS: Preventing Another Tower of Babel? Ann Surg 2016;263:837-8. 10.1097/SLA.0000000000001632 [DOI] [PubMed] [Google Scholar]
- 3.de Santibanes E, Alvarez FA, Ardiles V, et al. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini-ALPPS technique. Langenbecks Arch Surg 2016;401:557-63. 10.1007/s00423-016-1424-1 [DOI] [PubMed] [Google Scholar]
- 4.Machado MA, Makdissi FF, Surjan RC. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann Surg 2012;256:e13; author reply e16-9. [DOI] [PubMed]
- 5.Truant S, El Amrani M, Baillet C, et al. Laparoscopic Partial ALPPS: Much Better Than ALPPS! Ann Hepatol 2019;18:269-73. 10.5604/01.3001.0012.7937 [DOI] [PubMed] [Google Scholar]
- 6.de Graaf W, van Lienden KP, van den Esschert JW, et al. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg 2011;98:825-34. 10.1002/bjs.7456 [DOI] [PubMed] [Google Scholar]
- 7.Rassam F, Olthof PB, van Lienden KP, et al. Functional and volumetric assessment of liver segments after portal vein embolization: Differences in hypertrophy response. Surgery 2019;165:686-95. 10.1016/j.surg.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 8.Cieslak KP, Bennink RJ, de Graaf W, et al. Measurement of liver function using hepatobiliary scintigraphy improves risk assessment in patients undergoing major liver resection. HPB (Oxford) 2016;18:773-80. 10.1016/j.hpb.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Graaf W, van den Esschert JW, van Lienden KP, et al. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol 2009;16:423-30. 10.1245/s10434-008-0222-6 [DOI] [PubMed] [Google Scholar]
- 10.Hoekstra LT, van Lienden KP, Verheij J, et al. Enhanced tumor growth after portal vein embolization in a rabbit tumor model. J Surg Res 2013;180:89-96. 10.1016/j.jss.2012.10.032 [DOI] [PubMed] [Google Scholar]
- 11.van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. 10.1007/s00270-012-0440-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pommier R, Ronot M, Cauchy F, et al. Colorectal liver metastases growth in the embolized and non-embolized liver after portal vein embolization: influence of initial response to induction chemotherapy. Ann Surg Oncol 2014;21:3077-83. 10.1245/s10434-014-3700-z [DOI] [PubMed] [Google Scholar]
- 13.Croome KP, Hernandez-Alejandro R, Parker M, et al. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB (Oxford) 2015;17:477-84. 10.1111/hpb.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandstrom P, Rosok BI, Sparrelid E, et al. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg 2018;267:833-40. 10.1097/SLA.0000000000002511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schadde E, Schnitzbauer AA, Tschuor C, et al. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol 2015;22:3109-20. 10.1245/s10434-014-4213-5 [DOI] [PubMed] [Google Scholar]
- 16.Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J Surg 2018;42:806-15. 10.1007/s00268-017-4181-6 [DOI] [PubMed] [Google Scholar]
- 17.Linecker M, Bjornsson B, Stavrou GA, et al. Risk Adjustment in ALPPS Is Associated With a Dramatic Decrease in Early Mortality and Morbidity. Ann Surg 2017;266:779-86. 10.1097/SLA.0000000000002446 [DOI] [PubMed] [Google Scholar]
- 18.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. 10.1097/SLA.0b013e31824856f5 [DOI] [PubMed] [Google Scholar]
- 19.van der Poel MJ, Huisman F, Busch OR, et al. Stepwise introduction of laparoscopic liver surgery: validation of guideline recommendations. HPB (Oxford) 2017;19:894-900. 10.1016/j.hpb.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 20.Couinaud C. Liver lobes and segments: notes on the anatomical architecture and surgery of the liver. Presse Med 1954;62:709-12. [PubMed] [Google Scholar]
- 21.Rassam F, Olthof PB, Richardson H, et al. Practical guidelines for the use of technetium-99m mebrofenin hepatobiliary scintigraphy in the quantitative assessment of liver function. Nucl Med Commun 2019;40:297-307. 10.1097/MNM.0000000000000973 [DOI] [PubMed] [Google Scholar]
- 22.de Graaf W, van Lienden KP, Dinant S, et al. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg 2010;14:369-78. 10.1007/s11605-009-1085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. 10.1016/j.surg.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 24.Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829-36; discussion 836-8. 10.1097/SLA.0000000000000947 [DOI] [PubMed] [Google Scholar]
- 25.Alvarez FA, Ardiles V, de Santibanes M, et al. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg 2015;261:723-32. 10.1097/SLA.0000000000001046 [DOI] [PubMed] [Google Scholar]
- 26.Truant S, Scatton O, Dokmak S, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol 2015;41:674-82. 10.1016/j.ejso.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 27.Petrowsky H, Gyori G, de Oliveira M, et al. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg 2015;261:e90-2. 10.1097/SLA.0000000000001087 [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, et al. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery 2015;157:194-201. 10.1016/j.surg.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 29.Linecker M, Kambakamba P, Reiner CS, et al. How much liver needs to be transected in ALPPS? A translational study investigating the concept of less invasiveness. Surgery 2017;161:453-64. 10.1016/j.surg.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 30.Wei W, Zhang T, Zafarnia S, et al. Establishment of a rat model: Associating liver partition with portal vein ligation for staged hepatectomy. Surgery 2016;159:1299-307. 10.1016/j.surg.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 31.Schlegel A, Lesurtel M, Melloul E, et al. ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg 2014;260:839-46; discussion 846-7. 10.1097/SLA.0000000000000949 [DOI] [PubMed] [Google Scholar]
- 32.Olthof PB, Tomassini F, Huespe PE, et al. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: Liver volume overestimates liver function. Surgery 2017;162:775-83. 10.1016/j.surg.2017.05.022 [DOI] [PubMed] [Google Scholar]
- 33.Truant S, Baillet C, Deshorgue AC, et al. Contribution of hepatobiliary scintigraphy in assessing ALPPS most suited timing. Updates Surg 2017;69:411-9. 10.1007/s13304-017-0481-5 [DOI] [PubMed] [Google Scholar]
- 34.Sparrelid E, Jonas E, Tzortzakakis A, et al. Dynamic Evaluation of Liver Volume and Function in Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. J Gastrointest Surg 2017;21:967-74. 10.1007/s11605-017-3389-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olthof PB, Coelen RJS, Bennink RJ, et al. (99m)Tc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB (Oxford) 2017;19:850-8. 10.1016/j.hpb.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 36.Tong YF, Meng N, Chen MQ, et al. Maturity of associating liver partition and portal vein ligation for staged hepatectomy-derived liver regeneration in a rat model. World J Gastroenterol 2018;24:1107-19. 10.3748/wjg.v24.i10.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuo K, Murakami T, Kawaguchi D, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 2016;159:1289-98. 10.1016/j.surg.2015.12.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as