Abstract
Background
The utility of postmastectomy radiotherapy (PMRT) in breast cancer patients with T1–2 (tumor size ≤5 cm) and N1 (one to three lymph nodes involved) disease remains controversial. The aim of this population-based study was to investigate the effectiveness of PMRT in this patient subset in the current clinical practice.
Methods
We included T1–2N1 breast cancer patients treated with mastectomy from 2004 to 2012 using the data form the Surveillance, Epidemiology, and End Results program. The association of PMRT administration with breast cancer-specific survival was determined using multivariable Cox analysis.
Results
We identified 10,248 patients of this study, including 3,725 (36.3%) received PMRT and 6,523 (63.7%) patients did not receive PMRT. Use of PMRT showed increase from 2008 onward; the percentage of patients receiving PMRT was 30.6% in 2004 and was 47.1% in 2012 (P<0.001). Patients diagnosis after 2008, aged <50 years, high tumor grade, T2 stage, and ≥2 positive lymph nodes were independently related to PMRT receipt. Multivariate analysis indicated that PMRT was not related to better breast cancer–specific survival compared to those without PMRT both before (P=0.186) and after propensity score matching (P=0.137).
Conclusions
In breast cancer with T1–2N1 disease, PMRT does not appear to improve survival in the era of modern systemic therapy.
Keywords: Breast cancer, mastectomy, radiotherapy, 1–3 lymph node metastases
Introduction
There is a generally accepted consensus that postmastectomy radiotherapy (PMRT) should be administered to breast cancer patients with ≥4 axillary nodes involved or tumor size larger than 5 cm. However, the benefit of PMRT in patients with T1–2 tumors (tumor size ≤5 cm) and one to three axillary nodes involved (N1) has not been fully elucidated. The findings from Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) found that PMRT could provide survival benefit in T1–2N1 patients. There was absolute benefit of 16.7% for locoregional recurrence (LRR) and 7.9% for breast cancer-specific survival (BCSS) (1). However, cyclophosphamide, methotrexate, and fluorouracil was the main chemotherapy regimen in patients enrolled in the EBCTCG study. The findings from the Danish Breast Cancer Cooperative Group (DBCG) 82 b and c trials showed that the addition PMRT reduced the LRR and translated to a larger decrease of breast cancer specific death in T1–2N1 patients than those with ≥4 lymph node metastases (2). However, the DBCG trials were limited by suboptimal chemotherapy intensity and lymph node dissection.
Two recent randomized trials, the European Organisation for Research and Treatment of Cancer (EORTC) 22922/10952 trial and National Cancer Institute of Canada MA.20 trial, which were more representative of modern systemic therapy, also supported the utility of PMRT and/or irradiation to regional lymph node for N1 disease (3,4). However, mastectomy was performed in only one quarter of patients in EORTC 22922/10952 trial and none of whom received mastectomy in the MA.20 trial.
The outcomes of N1 disease after mastectomy have been improved in recent studies, but this mostly reflects improvements in cancer screening, surgical approaches, and systemic therapy (5-7). In the modern series, the 5-year LRR in patients without PMRT was in the range of 3.2–6.1%, and remained <10% even after 10 years of follow-up (5,8-15). Therefore, the absolute benefits of PMRT for T1–2N1 disease may actually be small. Recently updated guidelines from the American Society of Clinical Oncology, American Society of Radiation Oncology, and Society of Surgical Oncology, the panel unanimously agreed that the administration of PMRT could decrease the risk of LRR and improve BCSS of T1–2N1 patients. However, some T1–2N1 patients may have a lower risk of LRR that any survival benefit with PMRT will be outweighed by the adverse effects (16). This knowledge can guide the clinician and patients for the decision making of PMRT due to the detrimental impacts including the associated costs, worse self-reported chest wall symptoms, and late toxicity by PMRT (17,18). In light of this, the aims of this population-based study were to investigate the clinical value of PMRT in T1–2N1 breast cancer and to perform stratified analyses based on patient characteristics.
Methods
Surveillance, epidemiology, and end results (SEER) and patients
The present study retrospectively collected data for patients between 2004 and 2012 using the SEER program. The SEER program of the National Cancer Institute is a cancer statistics resource that includes data on cancer incidence, demographics, clinicopathological variables, treatment, and vital status derived from 18 cancer registries in the United States (US) (19). Women who met the following inclusion criteria were considered: (I) they had a pathologically confirmed diagnosis of T1–2N1 invasive ductal carcinoma; (II) they had undergone mastectomy with lymph node dissection and removal of ≥10 lymph nodes; (III) they had received chemotherapy with or without PMRT; and (IV) available data on race/ethnicity, hormone receptor (HoR) status, tumor grade, as well as marital status. Patients were excluded if they (I) did not have a positive pathology diagnosis, (II) had a metastatic disease at the time of initial diagnosis; (III) had received radiotherapy before mastectomy, or (IV) had been treated with non-beam radiotherapy. The patients were separated into two groups according to whether or not they were treated with PMRT and outcomes were compared between the groups. In addition, the impact of PMRT on different patient subgroups was examined. Our study was exempt from the approval process by ethics committee because the SEER program only contains anonymous data.
Variables
The following patients characteristics were included in this study: years of diagnosis, age at diagnosis, race/ethnicity, tumor stage, HoR status, tumor grade, number of axillary nodes involved, marital status, and receipt/non-receipt of PMRT. The primary outcome examined in this study was BCSS, which was estimated from the time of diagnosis of breast cancer to the time of death from breast cancer or the follow-up cutoff.
Statistical analysis
The χ2 test was conducted to compare the demographic and clinicopathological variables between the PMRT and non-PMRT groups. Binomial logistic regression was performed to determine the independent predictors related to the administration of PMRT. We used a 1:1 propensity score matching (PSM) to decrease the selection bias between PMRT and Non-PMRT cohorts (20,21). BCSS curves were plotted by the Kaplan-Meier method and log-rank test was conducted to compare BCSS between PMRT and Non-PMRT cohorts. Multivariate Cox proportional hazard survival analyses were performed to examine the effectiveness of PMRT in terms of BCSS. P values less than 0.05 were defined as statistical significance. All data were analyzed using SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Results
We identified 10,248 patients in the analysis. Of all the patients, 53.1% (n=5,437), 58.7% (n=6,109), 72.7% (n=7,454), and 62.3% (n=6,387) were poorly/undifferentiated, T2 stage, estrogen receptor (ER) positive, and progesterone receptor (PR) positive disease, respectively. In addition, 4,962 (48.4%), 3,235 (31.6%), and 2,051 (20.0%) patients were one, two, and three axillary nodes involved, respectively. Table 1 summarizes the patients’ characteristics.
Table 1. Characteristics of patients in the two treatment cohorts before and after PSM.
| Variables | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | No, n (%) | Yes, n (%) | P | n | No | Yes | P | ||
| Year of diagnosis | <0.001 | 1 | |||||||
| 2004 | 1,018 | 706 (10.8) | 312 (8.4) | 472 | 236 | 236 | |||
| 2005 | 942 | 671 (10.3) | 271 (7.3) | 398 | 199 | 190 | |||
| 2006 | 1,021 | 716 (11.0) | 305 (8.2) | 480 | 240 | 240 | |||
| 2007 | 1,229 | 827 (12.7) | 402 (10.8) | 614 | 307 | 307 | |||
| 2008 | 1,287 | 814 (12.5) | 473 (12.7) | 730 | 365 | 365 | |||
| 2009 | 1,306 | 848 (13.0) | 458 (12.3) | 698 | 349 | 349 | |||
| 2010 | 1,270 | 773 (11.9) | 497 (13.3) | 738 | 369 | 369 | |||
| 2011 | 1,138 | 619 (9.5) | 519 (13.9) | 674 | 337 | 337 | |||
| 2012 | 1,037 | 549 (8.4) | 488 (13.1) | 618 | 309 | 309 | |||
| Age (years) | <0.001 | 1 | |||||||
| <50 | 4,468 | 2,587 (39.7) | 1,881 (50.5) | 2,500 | 1,250 | 1,250 | |||
| ≥50 | 5,780 | 3,936 (60.3) | 1,844 (49.5) | 2,922 | 1,461 | 1,461 | |||
| Race/ethnicity | 0.029 | 1 | |||||||
| Non-Hispanic White | 6,638 | 4,263 (65.4) | 2,375 (63.8) | 3,886 | 1,943 | 1,943 | |||
| Non-Hispanic Black | 1,242 | 744 (11.4) | 498 (13.4) | 542 | 271 | 271 | |||
| Hispanic (all races) | 1,277 | 825 (12.6) | 452 (12.1) | 570 | 285 | 285 | |||
| Other | 1,091 | 691 (10.6) | 400 (10.7) | 424 | 212 | 212 | |||
| Grade | <0.001 | 1 | |||||||
| Well-differentiated | 864 | 657 (10.1) | 207 (5.6) | 272 | 136 | 136 | |||
| Moderately differentiated | 3,947 | 2,599 (39.8) | 1,348 (36.2) | 2,036 | 1,018 | 1,018 | |||
| Poorly/undifferentiated | 5,437 | 3,267 (50.1) | 2,170 (58.3) | 3,114 | 1,557 | 1,557 | |||
| Tumor stage | <0.001 | 1 | |||||||
| T1 | 4,139 | 2,938 (45.0) | 1,201 (32.2) | 1,756 | 878 | 878 | |||
| T2 | 6,109 | 3,585 (55.0) | 2,524 (67.8) | 3,666 | 1,833 | 1,833 | |||
| Number of positive lymph nodes (n) | <0.001 | 1 | |||||||
| 1 | 4,962 | 3,534 (54.2) | 1,428 (38.3) | 2,396 | 1,198 | 1,198 | |||
| 2 | 3,235 | 2,008 (30.8) | 1,227 (32.9) | 1,872 | 936 | 936 | |||
| 3 | 2,051 | 981 (15.0) | 1,070 (28.7) | 1,154 | 577 | 577 | |||
| ER status | 0.002 | 1 | |||||||
| Negative | 2,794 | 1,711 (26.2) | 1,083 (29.1) | 1,488 | 744 | 744 | |||
| Positive | 7,454 | 4,812 (73.8) | 2,642 (70.9) | 3,934 | 1,967 | 1,967 | |||
| PR status | 0.007 | 1 | |||||||
| Negative | 3,861 | 2,394 (36.7) | 1,467 (39.4) | 1,966 | 983 | 983 | |||
| Positive | 6,387 | 4,129 (63.3) | 2,258 (60.6) | 3,456 | 1,728 | 1,728 | |||
| Marital status | 0.981 | ||||||||
| Unmarried | 3,556 | 2,264 (34.7) | 1,292 (34.7) | – | – | – | – | ||
| Married | 6,692 | 4,259 (65.3) | 2,433 (65.3) | – | – | – | – | ||
ER, estrogen receptor; PR, progesterone receptor; PSM, propensity score matching.
PMRT was administered to 3,725 (36.3%) patients and not administered to 6,523 (63.7%) patients. Patients diagnosed after 2008, aged <50 years, poorly differentiated/undifferentiated tumors, ≥2 lymph node metastases, and ER negative as well as PR negative status were more likely to receive postoperative PMRT. Figure 1 shows the change in PMRT use from 2004 to 2012, notably that the percentage of PMRT receipt was significantly increased after 2008. The percentage of patients treated with PMRT was 30.6% in 2004 and was 47.1% in 2012 (P<0.001). Binomial regression analysis showed that patients diagnosed after 2008, aged <50 years, higher tumor grade, T2 stage disease as well as ≥2 positive lymph nodes were independent predictors associated with the administration of PMRT (Table 2).
Figure 1.
Change in use of PMRT during the study period. PMRT, postmastectomy radiotherapy.
Table 2. Independent predictors of PMRT receipt.
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Year of diagnosis | |||
| 2004 | 1 | ||
| 2005 | 0.929 | 0.760–1.135 | 0.471 |
| 2006 | 0.991 | 0.815–1.205 | 0.928 |
| 2007 | 1.183 | 0.983–1.423 | 0.075 |
| 2008 | 1.369 | 1.142–1.640 | 0.001 |
| 2009 | 1.291 | 1.077–1.547 | 0.006 |
| 2010 | 1.548 | 1.293–1.855 | <0.001 |
| 2011 | 1.986 | 1.653–2.386 | <0.001 |
| 2012 | 2.142 | 1.776–2.582 | <0.001 |
| Age (years) | |||
| <50 | 1 | ||
| ≥50 | 0.623 | 0.573–0.678 | <0.001 |
| Race/ethnicity | |||
| Non-Hispanic White | 1 | ||
| Non-Hispanic Black | 1.101 | 0.967–1.253 | 0.147 |
| Hispanic (all races) | 0.853 | 0.748–0.972 | 0.017 |
| Other | 0.978 | 0.852–1.124 | 0.759 |
| Grade | |||
| Well differentiated | 1 | ||
| Moderately differentiated | 1.409 | 1.182–1.679 | <0.001 |
| Poorly/undifferentiated | 1.745 | 1.468–2.073 | <0.001 |
| Tumor stage | |||
| T1 | 1 | ||
| T2 | 1.583 | 1.449–1.729 | <0.001 |
| Number of positive lymph nodes (n) | |||
| 1 | 1 | ||
| 2 | 1.497 | 1.360–1.648 | <0.001 |
| 3 | 2.640 | 2.367–2.948 | <0.001 |
| ER status | |||
| Negative | 1 | ||
| Positive | 0.963 | 0.838–1.106 | 0.595 |
| PR status | |||
| Negative | 1 | ||
| Positive | 0.961 | 0.847–1.091 | 0.543 |
| Marital status | |||
| Unmarried | 1 | ||
| Married | 1.004 | 0.917–1.099 | 0.932 |
PMRT, postmastectomy radiotherapy; CI, confidence interval; ER, estrogen receptor; OR, odds ratio; PR, progesterone receptor.
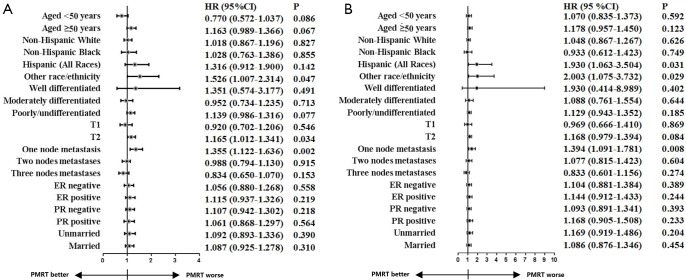
At median follow-up of 76 months, Kaplan-Meier analysis indicated that the PMRT cohort had worse 5-year BCSS than those in non-PMRT cohort before PSM (90.6% vs. 92.5%; log-rank test, P<0.001) (Figure 2). However, after adjustment for age, race/ethnicity, tumor stage, number of lymph nodes involved, tumor grade, HoR status, and marital status, comparable BCSS was observed between PMRT and Non-PMRT groups [hazard ratio (HR) =1.089, 95% confidence interval (CI): 0.960–1.235; P=0.186] (Table 3). Similar results were found after stratification by age category, HoR status, tumor grade, and marital status using multivariate Cox analysis (Figure 3A).
Figure 2.
Comparison of BCSS between the non-PMRT and PMRT cohorts before (A) and after (B) PSM. BCSS, breast cancer-specific survival; PMRT, postmastectomy radiotherapy; PSM, propensity score matching.
Table 3. Multivariate analysis of prognostic factors (before and after PSM).
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) | |||||||
| <50 | 1 | 1 | |||||
| ≥50 | 1.119 | 0.989–1.268 | 0.075 | 1.142 | 0.969–1.347 | 0.113 | |
| Race/ethnicity | |||||||
| Non-Hispanic White | 1 | 1 | |||||
| Non-Hispanic Black | 1.254 | 1.062–1.482 | 0.008 | 1.141 | 0.899–1.448 | 0.278 | |
| Hispanic (all races) | 0.943 | 0.777–1.144 | 0.550 | 0.730 | 0.540–0.987 | 0.041 | |
| Other | 0.839 | 0.676–1.040 | 0.109 | 0.880 | 0.645–1.201 | 0.420 | |
| Grade | |||||||
| Well differentiated | 1 | 1 | |||||
| Moderately differentiated | 1.905 | 1.281–2.832 | 0.001 | 1.825 | 0.891–3.739 | 0.100 | |
| Poorly/undifferentiated | 2.857 | 1.929–4.233 | <0.001 | 3.060 | 1.501–6.238 | 0.002 | |
| Tumor stage | |||||||
| T1 | 1 | 1 | |||||
| T2 | 1.846 | 1.607–2.120 | <0.001 | 1.925 | 1.564–2.369 | <0.001 | |
| Number of positive lymph nodes (n) | |||||||
| 1 | 1 | 1 | |||||
| 2 | 1.125 | 0.979–1.292 | 0.097 | 0.968 | 0.804–1.164 | 0.727 | |
| 3 | 1.234 | 1.059–1.438 | 0.007 | 1.105 | 0.901–1.355 | 0.338 | |
| ER status | |||||||
| Negative | 1 | 1 | |||||
| Positive | 0.805 | 0.676–0.958 | 0.015 | 0.796 | 0.611–1.039 | 0.093 | |
| PR status | |||||||
| Negative | 1 | 1 | |||||
| Positive | 0.555 | 0.466–0.661 | <0.001 | 0.553 | 0.422–0.724 | <0.001 | |
| Marital status | |||||||
| Unmarried | 1 | 1 | |||||
| Married | 0.862 | 0.761–0.977 | 0.020 | 0.845 | 0.718–0.995 | 0.044 | |
| PMRT | |||||||
| No | 1 | 1 | |||||
| Yes | 1.089 | 0.960–1.235 | 0.186 | 1.129 | 0.962–1.324 | 0.137 | |
CI, confidence interval; ER, estrogen receptor; HR, hazard ratios; PMRT, postmastectomy radiotherapy, PR, progesterone receptor; PSM, propensity score matching.
Figure 3.
Adjusted hazard ratios for BCSS between non-PMRT and PMRT cohorts in different subgroups before (A) and after (B) PSM. BCSS, breast cancer-specific survival; PMRT, postmastectomy radiotherapy; PSM, propensity score matching.
The matching variables in PSM included years of diagnosis, age at diagnosis, race/ethnicity, tumor grade, tumor stage, number of lymph nodes involved, and HoR status. A total of 2,711 pairs of patients were completely matched using PSM (Table 1). In the PSM cohort, comparable BCSS was found between Non-PMRT and PMRT groups, the 5-year BCSS was 91.5% and 90.6%, respectively (log-rank test, P=0.174) (Figure 2B). Multivariate Cox analysis also showed that the receipt of PMRT was not correlated with better BCSS (HR =1.129, 95% CI: 0.962–1.324; P=0.137) (Table 3). Similar results were found after stratification by age category, tumor stage, tumor grade, HoR status, and marital status (Figure 3B).
Discussion
The utility of PMRT remains controversial for T1–2N1 breast cancer in recent decades. In the present study, we assessed the clinical value of PMRT in this patient subset using the data available in the SEER database and found that the addition of PMRT was not correlated with better BCSS in the current practice setting.
The trend in the receipt of PMRT has varied significantly over the years. A study from the National Cancer Database indicated that the utilization of PMRT increased significantly from 23.9% in 2003 to 36.4% in 2011, with an annual percentage change of 6.2% (22). The proportion of patients receiving PMRT was lower in Asia, but the utilization of PMRT increased in recent years (from 7.4% to 9.3% in earlier years to 14.1% to 23.5% in recent years) (8,23). In our study, the use of PMRT started increasing after 2008; while 30.6% of patients received PMRT in 2004 and 47.1% received PMRT in 2012. The significantly increasing of PMRT receipt from 2008 onward may be related to the publication of findings from the DBCG trials, which demonstrated a survival benefit from PMRT for this population (2). The National Comprehensive Cancer Network guidelines also started strongly recommending PMRT in this population.
In addition to the years of diagnosis, there were several indicator associated with PMRT administration including young age, large tumor size, high tumor grade, higher number of positive axillary nodes, extracapsular nodal extension, lymphovascular invasion (LVI), invasive lobular histology, and triple negative disease (22-24). In our study, patients with aged <50 years, higher tumor grade, T2 stage as well as ≥2 lymph node metastases were found to be independent predictors of PMRT administration. However, ER status, PR status, and marital status were not related to PMRT administration.
The EBCTCG meta-analysis showed that PMRT could improve locoregional control and survival outcomes in T1–2N1 breast cancer when systemic therapy was given. However, most patients in that study received insufficient systemic therapy. The 10-year LRR rates in patients receiving systemic therapy were 21.0% and 4.3% in the non-PMRT and PMRT groups, respectively (1). The findings from DBCG 82 b&c trials also showed lower risk of LRR and better survival outcomes with the addition of PMRT (2). However, the suboptimal axillary management and systemic treatment in the DBCG trials may have been responsible for the higher risk of LRR and breast cancer related death. It should be noted that modern systemic treatments were more effective for reducing the risk of LRR and distant metastases (25,26). In modern series, the 5- and 10-year LRR rate was only 3.2% to 6.1% and less than 10% for this population without PMRT, respectively (5,8-15). As we demonstrated in our study, PMRT did not appear to be associated with better BCSS in the era of modern systemic therapy. All patients in our study received chemotherapy and had ≥10 lymph nodes removed, which may decrease the potential bias of patients selection and strengthen our results. Therefore, for women considering PMRT today, the absolute risk of LRR may be lower than that of women in the trials cited above, and the absolute risk reduction achieved with the administration of PMRT may be smaller. The secondary analyses of Breast Cancer International Research Group (BCIRG)-005 trial and Breast International Group 02-98 trial showed that the receipt of PMRT was associated with lower risk of LRR (10-year LRR: 2–2.5% vs. 6.5–9.0% in PMRT vs. no PMRT cohorts), though survival outcomes were comparable between the treatment arms (14,15). Muhsen et al. showed that the receipt of PMRT was not related to better locoregional control and lower breast cancer related death (24). Several recent studies also indicated that PMRT was not correlated with lower risk of LRR in this population treated in the modern era (5,6). However, Chang et al. showed a significantly improved disease-free survival (DFS) in patients with extremely low risk of LRR who received PMRT, suggesting that the progress of systemic treatment might not mitigate the survival benefit of PMRT in this patient subset (8). However, their study only had 97 patients in the PMRT cohort, which may limit the generalizability of their findings.
Several studies have attempted to identify the subgroups likely to derive survival benefit with PMRT. Our previous study have showed that PMRT was related to better locoregional control and DFS in high-risk subsets of patients [i.e., T2 stage, ≥2 axillary nodes involved, and human epidermal growth factor receptor 2 (HER2)-enriched subtype] (27). Another SEER study that included patients diagnosed between 1998 and 2008 showed a reduction in breast cancer related death after PMRT only in patients with three axillary nodes involved or those with T2 stage and two axillary nodes involved (28). However, 40.2% of patients in the above study had <10 lymph nodes removed, and the information of chemotherapy was also not included in the analysis. The common high-risk factors for LRR in T1–2N1 patients including young age, large tumor size, higher number of positive lymph nodes, LVI, HoR negative, extracapsular extension, and close resection margin (29). However, the results regarding to high-risk factors of LRR in T1–2N1 patients were inconsistent. With the development of genetic testing, the association between genetic testing and the risk of LRR may help guide to the decision-making of PMRT in this patient subset.
Subgroup analysis in this study indicated that the administration of PMRT was associated with lower BCSS in patients with T2 stage, one positive lymph node, and other race (Figure 3). This was probably due to selection bias, as patients with adverse factors were more likely to receive PMRT.
The primary strengths in the present study include the large sample size and the subgroup analysis. In addition, our study arises from a real-world database that is more generalizability and represents the current population. However, several limitations should be recognized of our study. First, this was a retrospective study, and the two treatment cohorts were probably not comparable in all respects. Patients in the PMRT cohort had more adverse prognostic factors, and this might have influenced the results. Second, details on chemotherapy regimen, endocrine therapy, and targeted therapy are not recorded in the SEER database. In the US, 50–80% of patients with node-positive breast cancer were treated with anthracycline-based chemotherapy from 1999 to 2005, and taxane-based chemotherapy regimens were increased after 2005 (30). Therefore, we assumed that taxanes and anthracyclines were the main systemic treatment regimens used in our study. As all patients in the present study was received chemotherapy and the 5-year BCSS was over 90%, we can assume that these patients may have better compliance with endocrine therapy and targeted therapy. Third, the SEER database does not have information on Ki-67, HER2, and LVI status; the dose and target volume of PMRT; and the patterns of LRR. Finally, we recognized that there was potential underreporting of PMRT in the SEER database (31).
Conclusions
In conclusion, PMRT does not appear to improve survival of T1–2N1 breast cancer patients treated in the modern era. We expect the results of the Selective Use of Postoperative Radiotherapy After Mastectomy (SUPREMO) trial to clarify the indications of PMRT in this patient subset.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Funding: This work was partly supported by the National Natural Science Foundation of China (81872459, 81803050), the Commission Young and Middle-aged Talents Training Project of Fujian Health Commission (No. 2019-ZQNB-25), and the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The approval process of Institutional Review Board was waived because of the de-identified information of the patients included in the SEER.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.49). The authors have no conflicts of interest to declare.
References
- 1.EBCTCG (Early Breast Cancer Trialists' Collaborative Group) , McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. 10.1016/S0140-6736(14)60488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol 2007;82:247-53. 10.1016/j.radonc.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Poortmans PM, Collette S, Kirkove C, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 2015;373:317-27. 10.1056/NEJMoa1415369 [DOI] [PubMed] [Google Scholar]
- 4.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:307-16. 10.1056/NEJMoa1415340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asaga S, Kinoshita T, Shiino S, et al. Prognostic factors for breast cancer patients with T1–2 tumor and 1–3 positive axillary nodes treated using total mastectomy without radiotherapy. Breast J 2019;25:26-33. 10.1111/tbj.13148 [DOI] [PubMed] [Google Scholar]
- 6.Miyashita M, Tada H, Suzuki A, et al. Minimal impact of postmastectomy radiation therapy on locoregional recurrence for breast cancer patients with 1 to 3 positive lymph nodes in the modern treatment era. Surg Oncol 2017;26:163-70. 10.1016/j.suronc.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Rahman O. Impact of postmastectomy radiotherapy on the outcomes of breast cancer patients with T1–2 N1 disease: An individual patient data analysis of three clinical trials. Strahlenther Onkol 2019;195:297-305. 10.1007/s00066-018-1343-x [DOI] [PubMed] [Google Scholar]
- 8.Chang JS, Lee J, Kim KH, et al. Do Recent Advances in Diagnostic and Therapeutic Procedures Negate the Benefit of Postmastectomy Radiotherapy in N1 Patients With a Low Risk of Locoregional Recurrence? Medicine (Baltimore) 2015;94:e1259. 10.1097/MD.0000000000001259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HJ, Shin KH, Kim JH, et al. Incorporating Risk Factors to Identify the Indication of Post-mastectomy Radiotherapy in N1 Breast Cancer Treated with Optimal Systemic Therapy: A Multicenter Analysis in Korea (KROG 14-23). Cancer Res Treat 2017;49:739-47. 10.4143/crt.2016.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazan JG, Majithia L, Quick AM, et al. Heterogeneity in Outcomes of Pathologic T1–2N1 Breast Cancer After Mastectomy: Looking Beyond Locoregional Failure Rates. Ann Surg Oncol 2018;25:2288-95. 10.1245/s10434-018-6565-8 [DOI] [PubMed] [Google Scholar]
- 11.Chang JH, Shin KH, Ahn SD, et al. Chest wall recurrence in pT1–2N0-1 breast cancer patients after mastectomy without radiotherapy. Breast Cancer Res Treat 2018;169:507-12. 10.1007/s10549-018-4707-0 [DOI] [PubMed] [Google Scholar]
- 12.Abi Jaoude J, de Azambuja E, Makki M, et al. Post-Mastectomy Radiation Therapy in HER-2 Positive Breast Cancer Patients: Analysis of the HERA Trial. Int J Radiat Oncol Biol Phys 2020;106:503-10. 10.1016/j.ijrobp.2019.10.022 [DOI] [PubMed] [Google Scholar]
- 13.Joo JH, Kim SS, Son BH, et al. Axillary Lymph Node Dissection Does Not Improve Post-mastectomy Overall or Disease-Free Survival among Breast Cancer Patients with 1–3 Positive Nodes. Cancer Res Treat 2019;51:1011-21. 10.4143/crt.2018.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam MM, Wu SP, Perez C, et al. The effect of post-mastectomy radiation in women with one to three positive nodes enrolled on the control arm of BCIRG-005 at ten year follow-up. Radiother Oncol 2017;123:10-4. 10.1016/j.radonc.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 15.Zeidan YH, Habib JG, Ameye L, et al. Postmastectomy Radiation Therapy in Women with T1-T2 Tumors and 1 to 3 Positive Lymph Nodes: Analysis of the Breast International Group 02-98 Trial. Int J Radiat Oncol Biol Phys 2018;101:316-24. 10.1016/j.ijrobp.2018.01.105 [DOI] [PubMed] [Google Scholar]
- 16.Recht A, Comen EA, Fine RE, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol 2016;34:4431-42. 10.1200/JCO.2016.69.1188 [DOI] [PubMed] [Google Scholar]
- 17.Wennstig AK, Wadsten C, Garmo H, et al. Long-term risk of ischemic heart disease after adjuvant radiotherapy in breast cancer: results from a large population-based cohort. Breast Cancer Res 2020;22:10. 10.1186/s13058-020-1249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velikova G, Williams LJ, Willis S, et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2-year follow-up results of a randomised controlled trial. Lancet Oncol 2018;19:1516-29. 10.1016/S1470-2045(18)30515-1 [DOI] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program. Available online: www.seer.cancer.gov
- 20.Moons P. Propensity weighting: how to minimise comparative bias in non-randomised studies? Eur J Cardiovasc Nurs 2020;19:83-8. 10.1177/1474515119888972 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC, Jembere N, Chiu M. Propensity score matching and complex surveys. Stat Methods Med Res 2018;27:1240-57. 10.1177/0962280216658920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao K, Liederbach E, Lutfi W, et al. Increased utilization of postmastectomy radiotherapy in the United States from 2003 to 2011 in patients with one to three tumor positive nodes. J Surg Oncol 2015;112:809-14. 10.1002/jso.24071 [DOI] [PubMed] [Google Scholar]
- 23.Chang JS, Choi JE, Park MH, et al. Trends in the Application of Postmastectomy Radiotherapy for Breast Cancer With 1 to 3 Positive Axillary Nodes and Tumors ≤5 cm in the Modern Treatment Era: A Retrospective Korean Breast Cancer Society Report. Medicine (Baltimore) 2016;95:e3592. 10.1097/MD.0000000000003592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhsen S, Moo TA, Patil S, et al. Most Breast Cancer Patients with T1–2 Tumors and One to Three Positive Lymph Nodes Do Not Need Postmastectomy Radiotherapy. Ann Surg Oncol 2018;25:1912-20. 10.1245/s10434-018-6422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire A, Lowery AJ, Kell MR, et al. Locoregional Recurrence Following Breast Cancer Surgery in the Trastuzumab Era: A Systematic Review by Subtype. Ann Surg Oncol 2017;24:3124-32. 10.1245/s10434-017-6021-1 [DOI] [PubMed] [Google Scholar]
- 26.Pondé NF, Zardavas D, Piccart M. Progress in adjuvant systemic therapy for breast cancer. Nat Rev Clin Oncol 2019;16:27-44. 10.1038/s41571-018-0089-9 [DOI] [PubMed] [Google Scholar]
- 27.He ZY, Wu SG, Zhou J, et al. Postmastectomy radiotherapy improves disease-free survival of high risk of locoregional recurrence breast cancer patients with T1–2 and 1 to 3 positive nodes. PLoS One 2015;10:e0119105. 10.1371/journal.pone.0119105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo D, Hou N, Jaskowiak N, et al. Use of Postmastectomy Radiotherapy and Survival Rates for Breast Cancer Patients with T1-T2 and One to Three Positive Lymph Nodes. Ann Surg Oncol 2015;22:4295-304. 10.1245/s10434-015-4528-x [DOI] [PubMed] [Google Scholar]
- 29.Kaššák F, Rossier C, Picardi C, et al. Postmastectomy radiotherapy in T1–2 patients with one to three positive lymph nodes - Past, present and future. Breast 2019;48:73-81. 10.1016/j.breast.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 30.Giordano SH, Lin YL, Kuo YF, et al. Decline in the use of anthracyclines for breast cancer. J Clin Oncol 2012;30:2232-9. 10.1200/JCO.2011.40.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care 2016;54:e55-64. 10.1097/MLR.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as