Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of cirrhosis in the USA.
Objectives
We aimed to determine the time to develop hepatic events in patients with NAFLD and develop a simple model to identify patients at risk for hepatic decompensation.
Design
Retrospective cohort study.
Patients
Seven hundred patients with NAFLD met inclusion criteria for the study. Patients were divided into model construction (n = 450) and validation (n = 250) cohorts.
Main Measures
Demographic, clinical, and laboratory variables were gathered at the time of diagnosis of NAFLD. Kaplan-Meier analysis determined the time to development of hepatic events from initial diagnosis. A time-to-event prediction model was established in the model construction cohort using the multivariate Cox proportional hazards model and was then internally validated.
Key Results
Forty-nine (7%) patients developed hepatic events at a mean duration of 6.2 ± 4.2 years from initial diagnosis. Kaplan-Meier probability of developing a hepatic event at 5-, 10-, and 12-year intervals was 4.8%, 10.6%, and 11.3%, respectively. Age, presence of diabetes, and platelet count were identified as significant variables to predict hepatic events. NAFLD decompensation risk score was developed as “age × 0.06335 + presence of diabetes (yes = 1, no = 0) × 0.92221 − platelet count × 0.01522” to predict the probability of hepatic decompensation. Risk score model had an area under the curve of 0.89 (95% CI = 0.92, 0.86) and it performed well in both the validation (0.91, 0.87–0.94) and the overall cohort (0.89, 0.87–0.91).
Conclusions
A significant proportion of patients with NAFLD developed hepatic decompensation. We have provided a simple, objective model to help identify “at-risk” patients.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-05725-1) contains supplementary material, which is available to authorized users.
KEY WORDS: liver disease, fatty liver, cirrhosis, prevention, metabolic syndrome
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is among the most common liver disorders seen in the world.1–3 Estimates of prevalence in the USA have ranged between 10 and 46%2, 4–6 with incidence rising in the last decade.5 This is likely secondary to the worsening obesity epidemic, as key NAFLD risk factors include obesity, insulin resistance, and type II diabetes.7 NAFLD is a spectrum of liver disease divided into non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH).8 The majority of NAFLD patients have a good long-term prognosis and will likely not see progression of disease.9 However, some patients are at increased risk of developing decompensated cirrhosis and hepatocellular carcinoma (HCC). The incidence of decompensated cirrhosis from NAFLD is on the rise10–12 with liver-related complications seen in up to 20% of NAFLD patients, and liver-related death or need for liver transplantation in up to 13%.11 NAFLD cirrhosis is becoming the most common indication for liver transplant in North America and Europe. Patients frequently present to hepatologists with advanced fibrosis and decompensated cirrhosis, raising the need for identification of at-risk patients as early as possible.
The degree of liver fibrosis is one of the most significant predictors of risk of cirrhosis and hepatic decompensation. A 2015 longitudinal study of NAFLD patients found an association between fibrosis stage and cirrhotic decompensation, mortality, and liver transplantation.13 The current gold standard for estimating the degree of fibrosis is liver biopsy. However, this is an invasive procedure associated with several complications,14 and sampling error can lead to inaccurate staging.15 Non-invasive testing by elastography can reliably predict the degree of liver fibrosis, but is not readily available in non-specialty settings.16 Other non-invasive approaches utilizing serum laboratory tests such as the NAFLD fibrosis score, the BARD score, the neutrophil-to-lymphocyte ratio, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) ratio, AST-to-platelet ratio index (APRI), and the FIB-4 score have been designed to rule out advanced liver fibrosis in the NAFLD population.17–21 Currently available models, however, have not been shown to reliably predict hepatic events or hepatic event-free survival. This is important as survival decreases dramatically in patients with hepatic decompensation.22 Patients with advanced fibrosis and cirrhosis may remain compensated for years without disease progression, while some patients with NASH but with no clinical evidence of cirrhosis may be at risk for developing hepatic events. Thus, in this study, we aimed to (1) determine the time-to-development of hepatic events (defined as ascites, hepatic encephalopathy, esophageal varices, and/or hepatocellular carcinoma) and hepatic event-free survival in NAFLD patients without obvious evidence of cirrhosis, (2) identify easily available clinical and laboratory variables that are predictors of development of hepatic events, and (3) develop a simple objective model to identify NAFLD patients at high risk of developing hepatic events, which is the strongest clinical predictor of liver-related survival in NAFLD patients.22
METHODS
Patient Population
A database of patients seen at the University of Iowa Hospitals and Clinics between 2000 and 2016 was created by obtaining ICD codes that contained NAFLD, NASH, fatty liver, NASH cirrhosis, cryptogenic cirrhosis, or unspecified cirrhosis. Patient charts were individually reviewed and patients with NAFLD were included in the study. Patients seen at both primary care and specialty clinics were included in the study. Clinical data was gathered at the time of diagnosis of fatty liver disease through the electronic medical record (EMR), including referring hospital media when available.
Patient Selection
The diagnosis of NAFLD was defined as per the current American Association for the Study of Liver Diseases (AASLD) guidelines as (i) presence of hepatic steatosis in imaging (ultrasound, elastography, CT scan, or MRI) and/or liver histology (ii) lack of significant alcohol use (as defined below), or the chronic use of a medication that can cause steatosis, or lack of a condition that can secondarily cause steatosis.23 Patients were excluded if they had other risk factors for chronic liver disease or fatty liver,8 such as heavy alcohol use (defined as > 7 drinks per week for females, > 14 drinks per week for males, or documentation of alcohol abuse), viral hepatitis, drug-induced liver injury, autoimmune liver disease, or genetic liver diseases. Patients with known malignancy were excluded from the study. Patients with evidence of cirrhosis on imaging and/or elastography, and patients with evidence of cirrhosis on liver biopsy, at the time of diagnosis were excluded. Finally, patients who developed hepatic decompensation/hepatic event within 12 months of NAFLD diagnosis (n = 7) were excluded as they likely had unrecognized cirrhosis and portal hypertension at initial diagnosis.22
Data Collection
Demographic, clinical, and biochemical data was gathered through the EMR at times of initial diagnosis of NAFLD, hepatic decompensation/event, liver transplantation, and death. Clinical data included gender, age, ethnicity, body mass index (BMI), and presence of diabetes (defined by hemoglobin A1c (HgbA1c) ≥ 6.5%, inclusion in problem list, or use of diabetic medication). Biochemical values included serum sodium, creatinine, total bilirubin, alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), aspartate transaminase (AST), alanine transaminase (ALT), prothrombin time, serum albumin, platelet count, hemoglobin level, white blood cell count (WBC), neutrophil count, lymphocyte count, monocyte count, ferritin, and transferrin saturation. Laboratory values were not recorded in the setting of acute illness to avoid entering spurious values of acute phase reactants. The NAFLD fibrosis score was calculated for patients with the following formula: − 1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2), + 1.13 × impaired fasting glucose or presence of diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet (× 109/l) − 0.66 × albumin (g/dl).14 APRI was calculated with the following formula: AST level/ upper limit of normal (IU/l)/platelet count (× 109/l) × 100.24
Statistical Analysis
All data (n = 700) in the study was used to analyze the time-to-develop hepatic decompensation/events. Kaplan-Meier analysis was used to determine the time from initial diagnosis of NAFLD to the development of hepatic events and hepatic event-free survival at 5-, 10-, and 12-year intervals. Cox proportional hazards analysis was used to develop the model to predict hepatic events in patients with cirrhosis (NAFLD decompensation model). Time was measured from the date of diagnosis of NAFLD to the date of development of hepatic event, death, or date of last follow-up. Patients who remained alive without any hepatic events were censored at the time of last follow-up.
All 700 patients in the study were then randomly divided into two cohorts: a model construction cohort (n = 450), and a model validation cohort (n = 250). In the construction cohort, univariate analysis using Cox regression was performed to identify variables that predict the development of hepatic events in NAFLD patients. Covariates with a P value ≤ 0.10 were considered significant. Significant variables on univariate analysis and variables with clinical justification for inclusion in the model were included in multivariate Cox regression analysis with stepwise backward elimination approach until all remaining variables had a P value ≤ 0.05.
The Cox proportional hazards model was used to determine the risk score for the development of hepatic events in NAFLD patients using the adjusted regression parameters of the predictive variables. Efron method was used to handle tied events in the model, and test of proportional hazards assumption was performed based on Schoenfeld residuals. Predictive power and the area under the receiver operating characteristic curve (AUROC) of the survival model was performed using the Harrell’s “c” concordance test (c-statistic) which provides time-dependent AUROC taking into account the censoring distribution.25, 26 The model was then cross-validated for predictive accuracy in both the validation cohort (n = 250) and the overall cohort (n = 700). We developed the formula to estimate the predicted probability of an individual NAFLD patient to develop a hepatic event at 5-, 10-, and 12-year intervals from the time of diagnosis, with the Cox proportional hazards model taking the standard form, S(t,P) = S0(t)exp (aP1 + bP2+ … .zPn), where S(t,P) is the predicted event-free survival at time t of a patient with values P1 to Pn for each predictor of hepatic event as determined on multivariate analysis, and S0(t) is the “baseline” event-free survival as estimated in our population at different times. The percent probability of development of hepatic events can then be determined as “1-predicted probability.” All statistical analyses were performed using the Statistical Analysis Software (SAS), version 9.4. SAS Institute Inc., Cary, NC, USA, and the Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.
RESULTS
Patient Population Characteristics
A total of 3086 patients from the original database were screened, with 700 patients meeting the inclusion criteria of NAFLD without clinical evidence of cirrhosis at the time of diagnosis. Age at diagnosis varied from 19 to 81; mean age was 48.6 ± 12.5. There were 267 (38.1%) males, and 223 (31.8%) patients had diabetes mellitus. BMI at diagnosis ranged from 18.1 to 75.4, with mean BMI was 36.2 ± 7.3. Six hundred twenty-two (89%) patients identified as Caucasian or White, 50 (7%) as Hispanic, 12 as Black (2%), 11 (2%) as Asian, and 5 (< 1%) as Native American. Mean follow-up for the patient population was 6.6 ± 4.2 years, with a total of 4620 person-years. Baseline characteristics are provided in Table 1.
Table 1.
Baseline Characteristics of All 700 Patients
| Variable | No hepatic events (n = 651) | Hepatic events (n = 49) |
|---|---|---|
| Age | 46.9 ± 12.4 | 57 ± 9.5 |
| Male sex | 244 (38%) | 23 (47%) |
| BMI | 36.3 ± 7.3 | 35.8 ± 6.7 |
| Diabetes mellitus | 196 (30%) | 27 (55%) |
| HbgA1c | 6.6 ± 1.7 | 7.7 ± 2.1 |
| Serum sodium | 139.3 ± 5.2 | 139.1 ± 2.5 |
| Serum creatinine | 0.86 ± 0.2 | 1.0 ± 0.2 |
| WBC count | 8.0 ± 3.6 | 7.1 ± 2.4 |
| Neutrophil count | 5012 ± 2076 | 4084 ± 1777 |
| Lymphocyte count | 2162 ± 1212 | 1711 ± 749 |
| Neutrophil/lymphocyte ratio | 4.2 (1.7, 6.7) | 2.6 (2.2, 3.0) |
| Monocyte count | 521 ± 236 | 456 ± 183 |
| Platelet count | 263 (244–283) | 183 (164–201) |
| Total bilirubin | 0.50 (0.47–0.53) | 0.68 (0.58–0.77) |
| ALP | 92.0 (88.6–95.5) | 126.8 (64.5–189.0) |
| GGT | 87.3 (76.6–98.1) | 114 (79.8–144) |
| AST | 48.5 (44.5–54.6) | 61.1 (47.8–74.4) |
| ALT | 63.9 (59.7–68.2) | 71.4 (51.5–91.2) |
| Serum albumin | 4.4 (4.3–4.5) | 4.1 (4.0–4.2) |
| Prothrombin time | 10.9 ± 1.8 | 11.7 ± 3.2 |
| Serum transferrin | 25.8 (24.0–27.5) | 30.2 (24.0–36.5) |
| Serum ferritin | 219 (189–251) | 256 (135–378) |
| APRI Index | 0.63 (0.57–0.69) | 1.1 (0.89–1.4) |
| NAFLD fibrosis score | − 1.6 (− 1.8, − 1.3) | − 0.56 (− 1.3, − 0.17) |
BMI: body mass index; HgbA1c: hemoglobin A1c; WBC: white blood cell; ALP: alkaline phosphatase; GGT: gamma-glutamyl transferase: AST: aspartate aminotransferase; ALT: alanine aminotransferase; APRI: AST-to-platelet ratio index: NAFLD: non-alcoholic fatty liver disease
Time to Hepatic Decompensation
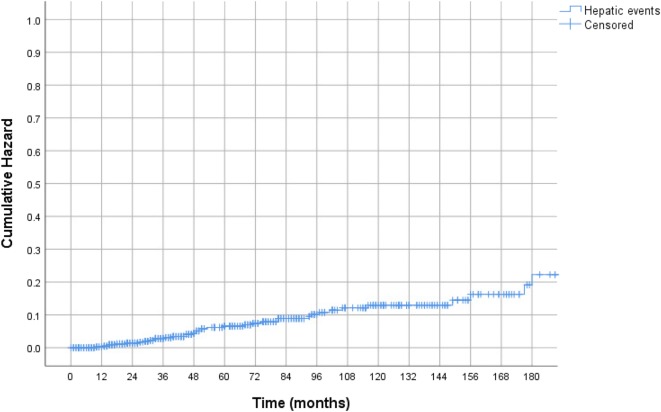
Forty-nine patients (7%) developed hepatic events at a mean duration of 6.2 ± 4.2 years from initial diagnosis of NAFLD. Among these patients, 26 developed esophageal varices, 15 developed ascites, 6 developed hepatic encephalopathy, and 1 developed HCC; 1 patient presented with both esophageal varices and hepatic encephalopathy. Kaplan-Meier probability of developing a hepatic event at 5-, 10-, and 12-year intervals was 4.8%, 10.6%, and 11.3%, respectively (Fig. 1). All-cause mortality was 5.1% (36 out of 700 patients). Liver transplantation was performed in nine patients. Kaplan-Meier probability of hepatic event-free survival at 5-, 10-, 12-year intervals was 94.7%, 86.2%, and 82.4%, respectively (Appendix Fig. 1).
Fig. 1.
Kaplan-Meier “time-to-hepatic event” curve of the entire cohort.
Predictors of Hepatic Decompensation
In the construction cohort (n = 450), univariate analysis identified age, presence of diabetes mellitus, HgbA1c, platelet count, total bilirubin, albumin, AST/ALT ratio, INR, APRI, and NAFLD fibrosis score as significant predictors (Table 2). BMI, sodium, creatinine, ferritin, and neutrophil/lymphocyte ratio were also included in multivariate analysis as they were thought to be clinically significant. On multivariate Cox regression analysis of admission variables, only advanced age (P < 0.001), presence of diabetes mellitus (P = 0.011), and low platelet count (P < 0.001) were significant predictors of development of hepatic events in patients with NAFLD (Table 3). Addition of other variables to this model did not significantly improve the “c” statistic.
Table 2.
Univariate Analysis by Cox Regression
| Variable | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| Age | 1.084 (1.047–1.123) | < 0.0001 |
| Male sex | 1.082 (0.557–2.104) | 0.816 |
| BMI | 1.023 (0.974–1.074) | 0.3673 |
| Diabetes mellitus | 2.831 (1.417–2.657) | 0.003 |
| HgbA1c | 1.531 (1.217–1.926) | 0.0003 |
| Serum creatinine | 3.498 (0.648–18.875) | 0.1454 |
| WBC count | 0.940 (0.803–1.099) | 0.4365 |
| Neutrophil count | 0.999 (0.9997–1.000) | 0.7391 |
| Lymphocyte count | 0.999 (0.998–1.000) | 0.154 |
| Neutrophil/lymphocyte ratio | 0.996 (0.949–1.047) | 0.8874 |
| Monocyte count | 1.000 (0.999–1.002) | 0.6761 |
| Platelet count | 0.983 (0.979–0.988) | < 0.0001 |
| Total bilirubin | 5.407 (2.576–11.351) | < 0.0001 |
| ALP | 1.001 (1.000–1.003) | 0.0605 |
| GGT | 1.001 (0.998–1.004) | 0.6634 |
| AST | 1.004 (0.999–1.009) | 0.1533 |
| ALT | 1.001 (0.996–1.006) | 0.7791 |
| AST/ALT ratio | 1.713 (1.075–2.730) | 0.023 |
| Serum Albumin | 0.354 (0.193–0.649) | 0.0008 |
| Prothrombin time | 2.067 (0.947–1.204) | 0.285 |
| Serum transferrin | 1.018 (0.988–1.049) | 0.245 |
| Serum ferritin | 1 (0.998–1.001) | 0.9205 |
| APRI index | 1.678 (1.316–2.140) | <0.0001 |
| NAFLD fibrosis score | 1.306 (1.080–1.580) | 0.006 |
BMI: body mass index; HgbA1c: hemoglobin A1c; WBC: white blood cell; ALP: alkaline phosphatase; GGT: gamma-glutamyl transferase; AST: aspartate aminotransferase; ALT: alanine aminotransferase, APRI: AST-to-platelet ratio index; NAFLD: non-alcoholic fatty liver disease
Table 3.
Final Multivariate Cox Regression Model
| Variable | Hazard’s ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age | 1.064 | 1.028–1.103 | < 0.001 |
| Diabetes mellitus | 2.549 | 1.249–5.206 | 0.011 |
| Platelet count | 0.985 | 0.979–0.989 | < 0.001 |
Model Development
We developed the predictive model using the three variables significant on multivariate analysis—age, presence of diabetes, and platelet count. No statistically significant interactions were identified between the three variables. The test of proportional-hazard assumption had a χ2 of 0.4 and a P > χ2 of 0.9398, suggesting no violation of the proportional-hazard assumption. The c-statistic for the model was 0.89 (95% CI = 0.86, 0.92). In comparison, the c-statistic for APRI was 0.76 (0.72, 0.79), and for NAFLD fibrosis score was 0.60 (0.56, 0.64). Time-variant AUROC was also higher for the model as compared to the APRI and NAFLD fibrosis score (Appendix Figs. 2, 3, and 4). The Iowa NAFLD Decompensation Risk Score was calculated as = age × 0.06335 + presence of diabetes (yes = 1, no = 0) × 0.92221 − platelet count × 0.01522. The hazard ratio was calculated as exp(decompensation risk score).
The predicted probability of an individual patient P with NAFLD to have an event-free status at 5-, 10-, and 12-year intervals was calculated using the formula S(t,P) = S0(t)(Hazard ratio (P)) where S0(t) is the baseline survival. The estimated baseline event-free survival for our cohort at 5-, 10-, and 12-year intervals was 0.98, 0.95, and 0.94, respectively. The probability of an individual NAFLD patient to develop hepatic event at time “t” can then be calculated as 1-predicted probability at time “t.” For example, using our model, the probability of developing a hepatic event for a 60-year-old NAFLD patient with diabetes and a platelet count of 160,000 cells/mm3 at 5-, 10-, and 12-year intervals are 18%, 40%, and 46% respectively; the probability of a 50-year-old NAFLD patient without diabetes and a platelet count of 250,000 cells/mm3 5-, 10-, and 12-year intervals are 1%, 2.7%, and 3.2% respectively.
Validation of the Model
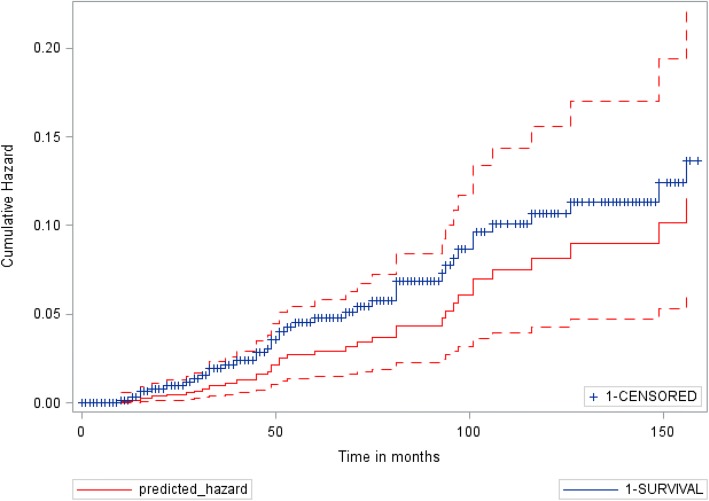
The diagnostic accuracy of the model was cross-validated in the validation cohort (n = 250) and overall cohort (n = 700), with c-statistics of 0.91 (0.87, 0.94) and 0.89 (0.87, 0.91), respectively. In comparison, the c-statistic for APRI was 0.86 (0.82, 0.90) and 0.78 (0.75, 0.81), and for the NAFLD fibrosis score was 0.71 (0.65, 0.77) and 0.63 (0.59, 0.66), respectively. Similarly, the AUROC for the model was better than APRI and NAFLD fibrosis score (Appendix Figs. 5, 6, and 7). Figure 2 shows comparison of the “observed” Kaplan-Meier “time-to-hepatic event” curve for the entire cohort of 700 patients to the “expected” cumulative hazard as predicted by our Cox proportional hazard model derived from the model development cohort of 450 patients.
Fig. 2.
Observed Kaplan-Meier “time-to-hepatic event” curve (blue line) versus the predicted cumulative hazard curve (solid red line) with interval bounds (dotted red line) for the entire cohort of 700 patients.
Analysis of NAFLD Patients Stratified by NAFLD Fibrosis Score
Five hundred eighty-five (84%) patients had a “low” or “indeterminate” NAFLD fibrosis score and 115 (16%) patients had a high NAFLD fibrosis score. Of the 585 patients with low or indeterminate score, 31 (5.3%) developed hepatic events, while 18 (15.6%) of 115 patients with a high NAFLD fibrosis score developed hepatic events (P < 0.01). We tested our NAFLD decompensation model for diagnostic accuracy in the 585 patients with low or indeterminate scores. Our model had c-statistic of 0.89 (95% CI = 0.86, 0.91); APRI had c-statistic 0.78 (95% CI = 0.75, 0.81).
Analysis of NAFLD Patients Stratified by Abnormal ALT Level
Three hundred and eighty-six patients (55%) had ALT level above the upper limit of normal (> 42 IU), of which 28 (7.2%) developed hepatic events, while 21 (6.7%) of 314 patients with normal ALT developed hepatic events. There was no difference in the Kaplan-Meier time to hepatic events among patients with or without abnormal ALT (P = 0.734) (Appendix Fig. 8).
DISCUSSION
NAFLD is the most common chronic liver disorder in the USA. The incidence of decompensated NASH cirrhosis is rising, becoming one of the most common indications for liver transplantation. Based on our study, the incidence of hepatic decompensation in NAFLD patients without cirrhosis is around 5% at 5 years and 10% at 10 years. We have developed and internally validated a simple objective model composed of age, presence of diabetes, and platelet count—three readily available variables. Our model has excellent predictive ability to identify the development of hepatic decompensation in NAFLD patients up to 12 years from the time of diagnosis. We thus believe that our model would help clinicians identify the small number of “at-risk” patients who need close observation and/or referral to liver clinics from the vast majority of NAFLD patients who have a benign course.
In a 2013 study of 320 NAFLD patients, Angulo et al. showed that the NAFLD fibrosis score and the APRI performed well to predict liver-related events in NAFLD patients.27 However, the NAFLD fibrosis score and the APRI index did not perform as well in our cohort. This is likely explained by the fact that more than 50% of patients in their study population already had advanced fibrosis/cirrhosis and prediction scores may not be needed in patients with clinically obvious cirrhosis. In comparison, only a small percentage of patients were likely to have advanced fibrosis/cirrhosis in our cohort (only 16% of patients in our study had a high NAFLD fibrosis score). Thus, currently available non-invasive models that have been developed and validated to detect advanced fibrosis may not necessarily predict the development of hepatic events in NAFLD patients with early disease.
The predictive ability of age, presence of diabetes, and platelet count is not a new finding, and is likely not unique to our population. In a 2011 study by Bhala et al. of 247 NAFLD patients, 19.4% developed hepatic events with age, low platelet count, and low ALT as independent predictors of developing liver-related complications. However, all the patients in their study had advanced fibrosis/cirrhosis.11 Similarly, in a 2015 study of 619 patients with biopsy-proven NAFLD, Angulo et al. showed that age, presence of diabetes, and current smoking was associated with mortality and need for liver transplantation.13 Thus, our model is likely to perform well outside our population.
Our study has several strengths. This is the largest NAFLD cohort to date to evaluate common objective tests and develop a simple objective model that identifies patients at increased risk of developing hepatic decompensation. Our model identifies NAFLD patients with early disease who may benefit from interventions or referral to specialty clinics prior to development of clinically obvious cirrhosis. Additionally, our NAFLD decompensation model can be used easily in all clinical settings, and our model can be presented as a web page or smartphone application. Clinicians will be able to enter the data points for the three variables for their patients (age, presence and absence of diabetes, and platelet count) to automatically obtain the predicted probability of developing hepatic events in their patient.
Our study has several limitations. This is a retrospective study and the inherent challenges attributed to retrospective design are applicable to our study. Sample size is a limitation as the small number of events in our cohort limits the number of variables included in the model. Liver biopsy was not performed routinely in our patients, and thus we cannot provide histological confirmation of the diagnosis of NAFLD, differentiate NAFL from NAFLD, or estimate baseline fibrosis score. However, all patient charts were reviewed in detail and the diagnosis of NAFLD was made as per current AASLD guidelines.24 The majority of our patients likely had early disease as only 16% of our population had a high NAFLD fibrosis score and only 7% of our patients developed hepatic events, significantly lower than that of prior studies which predominantly had patients with advanced fibrosis (19.4% Bhala et al., 18.7% Angulo et al.).11, 27
In conclusion, a simple objective model composed of age, presence of diabetes, and platelet count may reliably help identify NAFLD patients at high risk of developing hepatic events before the development of clinically obvious cirrhosis. Offering early intervention—such as bariatric surgery, dietary specialists, and/or enrollment in NAFLD therapeutic clinical trials—may help improve survival and decrease the need for liver transplantation.
Electronic supplementary material
(DOC 378 kb)
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Prior Presentations
a. Digestive Disease Week. Washington, DC. May 2018.
b. The Liver Meeting, AASLD. San Francisco, CA. November 2018.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Heidi S. Ahmed, Email: heidi.s.ahmed@gmail.com.
Arvind R. Murali, Email: arvind-murali@uiowa.edu.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132(2):112–7. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 4.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178(1):38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.Corey KE, Kaplan LM. Obesity and liver disease: the epidemic of the twenty-first century. Clin Liver Dis. 2014;18(1):1–18. doi: 10.1016/j.cld.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Wang XJ, Malhi H. Nonalcoholic Fatty Liver Disease. Ann Intern Med. 2018;169(9):ITC65–ITC80. doi: 10.7326/AITC201811060. [DOI] [PubMed] [Google Scholar]
- 9.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129(1):375–8. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–2. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54(4):1208–16. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51(2):371–9. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–97. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Knawy B, Shiffman M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007;27(9):1166–73. doi: 10.1111/j.1478-3231.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 15.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 16.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66(5):1486–501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 18.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441–7. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 19.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–9. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 20.Xun YH, Guo JC, Lou GQ, Jiang YM, Zhuang ZJ, Zhu MF, et al. Non-alcoholic fatty liver disease (NAFLD) fibrosis score predicts 6.6-year overall mortality of Chinese patients with NAFLD. Clin Exp Pharmacol Physiol. 2014;41(9):643–9. doi: 10.1111/1440-1681.12260. [DOI] [PubMed] [Google Scholar]
- 21.Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32(2):297–302. doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 22.D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39(10):1180–93. doi: 10.1111/apt.12721. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 24.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cardenas E, Sanchez-Avila F, Vargas-Vorackova F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7(4):350–7. [PubMed] [Google Scholar]
- 25.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3(2):143–52. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782–9. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 378 kb)