Abstract
Background
After non-fatal opioid overdoses, opioid prescribing patterns are often unchanged and the use of medications for opioid use disorder (MOUDs) remains low. Whether such prescribing differs by race/ethnicity remains unknown.
Objective
To assess the association of race/ethnicity with the prescribing of opioids and MOUDs after a non-fatal opioid overdose.
Design
Retrospective cohort study.
Participants
Patients prescribed ≥ 1 opioid from July 1, 2010, to September 30, 2015, with a non-fatal opioid overdose in the Veterans Health Administration (VA).
Main Measures
Primary outcomes were the proportion of patients prescribed: (1) any opioid during the 30 days before and after overdose and (2) MOUDs within 30 days after overdose by race and ethnicity. We conducted difference-in-difference analyses using multivariable regression to assess whether the change in opioid prescribing from before to after overdose differed by race/ethnicity. We also used multivariable regression to test whether MOUD prescribing after overdose differed by race/ethnicity.
Key Results
Among 16,210 patients with a non-fatal opioid overdose (81.2% were white, 14.3% black, and 4.5% Hispanic), 10,745 (66.3%) patients received an opioid prescription (67.1% white, 61.7% black, and 65.9% Hispanic; p < 0.01) before overdose. After overdose, the frequency of receiving opioids was reduced by 18.3, 16.4, and 20.6 percentage points in whites, blacks, and Hispanics, respectively, with no significant difference-in-difference in opioid prescribing by race/ethnicity (p = 0.23). After overdose, 526 (3.2%) patients received MOUDs (2.9% white, 4.6% black, and 5.5% Hispanic; p < 0.01). Blacks (adjusted OR (aOR) 1.6; 95% CI 1.2, 1.9) and Hispanics (aOR 1.8; 95% CI 1.2, 2.6) had significantly larger odds of receiving MOUDs than white patients.
Conclusions
In a national cohort of patients with non-fatal opioid overdose in VA, there were no racial/ethnic differences in changes in opioid prescribing after overdose. Although blacks and Hispanics were more likely than white patients to receive MOUDs in the 30 days after overdose, less than 4% of all groups received such therapy.
KEY WORDS: opioid, overdose, race/ethnicity, disparities, veteran
BACKGROUND
Opioid overuse and misuse remain significant problems in the USA, impacting a broad range of communities and claiming nearly 400,000 lives from 1999 to 2017.1 This opioid crisis is of particular importance in the Veterans Health Administration (VA), the largest integrated health-care delivery system in the USA, that serves a patient population at increased risk of opioid misuse and fatal opioid overdose.2, 3
Recent evidence suggests that interventions to reduce the harms of opioid misuse, addiction, and overdose are markedly underutilized.4 This undertreatment includes low rates of prescribing of medications for opioid use disorder (MOUDs), such as methadone, buprenorphine, and naltrexone.5, 6 This is due, in part, to the limited number of medical providers credentialed to prescribe buprenorphine, both within and outside VA.7, 8 More concerning among patients with non-fatal opioid overdoses, few individuals receive MOUD, and opioid prescribing patterns frequently remain unchanged following such overdoses based on studies performed outside VA.9, 10 These serious sentinel overdose events among opioid users represent opportune windows to taper or discontinue chronic, high-dose opioid use or to initiate MOUDs as effective forms of harm reduction.
Whereas previous studies have reported lower prescribing of prescription opioids in racial and ethnic minorities compared to white individuals, recent evidence has demonstrated similar racial disparities in receipt of MOUD.11 However, there have not been previous studies examining whether these disparities extend to opioid and MOUD prescribing around the period of a non-fatal overdose, and none in VA, where insurance status and provider access would not be expected to influence such disparities.6, 12 The primary aim of this study was to assess the association between race and ethnicity and patterns of opioid prescribing before and after a non-fatal opioid overdose in a national cohort of patients managed within VA. A secondary aim was to assess the receipt of MOUD (i.e., buprenorphine, methadone, and naltrexone) following such overdose events. We hypothesized that racial and ethnic minorities would experience a more drastic reduction in prescribing of opioids after overdose and be less likely to receive MOUD compared to white individuals, even when adjusting for well-known factors associated with availability of opioids and opioid use treatment including geographic region and rurality.
METHODS
Data Sources
We obtained VA administrative and clinical data from the VA Corporate Data Warehouse (CDW), which contains information on patient demographics, all outpatient and inpatient clinical encounters, diagnosis codes for all encounters, and all prescribed medications dispensed within VA. We obtained data from the Centers for Medicare & Medicaid Services (CMS) for all dispensed prescriptions from the Medicare Part D prescription drug event files for patients with dual VA and Medicare insurance enrollment. We also obtained death data from the National Death Index (NDI), considered the “gold standard” for vital status assessment among national mortality databases.13
Study Cohort
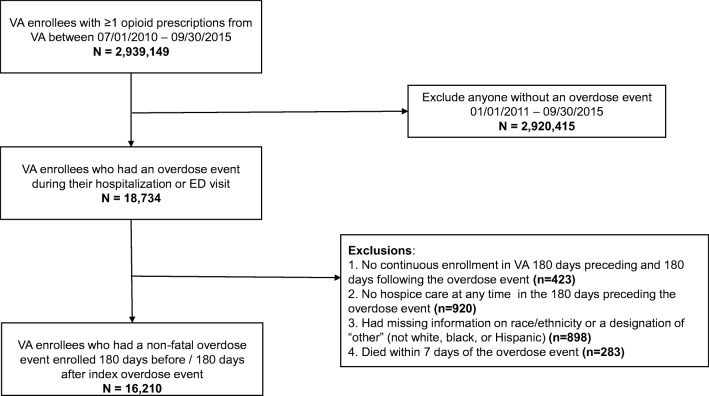
We identified all patients enrolled in VA from July 1, 2010, to September 30, 2015, who filled at least 1 opioid prescription from VA during this period (n = 2,939,149) (Fig. 1). Next, based on established ICD-9 codes,14 we identified patients who had a hospitalization or ED visit for an opioid overdose from 2011 to September 30, 2015 (n = 18,734), and we assigned an index date for the first overdose event. We excluded patients who did not have continuous enrollment in VA 180 days preceding and 180 days following the index date (n = 423), were in hospice care at any time in the 180 days preceding the index date (n = 920), or had missing information on race/ethnicity or a designation of “other” (not white, black, or Hispanic) race/ethnicity (n = 898). To identify patients with non-fatal opioid overdoses, we excluded patients who died within 7 days of their overdose event (n = 283), attributing these deaths to the proximate overdose event. Our final analysis cohort comprised 16,210 patients.
Fig. 1.
Study flow diagram. After the initial exclusion of patients without an overdose event, there were 18,734 patients who had an opioid overdose event. After further exclusions including continuous Veterans Health Administration (VA) enrollment, hospice care receipt, missing or other race/ethnicity, and death within 7 days, the final cohort of patients with non-fatal opioid overdose was 16,210.
Study Outcomes
We examined several outcomes capturing changes in the use of opioids from before to after overdose. Specifically, we examined (1) the proportion of patients who received any opioid fills, (2) the mean number of days receiving an opioid, and (3) the proportion of patients who received at least 1 day of high-dose opioid (> 120 morphine milligram equivalents (MME)), in the 30 days before and 30 days after the index overdose. For these opioid use outcomes, we used a selected list of common opioids and based our high-dose measure on Pharmacy Quality Alliance measures, as previously described.14 We also examined receipt of a prescription for MOUD (i.e., buprenorphine, naltrexone, liquid methadone, or at least 1 methadone clinic visit) within 30 days after the index overdose. We conducted sensitivity analyses, where, in addition to VA prescriptions, we considered prescriptions for opioids and/or MOUD from Medicare Part D.
Primary Independent Variable and Baseline Covariates
Our key independent variable was race and ethnicity. We categorized this variable as non-Hispanic white (white), non-Hispanic black (black), or Hispanic. Baseline covariates consisted of sociodemographic, clinical, and other patient-level characteristics. The demographic variables consisted of age in years, sex (male, female), and Medicare Part D insurance status for prescription medications (VA only, Medicare Part D, or Medicare Part D with Medicaid or low-income subsidy). The clinical variables consisted of a combined measure of comorbidity captured by the Gagne Index (a clinical risk adjustment score based on 20 conditions derived from the Charlson and Elixhauser indices that predicts 1-year all-cause mortality)15 in the year prior to overdose, and a measure of VA disability status (based on VA priority enrollment group status and defined as high disability, low/moderate disability, low income, and no service-connected disability).16 Finally, we used the patients’ Federal Information Processing Standards (FIPS) code of residence to assess driving distance to the nearest VA primary care clinic and geographic census region (Northeast, Midwest, South, West, outside the 50 states and DC). The analysis also included patient-level rurality (large metro, small metro, micropolitan, and non-core rural).
Statistical Analyses
We compared patient characteristics across racial and ethnic groups using chi-square tests for categorical variables and analysis of variance tests or Kruskal-Wallis tests for continuous variables. For the three opioid use outcomes, we summarized the descriptive statistics of proportion and mean before and after overdose. We then conducted difference-in-difference analyses to test whether the change from before to after overdose differed by race and ethnicity. We used multivariable logistic regression models with generalized estimating equations (GEEs) for the two binary outcomes of any opioid use and high-dose opioid use, and linear regression with GEE for the continuous outcome of the number of days receiving an opioid, accounting for a correlation of the two observations within a patient. Our main estimates of interest (adjusted difference by race and ethnicity and adjusted difference-in-difference across race and ethnicity) were measured by including in the models the interaction term between race/ethnicity and time period (before versus after overdose). For the MOUD outcome, we summarized the proportion of any MOUD use and specific types of MOUD, overall and by race and ethnicity. We further used multivariable logistic regression to test whether MOUD receipt after overdose differed by race and ethnicity.
We decided a priori to adjust all analyses for baseline characteristics including categorical age, sex, Gagne Index, prescription insurance status, driving distance to the nearest VA primary clinic, region, rurality, and VA disability status. We conducted sensitivity analyses for the two outcomes of any opioid use and receipt of MOUD, where, in addition to VA prescriptions, we considered prescriptions from Medicare Part D in the outcome measurement. We addressed missing observations (< 1% total) using single imputation by chained equations adjusting for all covariates from Table 1. Statistical significance was set at p value < 0.05. We used Stata’s margins option after the xtgee command to estimate the difference and difference-in-difference. We conducted all analyses using Stata 14 (College Station, TX).
Table 1.
Baseline Characteristics of Patients with a Non-Fatal Opioid Overdose, Overall and by Race and Ethnicity
| Characteristics | Overall (n = 16,210) | White (n = 13,171) | Black (n = 2314) | Hispanic (n = 725) |
|---|---|---|---|---|
| Age (years), mean (SD)*† | 60.8 (13.8) | 61.1 (14.2) | 59.7 (11.1) | 59.9 (14.3) |
| Age (years), n (%)*† | ||||
| 18–39 | 1385 (8.6) | 1204 (9.2) | 108 (4.7) | 73 (10.1) |
| 40–64 | 8612 (53.2) | 6686 (50.8) | 1537 (66.5) | 389 (54.0) |
| ≥ 65 | 6192 (38.2) | 5267 (40.0) | 666 (28.8) | 259 (35.9) |
| Male sex, n (%)*† | 14,892 (92.0) | 12,172 (92.5) | 2041 (88.4) | 679 (93.8) |
| Gagne Index, median (Q1, Q3) | 2 (1, 5) | 2 (1, 4) | 2 (1, 5) | 2 (1, 4) |
| Medicare Part D prescription insurance status, n (%)* | ||||
| VA only | 13,349 (82.4) | 10,922 (82.9) | 1834 (79.3) | 593 (81.8) |
| Medicare Part D | 1410 (8.7) | 1144 (8.7) | 188 (8.1) | 78 (10.8) |
| Medicare Part D with Medicaid or LIS | 1451 (8.9) | 1105 (8.4) | 292 (12.6) | 54 (7.4) |
| Driving distance to the nearest VA primary care (miles), median (Q1, Q3)*† | 10 (5, 20) | 11 (5, 21) | 6 (3, 11) | 8 (4, 14) |
| Region, n (%)*† | ||||
| Midwest | 3813 (23.7) | 2879 (21.9) | 525 (22.7) | 59 (8.1) |
| Northeast | 1921 (11.9) | 1428 (10.8) | 262 (11.3) | 53 (7.3) |
| South | 6133 (38.1) | 4407 (33.5) | 989 (42.7) | 130 (17.9) |
| West | 4126 (25.7) | 3095 (23.5) | 284 (12.3) | 337 (46.5) |
| Outside the 50 states and outside DC | 91 (0.6) | 5 (0.04) | 0 (0.0) | 86 (11.9) |
| Rurality, n (%)*† | ||||
| Large metro | 7424 (46.2) | 5492 (42.0) | 1531 (66.7) | 401 (55.6) |
| Small metro | 5551 (34.5) | 4695 (35.9) | 590 (25.7) | 266 (36.9) |
| Micropolitan | 1740 (10.8) | 1624 (12.4) | 83 (3.6) | 33 (4.6) |
| Non-core rural | 1367 (8.5) | 1256 (9.6) | 90 (3.9) | 21 (2.9) |
| VA priority status, n (%)‡ | ||||
| High disability | 6850 (42.3) | 5501 (41.8) | 1003 (43.3) | 346 (47.7) |
| Low or moderate disability | 2531 (15.6) | 2086 (15.8) | 341 (14.7) | 104 (14.3) |
| Low income | 5931 (36.6) | 4827 (36.7) | 862 (37.3) | 242 (33.4) |
| No service-connected disability | 898 (5.5) | 757 (5.7) | 108 (4.7) | 33 (4.6) |
Patient characteristics are compared across racial and ethnic groups using chi-square tests for categorical variables and analysis of variance tests or Kruskal-Wallis tests for continuous variables
SD standard deviation, Q1 quartile 1, Q3 quartile 3, VA Veterans Health Administration, LIS low income subsidy
*p value < 0.001
†Variables with missing data < 1.0%
‡p value < 0.05
RESULTS
Overall, 16,210 patients enrolled in VA who received at least 1 opioid prescription between July 2010 and September 2015 had a non-fatal opioid overdose event (Table 1). Their mean age was 60.8 (standard deviation (SD) 13.8) years, and 92.0% were male, 14.3% were black, and 4.5% were Hispanic. There were statistically significant differences in all sociodemographic and clinical factors across race and ethnicity (Table 1).
Frequency of Opioid Prescribing Before and After Non-Fatal Overdose
Overall, 10,745 (66.3%) patients received an opioid prescription in the 30 days before their index opioid overdose event, including 67.1% white, 61.7% black, and 65.9% Hispanic patients (p value < 0.01) (Table 2). In the 30 days after overdose, the proportion of patients receiving opioids was reduced by 18.3 percentage points in white patients, 16.4 percentage points in black patients, and 20.5 percentage points in Hispanic patients, but still exceeded 45% for all three groups (Table 2). In adjusted difference-in-difference models, there were no statistically significant differences by race and ethnicity in changes in opioid prescribing before and after overdose. The proportions of patients receiving an opioid prescription before and after overdose were similar in sensitivity analyses, in which, in addition to VA prescriptions, we considered prescriptions for opioids from Medicare Part D. In this subset, there were an additional 298 patients prescribed opioids before overdose and 289 patients prescribed opioids after overdose.
Table 2.
Frequency and Patterns of Opioids Prescribed 30 Days Before and 30 Days After Non-Fatal Overdose
| Opioid use measures | Before overdose | After overdose | Adjusted difference | Adjusted difference-in-difference |
|---|---|---|---|---|
| Any opioid use, n (%) | 10,745 (66.3) | 7845 (48.4) | ||
| White (n = 13,171) | 8839 (67.1) | 6462 (49.1) | − 18.3% (− 19.3, − 17.3)* | Reference |
| Black (n = 2314) | 1428 (61.7) | 1051 (45.4) | − 16.4% (− 18.8, − 14.1)* | 1.8% (− 0.7, 4.4)† |
| Hispanic (n = 725) | 478 (65.9) | 332 (45.8) | − 20.5% (− 24.7, − 16.3)* | − 2.2% (− 6.5, 2.1)† |
| High-dose opioid use, n (%)‡ | 2577 (15.9) | 1809 (11.2) | ||
| White (n = 13,171) | 2197 (16.7) | 1536 (11.7) | − 4.9% (− 5.5, − 4.3)* | Reference |
| Black (n = 2314) | 294 (12.7) | 215 (9.3) | − 3.2% (− 4.5, − 2.0)* | 1.7% (0.3, 3.1)§ |
| Hispanic (n = 725) | 86 (11.9) | 58 (8.0) | − 3.4% (− 5.4, − 1.4)* | 1.5% (− 0.6, 3.6)† |
| Number of days receiving an opioid, mean (SD)‖ | 9.1 (10.1) | 7.9 (10.1) | ||
| White (n = 13,171) | 9.3 (10.1) | 8.0 (10.1) | − 1.26 (− 1.48, − 1.03* | Reference |
| Black (n = 2314) | 8.3 (10.0) | 7.4 (9.9) | − 0.94 (− 1.46, − 0.43* | 0.31 (− 0.25, 0.88)† |
| Hispanic (n = 725) | 9.0 (10.0) | 7.5 (10.0) | − 1.48 (− 2.40, − 0.56)§ | − 0.22 (− 1.18, 0.74)† |
Adjusted models control for categorical age, sex, Gagne Index, prescription insurance status, driving distance to the nearest VA primary clinic, region, rurality, and VA priority status. To estimate the adjusted difference and adjusted difference-in-difference, the interaction term between race/ethnicity and the dichotomized variable of before versus after overdose was included with the Stata margin option after the xtgee command. All p values for comparisons across the 3 racial groups were statistically significant at < 0.003 for both before and after overdose comparisons
SD standard deviation
*p value < 0.001
†Not statistically significant
‡High-dose opioid use is defined as having at least 1 day with > 120 MME
§p value < 0.05
‖The number of days receiving an opioid ranges from 0 to 30 days
Patterns of High-Dose Opioid Prescribing Before and After Non-Fatal Overdose
Before overdose, black (12.7%) and Hispanic (11.9%) patients were less likely to have high-dose opioid use (at least 1 day with > 120 MME) compared to white patients (16.7%) (p value < 0.01) (Table 2). After overdose, all groups had a reduction in the proportion of high-dose opioid use, although the reduction was statistically significantly smaller for black versus white patients (adjusted difference-in-difference 1.7 percentage points, 95% CI 0.3, 3.1). Before overdose, black (8.3 days, SD 10.0) and Hispanic (9.0 days, SD 10.0) patients had a smaller mean number of days receiving an opioid than white patients (9.3 days, SD 10.1) (p value < 0.01) (Table 2). After overdose, the mean number of days receiving an opioid decreased by one or more days for all groups, with no statistically significant differences by race or ethnicity.
Frequency of MOUD Prescribing After Non-Fatal Overdose
Overall, only 526 (3.2%) patients received any form of MOUD within 30 days after the index overdose event, with annual rates ranging from 2.7% in 2011 to 3.7% in 2015. Compared to white patients, black (adjusted OR (aOR) 1.55; 95% CI 1.22, 1.96) and Hispanic (aOR 1.76; 95% CI 1.21–2.56) patients had significantly larger adjusted odds of receiving any form of MOUD after overdose (Table 3). When examining individual types of MOUD, compared to white patients, Hispanic patients had a significantly larger adjusted odds of receiving suboxone/buprenorphine (aOR 1.96; 95% CI 1.16–3.32) and black patients had significantly larger adjusted odd of receiving methadone (aOR 1.91; 95% CI 1.44–2.53) (Table 3). Naltrexone prescribing was least frequent and was similar across all racial and ethnic groups. In sensitivity analyses, including Medicare Part D prescribing data had minimal influence on these findings, as < 10 patients received MOUD through Medicare Part D after overdose. Including overdose year in the statistical model did not impact any of these outcomes.
Table 3.
Medications for Opioid Use Disorder After Non-Fatal Overdose by Medication Type
| Medication type | After overdose, n (%) | Unadjusted odds ratio | p value | Adjusted odds ratio | p value |
|---|---|---|---|---|---|
| Any MOUD (n = 526, 3.2%) | |||||
| White | 379 (2.9) | Reference | Reference | ||
| Black | 107 (4.6) | 1.64 (1.31, 2.04) | < 0.001 | 1.55 (1.22, 1.96) | < 0.001 |
| Hispanic | 40 (5.5) | 1.97 (1.41, 2.75) | < 0.001 | 1.76 (1.21, 2.56) | 0.003 |
| Buprenorphine (n = 212, 1.3%) | |||||
| White | 168 (1.3) | Reference | Reference | ||
| Black | 25 (1.1) | 0.85 (0.55, 1.29) | 0.44 | 0.97 (0.62, 1.51) | 0.90 |
| Hispanic | 19 (2.6) | 2.08 (1.29, 3.37) | 0.003 | 1.96 (1.16, 3.32) | 0.01 |
| Methadone (n = 307, 1.9%) | |||||
| White | 202 (1.5) | Reference | Reference | ||
| Black | 84 (3.6) | 2.42 (1.87, 3.13) | < 0.001 | 1.91 (1.44, 2.53) | < 0.001 |
| Hispanic | 21 (2.9) | 1.92 (1.21, 3.02) | 0.005 | 1.59 (0.96, 2.63) | 0.07 |
| Naltrexone (n = 61, 0.4%) | |||||
| White | 49 (0.4) | Reference | Reference | ||
| Black | 5 (0.2) | 0.58 (0.23, 1.46) | 0.25 | 0.61 (0.24, 1.57) | 0.30 |
| Hispanic | 7 (1.0) | 2.61 (1.18, 5.78) | 0.02 | 2.22 (0.93, 5.30) | 0.07 |
Adjusted models control for categorical age, sex, Gagne Index, prescription insurance status, driving distance to the nearest VA primary clinic, region, rurality, and VA priority status
DISCUSSION
In a national cohort of patients enrolled in VA with a non-fatal opioid overdose, receipt of an opioid prescription decreased by 16–21 percentage points in the 30 days after overdose but remained high, with no significant differences across racial and ethnic groups. Overall, MOUD prescribing in VA was very low in all racial groups in the 30 days after overdose, though statistically significantly higher in black and Hispanic patients. As health systems, including VA, devise programs to reduce harms of opioid use and misuse, decreasing unsafe opioid prescribing and increasing access to MOUD following non-fatal overdose events, represent critical opportunities for intervention.
This study adds to the current evidence on provider responses to a non-fatal opioid overdose. To our knowledge, this is the first study examining racial and ethnic differences in opioid or MOUD prescribing inside or outside VA during this opportune time window to intervene after overdose. In an analysis of patients in Massachusetts who survived an opioid overdose from 2012 to 2014, LaRochelle et al. found that 30% of patients received treatment for opioid use disorder with methadone, buprenorphine, or naltrexone within 12 months of overdose.10 The higher rate of MOUD use in the prior non-VA study may represent state-specific harm reduction treatment opportunities and policies in a state affected by high rates of opioid overdose fatalities17 as well as a trend towards higher rates of MOUD receipt with time from the index overdose event (e.g., 12 months compared to 30 days in the present analysis). In a study of Medicaid enrollees in Pennsylvania with prescription opioid overdose from 2000 to 2012, Frazier et al. observed lower rates of MOUD, with 6.7% of enrollees receiving buprenorphine and 8.3% receiving methadone.9 Similar to the present study, opioid prescribing after overdose remained high at 59.6%, suggesting a potentially inadequate response to a life-threatening overdose event. In an analysis of 2468 commercially insured patients who had an opioid overdose from 2000 to 2012, LaRochelle and colleagues found that opioids were dispensed to 91% of patients within 1 year after an overdose, with up to 34% of patients receiving large morphine equivalent dosages of opioids within 90 days of an overdose event.5 Although the rates of overall and high-dose opioid receipt were lower in this VA analysis, the persistently high rates of opioid prescribing after an overdose event represent an opportunity to identify alternative pain management strategies in this high-risk population.
The VA population is particularly at risk of opioid misuse and overdose. VA patients have a higher rate of service- or trauma-related disability (as reflected in our patient cohort) and chronic pain compared to the general population,18 which likely influence opioid prescribing, even after a non-fatal overdose. To address this high-risk population, along with rising opioid misuse nationally, the VA instituted its Opioid Safety Initiative (OSI) in 2013, a system-wide initiative which provides monthly metrics on opioid prescribing to VA facility and regional leaders.19 The OSI has since resulted in lower prescribing of high-dosage opioids and concomitant opioid and benzodiazepine prescribing in VA patients while increasing the use of alternative pain therapies.20 VA has also developed a predictive modeling tool, the Stratification Tool for Opioid Risk Management (STORM), to provide VA facilities and clinicians with targeted strategies to reduce at-risk opioid use.21 Additionally, in 2015, VA updated its clinical practice guidelines for the treatment of substance use disorders, strongly recommending buprenorphine or methadone as a first-line treatment for patients with OUD.22 Furthermore, over the past several years, there has been greater use of state prescription drug monitoring programs (PDMPs) across the country, including within VA, which has been associated with decreased high-risk opioid prescribing.23 Our study shows that there was still an opportunity for improvement at the end of our study time period in the management of opioid use disorder. In future work, it will be important to understand how these national initiatives may have influenced racial and ethnic disparities in opioid and MOUD prescribing after overdose more recently in VA.
In contrast to previous non-VA studies examining racial variation in MOUD use in general,24 we focused on the critical time period following an overdose and found that black and Hispanic patients were more likely to receive MOUD than white patients, although overall rates were very low. These findings were supported in a recent analysis of veterans with HIV, which showed significantly higher rates of timely MOUD initiation (methadone and buprenorphine) in black than white patients following a clinical encounter for opioid use disorder.25 However, our findings differ from those demonstrated in a recently published analysis of National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey data from 2004 to 2015 by Lagisetty and colleagues,11 which revealed that in ambulatory care settings, black patients had significantly lower adjusted odds than white patients (aOR 0.23; 95% CI 0.13–0.44) of receiving buprenorphine treatment for opioid use disorder.
The somewhat paradoxical finding of more frequent treatment with MOUD for black and Hispanic than white patients with opioid use in our cohort may reflect sociodemographic differences in the VA patient population compared to the civilian patient population and the generally equal access to MOUD for patients in VA compared to outside VA. Another possible explanation for greater use of MOUD among black and Hispanic patients is that after a non-fatal overdose, providers may have higher concern for substance use disorder in minority patients receiving opioids compared to white patients. This hypothesis is supported by a study of racial differences in the management of patients on chronic opioid therapy for non-cancer pain in a single VA medical center, which found that black patients were almost twice as likely as white patients to be referred for substance abuse assessment, and almost half as likely as white patients to be referred to a pain specialist for additional consultation.26
Our analysis demonstrated greater MOUD receipt among black patients, driven by a higher rate of methadone prescribing compared to buprenorphine (which has been shown to be prescribed more in white patients compared to non-whites in previous studies).27 Prior work has also shown that opioid prescribing is higher, and MOUD prescribing (particularly methadone)28 is lower in rural regions compared to urban areas in the USA.29 While our analysis found that only a small percentage of total patients with non-fatal overdose (8.5%) resided in rural areas, these patients were predominantly white (91.9%) which may have influenced our unique racial observations. We adjusted our findings for rurality, which did not change the outcomes, thus making rurality unlikely to explain the racial and ethnic differences observed in our analysis. The higher rates of buprenorphine use in Hispanic compared to white patients in our analysis has not been reported in previous studies to our knowledge and may represent a difference in the Hispanic patient population in VA compared to the Hispanic civilian population. Additionally, while geographic region was controlled for in this analysis, Hispanic patients were predominantly from the Western region where prescribing practices may differ from other parts of the country. Perhaps most importantly, the overall low rates of MOUD prescribing across all patient subgroups in our analysis highlight the opportunities and challenges of universally providing MOUD to a high-risk group of patients likely to benefit from such therapy.30
Our study adds to the well-described literature reporting racial and ethnic disparities in opioid treatment, demonstrating that even after non-fatal overdose, white patients are more likely to receive higher doses and longer durations of opioid prescriptions compared to non-whites. Prior studies have attributed these differences in prescribing patterns to various patient-level factors including differential access to health care, socioeconomic status (such as employment), insurance status, and cultural beliefs about opioid medications.31 Provider bias and historical mistrust are also potential underexamined explanatory factors that may influence racial and ethnic variation in pain treatment.32, 33 Nevertheless, we are unable to fully capture the reasons for the racial variation in opioid prescribing observed in our analysis using retrospective administrative data.
This study has certain limitations. First, we examined overdoses from 2011 until 2015, and opioid and MOUD prescribing has both changed since that time due to increasing patient and provider recognition of the opioid crisis and programs and policies to address it. Notably, when we added overdose year to our statistical model, our findings remained the same. Second, despite careful adjustment for baseline sociodemographic and clinical factors, the potential for unmeasured confounding of the relationship between race/ethnicity and post-overdose treatment is possible, given the observational design of our study. However, the use of difference-in-difference analyses allowed for accounting of any unobserved but fixed confounding. Third, our data do not capture medications purchased without insurance or through non-VA insurance other than Medicare, or MOUD provided by non-VA facilities, and thus may underestimate the frequency of opioid use and MOUD use before and after opioid overdoses. Finally, our findings were generated for individuals with opioid overdoses diagnosed within VA and thus may not generalizable to those who have such events in other health-care systems or entirely outside of a health-care system.
In conclusion, among a national cohort of patients enrolled in VA with a non-fatal opioid overdose between 2011 and 2015, a critical time period in the opioid epidemic, opioid prescribing remained similarly high across all racial and ethnic groups after overdose. Prescribing of medications for opioid use disorder was very low after overdose, with statistically higher rates in black and Hispanic patients. These findings demonstrate an opportunity to improve the quality of care for all patients with opioid use disorder, particularly in the vulnerable period around a non-fatal overdose event.
Compliance with Ethical Standards
Conflict of Interest
Dr. Gellad, Dr. Hausmann, and Dr. Thorpe report received grants from the Department of Veterans Affairs during the conduct of this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hedegaard H, Warner M, Miniño AM. Drug Overdose Deaths in the United States, 1999-2016. 2017. https://www.cdc.gov/nchs/products/databrieds/db294.htm. Accessed 2 June 2019. [PubMed]
- 2.Moyo P, Zhao X, Thorpe CT, et al. Patterns of opioid prescriptions received prior to unintentional prescription opioid overdose death among Veterans. Res Social Adm Pharm. 2018. 10.1016/j.sapharm.2018.10.023 [DOI] [PMC free article] [PubMed]
- 3.Wilder CM, Miller SC, Tiffany E, Winhusen T, Winstanley EL, Stein MD. Risk factors for opioid overdose and awareness of overdose risk among veterans prescribed chronic opioids for addiction or pain. J Addict Dis. 2016. 10.1080/10550887.2016.1107264 [DOI] [PMC free article] [PubMed]
- 4.Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and Treatment of Opioid Misuse and Addiction. JAMA Psychiatry. 2019;76(2):208. doi: 10.1001/jamapsychiatry.2018.3126. [DOI] [PubMed] [Google Scholar]
- 5.Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose: A cohort study. Ann Intern Med. 2016. 10.7326/M15-0038 [DOI] [PubMed]
- 6.Hadland SE, Bagley SM, Rodean J, et al. Receipt of Timely Addiction Treatment and Association of Early Medication Treatment with Retention in Care among Youths with Opioid Use Disorder. JAMA Pediatr. 2018;02118:E1–E9. doi: 10.1001/jamapediatrics.2018.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenstein-Mah H, Hagedorn H, Kay CL, Christopher ML, Gordon AJ. Underutilization of the current clinical capacity to provide buprenorphine treatment for opioid use disorders within the Veterans Health Administration. Subst Abus. 2018. 10.1080/08897077.2018.1509251 [DOI] [PubMed]
- 8.Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018. 10.1080/08897077.2018.1452327 [DOI] [PMC free article] [PubMed]
- 9.Frazier W, Cochran G, Lo-Ciganic WH, et al. Medication-assisted treatment and opioid use before and after overdose in Pennsylvania Medicaid. J Am Med Assoc. 2017. 10.1001/jama.2017.7818 [DOI] [PMC free article] [PubMed]
- 10.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. Ann Intern Med. 2018;169(3):137–145. doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagisetty PA, Ross R, Bohnert A, Clay M, Maust DT. Buprenorphine Treatment Divide by Race/Ethnicity and Payment. JAMA Psychiatry. 2019. 10.1001/jamapsychiatry.2019.0876 [DOI] [PMC free article] [PubMed]
- 12.Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White Opioid Mortality in the United States, 1979-2015. Epidemiology. 2018. 10.1097/EDE.0000000000000858 [DOI] [PMC free article] [PubMed]
- 13.Cowper DC, Kubal JD, Maynard C, Hynes DM. A Primer and Comparative Review of Major U.S. Mortality Databases. Ann Epidemiol. 2002;12(7):462–468. doi: 10.1016/S1047-2797(01)00285-X. [DOI] [PubMed] [Google Scholar]
- 14.Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD, Coplan PM. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol Drug Saf. 2017. 10.1002/pds.4157 [DOI] [PubMed]
- 15.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011. 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed]
- 16.Thorpe CT, Gellad WF, Mor MK, et al. Effect of Dual Use of Veterans Affairs and Medicare Part D Drug Benefits on Antihypertensive Medication Supply in a National Cohort of Veterans with Dementia. Health Serv Res. 2018. 10.1111/1475-6773.13055 [DOI] [PMC free article] [PubMed]
- 17.Massachusetts Department of Public Health. MA Opioid-Related EMS Incidents 2013-September 2018. 2019. https://www.mass.gov/files/documents/2019/02/12/Emergency-Medical-Services-Data-February-2019.pdf. Accessed April 17, 2019.
- 18.Nahin RL. Severe Pain in Veterans: The Effect of Age and Sex, and Comparisons With the General Population. J Pain. 2017. 10.1016/j.jpain.2016.10.021 [DOI] [PMC free article] [PubMed]
- 19.Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: Lessons from the department of veterans affairs. JAMA Intern Med. 2017. 10.1001/jamainternmed.2017.0147 [DOI] [PubMed]
- 20.Bickell NA, Weidmann J, Fei K, Lin JJ, Leventhal H. Underuse of breast cancer adjuvant treatment: patient knowledge, beliefs, and medical mistrust. J Clin Oncol. 2009;27(31):5160–5167. doi: 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinman M, Gellad WF, McCarthy S, et al. Protocol for evaluating the nationwide implementation of the VA Stratification Tool for Opioid Risk Management (STORM). Implement Sci. 2019. 10.1186/s13012-019-0852-z [DOI] [PMC free article] [PubMed]
- 22.The Management of Substance Use DIsorders. VA / DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Assessment. 2015.
- 23.Wen H, Hockenberry JM, Jeng PJ, Bao Y. Prescription Drug Monitoring Program Mandates: Impact On Opioid Prescribing And Related Hospital Use. Health Aff. 2019. 10.1377/hlthaff.2019.00103 [DOI] [PMC free article] [PubMed]
- 24.Hansen HB, Siegel CE, Case BG, Bertollo DN, DiRocco D, Galanter M. Variation in use of buprenorphine and methadone treatment by racial, ethnic, and income characteristics of residential social areas in New York City. J Behav Health Serv Res. 2013;40(3):367–377. doi: 10.1007/s11414-013-9341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019. 10.1016/j.drugalcdep.2019.01.038 [DOI] [PMC free article] [PubMed]
- 26.Hausmann LRM, Gao S, Lee ES, Kwoh CK. Racial disparities in the monitoring of patients on chronic opioid therapy. Pain. 2013. 10.1016/j.pain.2012.07.034 [DOI] [PubMed]
- 27.Manhapra A, Quinones L, Rosenheck R. Characteristics of veterans receiving buprenorphine vs. methadone for opioid use disorder nationally in the Veterans Health Administration. Drug Alcohol Depend. 2016. 10.1016/j.drugalcdep.2015.12.035 [DOI] [PMC free article] [PubMed]
- 28.Joudrey PJ, Edelman EJ, Wang EA. Drive Times to Opioid Treatment Programs in Urban and Rural Counties in 5 US States. JAMA. 2019;322(13):1310. doi: 10.1001/jama.2019.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dick A, Pacula R, Gordon AJ, et al. Increasing access to opioid agonist treatment in U.S. treatment shortage areas. Drug Alcohol Depend. 2015. 10.1016/j.drugalcdep.2014.09.189
- 30.Oliva EM, Trafton JA, Harris AHS, Gordon AJ. Trends in opioid agonist therapy in the veterans health administration: Is supply keeping up with demand? Am J Drug Alcohol Abuse. 2013. 10.3109/00952990.2012.741167 [DOI] [PubMed]
- 31.Meints SM, Cortes A, Morais CA, Edwards RR. Racial and ethnic differences in the experience and treatment of noncancer pain. Pain Manag. 2019. 10.2217/pmt-2018-0030 [DOI] [PMC free article] [PubMed]
- 32.Armstrong K, McMurphy S, Dean LT, et al. Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med. 2008;23(6):827–833. doi: 10.1007/s11606-008-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whetten K, Leserman J, Whetten R, et al. Exploring lack of trust in care providers and the government as a barrier to health service use. Am J Public Health. 2006. doi:10.2105/AJPH.2005.063255 [DOI] [PMC free article] [PubMed]