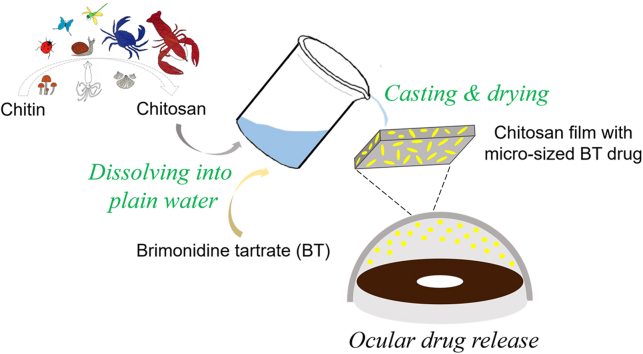
Abstract
Chitosan is a nature-based polymer with low toxicity, excellent biocompatibility and biodegradability. However, the intractable solubility of chitosan in water and most conventional solvents hampers its biomedical applications. Following the dissolution method for dissolving chitosan in plain water developed by us, chitosan was dissolved in ionic liquid followed by overnight freezing at −20 °C and subsequent solvent exchange with plain water at room temperature. In this study, we fabricated a drug-carrying chitosan film via solution casting and air-drying by using the plain water-based chitosan solution. Specifically, brimonidine tartrate (BT), an antiglaucoma drug, was dissolved in the plain-water based solution and used to prepare BT-loaded chitosan film, i.e., chitosan-BT film. The resulting film is transparent, structurally stable, and mucoadhesive. Micro-sized antiglaucoma BT drug crystals form and are well dispersed in the chitosan film. The chitosan-BT film enables BT to have a high corneal permeability with fast drug release kinetics for potential ocular drug delivery.
Keywords: Chitosan, Plain water, Brimonidine, Glaucoma, Ionic liquid
Graphical abstract
Highlights
-
•
A drug-carrying chitosan film is made via solution casting and air-drying of plain water-based chitosan solution.
-
•
The antiglaucoma drug brimonidine tartrate loaded into the chitosan film forms micro-sized crystals with uniform dispersion.
-
•
The chitosan film enables BT to have a high corneal permeability with fast drug release for potential ocular drug delivery.
1. Introduction
Chitosan is a nature-based polymer commonly obtained from the deacetylation of chitin, one of the most abundant and renewable biopolymers on the earth after cellulose. Due to good biocompatibility and biodegradability, chitosan has broad applications in the fields of cosmetic, food, pharmaceutical and biomedicine [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]]. Food and Drug Administration (FDA) has approved chitosan-based wound dressings [11]. Inheriting biologically desired characteristics such as anti-bacteria and bioadhesion, chitosan films have been used as food wraps, artificial skins, contact lenses and so on [[12], [13], [14], [15]]. However, the poor solubility of chitosan is still an issue for fabrication. It is difficult to dissolve chitosan into water and most solvents because of the intermolecular and intramolecular hydrogen-bonded network [16].
Acids like acetic acid are commonly used to dissolve chitosan [17]. However, the use of acids may involve many corrosive processes and the acid residues may affect the skin, mucosa, tissue and so on. Alkali neutralization and water washing usually follow the acid treatment to remove acids from chitosan. Nevertheless, the residues of acids and alkalis may remain in products and have adverse biological effects. In addition, it remains a challenge to incorporate water-soluble compounds into chitosan during the solubilization process. Recently, ionic liquids (ILs) have received much attention as solvents for chitosan solubilization because of their compelling features, such as non-volatile, high thermal and chemical stability, and easy to recycle [18]. ILs are more environmentally friendly and the chitosan/ILs systems have better processability to develop chitosan materials [18]. Although previous studies showed that some ionic liquids are relatively safe for industrial applications, the toxicity of ionic liquids still needs to be further elucidated to support the biological use of chitosan/ILs systems [19,20]. Plain water can be the safest and the most applicable solvent to develop chitosan-based biomedical materials. In our previous study, we developed an ecofriendly method for dissolving chitosan in plain water [21]. In the method, chitosan was first dissolved in the ionic liquid EMIM Ac and then the chitosan/ionic liquid mixture was kept at −20 °C overnight. Then, the extensive solvent exchange with plain water at room temperature was conducted to obtain a stable dispersion of nanosized chitosan in plain water. In the process, the hydrogen-bonded network of chitosan is disrupted by the ionic liquid to free the amines that participate in hydrogen bonding. The intermediate freezing step prevents the dissolved chitosan from reconnecting with hydrogen-bonding interactions. The solvent-exchange step leads to the protonation of chitosan's amine groups, which in turn promotes chitosan solvation in plain water. This method eliminates solvent effects and skips tedious post-solubilization processes. Furthermore, it opens up opportunities to develop and apply chitosan-based products for those biomedical applications that depend critically on safer formulations such as ocular drug delivery.
In this work, we developed an ocular drug-loaded chitosan film by using the plain water-based chitosan solution. Drug-carrying film materials such as therapeutic contact lenses have been applied to treat eye diseases recently [[22], [23], [24], [25], [26]]. Brimonidine tartrate (BT) is an antiglaucoma drug commonly taken in the form of eye drops. The efficacy of BT can be improved in many aspects through the use of polymer carriers [[27], [28], [29], [30], [31]]. In our new formulation, micro-sized brimonidine tartrate crystals formed within the chitosan film through the solution casting and air-drying process. The SEM, swelling, stability and light transmittance experiments were conducted to characterize the chitosan film. The cytotoxicity and mucoadhesion for the obtained film were also investigated. The drug release kinetics and permeability of BT in the form of the chitosan-BT film were studied. With excellent biocompatibility and high corneal permeability of the drug, this drug-carried chitosan film can be potentially used for ocular drug delivery.
2. Materials and methods
2.1. Materials
Chitosan with high molecular weight (HMW) (310,000–375,000 Da), ionic liquid 1-ethyl-3-methylimidazolium acetate (EMIM Ac, ≥95.0%) and cell proliferation reagent WST-1 were purchased from Sigma-Aldrich (St. Louis, MO). Brimonidine tartrate (BT) (salt form) was purchased from AvaChem Scientific (San Antonio, TX). Water (HPLC grade), acetonitrile (ACN, HPLC grade), trifluoroacetic acid (TFA), phosphate-buffered saline (PBS, 10 × ), ELISA kits of human IL-6, IL-β and TNF-α were purchased from Thermo Fisher Scientific (Logan, UT). Gastric porcine mucin was purchased from PFALTZ&BAUER, Inc. (CT, USA). The concentration of mucin was measure by Pierce™ BCA protein assay kit purchased from Thermo Fisher. Fresh rabbit whole eyes were purchased from Pel-Freeze Biologicals (Rogers, AR). Rhodamine B (RB) was purchased from Fluka.
2.2. Methods
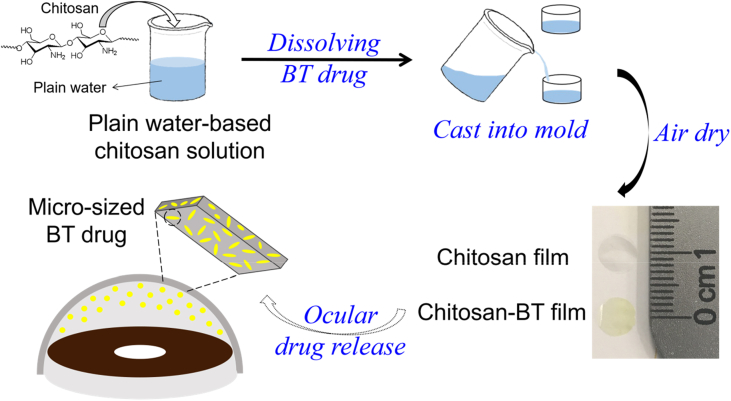
Preparation of Chitosan Films. The plain water-based chitosan solution was prepared following our previous method [21]. Briefly, the HMW chitosan was dissolved in ionic liquid EMIM Ac and frozen overnight, then solvent exchanged with plain water to form the plain water-based chitosan solution with a concentration of about 2.4 mg/mL [21]. Chitosan film was prepared by casting 2 mL of the plain water-based chitosan solution into a rounded polytetrafluoroethylene (PTFE) mold with a diameter of 32 mm followed by air-drying overnight. Brimonidine tartrate (BT) was dissolved in the plain water-based chitosan solution with a concentration of 1 mg/mL. Following the same casting-drying method, chitosan-BT film was prepared. Rose-colored chitosan-RB film was also prepared. The obtained films were punched by a 6 mm-diameter puncher for further use (Fig. 1).
Fig. 1.
Preparation of chitosan film and chitosan-BT film.
Scanning Electron Microscopy (SEM). Chitosan film and chitosan-BT films (before and after drug release) were sputter-coated with platinum for 90 s. Then, SEM images were taken on a scanning electron microscope (Hitachi FE-SEM Su-70, Japan).
Swelling Studies. The swelling behavior of the chitosan film was determined by immersing chitosan film in PBS (pH 7.4, 1 × ) at 37 °C. The swollen chitosan film was gently taken out and weighted at different time intervals. The measurement duration was up to 24 h in order to reach the maximum absorption. The swelling ratio was calculated by: Swelling ratio (%) = (Wt - W0)/W0 × 100, where Wt represents the mass of the swollen sample at time point t and W0 represents the initial mass of the sample.
Stability Studies. The stability of chitosan film was investigated by monitoring the mass of the chitosan film after swelling equilibrium in PBS (pH 7.4, 1 × ) at 37 °C. The chitosan film was immersed in PBS (pH 7.4, 1 × ) at 37 °C and the mass of the swollen chitosan film at 24 h was taken as the initial mass (ms). The mass of the swollen sample at time point t is defined as mt. The ratio of mt/ms represents the weight change and the stability of the chitosan film.
Light Transmittance. The visible light transmittance of the chitosan film was measured with a UV–Vis spectrophotometer (Evolution 201) in the wavelength range from 400 to 800 nm.
Cytotoxicity Assessment. Cytotoxicity of the chitosan film was evaluated on both NIH3T3 mouse embryo fibroblasts cells and primary human corneal epithelial (HCE) cells. HCE cells, derived from normal human corneal tissue, were cultured following the manufacturer's instructions in complete corneal epithelial cell growth medium. HCE cells within third passages were used. Chitosan film was sterilized by UV irradiation before use. NIHT3T3 cells were seeded in a 6-well plate at a density of 5 × 105 cells/well. HCE cells were seeded in a 12-well plate at a density of 5 × 104 cells/well. The cells were cultured for 24 h to allow attachment. Sterilized chitosan film was put in an insert with different doses. The inserts loaded with chitosan films were placed in the wells, immersed by cell culture medium without contacting with the cells. After 24-h culture, the cytotoxicity was analyzed by WST-1 assay. For quantification of cytokines, 600 μL of the culture media of HCE cells was collected and centrifuged after 6-h culturing. The supernatants were subjected to ELISA analysis following the manufacturer's instructions.
Mucoadhesion Tests. To assess mucoadhesive properties of the chitosan film, a series of mucin aqueous solutions were prepared for quantitative analysis of the interaction between mucin particles and the chitosan film [32]. A standard curve was created by using Pierce™ BCA protein assay kit following the manufacture's procedure with different mucin concentrations (2000, 1500, 1000, 500, 200, 100, and 20 μg/mL). Chitosan film (~1.43 mg, ~2.30 cm2) was added to 1 mL of as prepared mucin solution (2 mg/mL). After determined time points (1 h, 2 h, and 24 h), the concentration of the mucin solution was analyzed using the same assay kit as the standard curve. The mucoadhesive property of the chitosan film was calculated by (m0-mt)/s, where m0 is the mass of mucin in the solution before chitosan film adsorption, mt is the mass of mucin in the solution after chitosan film adsorption, s is the adsorption area of the chitosan film. The adsorption area of 1.43 mg chitosan film is about 4.60 cm2.
In Vitro Drug Release Kinetics. Drug release from chitosan-BT film was performed. Briefly, 0.8 mg of chitosan-BT film containing about 222 μg of BT was immersed in 1 mL of PBS in a centrifuge tube. The tube was maintained at 37 °C. At predetermined time intervals, 1 mL of PBS medium was withdrawn, and 1 mL of fresh PBS was added to maintain a constant volume. The BT concentration in the withdrawn PBS medium was analyzed on HPLC based on a standard curve of BT. All experiments were performed in triplicate.
Ex Vivo Permeation Studies. The corneal permeability of BT in the chitosan-BT film was evaluated in a Franz diffusion cell system (PermeGear, Hellertown, PA) [33]. The Franz diffusion cell had a diffusion area of 0.785 cm2 with a donor chamber and a receiver chamber. Cornea wasextracted from fresh rabbit eye and immediately mounted in the diffusion cell with the epithelial surface facing the donor chamber and the endothelial surface facing the acceptor chamber. A rounded chitosan-BT film with a 6 mm diameter attached to the cornea epithelial surface and loaded in the donor chamber with 200 μL of PBS. The receiver chamber of the diffusion cell was filled with 5 mL of PBS. At predetermined time points up to 1 h, an aliquot of 500 μL PBS was withdrawn from the receiver chamber and analyzed by HPLC, and fresh PBS (500 μL) was added to the receiver chamber after each sampling. All the experiments were conducted in triplicate. The permeability coefficient, P, was calculated as follows: P = (dQ/dt)/AC, where dQ/dt is the steady-state slope of a cumulative flux curve, C is the loading concentration of BT in the donor chamber, and A is the effective cross-sectional area (0.785 cm2) for diffusion. Flux (μg/cm2/h) is determined by (dQ/dt)/A [33].
Statistical Analysis. The data were reported as means ± standard deviation (SD). Student's t-test was conducted for statistical analysis. P values less than 0.05 were considered statistically significant.
3. Results and discussion
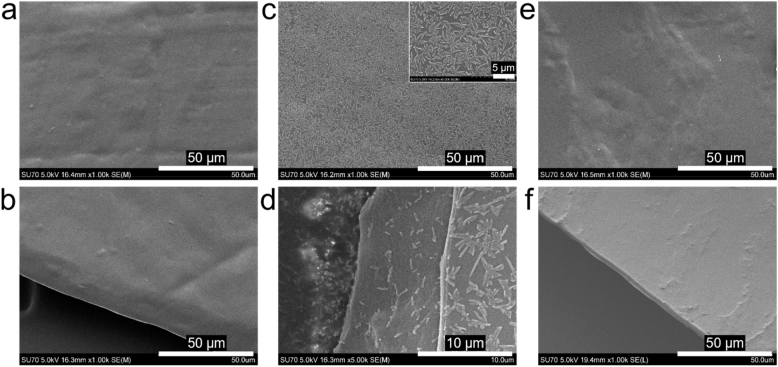
The chitosan film and chitosan-BT film were prepared through a solution casting and air-drying process with plain water as the solvent (Fig. 1). Fig. 2 shows the SEM images of the chitosan film and chitosan-BT film before and after drug release. As shown in Fig. 2a and b, a smooth chitosan film was obtained after evaporating the solvent of plain water at room temperature. The thickness of the chitosan film is about 3.5 μm. After dissolving of brimonidine tartrate (BT) in the plain water-based chitosan solution, a chitosan-BT film was prepared. BT crystals are uniformly formed and well dispersed in the chitosan film, as shown in Fig. 2c. Submicron/micro-sized BT particles (Fig. 2c inset) are loaded on the surface and inserted into the inside of the chitosan film (Fig. 2d). After drug release from the chitosan-BT film, micro-sized BT crystals disappear from the smooth chitosan film (Fig. 2e and f).
Fig. 2.
SEM images of chitosan film (a, b), chitosan-BT film (c, d), and chitosan-BT film after drug release (e, f).
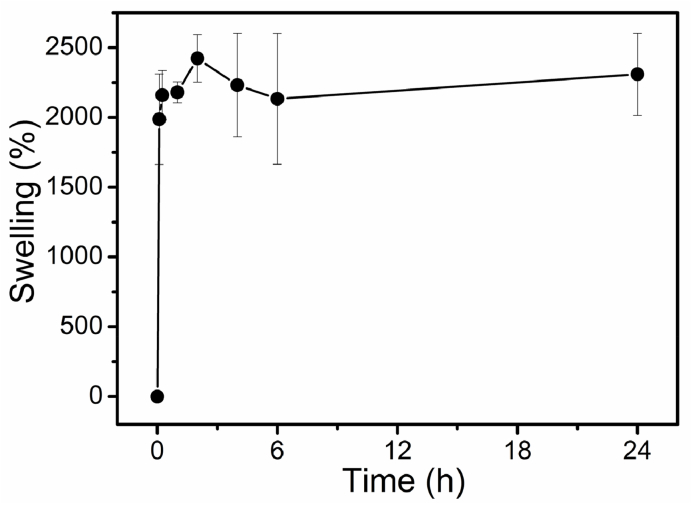
As shown in Fig. 3, the swelling ratio of the chitosan film reaches to 1987% at 6 min and exceeds 2000% within 15 min. Then, the swelling ratio fluctuates around 2300% for a swelling equilibrium. The high swelling capacity of the chitosan film in PBS is attributed to the extremely hydrophilic nature and the high affinity to the salt solutions of chitosan due to the hydroxy and amino groups [34,35]. In this study, a chitosan film formed following solution casting and air-drying of the plain water-based chitosan solution. During the chitosan solution preparation, the hydrogen-bonded network of chitosan was disrupted, and the chitosan system experienced a significant volume expansion during solvation [21]. Upon drying, the hydrogen-bonded network restores and the chitosan film forms, in which chitosan molecules are connected via a relatively extended chain conformation, leading to increased exposure of hydrophilic functional groups (hydroxy and amino groups). This is presumably attributed to the unusually high swelling ratio for the film prepared using our method compared with chitosan film prepared using the traditional method [36]. The chitosan film has a high swelling ratio in PBS, an attribute enabling drug carrier to efficiently release drug to the aqueous environment via swelling-controlled diffusion.
Fig. 3.
Swelling behavior of chitosan film in PBS at 37 °C.
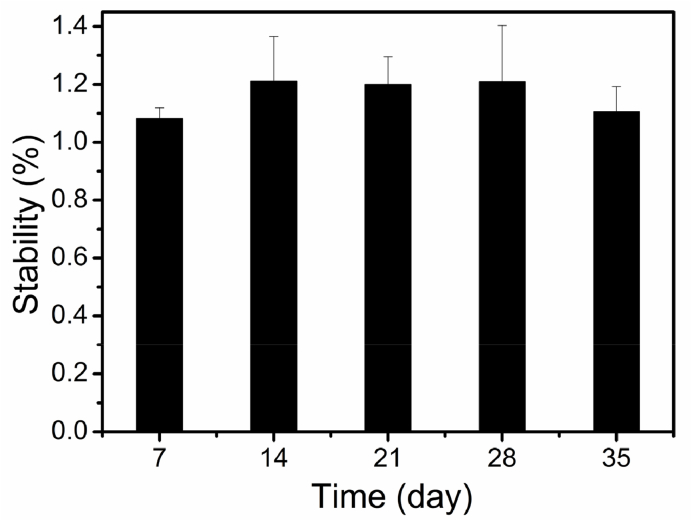
The stability of the chitosan film is shown in Fig. 4. Good stability has been found for the chitosan film with no obvious change of the weight ratio over 35 days. By using an ecofriendly method in our previous study, a plain water-based chitosan solution was prepared through breaking the hydrogen-bonded network of chitosan and the chitosan amino groups are protonated by water molecules [21]. As the removal of plain water by air-dying, the hydrogen-bonded interactions among chitosan macromolecules restore, and a stable chitosan film forms with the recovered hydrogen-bonded network. The obtained chitosan film is stable and insoluble in PBS. It is possible to develop this chitosan film as a stable drug-carrier such as ocular insert.
Fig. 4.
Stability of chitosan film in PBS at 37 °C.
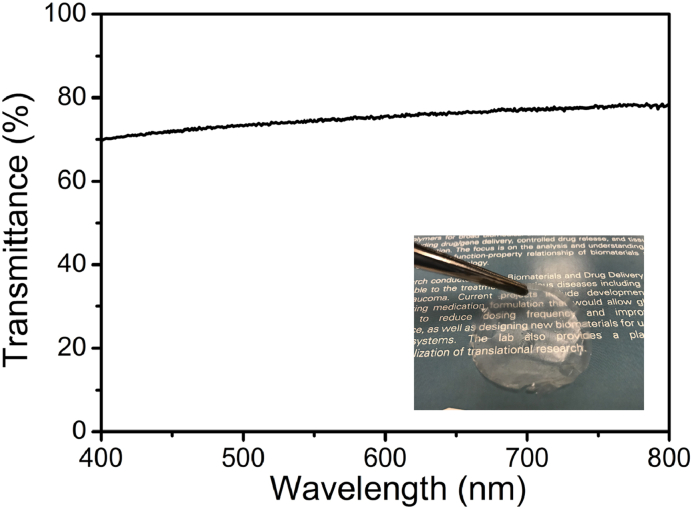
Fig. 5 shows the transmittance spectrum and photograph of the chitosan film. The chitosan film is flexible and transparent (Fig. 5, inset). Good light transmittance was exhibited by the chitosan film with a light transmittance value of over 70% from 400 nm to 800 nm. Many transparent film materials as functional contact lenses showed improved effects for eye treatment [15,[22], [23], [24], [25], [26]]. As a transparent film, this chitosan material can be potentially composed of a contact lens for ocular drug release.
Fig. 5.
Transmittance spectrum of the chitosan film. Inset: chitosan film shows excellent optical transparency.
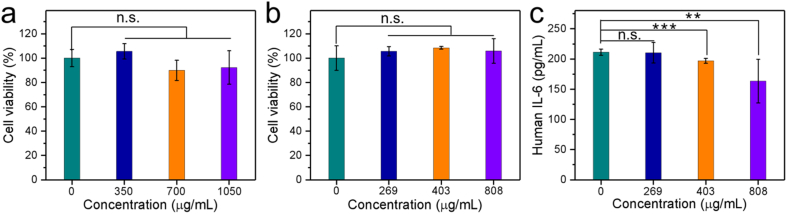
The compatibility of the chitosan film was studied by cytotoxicity assessments on both NIH3T3 cells and HCE cells, as shown in Fig. 6. The cytotoxicity was not observed on NIH3T3 cells when the concentration of chitosan film is up to 1050 μg/mL (Fig. 6a). When the concentration of chitosan film is lower than 808 μg/mL, the chitosan film is also cytocompatible on HCE cells, as shown in Fig. 6b. IL-6, IL-β, and TNF-α cytokines on the HCE cells were analyzed following treatment with chitosan film at 269, 403, and 808 μg/mL. The chitosan film does not accelerate the secretion of IL-β and TNF-α. No IL-β and TNF-α signals were detected in the experiments. Chitosan film at concentrations of 403 and 808 μg/mL partly decreases the IL-6 levels on HCE cells (Fig. 6c). Generally, the cytotoxicity of the chitosan film was not observed according to the viability assessments of NIH3T3 cells and HCE cells. Chitosan is considered to be a biomaterial with very low toxicity [37]. Avoiding the solvent effects, chitosan products can practically meet the need of bioapplication. The chitosan film prepared from the solvent of plain water shows excellent compatibility for biomedical uses.
Fig. 6.
Cytotoxicity on (a) NIH3T3 cells, (b) HCE cells, and (c) cytokine IL-6 expression on HCE cells (n = 4). N.S. indicates not significant, ** indicates p < 0.01 and *** indicates p < 0.001.
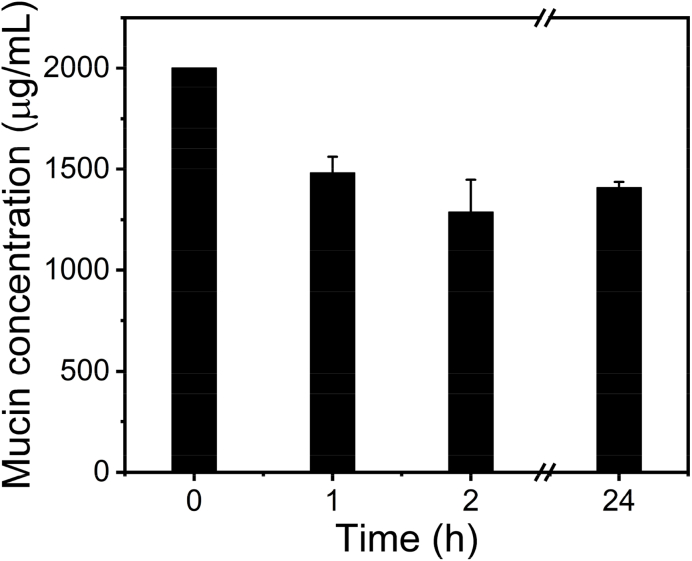
The mucoadhesive properties of the chitosan film were tested by its interactions with mucin [32]. When adding a polymer film into a mucin solution, the concentration of the mucin would decrease if the polymer film has the affinity to the mucin. As shown in Fig. 7, the concentration of the mucin solution drops from 2000 μg/mL to ~1500 μg/mL at 1 h, and to ~1400 μg/mL over 2 h. The absorption of mucin particles on chitosan film (~1.43 mg) accounts for the decrease of the mucin concentration, which indicates the mucoadhesive properties of the chitosan film. The mucoadhesive property of the chitosan film to mucin is about 128 μg/cm2. The mucoadhesive property of the chitosan to mucin is attributed to their many interactions, such as hydrogen bonding, electrostatic interactions, hydrophobic effects and so on [38]. The mucoadhesive chitosan film has a great potential for transmucosal administration.
Fig. 7.
Concentration of mucin in the aqueous solutions with chitosan film.
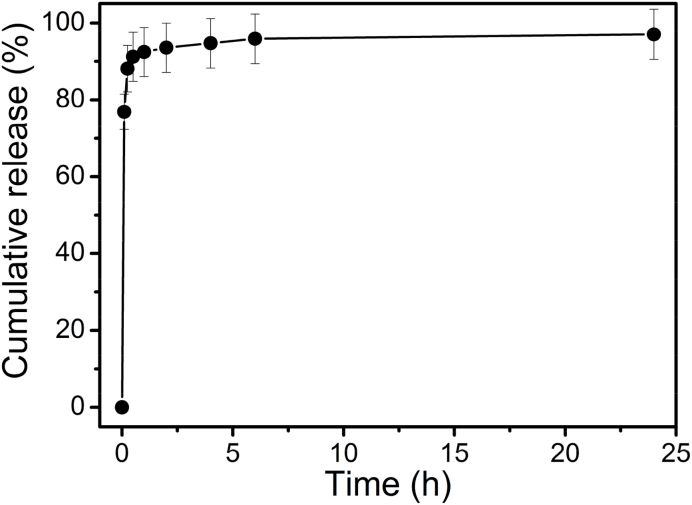
In vitro drug release kinetics of BT for the chitosan-BT film in PBS at 37 °C are shown in Fig. 8. BT is released quickly from the chitosan-BT film. More than 75% of the drug is released within 6 min, and about 90% of drug is released less than half an hour. The remaining BT drug shows a relatively slow release. The chitosan film is extremely hydrophilic, and water solution can be easily absorbed by the chitosan film as shown from the results of the swelling behavior (Fig. 3). With a large surface area, micro-sized BT drug would fast dissolve in the aqueous environment. According to the results of drug release (Fig. 8) and SEM (Fig. 2), the fast release is from the fast-dissolved BT particles loaded on the surface of the chitosan-BT film, and the relatively slow release is attributed to the remaining drug inserted into the inside of the chitosan-BT film.
Fig. 8.
In vitro release kinetics of brimonidine tartrate from chitosan-BT film in PBS at 37 °C.
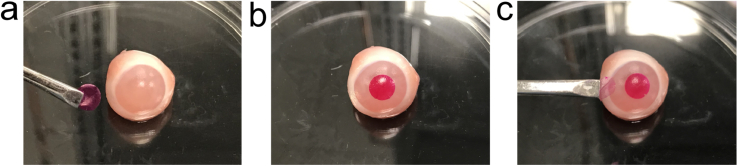
As a model to show the practical release to the eye globe, a rhodamine B (RB)-loaded chitosan film (chitosan-RB film) has been prepared using the same method as the chitosan-BT film. The chitosan-RB film and the rabbit eye globe are chosen to demonstrate the release behavior as shown in Fig. 9. A rose-colored chitosan film was obtained (Fig. 9a). As shown in Fig. 9b, the chitosan-RB film on the rabbit eye globe shows good mucoadhesion. The mucoadhesive property of the chitosan film is further supported by the mucin experiments (Fig. 7). The chitosan-RB film almost turns transparent after release, and RB is well released onto the rabbit globe as shown in Fig. 9c. The light transmission of the chitosan film (Fig. 5) would maintain after the release of RB. It is consistent with the fast release behavior of chitosan-BT film with about 75% BT drug having been released in 6 min as shown in Fig. 8.
Fig. 9.
Chitosan-RB film releases RB to the rabbit eye. (a) Before the application, (b) attachment of the chitosan-RB film onto the rabbit eye for 6 min, and (c) removal of the film.
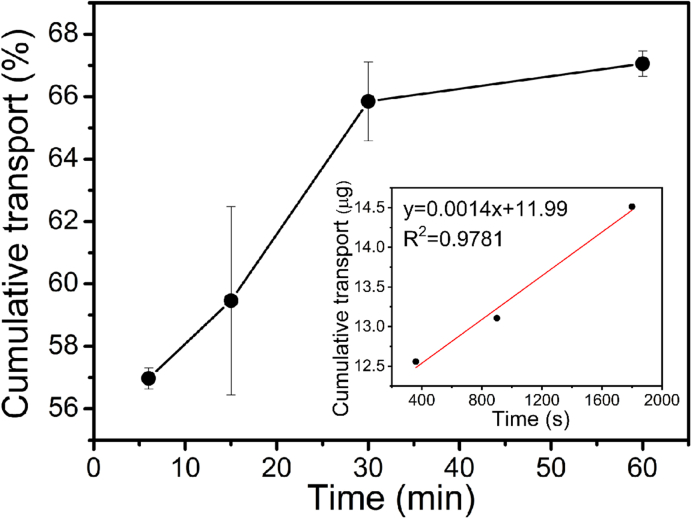
The transport of BT across the rabbit cornea was further studied with the chitosan-BT film. As shown in Fig. 10, the cumulative flux curve of BT displays a linear increase within the first 30 min and reaches a plateau afterward. The permeability is then calculated according to the linear range (Fig. 10 inset) of the cumulative flux curves. The cornea permeability of BT in the chitosan-BT film is determined to be 1.62 × 10−5 cm/s, which is much higher than the permeation coefficient from many previous systems [28,30]. Typical permeability coefficient for brimonidine range is between 2.1 × 10−7 and 3.6 × 10−7 cm/s [28,39]. Nano/micro-sized drug preparations can improve drug solubility and bioavailability due to the large surface area [40,41]. The micro-sized BT preparation through the chitosan film (Fig. 2) can significantly improve the BT transport across the cornea for ocular release. Although the release of BT is fast, this chitosan-BT film could possibly be an alternative of eye drops since the released BT can efficiently cross the cornea.
Fig. 10.
Ex vivo transport of brimonidine tartrate across the cornea from chitosan-BT film. Inset: linear range (30 min) of the cumulative flux curves used for permeability determination.
4. Conclusion
In this work, a chitosan film loaded with micro-sized drug was prepared using a simple and ecofriendly method based on solution casting and air-drying of plain water-based chitosan solution. Brimonidine tartrate (BT) as a model drug was incorporated to form a chitosan-BT film. Micro-sized BT crystals uniformly form and are dispersed in the chitosan film. The chitosan film is transparent and stable with good properties of mucoadhesion and biocompatibility. BT is released fast from the chitosan-BT film but has a high cornea permeability. This chitosan-BT film has a great potential to be used for ocular drug delivery.
Author contribution
BL, JW and HY conceptualized the project. BL and JW conducted most experiments and data analysis with equal contribution. QG conducted cell-based assays. HY was responsible for project administration.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgment
This work was supported, in part, by the National Institutes of Health (R01EY024072) (HY) and the Fundamental Research Funds for the Central Universities (Grant No. 3332019100, 2019PT320028) (BL).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Rinaudo M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006;31:603–632. [Google Scholar]
- 2.Kumar M., Muzzarelli R.A.A., Muzzarelli C., Sashiwa H., Domb A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 3.Aranaz I., Acosta N., Civera C., Elorza B., Mingo J., Castro C., de los Llanos Gandia M., Heras Caballero A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers. 2018;10(1–25):213. doi: 10.3390/polym10020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahidi F., Arachchi J.K.V., Jeon Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999;10:37–51. [Google Scholar]
- 5.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. (N. Y.) 1998;15:1326–1331. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- 6.Sinha V.R., Singla A.K., Wadhawan S., Kaushik R., Kumria R., Bansal K., Dhawan S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004;274:1–33. doi: 10.1016/j.ijpharm.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Dash M., Chiellini F., Ottenbrite R.M., Chiellini E. Chitosan—a versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011;36:981–1014. [Google Scholar]
- 8.Yang Y., Wang X., Yang F., Shen H., Wu D. A universal soaking strategy to convert composite hydrogels into extremely tough and rapidly recoverable double-network hydrogels. Adv. Mater. 2016;28:7178–7184. doi: 10.1002/adma.201601742. [DOI] [PubMed] [Google Scholar]
- 9.Zhu K., Duan J., Guo J., Wu S., Lu A., Zhang L. High-strength films consisted of oriented chitosan nanofibers for guiding cell growth. Biomacromolecules. 2017;18:3904–3912. doi: 10.1021/acs.biomac.7b00936. [DOI] [PubMed] [Google Scholar]
- 10.Zhu D., Jin X., Leng X., Wang H., Bao J., Liu W., Yao K., Song C. Local gene delivery via endovascular stents coated with dodecylated chitosan-plasmid DNA nanoparticles. Int. J. Nanomed. 2010;5:1095–1102. doi: 10.2147/IJN.S14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedmore I., McManus J.G., Pusateri A.E., Holcomb J.B. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J. Trauma Acute Care Surg. 2006;60:655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 12.Rabea E.I., Badawy M.E.T., Stevens C.V., Smagghe G., Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 13.Dutta P.K., Tripathi S., Mehrotra G.K., Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114:1173–1182. [Google Scholar]
- 14.Mao J.S., Zhao L.G., Yao K.D., Shang Q.X., Yang G.H., Cao Y.L. Study of novel chitosan-gelatin artificial skin in vitro. J. Biomed. Mater. Res. 2003;64A:301–308. doi: 10.1002/jbm.a.10223. [DOI] [PubMed] [Google Scholar]
- 15.Shi X.Y., Tan T.W. New contact lens based on chitosan/gelatin composites. J. Bioact. Compat Polym. 2004;19:467–479. [Google Scholar]
- 16.Tian Q., Liu S., Sun X., Sun H., Xue Z., Mu T. Theoretical studies on the dissolution of chitosan in 1-butyl-3-methylimidazolium acetate ionic liquid. Carbohydr. Res. 2015;408:107–113. doi: 10.1016/j.carres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Roberts G.A.F. Chitin Chemistry. Mcmillan Press Ltd.; London: 1992. Solubility and solution behaviour of chitin and chitosan; pp. 274–329. [Google Scholar]
- 18.Silva S.S., Mano J.F., Reis R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017;19:1208–1220. [Google Scholar]
- 19.Ostadjoo S., Berton P., Shamshina J.L., Rogers R.D. Scaling-up ionic liquid-based technologies: how much do we care about their toxicity? Prima facie information on 1-ethyl-3-methylimidazolium acetate. Toxicol. Sci. 2018;161:249–265. doi: 10.1093/toxsci/kfx172. [DOI] [PubMed] [Google Scholar]
- 20.Egorova K.S., Gordeev E.G., Ananikov V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017;117:7132–7189. doi: 10.1021/acs.chemrev.6b00562. [DOI] [PubMed] [Google Scholar]
- 21.Li B., Wang J., Moustafa M.E., Yang H. Ecofriendly method to dissolve chitosan in plain water. ACS Biomater. Sci. Eng. 2019;5:6355–6360. doi: 10.1021/acsbiomaterials.9b00695. [DOI] [PubMed] [Google Scholar]
- 22.Than A., Liu C., Chang H., Duong P.K., Cheung C.M.G., Xu C., Wang X., Chen P. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat. Commun. 2018;9(1–12):4433. doi: 10.1038/s41467-018-06981-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H.J., Zhang K., Moore L., Ho D. Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release. ACS Nano. 2014;8:2998–3005. doi: 10.1021/nn5002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasr F.H., Khoee S., Dehghan M.M., Chaleshtori S.S., Shafiee A. Preparation and evaluation of contact lenses embedded with polycaprolactone-based nanoparticles for ocular drug delivery. Biomacromolecules. 2016;17:485–495. doi: 10.1021/acs.biomac.5b01387. [DOI] [PubMed] [Google Scholar]
- 25.Deng J., Chen S., Chen J., Ding H., Deng D., Xie Z. Self-reporting colorimetric analysis of drug release by molecular imprinted structural color contact lens. ACS Appl. Mater. Interfaces. 2018;10:34611–34617. doi: 10.1021/acsami.8b11655. [DOI] [PubMed] [Google Scholar]
- 26.Liu X., Chen J., Qu C., Bo G., Jiang L., Zhao H., Zhang J., Lin Y., Hua Y., Yang P., Huang N., Yang Z. A mussel-inspired facile method to prepare multilayer-AgNP-loaded contact lens for early treatment of bacterial and fungal keratitis. ACS Biomater. Sci. Eng. 2018;4:1568–1579. doi: 10.1021/acsbiomaterials.7b00977. [DOI] [PubMed] [Google Scholar]
- 27.Yang H., Tyagi P., Kadam R.S., Holden C.A., Kompella U.B. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano. 2012;6:7595–7606. doi: 10.1021/nn301873v. [DOI] [PubMed] [Google Scholar]
- 28.Lancina M.G., Singh S., Kornpella U.B., Husain S., Yang H. Fast dissolving dendrimer nanofiber mats as alternative to eye drops for more efficient antiglaucoma drug delivery. ACS Biomater. Sci. Eng. 2017;3:1861–1868. doi: 10.1021/acsbiomaterials.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holden C.A., Tyagi P., Thakur A., Kadam R., Jadhav G., Kompella U.B., Yang H. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine. 2012;8:776–783. doi: 10.1016/j.nano.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Williamson G.S., Lancina M.G., Yang H. Mildly cross-linked dendrimer hydrogel prepared via aza-Michael addition reaction for topical brimonidine delivery. J. Biomed. Nanotechnol. 2017;13:1089–1096. doi: 10.1166/jbn.2017.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Williamson G.S., Yang H. Branched polyrotaxane hydrogels consisting of alpha-cyclodextrin and low-molecular-weight four-arm polyethylene glycol and the utility of their thixotropic property for controlled drug release. Colloids Surf., B. 2018;165:144–149. doi: 10.1016/j.colsurfb.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi H., Thongborisute J., Matsui Y., Sugihara H., Yamamoto H., Kawashima Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv. Drug Deliv. Rev. 2005;57:1583–1594. doi: 10.1016/j.addr.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Q., Fu Y., Kao W.J., Janigro D., Yang H. Transbuccal delivery of CNS therapeutic nanoparticles: synthesis, characterization, and in vitro permeation studies. ACS Chem. Neurosci. 2011;2:676–683. doi: 10.1021/cn200078m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aburahma M.H., Mahmoud A.A. Biodegradable ocular inserts for sustained delivery of brimonidine tartarate: preparation and in vitro/in vivo evaluation. AAPS PharmSciTech. 2011;12:1335–1347. doi: 10.1208/s12249-011-9701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salam A., Pawlak J.J., Venditti R.A., El-tahlawy K. Synthesis and characterization of starch citrate−chitosan foam with superior water and saline absorbance properties. Biomacromolecules. 2010;11:1453–1459. doi: 10.1021/bm1000235. [DOI] [PubMed] [Google Scholar]
- 36.Baskar D., Kumar T.S.S. Effect of deacetylation time on the preparation, properties and swelling behavior of chitosan films. Carbohydr. Polym. 2009;78:767–772. [Google Scholar]
- 37.Hu L., Sun Y., Wu Y. Advances in chitosan-based drug delivery vehicles. Nanoscale. 2013;5:3103–3111. doi: 10.1039/c3nr00338h. [DOI] [PubMed] [Google Scholar]
- 38.Sogias I.A., Williams A.C., Khutoryanskiy V.V. Why is chitosan mucoadhesive? Biomacromolecules. 2008;9:1837–1842. doi: 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- 39.Prausnitz M.R., Noonan J.S. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharmacol. Sci. 1998;87:1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 40.Sosnik A., das Neves J., Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog. Polym. Sci. 2014;39:2030–2075. [Google Scholar]
- 41.Paolicelli P., de la Fuente M., Sanchez A., Seijo B., Alonso M.J. Chitosan nanoparticles for drug delivery to the eye. Expet Opin. Drug Deliv. 2009;6:239–253. doi: 10.1517/17425240902762818. [DOI] [PubMed] [Google Scholar]