Highlights
-
•
High-risk multi-agent drug resistant GTN is a life threatening disease.
-
•
Majority of choriocarcinomas show intense PD-L1 immunoreactivity.
-
•
Pembrolizumab increases antitumor activity.
-
•
Effectiveness of Pembrolizumab in treating patients with high-risk multi-agent drug resistant GTN.
Keywords: Uterine choriocarcinoma, Gestational trophoblastic neoplasia (GTN), PD-L1, Pembrolizumab, High-risk multidrug resistance
1. Introduction
Gestational trophoblastic disease (GTD) is a spectrum of benign to malignant lesions following molar or non-molar pregnancies (Angiolo et al., 2019). Risk factors for gestational trophoblastic neoplasia (GTN) include age at the time of pregnancy, with GTN being more common in very young patients and patients over the age of 40, and history of a prior molar pregnancy. The incidence of GTN varies by race and ethnicity, but this is not well understood. In the United States, the incidence of choriocarcinoma following a gestational event, regardless of the patient’s race, is 1 in 41,094 pregnancies (Smith et al., 2003). Incidence also varies by country, with an increased risk of choriocarcinoma reported in Asia (Shanmugaratnam et al., 1971).
The diagnosis of GTN can occur after evacuation of a partial or complete mole in a patient with persistent elevated β-hCG or with abnormal uterine bleeding after delivery. The work-up of a patient with GTN includes serum β-hCG level, CT scan of the abdomen and pelvis, chest X-ray, and kidney and liver function tests. If imaging reveals liver metastasis, an MRI of the head should also be obtained to assess for brain metastasis. Using this information and a clinical exam, the patient is given a risk score to determine treatment (Table 1) (Figo Oncology Committee, 2002). Patients with high risk scores are more likely to develop drug resistance and are commonly treated with surgery followed by multi-agent chemotherapy with or without adjuvant radiation. The preferred multiagent chemotherapy EMA/CO (etoposide, methotrexate, dactinomycin, cyclophosphamide, vincristine) has remission rates reported as high as 91% (Kim, 1998). Yet, there are still 0.5–5% of patients who die from GTNs as a result of multidrug resistance often seen in conjunction with metastasis to the brain or liver (Ghorani, 2017). These patients require a new treatment approach.
Table 1.
GTN scoring according to risk score criteria developed by the World Health Organization (WHO) as used by FIGO. Low risk is a score < 6. High risk is a score ≥ 7 (Figo Oncology Committee, 2002).
| Prognostic Factor Score | 0 | 1 | 2 | 4 |
|---|---|---|---|---|
| Maternal age | Younger than 40 | 40 and/or older | --- | --- |
| Previous pregnancy | Hydatidiform mole | Abortion | Full-term pregnancy | --- |
| Months since last pregnancy | <4 | 4–6 | 7–12 | >12 |
| Pretreatment hCG (IU/mL) | <103 | 103-104 | >104-105 | ≥105 |
| Largest tumor size, including uterus | <3cm | 3 to < 5 cm | ≥5cm | --- |
| Site of metastases | Lung | Spleen or kidney | Gastrointestinal tract | Brain, liver |
| Number of metastases* | 0 | 1–4 | 5–8 | >8 |
| Number of drugs used to treat the tumor that have failed | 0 | 0 | 1 | ≥2 |
Chest X-ray is used to count number of lung metastases.
A new class of anti-cancer drugs, called immune checkpoint inhibitors, can be effective in restoring host immunity (Jørgensen, 2019). One way the human body regulates immunity is through the interaction between programmed cell death protein 1 (PD-1) on T-cells and its ligand PD-L1 on tumor cells. Among drugs targeting this interaction is Pembrolizumab. Since the incidence of GTN is rare, not much has been published on the treatment of patients with high-risk multidrug resistant GTN. Here we report on the treatment with Pembrolizumab of a woman with choriocarcinoma who showed consistent disease progression following six previous treatment therapies.
2. Case report
A 50-year-old Caucasian female (gravida 3, para 2, abortus 1) presented with an intra-uterine mass and hemorrhaged during an office endometrial biopsy requiring an emergent hysterectomy and bilateral salpingo-oopohrectomy. Pathology confirmed uterine choriocarcinoma with full-thickness myometrial involvement. A chest CT showed numerous small bilateral pulmonary nodules and her β-hCG was 28,725.0 mlU/mL. CT of the abdomen was negative for additional metastatic disease.
The patient was started on multi-agent chemotherapy with EMA/CO of which she completed eleven cycles. Her β-hCG decreased to a negative value as per our lab standard (<7 mlU/mL for post-menopausal women) after four cycles and remained negative for twenty-two weeks. Since the patient’s β-hCG was expected to decrease to ≤ 1 because she was on a high dose oral contraceptive, she was treated with three cycles of EMA/CO past her plateau at 1. Two months after the eleventh cycle, her β-hCG rose to 9.3 mlU/mL, and a chest CT showed persistent pulmonary nodules that were unchanged from three months prior. Consequently, the patient was started on EMA/EP (etoposide, methotrexate, actinomycin-D, cyclophosphamide, vincristine, substituting cyclophosphamide and vincristine for cisplatin and etoposide on Day 8) of which she completed five cycles, showing a decrease to a negative β-hCG following the first cycle.
Two months from her final cycle of EMA/EP, her β-hCG began to rise again, and in another two months it had reached 220 mlU/mL. Imaging showed a PET positive lesion at the top of the vagina. Although the final histology was negative for recurrence, this warranted an EUA and robotic upper vaginectomy which showed no disease.
After surgery her β-hCG initially normalized, but then increased for three months. She was started on TP/TE (paclitaxel, cisplatin/paclitaxel, etoposide) of which she completed seven cycles. Thirty-nine weeks after TP/TE initiation, a chest CT showed that at least three of her pulmonary nodules had increased in size and were amenable to pulmonary metastasectomy. The patient underwent two thoracotomy multiple lung wedge resections with mediastinal lymphadenectomy. Three resections in the right lung were positive for choriocarcinoma with clear margins. The left lung had one upper lobe nodule involved by metastatic choriocarcinoma. The patient’s post-operative β-hCG was elevated at 23 mlU/mL.
A month later, the patient was started on FAEV chemotherapy (floxuridine, dactinomycin, etoposide, vincristine) of which she completed four cycles. Two months after the patient’s fourth cycle, her β-hCG had increased again and continued increasing over the following three months to 385 mlU/mL. She underwent a PET scan which showed multiple enlarged nodules in the left lower lobe and one in the right lung. A new punctate nodule was found in the left lower lobe.
Five months after her fourth cycle of FAEV, the patient was switched to ICE (ifosfamide, carboplatin and etoposide). She completed four cycles, but before her final cycle, a PET-CT showed her left lower lung nodule had enlarged and a new right mediastinal node had appeared.
A month after completing the ICE regimen, she was put on a compassionate use treatment with TRC105, a monoclonal antibody that binds an angiogenic target called endoglin which is highly expressed on GTN tumor vessels and cells. Despite an initial drop in β-hCG, imaging showed signs of disease progression only eight weeks after initiating this treatment. Avastin was added to cycles three and four, but her β-hCG continued to increase to 283 mlU/mL by the fourth cycle.
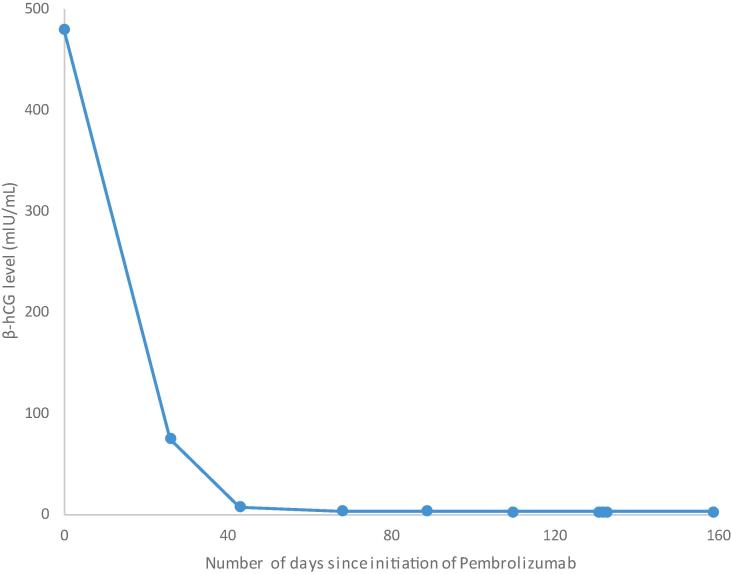
After a team discussion of chemotherapy options following disease progression after her sixth treatment regimen, the patient was started on Pembrolizumab. Her tumor was 100% PD-L1 positive, and she underwent six cycles of Pembrolizumab. After three cycles, her β-hCG became negative (Fig. 1). One month after initiating Pembrolizumab, the patient’s chest CT showed a slight decrease in her left lower lobe nodule and no signs of disease progression. After cycle six, a chest CT showed continual decrease of disease. The Pembrolizumab regimen was complicated by peripheral grade 3 neuropathy requiring discontinuance after cycle six.
Fig. 1.
Patient’s β-hCG level from the first (Cycle 1 initiation = Day 0) through final cycle (Cycle 6 initiation = Day 131) of Pembrolizumab treatment. The β-hCG level decreased to a negative value as per our lab standard (<7 mlU/mL for post-menopausal women) after 3 cycles (Cycle 3 initiation = Day 43) of Pembrolizumab.
Starting eight months after her final cycle, the patient’s β-hCG level began to increase from a negative value to 46 mlU/mL over the next seven months. Sixteen months after her final cycle, a PET-CT showed no evidence of new disease. At twenty-two months from her final cycle, disease progression was detected from a PET scan showing a new hilar lymphadenopathy and a chest CT showed multiple pulmonary nodules suspicious for disease recurrence. The patient recently reinitiated Pembrolizumab.
3. Discussion
High-risk multi-agent drug resistant GTN is a life threatening disease, and PD-1 immunotherapy has the potential to be an effective treatment. When PD-1 and PD-L1 interact there is an inhibitory signal which reduces T-cell proliferation (Alsaab et al., 2017). The antibodies in Pembrolizumab block PD-1 from interacting with PD-L1 which increases T-cell proliferation thereby increasing antitumor activity (Jørgensen, 2019). The majority of choriocarcinomas show intense PD-L1 immunoreactivity (Veras et al., 2017).
Our case presents a patient diagnosed with choriocarcinoma who was resistant to six prior treatment regimens, and achieved her longest period of remission following six cycles of Pembrolizumab treatment (Table 2). The patient’s β-hCG remained negative for eight months after her final Pembrolizumab cycle before a subsequent increase, and no new nodularity had been detected until twenty-two months after her final cycle. This case is also unique because it is one of the first published compassionate uses of Pembrolizumab to treat GTN in the United States.
Table 2.
Summarizes patient’s treatment regimens.
| Name of treatment regimen | Number of cycles | β-hCG level (mlU/mL) * | β-hCG level (mlU/mL) ** | Persistent disease detected by imaging |
|---|---|---|---|---|
| EMA/CO | 11 | 28,725.0 | 1.1 |
|
| EMA/EP | 5 | 22 | <0.6 |
|
| TP/TE | 6 | 799 | <0.6 |
|
| FAEV | 4 | 23 | <0.6 |
|
| ICE | 4 | 385 | 70 |
|
| TRC105 | 4 | 70 | 354 |
|
| Pembrolizumab | 6 | 480 | 2.6 |
EMA/CO etoposide, methotrexate, actinomycin-D, cyclophosphamide and vincristine, EMA/EP etoposide, methotrexate, actinomycin-D, cyclophosphamide, vincristine, substituting cyclophosphamide and vincristine for cisplatin and etoposide on Day 8, TP/TE paclitaxel, cisplatin/paclitaxel, etoposide, FAEV floxuridine, dactinomycin, etoposide and vincristine, ICE ifosfamide, carboplatin and etoposide.
Upon initiation of treatment [results from first treatment day or prior lab test].
Upon when treatment was ended [results from the final treatment day or subsequent lab test].
Detected twenty-two months from final Pembrolizumab cycle.
Another article published specifically on treatment of GTN with Pembrolizumab, a multicenter study conducted in the United Kingdom and Sweden by Ghorani et al. in 2017, reported on four patients between the ages of 37–47 when diagnosed. Three out of the four patients with metastatic drug-resistant GTN to the lung, liver, and or brain achieved remission with Pembrolizumab after recurrence of the disease following prior chemotherapy regimens (Ghorani, 2017).
Immune checkpoint inhibitors have the possibility to be a useful treatment for women showing multidrug resistance and may even be an alternative to high dose chemotherapy considering its favorable low toxicity.
Based on this rationale, a French group started an ongoing phase II trial (NCT03135769) investigating Avelumab in Chemo-resistant GTN. At the end of the study (estimated February 2023) they plan to demonstrate the clinical efficacy of Avelumab evaluated by the rate of patients with successful normalization of hCG assays and the oncologic outcomes (You, 2020).
In conclusion, our patient showed 100% PD-L1 tumor expression which is consistent with the literature that suggests choriocarcinomas express high PD-L1 immunoreactivity (Veras et al., 2017). Our treatment of this patient adds to the current scarcity of literature regarding the effectiveness of Pembrolizumab in treating patients with high-risk multi-agent drug resistant GTN, as we managed to show that Pembrolizumab can be an effective therapy in a case of a patient with this disease. This treatment approach should be further investigated in wider studies to be considered as a valid treatment for patients with high-risk multi-agent drug resistant GTN.
CRediT authorship contribution statement
Jennifer A. Goldfarb: Writing - original draft, Methodology, Formal analysis, Visualization. Giorgia Dinoi: Writing - review & editing, Methodology. Andrea Mariani: Conceptualization, Writing - review & editing. Carrie L. Langstraat: Conceptualization.
Declaration of Competing Interest
Dr. Langstraat reports grants from Medtronic, grants from Eight Medical, grants from ConMed, outside the submitted work. The other three authors have nothing to disclose.
References
- Angiolo G., Silvestro C., Guerrieri M.E., Damiano A.G. Placental site trophoblastic tumor and epithelioid trophoblastic tumor: Clinical and pathological features, prognostic variables and treatment strategy. Gynecol. Oncol. 2019;153(3):684–693. doi: 10.1016/j.ygyno.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Smith H.O., Qualls C.R., Prairie B.A., Padilla L.A., Rayburn W.F., Key C.R. Trends in gestational choriocarcinoma: A 27-year perspective. Obstet. Gynecol. 2003;102(5):978–987. doi: 10.1016/s0029-7844(03)00669-0. [DOI] [PubMed] [Google Scholar]
- Shanmugaratnam K., Muir C.S., Tow S.H., Cheng W.C., Christine B., Pedersen E. Rates per 100,000 births and incidence of choriocarcinoma and malignant mole in Singapore Chinese and Malays. Comparison with connecticut, Norway and Sweden. Int. J. Cancer. 1971;8(1):165–175. doi: 10.1002/ijc.2910080120. [DOI] [PubMed] [Google Scholar]
- Figo Oncology Committee FIGO staging for gestational trophoblastic neoplasia 2000. Int. J. Gynecol. Obstet. 2002;77(3):285–287. doi: 10.1016/S0020-7292(02)00063-2. [DOI] [PubMed] [Google Scholar]
- Kim S.J. Effects of multiagent chemotherapy and independent risk factors in the treatment of high-risk GTT - 25 years experiences of KRI-TRD. Int. J. Gynecol. Obstet. 1998;60(SUPPL) doi: 10.1016/S0020-7292(98)80010-6. pp. 1, pp. 85–96. [DOI] [PubMed] [Google Scholar]
- Ghorani E. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. 2017;390(10110):2343–2345. doi: 10.1016/S0140-6736(17)32894-5. [DOI] [PubMed] [Google Scholar]
- Jørgensen J.T. A paradigm shift in biomarker guided oncology drug development. Ann. Transl. Med. 2019;7(7) doi: 10.21037/atm.2019.03.36. 148–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaab Hashem O., Sau Samaresh, Alzhrani Rami, Tatiparti Katyayani, Bhise Ketki, Kashaw Sushil K., Iyer Arun K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras Emanuela, Kurman Robert J., Wang Tian-Li, Shih Ie-Ming. PD-L1 Expression in Human Placentas and Gestational Trophoblastic Diseases: Int. J. Gynecol. Pathol. 2017;36(2):146–153. doi: 10.1097/PGP.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B. You, “Trial record 1 of 1 for : Saved Studies Avelumab in Chemo-resistant Gestational Trophoblastic Neoplasias (TROPHIMMUN),” NIH U.S. National Library of Medicine Clinical Trials, 2020. [Online]. Available: https://clinicaltrials.gov/ct2/show/NCT03135769?id=NCT03135769&draw=2&rank=1&load=cart.