Graphical abstract
Keywords: Biopreparation, Biological control, Mycopathogen, Broccoli
Highlights
-
•
Biopreparations using low cost solid substrates with adequate nutrients achieved the effective cell counts of Bacillus megaterium AB4.
-
•
Bacillus megaterium biopreparation presented similar control of Alternaria japonica compared to chemical product Mancozeb.
-
•
Bacillus megaterium AB4 reduced the Alternaria japonica disease index in broccoli leaves to <15.6 %.
Abstract
Alternaria japonica causes annual losses of up to 25 % of the world broccoli crops, for this reason this research focused on the development of biopreparations containing Bacillus megaterium to prevent the outbreak of this disease caused by Alternaria japonica in the crop of Brassica oleracea var. italica. During the laboratory phase two types of biopreparations were evaluated, the first biopreparation was obtained by liquid fermentation composed of 40 g.L−1 of fava bean flour and 5 g.L−1 of ground brown sugar. This showed a maximum cell growth of 3.8 × 108 CFU. mL−1; while the second biopreparation was obtained by solid fermentation composed of wheat bran and it achieved a maximum cell growth of 4.7 × 109 CFU. g−1. In the fieldwork phase the aforementioned biopreparations were applied in an open-field crop.
At the end of the cultivation period, the degree of the disease in leaves and in the inflorescences was measured and through the statistical analysis, a significant difference was evidenced (α = 0.05). On the broccoli leaves the disease index values do not exceed 15.56 % and the disease index for postharvest florets was around 38 %. The evaluated variables showed a statistical similarity with the chemical treatment, thus determining the effective effect of the biopreparations.
1. Introduction
Plant diseases are a significant yield and quality constraint for growers agricultural, the loss of crops due to the attack of fungi of the genus Alternaria, could represent up to 25 % of the world crop production per year [1]. Disease management of crops often represents an important component of agricultural production cost and usually, the way to control and treat diseases in agricultural ecosystems includes the use of pesticides. However, the growing concern about the effects of pesticides on the environment and residues in food has led to the reduction or elimination of the use of a series of agrochemicals, therefore, it becomes an urgent matter to find options that allow to replace the use of pesticides. Currently, in the field of chemistry and pharmacological research, there has been an increased interest in the biological control of plant diseases [2].
A biological control agent (BCA) is usually a fungus, bacteria or virus, or a mixture of these, in plant pathology, the term BCA applies to the use of microbial antagonists to suppress diseases [3], the main advantage of using a BCA is that they are highly specific for a pathogen and therefore are considered harmless for non-target species [2].
Alternaria japonica is a cosmopolitan phytopathogen that can survive as saprophyte and as weak parasite, this pathogen has multicellular pigmented spores that reproduce in chains or in branched forms. Its conidium body can gradually narrow into a tapered secondary conidiophore [4,5]. This fungus can cause the reduction of seed germination; when it attacks the morphology of the host leaves, it produces a series of yellow concentric rings around the initial site of attack [6]. The most important effect of this disease can be found in the inflorescence, where it causes a decay that in some cases affects up to 100 % of inflorescences [7].
The control of phytopathogenic fungi using environmentally friendly practices has been developed based on the use of antagonistic microorganisms that, through competitive mechanisms, antibiosis and induction of resistance, limit its development. One of the genus that could react as a biocontroller of fungal microorganisms is Bacillus [8]. The metabolites that this bacterium produces are lipopeptide-like, and are found within molecules of biological interest due to the inhibition of phytopathogenic growth [9]. Species of the genus Bacillus, including B. megaterium, are attractive not only due to the production of a necessary range of phytopathogenic inhibitor molecules, but also because of the formation of endospores, useful for the duration and effectiveness of the formulations [10].
Nowadays, there are some commercially available culture mediums that offer the necessary parameters of energy, carbon and nitrogen that microorganisms require for their growth, but those can be costly. This is how solid fermentation is a viable alternative, which takes place in the absence or near absence of free water, thus being close to the natural environment to which the selected microorganisms are naturally adapted [11]. Some agricultural waste can be used as solid substrates, which leads to a reduction of capital costs and potentially provides a superior productivity [12]. Another advantage of solid fermentation is that it is a well-known technology, because it is used in different fields such as the production of enzymes, antibiotics, organic acids, unicellular proteins, biopesticides, biofuels [13,14]. In Ecuador, the cultivation of broccoli is widespread because the demand for this vegetable from the European continent has grown in recent years [15]. Due to these factors and the ever increased demand for this product, it becomes necessary to find an environmentally friendly alternative to the massification of Bacillus megaterium, through the use of low-cost materials and easy acquisition.
2. Materials and methods
2.1. Microorganisms
The phytopathogenic fungus Alternaria japonica was isolated from sick broccoli leaves and the Bacillus megaterium was obtained from a collection of isolated strains of soils with broccoli crops in the province of Tungurahua-Ecuador [16]. These samples are currently cryopreserved in Criobank, in the “Centro de Investigación y Valoración de la Biodiversidad”, at the “Universidad Politécnica Salesiana” in Quito - Ecuador [17].
The AB4 strain was chosen because, in previous research, its capacity as a biocontroller of Alternaria japonica, was register in in vitro tests [16] and in greenhouse tests [17].
2.2. Molecular identification of microorganisms
2.2.1. DNA extraction
The bacterial microorganism was cultured in TSB (Tryptic Soy Broth) and incubated at 30 °C. The DNA (deoxyribonucleic acid) was extracted from cultures of 24 h following the methodology described by Sambrook et al. [18].
The fungal microorganism was cultured in PDA (Potato Dextrose Agar) for 7 days at 25 °C and the DNA was extracted following the methodology described in González-Mendoza et al. [19].
For the bacterial microorganism, the 16S region was amplified with the primers 27 F (5´AGAGTTTGATCCTGGCTCA 3´) and 1492R (5´ GGTTACCTTGTTACGACTT 3´) [20]. PCR was performed in the thermal cycler under the following conditions: initial denaturation at 95 °C for 2 min, 24 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 1 min, initial extension for 2 min at 72 °C and final extension of 10 min at 72 °C followed by maintenance at 4 °C [21].
For the fungal microorganism, the ITS region was amplified. The ITS 1 primer was used (5´CCGTAGGTGAACCTGCGG-3´) as well as the ITS4 (5´TCCTCCGCTTATTGATATGC-3´) [22]. PCR was performed in the thermal cycler under the following conditions: initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 1 min, annealing at 53 °C for 1 min, initial extension for 1 min at 72 °C and final extension of 10 min at 72 °C followed by maintenance at 4 °C [23].
The PCR products were purified and subjected to Sanger sequencing. Sequence similarity was searched using the BLAST program (N) in the NCBI Genbank.
2.3. Production of biopreparations of Bacillus megaterium
2.3.1. Preparation of the inoculum
A bacterial suspension was prepared from the preserved microorganisms. Strain AB4 was reactive in nutrient agar plates. Fresh biomass was transferred to a sterile saline phosphate buffer solution. This cell suspension was used for all experiments.
2.3.2. Fermentation in the liquid state
To make the biopreparation by fermentation in a liquid state, soy flour, fava bean flour and ground brown sugar were used.
Table 1 shows the composition and concentration of the culture media used in the experiments.
Table 1.
Composition and concentration of liquid culture medium.
| Treatments | Nitrogen Source | g.L−1 | Carbon Source | g.L−1 |
|---|---|---|---|---|
| T1 | Soybean flour + S.Sa | 40 | Ground brown sugar | 5 |
| T2 | Soybean flour + S.Sa | 40 | Ground brown sugar | 6 |
| T3 | Fava Bean flour + S.Sa | 40 | Ground brown sugar | 5 |
| T4 | Fava Bean flour + S.Sa | 40 | Ground brown sugar | 6 |
| T5 | TSB (Trypticase Soy Agar) | |||
S.S = Stock of mineral salts: 0.45 g.L−1 M40gSO4, 3.6 mg.L−1 MnSO4, 10 mg.L-1C6H8O7, 5 g.L-1 NaCl, 1.9 g.L-1 K2HPO4.
The experiments were carried out in 250 mL glass Erlenmeyer flasks with 100 mL of each culture medium, 100 μL of the bacterial suspension was inoculated, at an initial concentration of 1.5 × 108 CFU. mL−1, using the spectrophotometer it was verified that the inoculum has absorbance similar to the standard 0.5 Mac Farland. It was incubated at 35 °C and 100 rpm for 72 h. Each treatment had four replications and the fermentation process was repeated twice.
2.3.3. Fermentation in the solid state
To make the biopreparation by fermentation in the solid state, enriched substrates were used. Details are presented in Table 2. 50 g of each substrate were placed in propylene bags and sterilized at 121 °C, 1 atm for 60 min. They were inoculated with 50 μL of fresh cell suspension at an initial concentration of 1 × 108 CFU. g−1, incubated at 35 °C for 72 h. The fermentation process was repeated twice.
Table 2.
Composition of solid substrates and concentration of nutrients.
| Treatments | Substrates | Carbon Source | g.Kg−1 | Nitrogen Source | g.Kg−1 |
|---|---|---|---|---|---|
| T1 | Barley grain | Ground Brown Sugar | 40 | Soybean flour + S.Sa | 40 |
| T2 | Wheat bran | Ground Brown Sugar | 40 | Soybean flour + S.Sa | 40 |
| T3 | Peat | Ground Brown Sugar | 40 | Soybean flour + S.Sa | 40 |
| T4 | Rice | Ground Brown Sugar | 40 | Soybean flour + S.Sa | 40 |
S.S = Stock of mineral salts: 5 g.Kg−1CaO, 10 g.Kg−1 K2HPO4.
2.3.4. Total cell production
The cell concentration of each treatment was estimated by a serial of dilutions in sterile saline phosphate buffer solution (70 mL of 0.2 M KH2PO4, 30 mL of 0.2 M K2HPO4 and 300 mL of deionized water, pH 6.5) and plateaus in nutrient agar, taking samples from 24, 48 and 72 h.
2.3.5. Efficacy test of the liquid and solid biopreparations in a culture of Brassica oleracea var. Italica
The trial was conducted in the province of Tungurahua-Ecuador in the parish of Izamba (2500 m.a.s.l. average temperature 14 °C), in an area of 280 m2, establishing a total of 25 beds, with a dimension of 14m × 0.5m each one. Planting distance was 0.4 m between plants and 0.3 m between lines, with two drip irrigation lines per bed; the variety sown was Domador of the commercial house SEMINIS.
There were a total of 1750 plants, 70 plants/bed, of which 10 plants were chosen per bed. From each plant, two leaves were chosen at random, which together with the respective inflorescence, were evaluated throughout the entire trial. Each treatment had three replications, and a Completely Random Design (CRD) was made.
The application of the treatments was done in leaves and inflorescence through direct spraying, with the help of manual pumps. The selected biopreparations, one liquid and one solid, were suspended in water and applied at a concentration of 106 CFU. mL−1. Nonylphenoletylene oxide was added, at a dose of 0.5 cm3.L−1 to improve the adhesion of the mixture to the plants. For the chemical control, Mancozeb was used at a concentration of 2 g.L−1 and an absolute control was used in the water.
The field was already infested by the disease, therefore, it was not necessary to inoculate the pathogen.
2.4. Disease assessment
The application of the treatments was from the sixth week after the transplant to the field with a weekly frequency application.
2.4.1. Leaves assessment
To determine the Area Under the Disease Progress Curve (AUDPC), the number of injuries caused by Alternaria japonica per leaf of each treatment was evaluated, for five weeks. At the end of the fieldwork period, the leaves were collected, and using the free software ImageJ version 2016, the healthy area and the affected area were measured in order to determine the final severity percentage. Furthermore, the Horsfall and Barratt Scale [24] was used and the Disease Index (%) was calculated as described in Saharan et al. [25].
2.4.2. Inflorescence assessment
To evaluate the disease in the inflorescences, each of the inflorescences of the different treatments were harvested, at the end of the field cultivation period and then placed in humid chambers. Each inflorescence was labelled and stored for 7 days, after this period, the number of sick and healthy florets were quantified. The disease index (%) was then determined.
2.5. Statistical analysis
The cell growth data of Bacillus megaterium obtained in the laboratory were recorded and transformed into Log10 to achieve uniformity in the variance.
The results of the laboratory and field phase were analyzed using Infostat software version 2013. An ANOVA analysis was performed with a Tukey Post Hoc test at α = 0.05.
3. Results
3.1. Identity of the AB4 strain and the phytopathogenic fungus
The sequence of the 16S rDNA gene of the strain AB4 was compared to the NCBI database. The results indicated a 96 % similarity to the Bacillus megaterium species.
When analyzed in the NCBI database the sequence of the ITS region of the phytopathogenic fungus gave a 99 % similarity to the species Alternaria japonica.
3.2. Fermentation in the liquid state
The effect of the combination of different nitrogen sources, such as fava bean flour and soybean meal with ground brown sugar, in the growth of strain AB4 is shown in Table 3. Results showed a significant difference in at least one of the treatments: a lower level of growth was obtained in treatments T1, T2 and T4. The treatments with significantly higher growth levels were T3, composed of 40 g.L−1 fava bean flour and 5 g.L−1 ground brown sugar, and T5, composed of TSB. Results showed that T3 and T5 are the liquid biopreparations with the highest bacterial growth and statistical similarity at 72 h, with 8.71 and 8.64 log (CFU.mL−1) respectively.
Table 3.
Effect of the different treatments on the growth of Bacillus megaterium by fermentation in liquid state after 24, 48 and 72 h of incubation at 35 °C and 100 rpm.
| TREATMENT TIME |
T1 (UFC. mL−1) |
T2 (UFC. mL−1) |
T3 (UFC. mL−1) |
T4 (UFC. mL−1) |
T5 (UFC. mL−1) |
ANOVA |
|---|---|---|---|---|---|---|
| 24 h | 8.02 ± 0.3 c | 8.03 ± 0.2 c | 8.18 ± 0.3 b | 8.04 ± 0.4 c | 8.43 ± 0.3 a | <0.0001* |
| 48 h | 8.23 ± 0.4 d | 8.44 ± 0.1 c | 8.61 ± 0.3 b | 8.60 ± 0.3 b | 8.68 ± 0.4 a | <0.0001* |
| 72 h | 8.48 ± 0.2 c | 8.47 ± 0.3 c | 8.64 ± 0.2b | 8.54 ± 0.4bc | 8.71 ± 0.4 a | <0.0001* |
Note: T1: 40 g.L−1 soybean flour and 5 g.L−1 ground brown sugar, T2: 40 g.L-1 soybean flour and 6 g.L−1 ground brown sugar, T3: 40 g.L−1 fava bean flour and 5 g.L−1 ground brown sugar, T4: 40 g.L−1 fava bean flour and 6 g.L-1 panela, T5: TSB. Mean ± standard deviation. * Significant differences at p < 0.05. Equal letters there is no significant difference between treatments.
3.3. Fermentation in the solid state
The results of the analysis for the solid fermentation at 24, 48 and 72 h, showed that there was a significant difference in cell concentration between treatments. The substrate with the highest concentration of biomass was T2, which corresponds to the wheat bran matrix with 9.6 log (CFU.g-1) at 72 h. The results are summarized in Table 4.
Table 4.
Effect of the different treatments on the growth of Bacillus megaterium by fermentation in solid state after 24, 48 and 72 h of incubation at 35 °C and 100 rpm.
| | TREATMENT TIME |
T1 (UFC. g−1) |
T2 (UFC. g−1) |
T3 (UFC. g−1) |
T4 (UFC. g−1) |
ANOVA |
|---|---|---|---|---|---|
| 24 h | 8.38 ± 0.4a | 8.51 ± 0.2a | 8.18 ± 0.3ab | 8.03 ± 0.4b | 0.0082 * |
| 48 h | 9.51 ± 0.9a | 9.18 ± 1 b | 9.23 ± 0.8a | 8.60 ± 1.2b | 0.0068* |
| 72 h | 9.42 ± 0.3b | 9.67 ± 0.1a | 9.11 ± 0.4c | 8.81 ± 0.2d | <0.0001* |
Note: T1: Barley rice, T2: Wheat bran, T3: Peat, T4: Rice. Mean ± standard deviation. * Significant differences at p < 0.05.
Equal letters there is no significant difference between treatments.
3.4. Efficacy of Bacillus megaterium biopreparations against the disease caused by Alternaria japonica
3.4.1. Area under the curve for the progress of the disease (AUDPC) for the number of lesions on leaves
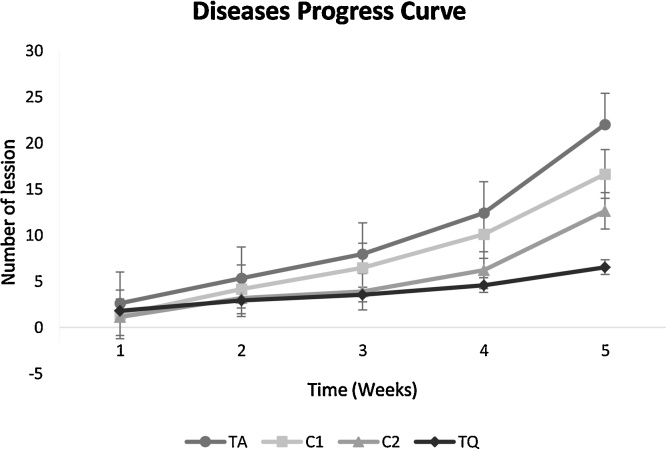
The variance analysis of the AUDPC, showed that there was a significant difference between the treatments evaluated in the field. The absolute control TA was the treatment with the highest number of lesions. The C2 treatment corresponding to the solid biopreparation and the chemical treatment TQ were statistically similar and with less affectation of the disease as it is shown in Table 5. Fig. 1 shows the evolution of the number of lesions during the different treatments.
Table 5.
Area under the disease progress curve, analysis of variance and Tukey's test of the rate of the disease in florets and leaves and number of lesions in leaves.
| TREATMENT PARAMETER |
C1 | C2 | TA | TQ | ANOVA |
|---|---|---|---|---|---|
| AUDPC for the number of lesions on leaves (% day-1) | 208.13 ± 5 b | 140.99 ± 2 c | 265.82 ± 8 a | 106.4 ± 5 c | <0.0001* |
| Disease index for florets in the post-harvest (%) | 37.78 ± 8 b | 33.89 ± 2 b | 51.39 ± 8 a | 38.89 ± 4 b | 0.0002* |
| Disease index in Leaves (%) | 15.56 ± 4 b | 14.82 ± 9 b | 21.94 ± 0 a | 13.33 ± 1 b | 0.0002* |
Note: C1 = Liquid Biopreparation, C2 = Solid Biopreparation, TA = Total Control, TQ = Chemical Control. Mean ± standard deviation. * Significant differences at p < 0.05. Equal letters there is no significant difference between treatments.
Fig. 1.
Curve of progress of the disease in the number of lesions per leaf.
Disease index (%)
3.4.1.1. Florets
As shown in Table 5, the treatments that were applied with the biocontroller based on Bacillus megaterium showed lower severity of the disease. The treatments C1, C2 and TQ had statistical similarity and the absolute control, TA, is the most susceptible to the disease.
3.4.1.2. Leaves
In the Tukey Post Hoc test, the C1 and C2 biopreparations were shown to be statistically similar to the chemical treatment, with values of the disease index not exceeding 15.56 %.
4. Discussion
In vivo biocontrol activity using Bacillus strains is reported in different crops during their growth and in postharvest fruit and roots [26,27].
For the elaboration of biopreparations, the selection of the source of nitrogen and carbon was based on Yánez-Mendizábal et al. [28] research, in which fermentation was carried out in the liquid state, using as a source of nitrogen soy flour. The use of flours as a substitute for microbiological culture medium has shown a favorable effect on the growth and sporulation of Bacillus strains [29].
Pastrana [30] mentions that in solid-state cultures the substrate can be transformed by microorganisms and this can also act as a source of nutrients [31]. Flours from unconventional sources, such as legumes, have not been exploited and contain proteins in regular quantity and quality [32]. That is why, the use of flour is a valid option to supplement bacterial nutrition due to its contribution of nutrients. In addition to the carbon source used in this study, wheat bran substrate, provided nutrients such as fiber, minerals, and vitamins. This facilitated the proliferation of bacteria and resulted in a higher concentration than that obtained in the biopreparation by fermentation in a liquid state. The use of solid fermentation enables the simulation of the natural growth conditions of microorganisms [33].
The production of lipopeptides can be achieved with both liquid and solid fermentation [34]. The metabolites of interest, previously reported by the Bacillus genus, include surfactin, fengicin, iturin A, B, and C, these lipopeptides and secondary metabolites are an important tool in the development of new effective products against plant pathogens of global interest [35].
In this study, the inhibition of the growth of the phytopathogen in the culture occurred because it was found that Bacillus megaterium produces at least 40 variants of antifungal type metabolites including Fengicin A and B and Bacillomycin D [36]. Pueyo [37] and Jung [38] have reported the use of these metabolites. They showed that these metabolites have prevented spore germination and the development of fungal mycelia. At the same time, endospores of Bacillus megaterium are reported as effective controllers of fungal diseases under greenhouse and field conditions [39].
The decrease of the disease index of fungal may have occurred because it was found that Bacillus megaterium is an antifungal of Alternaria in vitro and in vivo, by showing its ability to inhibit the growth of the pathogen mycelium [40].
Beneficial effects of a bacteria of the genus Bacillus can present several mechanisms of action. One of them is as a direct antagonism: when they colonize the rhizosphere of plants, they provide protection and deploy their entire arsenal of antibiotics to fight pathogenic microorganisms. The other mechanism is as an indirect pant protector: the bacteria provide an inducible systemic resistance, which makes the host more resistant to the future entry of pathogens. It has been shown that treatments with Bacillus can protect the aerial part of plants [41]. The disease index reported when using Bacillus megaterium in another Brassica species is 15.82 %, close to the value reported in this study [42].
5. Conclusions
The field evaluation of the Bacillus megaterium-based biopreparations showed significant differences between treatments. The biopreparation obtained by solid fermentation showed statistical similarity with the chemical treatment in the number of lesions per leaf and the index percentage of the disease in florets and leaves.
Due to the biocontrol potential of Bacillus megaterium, the symptoms of the disease caused by Alternaria japonica were controlled in leaves and inflorescences of Brassica oleracea var. italica.
The process of production of a biological control agent is a crucial stage, where research in obtaining low-cost biopreparations at the laboratory level, are relevant contributions for subsequent large-scale production processes.
Funding sources
This work was possible thanks to the support of the Universidad Politécnica Salesiana (UPS)
Declaration of Competing Interest
None.
Acknowledgements
Special thanks to BIOARN research group which is directed by the PhD María Elena Maldonado.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00454.
Contributor Information
Ramiro Daniel Acurio Vásconez, Email: racurio@ups.edu.ec.
Estefany Michelle Tenorio Moya, Email: etenorio@est.ups.edu.ec.
Leidy Andrina Collaguazo Yépez, Email: lcollaguazoy@est.ups.edu.ec.
Viviana Pamela Chiluisa-Utreras, Email: vchiluisa@ups.edu.ec.
Ivonne de los Ángeles Vaca Suquillo, Email: ivaca@ups.edu.ec.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Lugtenberg B. Principles of Plant-Microbe Interactions. Springer; Berlin: 2015. Introduction to plant-microbe-interactions; pp. 1–2. [Google Scholar]
- 2.O’Brien P. Biological control of plant diseases. Australas. Plant Pathol. 2017;vol. 46(no. 4):293–304. [Google Scholar]
- 3.Pal K., McSpadden B. Biological control of plant pathogens. Plant Health Instr. 2006:1–25. [Google Scholar]
- 4.Mamgain A., Roychowdhury R., Tah J. Alternariapathogenicity and its strategic controls. Res. J. Biol. 2013;vol. 1:1–9. [Google Scholar]
- 5.Woudenberg J., Groenewald J., Bindery M., Crous P. Alternaria redefined. Srudies in Mycol. 2013;vol. 75:171–212. doi: 10.3114/sim0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D. Chalkey, Invasive Fungi. Alternaria leaf spot of cole crops -Alternaria japonica, Systematic Mycology and Microbiology Laboratory, ARS, USDA, 25 Octubre 2010. [Online]. Available: https://nt.ars-grin.gov/taxadescriptions/factsheets/index.cfm?thisapp=Alternariajaponica. [Accessed 20 Abril 2019].
- 7.Cadena Yanchapaxi D. Efecto de tres dosis en tres épocas de aplicación de Pyraclostrobin (Comet) en el control de la mancha foliar (Alternaria brassicae Berk) y validación del efecto AgCelence en el rendimiento de un híbrido de brócoli (Brassica oleraceae oleracea) Cotopaxi. 2011 [Google Scholar]
- 8.Reinoso Y., Vaillant D., Casadesús L., García E., Pazos V. Selección de cepas de Bacillus y otros géneros relacionados para el control biológico de hongos fitopatógenos. Fitosanit. 2007;vol. 11(no. 1):35–40. [Google Scholar]
- 9.Ragazzo-Sánchez J., Robles-Cabrera A., Lomelí-González L., Luna-Solano G., Calderón-Santoyo M. Selección de cepas de Bacillus spp. productoras de antibióticos aisladas de frutos tropicales. Rev. Chapingo Ser. Hortic. 2011;vol. 27(no. 1):5–11. [Google Scholar]
- 10.Hu X., Roberts D., Xie L., Maul J., Yu C., Li Y., Zhang S., Xing L. Bacillus megaterium A6 suppresses Sclerotinia sclerotiorum on oilseed rape in the field and promotes oilseed rape growth. Crop Prot. 2013;vol. 52:151–158. [Google Scholar]
- 11.Hölkery U., Höfer M. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004;vol. 64(2):175–186. doi: 10.1007/s00253-003-1504-3. [DOI] [PubMed] [Google Scholar]
- 12.Verster B., Madonsela Z., Minnaar S., Coheny B., Harrison S. Water Research Commission; South Africa: 2014. Introducing the Wastewater Biorefinert Concept. [Google Scholar]
- 13.Bhargav S., Panda B., Aliy M., Javed S. Solid-state fermentation: An Overview. Chem. Biochem. Eng. Q. 2008;vol.22(1):49–70. [Google Scholar]
- 14.Lizardi-Jiménezy M., Hernández-Martínez R. Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech. 2017;vol. 7(1) doi: 10.1007/s13205-017-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MAGAP . 2014. Ministerio De Agricultura, Ganadería, Acuaculturay Pesca, 1 Octubre. [Online]. Available: http://sinagap.agricultura.gob.ec/phocadownloadpap/cultivo/2014/cboletin-situacional-brocoli-2014-actualizado.pdf. [Accessed 30 Abril 2018]. [Google Scholar]
- 16.Ñacatoy C., Valencia M.F. Aislamiento, identificación y pruebas in vitro de cepas autóctonas de Bacillus subtilis, como agente de biocontrol de Alternaria spp en Brassica oleracea var. italica. Quito. 2016 [Google Scholar]
- 17.Caicedoy S., Chacón J. Pruebas bajo invernadero de cepas de Bacillus subtilis como agente de biocontrol de Alternaria spp. En Brassica oleracea var. Italica y técnicas de conservación de cepas. Quito. 2017 [Google Scholar]
- 18.Sambrook J., Fritsch E., Maniantis T. second ed. Cold Spring Harbor Laboratory Press; New York: 1989. Molecular Cloning : a Laboratory Manual. [Google Scholar]
- 19.González-Mendoza D., Argumedo-Delira R., Morales-Trejo A., Pulido-Herrera A., Cervantes-Díaz L., Grimaldo-Juárez O., Alarcón A. A rapid method for isolation of total DNA from pathogenic filamentous plant fungi. Genet. Mol. Res. 2010;vol. 9(no. 1):162–166. doi: 10.4238/vol9-1gmr680. [DOI] [PubMed] [Google Scholar]
- 20.Weisburg W., Barns S., Pelletier D., Lane D. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;vol. 173(no. 2):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hongoh Y., Yuzawa H., Ohkuma M., Kudo T. Evaluation of primers and PCR conditions for the analysis of 16S rRNA genes from a natural environment. FEMS Microbiol. Lett. 2003;vol. 221(no. 2):299–304. doi: 10.1016/S0378-1097(03)00218-0. [DOI] [PubMed] [Google Scholar]
- 22.Siahmard O., Pableo R., Novero A. Molecular identification of rhizospheric Fungi associated with “Saba” banana via the amplification of internal transcribed spacer sequence of 5.8S ribosomal DNA. Asian J. Plant Sci. 2017;vol. 16(no. 2):78–86. [Google Scholar]
- 23.Umesha S., Manukumar H., Raghava S. A rapid method for isolation of genomic DNA from food-borne fungal pathogens. 3 Biotech. 2016;vol. 6(no. 2):1–9. doi: 10.1007/s13205-016-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horsfall J., Barratt R. An improved grading system for measuring plant diseases. Phytopathol. 1945;vol. 655(35) [Google Scholar]
- 25.Naresh M., Meena P., Saharan G. Springer Singapore; Singapore: 2015. Alternaria Diseases of Crucifers: Biology, Ecology and Disease Management. [Google Scholar]
- 26.Pertot I., Alabouvette C., Hinajeros E., Franca S. The use of microbial biocontrol agents against soil-borne diseases. Julio. 2015;19 [Online]. Available: https://ec.europa.eu/eip/agriculture/sites/agri-eip/files/8_eip_sbd_mp_biocontrol_final.pdf. [Accessed 6 Febrero 2019]. [Google Scholar]
- 27.Basurto-Cadena M., Vásquez-Arista M., García-Jiménez J., Salcedo-Hernández R., Bideshi D., Barboza-Corona J. Isolation of a new Mexican strain of Bacillus subtilis with antifungal and antibacterial activities. Sci. World J. 2012:1–7. doi: 10.1100/2012/384978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yánez-Mendizábal V., Viñas I., Usall J., Torres R., Solsona C. Production of the postharvest biocontrol agent Bacillus subtilis CPA-8 using low cost commercial products and by-products. Biol. Control. 2012;vol. 60(no. 3):280–289. [Google Scholar]
- 29.De la Cruz-De la Cruz E., Méndez-Luna D., Valera-Montero L. Cultivo de Bacillus subtilis CEPA QST 713 en reactor tipo airlift y su actividad antagónica contra Phytophthora capsici. Rev. Ing. Tecnol. Desarro. Sustent. 2016;vol. 1:38–42. [Google Scholar]
- 30.Pastrana L. Fundamentos de la fermentación en estado sólido y aplicación a la industria alimentaria. Cienc. Tecnol. Aliment. 1996;vol. 1(no. 3):4–12. [Google Scholar]
- 31.Stevenson L., Phillips F., O’sullivan K., Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012;vol. 63(no. 8):1001–1013. doi: 10.3109/09637486.2012.687366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres-González M., Jiménez-Munguía M., Bárcenas-Pozos M. Harinas de frutas y/o leguminosas y su combinación con harina de trigo. Rev. Temas Sel. Ing. Aliment. 2014;vol. 8(no. 1):94–102. [Google Scholar]
- 33.Hölkery U., Lenz J. Solid-state fermentation- are there any biotechnological advantages? Curr. Opinion Microbiol. 2005;vol. 8(3):301–306. doi: 10.1016/j.mib.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Beltrán-García E., Macedo-Raygoza G., Villafaña-Rojas J., Martínez-Rodríguez A., Chávez-Castrillon Y., Espinosa-Escalante F., Tetsuya P., Beltrán-García M. Fermentation Processes. IntechOpen; 2017. Production of lipopeptides by fermentation processes: endophytic bacteria, fermentation strategies and easy Methods for bacterial selection; pp. 199–222. [Google Scholar]
- 35.Ariza Y., Sánchez L. Determination of secondary metabolites from Bacillus subtilis with effect biological control on Fusarium sp. Nova. Publ. Cient. Cienc. Biomed. 2012;vol. 10(no. 18):149–155. [Google Scholar]
- 36.Ma Y., Kong Q., Qin C., Chen Y., Chen Y., Lv R., Zhou G. Identification of lipopeptides in Bacillus megaterium by two-step ultrafiltration and LC–ESI–MS/MS. AMB Express. 2016;vol. 6(no. 79):1–15. doi: 10.1186/s13568-016-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pueyo M., Bloch C., Carmona-Ribeiroy A., di Mascio P. Lipopeptides produced by a soli Bacillus megaterium strain. Microb. Ecol. 2009;vol. 57(2):367–378. doi: 10.1007/s00248-008-9464-x. [DOI] [PubMed] [Google Scholar]
- 38.Jungy H., Sang-Dal K. An antifungal antibiotic purified from Bacillus megaterium KL39, a biocontrol agent of red-pepper Phytophthora-blight disease. J. Microbiol. Biotechnol. 2005;vol. 15(5):1001–1010. [Google Scholar]
- 39.Chumthong A., Kanjanamaneesathian M., Pengnoo A., Wiwattanapatapee R. Water-soluble granules containing Bacillus megaterium for biological control of rice sheath blight: Formulation, bacterial viability and efficacy testing. World J. Microbiol. Biotechnol. 2008;vol. 24(no. 11):2499–2507. [Google Scholar]
- 40.Hammady A., Abbo A. The antifungal effects of four tomato rhizosphere Bacillus spp. against Alternaria alternata. Int. J. Sci. Res. 2014;vol. 3(7):1324–1328. [Google Scholar]
- 41.Tozlu E., Tekiner N., Kotany R., Örtücü S. Investigation on the biological control of Alternaria alternata. Indian J. Agric. Sci. 2018;vol. 88(8):1241–1248. [Google Scholar]
- 42.Yasin N. Application of rhizobacteria for induction of systemic resistance in Brassica campestris L. against Alternaria leaf spot disease caused by Alternaria brassicae. Res. Rev. J. Microbiol. Biotechnol. 2017;vol. 6(1):51–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.