Abstract
A 6-year-old girl presented with history of infantile onset epileptic encephalopathy and developmental delay. She had polymorphic seizures that were refractory to regular anti-seizure medication. Incomplete control of seizures was achieved on starting pyridoxine, riboflavin and thiamine. Clinical exome sequencing done at 4 years revealed PNPO deficiency with a homozygous mutation in the highly conserved exon 3:c.352G > A p.Gly118R region of the gene. Thereafter, pyridoxine was weaned and pyridoxal phosphate was added with resultant refractory status epilepticus, which necessitated our approach to start pyridoxine and stop pyridoxal phosphate. With two antiseizure medication and three vitamins, she had improved seizure control. At 6 years of age an attempt to wean off riboflavin resulted in break through seizures. After restarting riboflavin along with pyridoxal phosphate, pyridoxine in low doses and two antiseizure medications, the child achieved good seizure control. Though partial responsiveness to pyridoxine with gene mutation in the exon 3: c.352G > A p. Gly118R is known, riboflavin dependence and transient worsening of seizures off pyridoxine has not been described to our knowledge. Our case highlights the importance of identifying the precise gene mutationsequence to properly identify variants relative to individual phenotypic expression, treatment responsivness and need for added vitamin supplementation.
Keywords: Neonatal, Seizures, Pyridoxal N phosphate oxidase (PNPO), Pyridoxine, Riboflavin
Highlights
-
•
PNPO (pyridoxal N phosphate oxidase) deficiency is a treatable cause of infantile and neonatal onset epileptic encephalopathy
-
•
PNPO deficiency is classically responsive to pyridoxal phosphate but other agents like high dose pyridoxine and riboflavin are also useful depending upon the genetic mutation involved.
-
•
The present report describes a paradoxical worsening which occurred when switched from pyridoxine to pyridoxal phosphate.
1. Introduction
To determine an etiology for neonatal/infantile onset epileptic encephalopathy is challenging. This requires a great understanding of the many metabolic pathways, the most common one being the pathway involving pyridoxine. We know that pyridoxal phosphate oxidase deficiency responds to pyridoxal phosphate and in some cases a transient response is seen to pyridoxine [1].Here, we describe the challenges faced in treating a child with infantile onset epileptic encephalopathy, who was genetically proven to harbour pyridoxal-N-phosphate oxidase (PNPO) gene mutation but did not respond to pyridoxal phosphate initially.
2. Case report
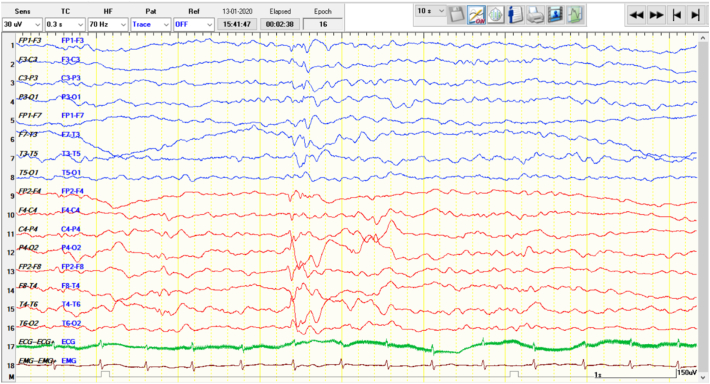
A six-year-old girl born to non-consanguineous parents, first in birth order presented with history of seizures from neonatal period. Antenatally, mother had pre-eclampsia and severe oligohydramnios. She was born at thirty-six weeks gestation by emergency lower segment caesarean section; cried immediately after birth with a birth weight was 3.2 kg. She had motor clonic seizures on third day of life which required hospitalization in the neonatal intensive care unit for one day following which she was discharged home. She was feeding and thriving well till her forty-fifth day, when she developed irritability and excessive crying following injection of the diphtheria-pertussis-tetanus (DPT) vaccine. Later that day following injection, she started to have flexor tonic spasms in clusters of 10 to 15 seizures daily, which responded well to intravenous adrenocorticotropic hormone (ACTH) and pyridoxine administered at 100 mg/day. She remained seizure-free untill her ninth month obtaining appropriate developmental milestones for her age. At her 9th month of age, she started to have seizures characterized by sudden onset of unexplained irritability, inconsolable cry, facial grimacing, abnormal eye movements, eye twitching and tonic posturing which were in clusters of 5 episodes per day and escalated to 30–40 episodes/day over the next few months with no diurnal variation. The seizures were precipitated by stimuli including touch, sound and fever. She was investigated with MRI Brain which showed periventricular leukomalacia and ventriculomegaly; EEG was suggestive of hypsarrhythmia later evolving into multifocal spike-and-slow wave discharges(Fig 2)[1], [2], [3]. Her initial blood lactate was 2.1 mg%, and serum ammonia was 42 mg%.
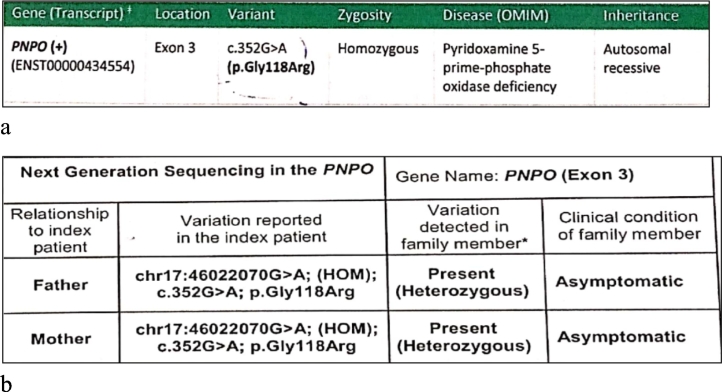
The seizures failed to respond to combinations of antiseizure medications before declining in frequency with the use of multivitamins (pyridoxine , riboflavin , thiamine) along with four antiseizure medications (phenobarbitone, clobazam, topiramate, valproate) at 2 years of age. An initial epileptic encephalopathy genetic panel was negative. In view of incomplete response to vitamin therapy, a mitochondrial encephalopathy like pyruvate dehydrogenase deficiency was suspected but all related investigations for the same were negative. She continued to have seizures occasionally. At 4 years of age, exome sequencing was done that revealed homozygous mutation in highly conserved area involving exon 3: c.352G > A p. Gly118R predicting a substitution of arginine for glycine in the PNPO gene. Both parents were identified as heterozygous carriers (Fig. 1a).
Fig. 1.
a The clinical exome report of the child that shows homozygous mutation in highly conserved area exon 3: c.352G > A p. Gly118R predicting a substitution of arginine for glycine in the PNPO gene.
b: Her parents report suggested they were heterozygous carriers of the same mutation.
Following this diagnosis, she was started on pyridoxal phosphate and an attempt was made to wean off pyridoxine and riboflavin. This resulted in a refractory status epilepticus requiring hospitalization for intensive care lasting one month. On recovery, she was initiated on three antiseizure medications along with pyridoxine (200 mg/day), riboflavin (100 mg/day), and three antiseizure medications: levetiracetam (40 mg/kg/day), sodium valproate(30 mg/kg/day) and clobazam (0.3 mg/kg/day). Despite this treatment, she continued to have febrile illness triggered myoclonic jerks until 6 years of age. She also attained complete neck control and sitting with support, recognising her mother and strangers, finger feeding and babbling, though also had spastic diplegia. During her latest visit to our clinic, an attempt was made to wean off riboflavin, when she again had one brief episode of seizures of similar semiology to the above seizure type. Therefore, riboflavin was reinstituted and pyridoxal phosphate (10 mg/kg/day) was added for continued seizures intermittently in addition to low dose of pyridoxine (3 mg/kg/day). This vitamin combination with 3 antiseizure medications have resulted in complete seizure freedom for 6 months. Further plans includetapering antiseizure medication slowly while the patient continues on vitamin supplementation. (See Fig. 2.)
Fig. 2.
EEG done at 3 years showing left parietotemporal spike-and-slow wave discharges. The record had shown multifocal spike-and slow-wave discharges.
3. Discussion
Neonatal and infantile epileptic encephalopathy poses a challenge to management because effectiveearly treatment of this neurological emergency has a huge bearing on the immediate as well as long term developmental and cognitive outcome of the patients. Apart from the use of stringent protocols for seizure management, suspecting, diagnosing and promptly treating the underlying cause especially metabolic etiologies are of the utmost priority [2].
Partial pyridoxine responsive or pyridoxine unresponsive neonatal epileptic encephalopathy could be the result of genetic perturbations in related metabolic pathways. Therefore, understanding these pathways is of utmost importance to formulate a management strategy that could ensure complete seizure control and restore neurological function.
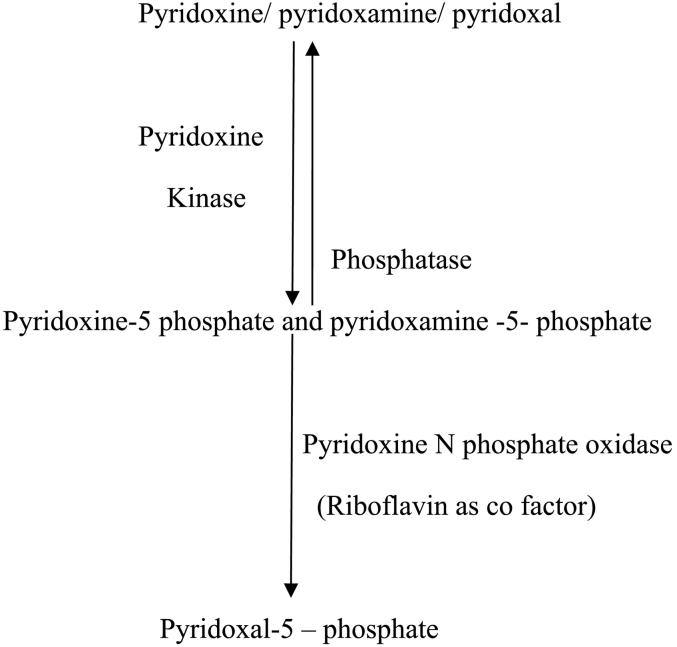
Pyridoxine and pyridoxamine obtained from dietary sources are transformed into the active form-pyridoxal phosphate (PLP) by PNPO (pyridoxal-N-phosphate oxidase). PNPO is a flavin mononucleotide-dependent enzyme, which requires riboflavin to convert pyridoxine and pyridoxamine to pyridoxal 5 phosphate as depicted in Fig. 3.
Fig. 3.
Metabolic pathway showing conversion of pyridoxine and pyridoxamine to the active form of vitamin B6 (Pyridoxal 5 phosphate) [3].
There are varied phenotypic presentations of the gene. One consistent feature reported in most series is neonatal/early infantile onset epilepsy. However, seizure onset can be delayed to mid or late infancy in a subset of patients [4]. The stormy course that ensues in the initial years of life is epitomised by polymorphic seizure types like myoclonic, tonic, clonic, generalized tonic–clonic, epileptic spasms with the notable exception of absences and astatic seizures thus distinguishing it from epileptic encephalopathies like GLUT-1 deficiency and Doose syndrome [4]. The common clinical phenotype that is mentioned is neonatal onset epileptic spasm that respond well to pyridoxal phosphate as opposed to pyridoxine.
Our child achieved reasonable seizure control with pyridoxine and a transient worsening of seizures when shifting treatment from pyridoxine to pyridoxal phosphate. Similar clinical scenarios that required continuation of pyridoxine therapy are described in Table 1 [5].
Table 1.
| Sl. no | Gene and mutation | Response to pyridoxine | Worsening on transition to pyridoxal phosphate | Concurrent use of antiseizure medication | Any need for additional vitamins |
|---|---|---|---|---|---|
| 1 child Jaeger et al. Neonatal onset seizures |
PNPO gene homozygous c.481C > T; p. (Arg161Cys) |
Yes | Not attempted | No | No |
| 8 children Plecko et al. Neonatal onset seizures |
PNPO gene homozygous Exon 7 c.674G > A; p(Arg225His) |
Yes | 1/8 children had worsening on transitioning | 4/8 children require additional antiseizure medications | No |
| 1 child Pearl et al. Neonatal onset seizures |
PNPO gene Homozygous Exon 3 c.352G > A; p. G118R |
Yes But response was only for 6 weeks |
No | No | No |
| 1 child Plecko et al. Neonatal onset seizures |
PNPO gene Compound heterozygous p. Arg141Cys in exon 5 and a deletion c.279_290del in exon 3 |
Yes | No | Required additional antiseizure medication | No |
However, there has been no mention of riboflavin dependency. The cases above mentioned were being treated as pyridoxine-dependent epilepsy and when the genetic evaluaton was found to be negative for ALDH7A1 gene, reanalysis was done that confirmed a PNPO gene mutation.
Mills et al. in their study classified patients with PNPO mutation into three groups, group 1 being neonatal onset seizures responding to pyridoxal phosphate, group 2 being spasms at presentation, group 3 being infantile onset seizures responding to pyridoxine. Out of 8 patients in group 3, 3 children had transient worsening of seizures as described in ours, and 1 child with mutation C.[98A4T] + c.[347G4A] + c.[673C4T] had achieved seizure control with addition of riboflavin supplementation [5,7].
Only the mutation described by Pearl et al. matched that of ours, but riboflavin dependence is not mentioned [8]. The fact that Riboflavin is a cofactor for pyridoxal-N-phosphate oxidase, can explain its role in the complete functioning of the enzyme and the subsequent therapeutic benefit. The worsening of seizures during transition from pyridoxine to pyridoxal phosphate could be explained by increased intracellular PLP levels that have the potential to precipitate seizures which has been described in experimental animal models/rats). The mutant (PNPO) enzyme having residual activity could have impaired inhibition by PLP, with resultant increase in pyridoxal-l-phosphate to toxic levels [9].
It is known that the treatment of the children PNPO deficiency requires supplementation of pyridoxal phosphate usually to a maximum of 60 mg/kg/day. Our case adds to the literature involving children with the novel mutation identified at exon 3: c.352G > A p. Gly118R of the PNPO gene. Furthermore, it also suggests that supplementation with riboflavin (maximum 100 mg/day) and a slow transition from pyridoxine to pyridoxal phosphate is warranted in addition to a gradual taper off of antiseizure medication.
The outcomes of children with PNPO deficiency have been variable with death in untreated cases and varying degrees of psychomotor retardation in appropriately treated children. However, the further developmental outcome in our child will be established in long-term follow-up.
4. Conclusion
Our child with PNPO deficiency had a novel gene mutation associated with transient pyridoxine responsiveness in addition to associated riboflavin dependency. She experiencedworsening of her seizures upon transitioning from pyridoxine to pyridoxal phosphate. This case stresses the importance of identifying genetic mutations and gene variants relative to phenotypic presentation. Using a treatment-based approach may imrpove seizure control and suggest the need for individualizing antiseizure medication and vitamin supplementation.
Disclosure
None of the authors report any conflicts of interest.
Ethical statement
We hereby declare that informed consent was obtained for the publication of the patient details without revealing the identity from the immediate family.
References
- 1.Chaves R.G. Successful screening for Gaucher disease in high-prevalence population in Tabuleiro do Norte. JIMD Rep. 2011;1(January):73–78. doi: 10.1007/8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plecko B., Stöckler S. Vitamin B6 dependent seizures. Can J Neurol Sci. 2009;36(suppl 2):S73–S77. [PubMed] [Google Scholar]
- 3.Marashly E.T., Bohlega S.A. Riboflavin has neuroprotective potential: focus on Parkinson’s disease and migraine. Front Neurol. 2017;8(JUL):1–12. doi: 10.3389/fneur.2017.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veerapandiyan A., Winchester S.A., Gallentine W.B. Electroencephalographic and seizure manifestations of pyridoxal 5′-phosphate-dependent epilepsy. Epilepsy Behav. 2011 doi: 10.1016/j.yebeh.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Plecko B., Paul K., Mills P. Pyridoxine responsiveness in novel mutations of the PNPO gene. Neurology. 2014 doi: 10.1212/WNL.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeger B., Abeling N.G., Salomons G.S. Pyridoxine responsive epilepsy caused by a novel homozygous PNPO mutation. Mol Genet Metab Reports. 2016;6:60–63. doi: 10.1016/j.ymgmr.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.M P.B., C S.S.M., F E.J. Epilepsy due to PNPO mutations: genotype, environment and treatment affect presentation and outcome. Brain. 2014;137:1350–1360. doi: 10.1093/brain/awu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip L.P., Keith H., C J., Colleen L.M., Yuezhou Y., Donald T. Partial pyridoxine responsiveness in PNPO deficiency. JIMD Rep. 2012 doi: 10.1007/8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills P.B., Footitt E.J., Mills K.A. Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (ALDH7A1 deficiency) Brain. 2010 doi: 10.1093/brain/awq143. [DOI] [PMC free article] [PubMed] [Google Scholar]