Abstract
Typically, biological systems are protected from the toxic effect of free radicals by antioxidant defense. Extracts from orchids have been reported to show high levels of exogenous antioxidant activity including Bulbophyllum orchids but so far, there have been no reports on antioxidant enzymes. Therefore, differences in protein expression from leaves and pseudobulbs of Bulbophyllum morphologlorum Kraenzl and Dendrobium Sonia Earsakul were studied using two-dimensional gel electrophoresis and mass spectrometry (LC/MS/MS). Interestingly, the largest group of these stress response proteins were associated with antioxidant defense and temperature stress, including superoxide dismutase (Cu–Zn) and heat shock protein 70. The high expression of this antioxidant enzyme from Bulbophyllum morphologlorum Kraenzl was confirmed by activity staining on native-PAGE, and the two Cu/Zn-SODs isoenzymes were identified as Cu/Zn-SOD 1 and Cu/Zn-SOD 2 by LC/MS/MS. The results suggested that Bulbophyllum orchid can be a potential plant source for medicines and natural antioxidant supplements.
Keywords: Bulbophyllum, Orchid, Proteomics, Stress response, Enzymatic antioxidant, Superoxide dismutase (Cu–Zn)
1. Introduction
The effect of oxidative stress and the process of autoxidation cause human diseases such as cardiovascular diseases, aging, cancers and diabetes [1]. Many antioxidants have been synthesized and used to prevent the process, but sometimes produced side effects [2]. As a result, natural antioxidants have been obtained from plants as potential medicines to prevent and/or treat such diseases [3]. The search for safe antioxidants from plants still continues. One of the most important enzymatic antioxidants that constitute the first line of antioxidant barrier against reactive oxygen species-induced damages is superoxide dismutase (SOD) [4,5]. Based on the catalytic metal ions at the active sites, SODs are classified into three distinct groups: Fe, Mn and Cu/Zn-SOD [6]. Diminished activities of SODs have been reported in various physiological and pathological conditions e.g. cancer, inflammatory diseases, aging and skin disorders. To date, several studies suggest that SODs are useful agents for prevention or treatment of various skin disorders, especially in melanoma cancer and skin inflammation. In plants, superoxide dismutases may contain different catalytic metal ions at the active site: Cu/Zn, Mn and Fe. The differences in type, number and distribution of metalloenzymes depend on the species, stage of development and environment [[7], [8], [9], [10], [11]]. In addition, SODs with the same metal cofactor can change roles in different species [12]. Iron-SODs are the oldest group of ubiquitous enzymes, found in chloroplasts and cytoplasm [13,14] Manganese-SODs are present in mitochondria and peroxisomes [15]. The Cu/Zn-SODs were reported to compose of two subunits with a combination of Cu and Zn atoms, respectively [16]. They are found in the chloroplasts, cytosol, peroxisomes and the apoplast [[17], [18], [19]]. In recent years, the SODs have been reported to play a role in plant protection against abiotic and biotic stress [20].
The Orchidaceae is a widely distributed flowering plant family, found in all types of habitats, and includes terrestrial, saprophytic, and epiphytic orchids. The Bulbophyllum orchid, an epiphyte, has some 1000 species in Africa and Asia, with the latter being mainly in China, Nepal, Sikkim, Bhutan, India, Burma, Thailand, Laos, and Vietnam [21]. Thailand has 154 known species of Bulbophyllum, making it the second most prevalent orchid genus after Dendrobium orchids [22]. Dendrobium and Bulbophyllum species have a long history and are commonly used as traditional Chinese medicines (TCM) in Asian countries [21,[23], [24], [25]]. Two known Bulbophyllum species, B. kwangtungense Schlecht (Shi dou-Ian) [21,26] and B. odoratissimum Lindl [27]. are used as medicinal orchids in the treatment of tuberculosis, chronic inflammation, and fever reduction [23,24]. Several reports have described the phytochemical constituents and biological effects of the chemical compounds extracted from the entire plant or plant parts (leaf, pseudobulb, or root) of Bulbophyllum used for various disease treatments [24]. The extracts from some orchids show high levels of exogenous antioxidant activity such as flavonoids in the leaves of Rhynchostylis retusa [28], and in the stems of Bulbophyllum kaitense [29], as well as the polyphenolics in the stems of Vanda cristata [28]. Dendrobium nobile was reported to be a potential source of antioxidants [30]. Orchids are therefore considered as good sources for antioxidants, but there is still no report on enzymatic antioxidants from Bulbophyllum orchids.
Proteomic techniques, using two-dimensional gel electrophoresis and nanoLC-mass spectrometry, is used worldwide to identify proteins from biological samples including plants and animals. Recently, proteomic studies of orchids have been reported to study various aspects, for example: the generation of the protocorm-like body of Vanilla planifolia Jacks. ex Andrews [31,32]; the browning in leaf culture of Phalaenopsis [33]; the pollination of the flower of Ophrys spp. [34], Cymbidium ensifolium (L.) Sw [35]. and Dendrobium chrysanthum [36]; the symbiotic reaction between fungi and the seeding of Oncidium sphacelatum Lindl [37,38]. and Dendrobium officinale Kimura and Migo herb [39,40]; the succinyl-proteome profile of the entire plant of Dendrobium officinale Kimura et Migo herb [41]; the adaptive drought strategies of Cymbidium sinense and C. tracyanum [42]; and the adaptive development of a tolerant mechanism to heavy metals by mycorrhizal Bipinnula fimbriata [43]. But there are still no data available in terms of the major proteins produced in the leaves and pseudobulbs of Bulbophyllum orchid.
Since our previous work (unpublished data) suggested that ethanol extracts of Bulbophyllum morphologlorum Kraenzl. (semi-epiphytic orchids) and Dendrobium Sonia Earsakul (epiphytic orchid) showed significant DPPH radical scavenging assay, as determined by the method of van Amsterdam et al. [44], we decide to investigate the endogenous enzymatic antioxidant activity of leaves and pseudobulbs of these orchids. Thus, comparative protein expression of Bulbophyllum morphologlorum Kraenzl and of Dendrobium Sonia Earsakul was studied by two-dimensional electrophoresis (2-DE) and nanoLC/MS/MS technology. In the present work, information was obtained on the differential expression of proteins and protein functions. The proteins involved in stress response were found in the highest amounts in Bulbophyllum orchid. SOD activity was detected by staining on native-PAGE and finally identified as Cu/Zn-SOD by nanoLC/MS/MS.
2. Materials and Methods
2.1. Plant materials and phenol protein extraction
Three-year-old Bulbophyllum morphologlorum Kraenzl. derived from seedlings were grown in a greenhouse at the Chulabhorn Research Institute, and Dendrobium Sonia Earsakul was purchased from the Chatuchak Sunday Market, Bangkok, Thailand. Ten grams of fresh leaf and pseudobulb samples were collected separately from mature orchids, and then immediately ground to a fine powder in liquid nitrogen prior to protein extraction with 50 mL of extraction buffer A (0.1 M Tris-HCl pH 8.8, 100 mM KCl, 0.4% 2-mercaptoethanol, 0.7 M sucrose), and the supernatant transferred to a new tube. After addition of 1 volume of extraction buffer B, consisting of the same buffer A with the addition of 2 mM phenylmethanesulfonyl fluoride (PMSF) and 50 mM ethylenediaminetetraacetic acid (EDTA) as protease inhibitors [45], the solution was mixed using a vortex, left at 4 °C for at least 30 min and centrifuged for 20 min, 4000 g at 4 °C. The supernatant was removed into a new tube and kept at 4 °C, and the pellet was extracted one more time using the same extraction buffer. The supernatant was combined with the first extraction and added with an equal volume of water-saturated phenol. The solution was mixed vigorously and kept on ice for 1 h, the solution was centrifuged for 20 min, 8000 g at 4 °C and the phenol phase was transferred to a new tube. The same phenol extraction was repeated one more time. Pooled phenol phase was added with 5 vol of 0.1 M of ammonium acetate in methanol and left overnight at −20 °C for protein precipitation. The sample was centrifuged as above and the protein pellet was dissolved immediately in cold water, sonicated for 3 min and then added with 9 vol of cold acetone. The solution was left at −20 °C for about 4 h to precipitate protein and centrifuged as above. The protein pellet was removed, dried and stored at −80 °C.
2.2. Two-dimensional gel electrophoresis (2-DE)
The protein pellet was resuspended in IEF buffer (7 M urea, 2 M thiourea, 4% CHAPS, 2% triton X-100, 100 mM DTT, 1% ampholytes pH 3–10, and 0.005% bromophenol blue). Then, pre-cast, 7 cm immobilized pH gradient strips (IPG strip), with a pH 4–7 linear gradient (GE Healthcare, UK), were loaded with 300 μg of protein in IEF buffer for each IPG strip, and rehydrated overnight. The 1st dimension was run in an EttanIPGphor II IEF Unit (GE Healthcare, UK) with these conditions: step 1, hold at 300 V for 30 min; step 2, gradient at 1000 V for 30 min; step 3, gradient at 5000 V for 90 min; and step 4, hold at 5000 V for 12–36 min. After the 1st dimension, proteins were reduced by incubating the IPG strips with 1% w/v DTT in equilibration buffer (6 M urea, 30% w/v glycerol, 2% SDS, and 50 mM Tris–HCl, pH 8.8), and alkylated with 2.5% w/v iodoacetamide in equilibration buffer (6 M urea, 30% w/v glycerol, 2% SDS, and 50 mM Tris–HCl, pH 8.8) [46]. The IPG strips were embedded within molten agarose directly on top of a 1.5 mm × 10 cm × 10.5 cm SDS-PAGE gel (4% stacking gel, 12.5% separating gel). Separation in the 2nd dimension involved SDS-PAGE with a constant current of a 12 μA/IPG strip for 3 h per gel. The protein spots were visualized by staining with 0.1% Coomassie brilliant blue R-250. The gel images were captured using the LabScan Image Scanner II software (GE Healthcare, UK), and the total protein spots were analyzed using the ImageMaster 2D Platinum 6.0 software (GE Healthcare, UK) by matching and comparing the differences in the % volume of the protein spots. The experiments were studied independently in triplicate. The protein spots that showed significant difference in volume ratio (P ≤ 0.05) were selected for further analysis using mass spectrometry.
2.3. Protein identification using mass spectrometry analysis
The selected protein spots from the 2-DE gels were excised and destained with 0.1 M NH4HCO3 and 50% acetonitrile. The disulphide bonds were reduced with 0.1 M NH4HCO3, 10 mM DTT, and 1 mM EDTA, alkylated with 100 mM iodoacetamide in 0.1 M NH4HCO3 and digested with trypsin. Liquid chromatography tandem-mass spectrometry (LC-MS/MS) analyses were carried out on a capillary LC system coupled to a Quadrupole-Time of flight tandem mass spectrometer (Waters Micromass, UK) equipped wih a Z-spray ion-source working in the nanoelectrospray mode. Glu-fibrinopeptide was used to calibrate the instrument in the MS/MS mode, and tryptic peptides were concentrated and desalted on a 75 μm ID × 150 mm C18 PepMap column (LC Packings, the Netherlands). Eluents A and B consisted of 0.1% formic acid in 97% water and 3% acetonitrile, and 0.1% formic acid in 97% acetonitrile, respectively. A 6 μL sample was injected into the nanoLC system, and the separation was performed with the following gradient: 0 min 7% B, 35 min 50% B, 45 min 80% B, 49 min 80% B, 50 min 7% B, and 60 min 7% B.
A database search using SWISS–PROT (http://www.ebi.ac.uk/uniprot/) and NCBI (http://www.ncbi.nlm.nih.gov/protein/) was performed with ProteinLynx (Waters Micromass, Manchester, UK). The Mascot search tool, available on the Matrix Science site (http://www.matrixscience.com), was used for some proteins which were not found in the previous databases [47]. The search parameters were used as follow: Database, Swiss-Prot; taxonomy, Viridiplantae (Green Plants), peptide mass tolerance was 1.2 Da, MS/MS ion mass tolerance was 0.6 Da, allowance was set to 1 missed cleavage, trypsin was set as the used enzyme and the peptide charge limit was set at 2+ and 3+. The identification of protein was analyzed by using p-value ≤0.05 and Mascot score >30 being considered as promising hits. Our criteria followed those of Kristiansenetal et al. [48], for example one matched peptide composed of at least 8 amino acids and a sequence tag of at least 3 amino acids would be considered as a good y-ion series. The peptide and Mascot score for proteins containing one matched peptide should be greater than 30. Protein function was obtained from the UniProt website (http://www.uniprot.org) [49]. Two-way statistical analysis of variance with Tukey's Honest Significant Difference post-hoc analysis was performed. Values were considered to indicate a statistically significant at p < 0.05 [50].
2.4. Protein-protein interaction analysis
STRING (the Search Tool for Retrieval of Interacting Genes/Proteins) database v 9.0 (string-db.org) was employed to obtain the interaction network. The confidence score was defined by STRING and the interaction confidence was calculated. The interaction network was constructed with a high confidence score>0.4. Cytoscape software (http://www.cytoscape.org) was used as a tool to visualize the protein-protein interaction network.
2.5. Protein precipitation by ammonium sulfate
Three grams of fresh leaf and pseudobulb samples from Bulbophyllum morphologlorum Kraenzl and Dendrobium Sonia Earsakul were collected from mature orchids, and then immediately ground separately to a fine powder in liquid nitrogen and left in 5 mL of extraction buffer (0.1 M NaCl, 20 mM phosphate buffer pH 7.2) at 4 °C. The mixture was stirred at 4 °C overnight and later centrifuged at 10,178×g for 30 min at 4 °C and the supernatant was collected. Then ammonium sulfate was added to the supernatant to 90% saturation, and the mixture was left overnight at 4 °C. Precipitated material was obtained by centrifugation (15,904×g, 30 min, 4 °C). The precipitate was dissolved in 400 μL deionized water, and dialyzed against 1000 mL of 20 mM phosphate buffer pH 7.2 (with 4 changes of the fresh buffer) over 18 h at 4 °C. The dialyzed material was then dried using speed-vac. The amount of protein was calculated by the Bradford assay [51].
2.6. Native polyacrylamide gel electrophoresis of SOD activity
The native-PAGE using 12.5% (w/v) polyacrylamide was prepared. The protein sample was dissolved in sample buffer without boiling. The gel was stained for SOD activity using the Chopra method [52]. Thirty micrograms of extracted proteins from leaves and pseudobulb of both orchids after ammonium sulfate precipitation were added with non-reducing sample buffer (62.5 mM Tris–HCl pH 6.8,10%, v/v glycerol and 1%, w/v bromophenol blue) and loaded onto native-PAGE. Electrophoresis was performed for 60 min at 4 °C and 10 mA. SOD activity was detected by incubating the gel in staining buffer (50 mM phosphate, pH 7.8), containing EDTA (1 mM) and riboflavin-NBT in the dark for 10 min. The riboflavin-NBT was replaced by 0.1%v/v TEMED and left in the dark for 15 min. Then the solution was removed and the gel was placed under a 25 W light bulb until SOD bands were visualized. The SOD bands were confirmed by in-gel tryptic digestion and LC/MS/MS using the above method.
3. Results
3.1. Protein profiles of leaves and pseudobulbs of Bulbophyllum morphologlorum Kraenzl and Dendrobium Sonia Earsakul
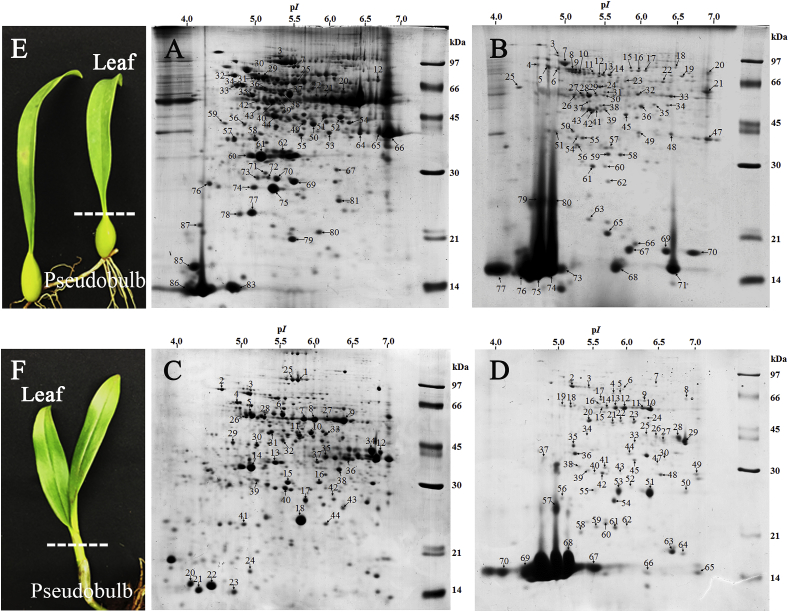
Three hundred micrograms of phenol extracted proteins from leaves and pseudobulb of Bulbophyllum morphologlorum Kraenzl and Dendrobium Sonia Earsakul were separately loaded in triplicate onto 2-DE gels. The results showed reproducible and clear proteomic maps with distinctive and intense spots ranging from 14 to 97 kDa as shown in Fig.1 (A-D). ImageMaster 2D Platinum software was used for analysis, showing that the Bulbophyllum leaf and pseudobulb extracts had 700 and 673 protein spots, respectively while the Dendrobium leaf and pseudobulb extracts had 679 and 551 protein spots, respectively. A total of 233 randomly selected protein spots of highly expressed proteins from both tissues of Bulbophyllum and Dendrobium were excised and trypsinized for identification of proteins by LC-MS/MS analysis.
Fig. 1.
Proteomic profiles of leaves (A) and pseudobulbs (B) of Bulbophyllum morphologlorum Kraenzl and of leaves (C) and pseudobulbs (D) of Dendrobium Sonia Earsakul. The 2-D electrophoresis was obtained using 300 μg phenol extracted proteins from both tissues of the orchids and 7 cm IPG with pH from 4 to 7 was used for the 1st dimension. E is Leaf and pseudobulb tissue of Bulbophyllum morphologlorum Kraenzl while F is Leaf and pseudobulb tissue of Dendrobium Sonia Earsakul.
3.2. Protein identification by LC-MS/MS analysis
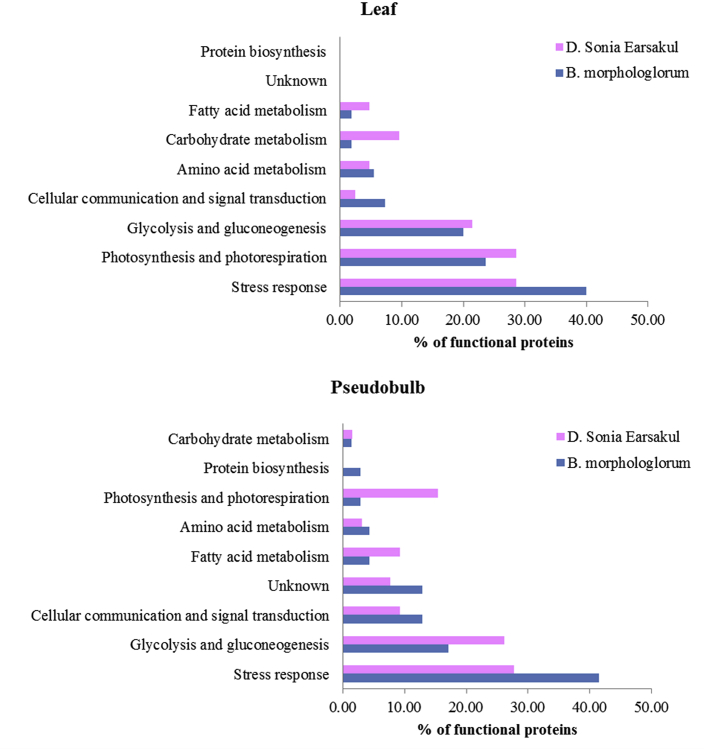
The highly expressed protein spots of interest, selected as representative proteins from the leaves and pseudobulbs of the Dendrobium and Bulbophyllum, were digested with trypsin and identified by LC-MS/MS. A total of 233 proteins were identified using SWISSPROT databases as annotated proteins (Table 1) including accession number, Mascot score, percent coverage, MW/pI (experimental and theoretical) and functions, using the criteria explained in the Materials and Methods. Since there is still no database for orchids, we searched by using viridiplantae (green plants) from the database. The identified proteins were from various types of plants that matched with the peptide sequences. Based on the Protein Analysis Through Evolutionary Relationships (PANTHER) Gene Ontology classification analyses, these 233 annotated proteins were categorized and displayed by the percent of proteins into 9 functional groups as follows: proteins involved in amino acid metabolism, carbohydrate metabolism, cellular communication and signal transduction, fatty acid metabolism, glycolysis and gluconeogenesis, photosynthesis and photorespiration, protein biosynthesis, stress response and unknown proteins. The functional proteins in the leaves of the Bulbophyllum were annotated into stress response (40%), photosynthesis and photorespiration (23.64%), and glycolysis and gluconeogenesis group (20%). In comparison, the proteins in pseudobulbs of the Bulbophyllum were dominated by stress response (41.43%), glycolysis and gluconeogenesis (17.14%), and cellular communication and signal transduction (12.86%) (Fig. 2).
Table 1.
Identified proteins of Bulbophyllum morphologorum Kranzl. (BM) and Dendrobium Sonia Earsakul (DE) by LC-MS/MS.
| Spot no. | Protein Identification | Accession no. | MASCOT score | % Coverage | (MW/pI) aTheoretical | (MW/pI)b Experimental | Functions |
|---|---|---|---|---|---|---|---|
| Bulbophyllum morphologorum's leaves | |||||||
| A3 | TMV resistance protein N-like (Eucalyptus grandis) | gi|702444611 | 38 | 2% | 39.64/7.53 | 97.00/5.4 | Stress response |
| A7 | Hydroquinone glucosyltransferase (Eucalyptus grandis) | gi|702327425 | 45 | 3% | 53.82/6.10 | 94.93/5.5 | Stress response |
| A12 | Auxin-binding protein ABP19a (Fragaria vesca subsp. vesca) | gi|470105207 | 31 | 3% | 22.98/5.90 | 74.26/6.6 | Stress response |
| A20 | ZG10 (Protein amino acid glycosylation) (Pisum sativum) | gi|37813069 | 45 | 3% | 28.26/7.25 | 61.16/6.3 | Glycolysis and gluconeogenesis |
| A21 | Centromeric histone H3 (Brassica juncea) | gi|134152527 | 42 | 4% | 19.48/11.60 | 58.73/5.8 | Cellular communication and signal transduction |
| A22 | Gastrodianin-4B (Gastrodia elata) | gi|62479957 | 86 | 11% | 18.21/8.58 | 58.73/5.7 | Stress response |
| A25 | Pyruvate, phosphate dikinase (Arabidopsis thaliana) | gi|79475768 | 45 | 7% | 95.332/5.36 | 74.27/5.5 | Glycolysis and gluconeogenesis |
| A29 | Heat shock cognate 70 kDa protein 2 (Zea mays) | gi|195616644 | 165 | 9% | 71.09/5.06 | 78.40/5.2 | Stress response |
| A30 | Heat shock protein 70 (Camellia sinensis) | gi|189380223 | 63 | 7% | 75.07/5.54 | 78.40/5.1 | Stress response |
| A31 | Heat shock cognate 70 kDa protein 2 (Zea mays) | gi|226500092 | 52 | 3% | 71.09/5.15 | 78.40/5.0 | Stress response |
| A32 | Heat shock protein 70 (Cucumis sativus) | gi|1143427 | 192 | 10% | 75.37/4.99 | 78.76/4.8 | Stress response |
| A33 | RuBisCO large subunit-binding protein subunit alpha, chloroplastic (Fragment) (Brassica napus) | gi|289365 | 86 | 8% | 57.66/4.84 | 70.13/4.8 | Photosynthesis and photorespiration |
| A34 | RuBisCO large subunit-binding protein subunit alpha, chloroplastic (Pisum sativum) | gi|219902505 | 65 | 3% | 61.94/5.15 | 70.13/4.8 | Photosynthesis and photorespiration |
| A35 | V-type proton ATPase subunit B1 (Vitis vinifera) | gi|225428086 | 197 | 17% | 54.25/5.04 | 61.16/5.0 | Cellular communication and signal transduction |
| A36 | RuBisCO large subunit-binding protein subunit beta (Pisum sativum) | gi|2506277 | 56 | 6% | 62.94/5.85 | 64.79/5.2 | Photosynthesis and photorespiration |
| A37 | 4-hydroxy-tetrahydrodipicolinate synthase, chloroplastic (Coix lacryma-jobi) | gi|300572573 | 37 | 1% | 41.05/6.84 | 62.37/5.5 | Amino acid metabolism |
| A38 | ATP synthase subunit alpha, chloroplastic (Lotus japonicus) | gi|13518443 | 32 | 9% | 55.75/5.22 | 59.95/5.3 | Photosynthesis and photorespiration |
| A39 | ATP synthase subunit beta, chloroplastic (Eucalyptus globulus subsp. Globulus) | gi|60460816 | 31 | 11% | 53.69/5.29 | 56.31/5.3 | Photosynthesis and photorespiration |
| A40 | Enolase 1 (Zea mays) | gi|162458207 | 202 | 6% | 48.03/5.20 | 53.89/5.3 | Glycolysis and gluconeogenesis |
| A42 | Nicotianamine synthase (Ricinus communis) | gi|255585344 | 42 | 10% | 75.81/7.29 | 55.10/5.0 | Stress response |
| A43 | DEAD-box ATP-dependent RNA helicase 31 (Arabidopsis thaliana) | gi|334188604 | 36 | 1% | 90.03/9.02 | 50.26/5.1 | Stress response |
| A44 | Actin (Glycine max) | gi|18532 | 56 | 2% | 41.57/5.23 | 44.21/5.2 | Stress response |
| A49 | Ribulose bisphosphate carboxylase/oxygenase activase 2 (Nicotiana tabacum) | gi|12643758 | 80 | 11% | 48.31/8.14 | 42.59/5.6 | Photosynthesis and photorespiration |
| A50 | Phosphoglycerate kinase (Musa acuminata) | gi|102139814 | 42 | 7% | 42.27/6.20 | 41.78/5.7 | Glycolysis and gluconeogenesis |
| A51 | Cytosolic 3-phosphoglycerate kinase activase 2 (Nicotiana tabacum) | gi|28172913 | 93 | 18% | 31.30/5.05 | 43.00/5.9 | Glycolysis and gluconeogenesis |
| A52 | Phosphoglycerate kinase (Ricinus communis) | gi|255544584 | 84 | 17% | 50.00/8.74 | 43.00/6.2 | Glycolysis and gluconeogenesis |
| A53 | Allyl alcohol dehydrogenase (Nicotiana tabacum) | gi|6692816 | 41 | 5% | 38.06/6.56 | 39.75/6.0 | Fatty acid metabolism |
| A54 | rbcL gene product (chloroplast) (Brassica napus) | gi|383930435 | 97 | 18% | 52.92/5.88 | 42.59/6.3 | Photosynthesis and photorespiration |
| A55 | Photosystem II stability/assembly factor HCF136 | gi|75252730 | 80 | 12% | 45.44/9.02 | 38.93/5.7 | Photosynthesis and photorespiration |
| A56 | Sedoheptulose-1,7-bisphosphatase (Ricinus communis) | gi|255579134 | 96 | 5% | 41.97/5.95 | 41.78/4.8 | Carbohydrate metabolism |
| A57 | Putative DNA damage repair toleration protein DRT102 (Trifolium pretense) | gi|84468444 | 62 | 3% | 32.95/5.06 | 38.53/4.7 | Stress response |
| A58 | B3 domain-containing transcription repressor VAL2-like (Cicer arietinum) | gi|828298615 | 35 | 2% | 44.49/5.75 | 38.53/5.0 | Stress response |
| A59 | Disease resistance protein (Theobroma cacao) | gi|16322949 | 42 | 3% | 15.13/6.66 | 42.59/4.6 | Stress response |
| A60 | Oxygen-evolving enhancer (Pisum sativum) | gi|131384 | 94 | 10% | 34.87/6.25 | 34.06/5.9 | Photosynthesis and photorespiration |
| A61 | Oxygen-evolving enhancer (Glycine max) | gi|356559442 | 105 | 23% | 35.04/6.66 | 34.06/6.0 | Photosynthesis and photorespiration |
| A64 | Glyceraldehyde-3-phosphate dehydrogenase (Knorringia sibirica) | gi|115371630 | 90 | 9% | 36.65/7.66 | 35.68/6.4 | Glycolysis and gluconeogenesis |
| A65 | Glyceraldehyde-3-phosphate dehydrogenase C subunit (Gossypium hirsutum) | gi|211906518 | 103 | 18% | 36.54/7.70 | 39.34/6.7 | Glycolysis and gluconeogenesis |
| A66 | Glyceraldehyde-3-phosphate dehydrogenase C1 (Pyrus x bretschneideri) | gi|381393064 | 142 | 23% | 36.92/8.24 | 39.34/6.8 | Glycolysis and gluconeogenesis |
| A67 | Putative alpha 7 proteasome subunit (Nicotiana tabacum) | gi|14594925 | 71 | 18% | 27.18/6.11 | 30.81/6.1 | Amino acid metabolism |
| A69 | Transcription factor (Vicia faba var minor) | gi|2104681 | 33 | 2% | 39.95/6.36 | 28.52/6.1 | Cellular communication and signal transduction |
| A70 | Triosephosphate isomerase (Coptis japonica) | gi|136057 | 75 | 8% | 27.07/5.54 | 29.20/5.3 | Glycolysis and gluconeogenesis |
| A71 | Putative disease resistance RPP13-like protein 1 (Pyrus x bretschneideri) | gi|694327264 | 47 | 1% | 200.00/5.64 | 30.00/5.2 | Stress response |
| A72 | Hypothetical protein SORBIDRAFT_02g031030 (Sorghum bicolor) | gi|242049978 | 49 | 13% | 32.34/6.45 | 29.43/5.2 | Glycolysis and gluconeogenesis |
| A73 | Cysteine proteinase COT44 (Brassica napus) | gi|118127 | 32 | 3% | 36.25/8.05 | 29.43/5.0 | Amino acid metabolism |
| A74 | Oxygen-evolving enhancer protein 2, chloroplastic (Helianthus annuus) | gi|302595736 | 36 | 5% | 28.12/8.67 | 27.85/5.0 | Photosynthesis and photorespiration |
| A75 | Oxygen-evolving enhancer protein 2 (Bruguiera gymnorhiza) | gi|8131593 | 41 | 6% | 17.58/4.91 | 27.855.2 | Photosynthesis and photorespiration |
| A76 | Heme-binding protein 2 (Cucumis sativus) | gi|449438953 | 57 | 5% | 24.48/4.65 | 28.52/4.4 | Photosynthesis and photorespiration |
| A77 | Superoxide dismutase (Cu–Zn) (Zantedeschia aethiopica) | SODCP_ZANAE | 73 | 12% | 22.06/6.17 | 24.48/4.9 | Stress response |
| A78 | Superoxide dismutase (Cu–Zn) (Zantedeschia aethiopica) | SODCO_ZANAE | 41 | 6% | 22.06/6.17 | 24.48/4.8 | Stress response |
| A79 | Superoxide dismutase (Cu–Zn) (Panax ginseng) | SODCP_PANGI | 78 | 8% | 15.25/5.45 | 21.10/5.5 | Stress response |
| A80 | Maturase K (Ferraria crispa) | gi|71060163 | 31 | 1% | 62.85/9.75 | 22.00/5.8 | Cellular communication and signal transduction |
| A81 | Mannose-binding lectin precursor (Tulipa hybrid cultivar) | gi|1141765 | 35 | 5% | 18.96/4.84 | 26.05/6.2 | Stress response |
| A83 | Peroxidase 27 (Arabidopsis thaliana) | PER27_ARATH | 42 | 3% | 34.93/9.19 | 14.10/4.7 | Stress response |
| A85 | Probable WRKY transcription factor 43 (Arabidopsis thaliana) | gi|1063699318 | 33 | 9% | 12.94/9.57 | 17.35/4.2 | Stress response |
| A86 | Mannose binding lectin AKA1 precursor (Amorphophallus konjac) | gi|30349401 | 76 | 7% | 14.42/10.20 | 14.10/4.1 | Stress response |
| Bulbophyllum morphologorum's pseudobulbs | |||||||
| B3 | Calcium calmodulin dependent protein kinase (Medicago truncatula var truncatula) | gi|163256950 | 58 | 7% | 22.85/5.30 | 103.64/4.9 | Stress response |
| B4 | Nuclease HARBI1 (Gossypium raimondii) | gi|823135887 | 42 | 2% | 42.00/9.70 | 90.35/4.6 | Cellular communication and signal transduction |
| B5 | 3-ketoacyl carrier protein synthase III (Allium ampeloprasum) | gi|1143069 | 32 | 1% | 42.62/6.40 | 88.14/4.7 | Fatty acid metabolism |
| B6 | Molecular chaperone hsp70b | gi|116061511 | 37 | 2% | 59.76/6.60 | 85.92/4.9 | Stress response |
| B7 | Heat shock protein 90 (Triticum aestivum) | gi|294717810 | 300 | 15% | 80.30/5.00 | 92.57/5.0 | Stress response |
| B8 | Heat shock cognate 70 kDa (Vitis vinifera) | gi|359486799 | 31 | 10% | 71.13/5.20 | 83.71/5.1 | Stress response |
| B9 | Heat shock cognate 70 kDa (Glycine max) | gi|356568992 | 83 | 20% | 71.19/5.10 | 81.50/5.2 | Stress response |
| B10 | High molecular weight heat shock protein (Malus x domestica) | gi|6969976 | 61 | 7% | 71.17/5.20 | 81.50/5.2 | Stress response |
| B11 | P-Protein-like protein (Arabidopsis thaliana) | gi|14596025 | 34 | 4% | 112.88/6.50 | 79.28/5.3 | Unknown |
| B12 | Heat shock protein 70 (Phaseolus vulgaris) | gi|399940 | 50 | 10% | 72.49/6.00 | 74.85/5.4 | Stress response |
| B13 | Phosphoglycerate mutase (Nicotiana attenuate) | gi|111162649 | 59 | 7% | 27.38/5.60 | 77.07/5.5 | Glycolysis and gluconeogenesis |
| B14 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase(Ricinus communis) | PMGI_RICCO | 54 | 3% | 60.78/5.40 | 77.07/5.6 | Glycolysis and gluconeogenesis |
| B15 | AsnC family transcriptional regulator (Propionispora sp. Iso 2/2) | gi|930608178 | 53 | 8% | 18.21/5.90 | 83.71/5.8 | Cellular communication and signal transduction |
| B16 | Chloroplast transketolase (Arabidopsis lyrata subsp. lyrata) | gi|297810173 | 107 | 5% | 79.53/6.50 | 83.71/5.9 | Stress response |
| B17 | Hypothetical protein SELMODRAFT_403066 (Selaginella moellendorffii) | gi|302754452 | 39 | 2% | 32.61/9.10 | 83.71/6.0 | Unknown |
| B18 | Methionine synthase (Solanum tuberosum) | gi|8439545 | 56 | 4% | 84.61/5.90 | 88.14/6.5 | Amino acid metabolism |
| B19 | Malate dehydrogenase (Cicer arietinum) | gi|4586606 | 38 | 4% | 17.74/5.09 | 74.85/6.6 | Carbohydrate metabolism |
| B20 | Histidine decarboxylase (Nicotiana tomentosiformis) | gi|697133277 | 32 | 1% | 52.36/7.20 | 77.07/6.9 | Amino acid metabolism |
| B21 | Catalase 2 (Elaeis guineensis) | gi|192910916 | 67 | 12% | 9.27/10.00 | 61.07/6.9 | Stress response |
| B22 | Succinate dehydrogenase (ubiquinone) flavoprotein subunit 1 (Glycine max) | gi|356498373 | 81 | 4% | 69.72/6.30 | 67.10/6.3 | Stress response |
| B23 | Predicted protein (Populus trichocarpa) | gi|224100535 | 53 | 4% | 67.54/6.60 | 67.10/5.7 | Unknown |
| B24 | Chaperonin CPN60 (Vitis vinifera) | gi|225433375 | 56 | 13% | 61.33/5.90 | 63.53/5.4 | Stress response |
| B25 | Calreticulin-like (Phoenix dactylifera) | gi|672144143 | 47 | 3% | 47.30/4.50 | 63.53/4.4 | Stress response |
| B26 | Enolase (Elaeis guineensis) | gi|353441078 | 90 | 14% | 23.00/4.80 | 58.60/5.1 | Glycolysis and gluconeogenesis |
| B27 | Enolase (Elaeis guineensis) | gi|192910834 | 67 | 9% | 47.73/5.98 | 58.60/5.2 | Glycolysis and gluconeogenesis |
| B28 | Enolase (Elaeis guineensis) | gi|192910834 | 105 | 11% | 47.73/5.98 | 58.60/5.3 | Glycolysis and gluconeogenesis |
| B29 | Enolase 1 (Zea mays) | gi|162458207 | 64 | 4% | 48.03/5.20 | 58.60/5.4 | Glycolysis and gluconeogenesis |
| B30 | Enolase (Oryza sativa Japonica Group) | gi|780372 | 56 | 8% | 47.96/5.40 | 58.60/5.6 | Glycolysis and gluconeogenesis |
| B31 | ATP synthase CF1 alpha subunit (Phalaenopsis aphrodite subsp. formosana) | gi|78103238 | 127 | 12% | 55.20/5.34 | 60.25/5.5 | Photosynthesis and photorespiration |
| B32 | S-adenosyl-l-homocysteine hydrolase (Hordeum vulgare subsp. vulgare) | gi|68655456 | 83 | 11% | 49.96/5.80 | 59.42/5.9 | Amino acid metabolism |
| B33 | Ribulosebisphosphate carboxylase large subunit chloroplast (Pogostemon cablin) | gi|349048 | 82 | 9% | 50.06/6.10 | 58.60/6.4 | Photosynthesis and photorespiration |
| B34 | Benzoate transporter (Pseudomonas sp. Os17) | gi|771840651 | 32 | 2% | 41.57/9.90 | 52.85/6.3 | Cellular communication and signal transduction |
| B35 | Unknown protein 18 (Pseudotsuga menziesii) | gi|205830697 | 78 | 100% | 1.39/5.80 | 52.85/6.2 | Unknown |
| B36 | Alcohol dehydrogenase 1(Solanum tuberosum) | gi|113365 | 91 | 12% | 41.07/5.92 | 52.85/6.0 | Stress response |
| B37 | Peroxisomal (S)-2-hydroxy-acid oxidase 2 (Aegilops tauschii) | gi|475560053 | 24 | 3% | 31.12/8.70 | 55.32/5.2 | Fatty acid metabolism |
| B38 | Elongation factor Tu (Glycine max) | gi|2494261 | 36 | 2% | 36.35/6.20 | 53.67/5.4 | Protein biosynthesis |
| B39 | Predicted protein (Populus trichocarpa) | gi|224109060 | 38 | 4% | 50.18/8.30 | 52.03/5.5 | Cellular communication and signal transduction |
| B41 | Cytosolic phosphoglycerate kinase (Pisum sativum) | gi|9230771 | 50 | 12% | 42.26/5.70 | 52.85/5.5 | Glycolysis and gluconeogenesis |
| B42 | Actin like protein (Phalaenopsis sp. True Lady) | AF246715_1 | 56 | 8% | 41.62/5.20 | 52.03/5.3 | Stress response |
| B43 | Monodehydroascorbate reductase (Oncidium Gower Ramsey) | gi|212896914 | 103 | 18% | 46.63/5.30 | 52.03/5.3 | Stress response |
| B45 | 3-phosphoglycerate kinase (Hordeum vulgare subsp. vulgare) | gi|21396683 | 39 | 11% | 31.32/4.90 | 47.921/5.2 | Glycolysis and gluconeogenesis |
| B47 | Glyceraldehyde-3-phosphate dehydrogenase (Ananas comosus) | gi|312192239 | 59 | 15% | 36.576.70 | 38.47/6.9 | Glycolysis and gluconeogenesis |
| B48 | Allyl alcohol dehydrogenase (Nicotiana tabacum) | gi|6692816 | 30 | 2% | 38.06/6.56 | 40.17/6.4 | Fatty acid metabolism |
| B49 | Plant invertase/pectin methylesterase inhibitor superfamily (Theobroma cacao) | gi|590708612 | 26 | 2% | 64.48/8.10 | 40.73/6.0 | Stress Response |
| B50 | Hydroxyacid dehydrogenase/reductase (Medicago truncatula) | gi|124359345 | 47 | 3% | 35.46/7.10 | 41.30/5.2 | Stress response |
| B51 | Quinone oxidoreductase (Helianthus annuus) | gi|14532287 | 34 | 2% | 33.17/4.80 | 41.30/4.8 | Stress response |
| B54 | TMV resistance protein N-like (Nicotiana sylvestris) | gi|698528100 | 32 | 1% | 129.67/7.80 | 39.60/5.1 | Stress response |
| B55 | la-related protein 6B-like isoform X1 (Musa acuminata subsp. malaccensis) | gi|695013984 | 48 | 2% | 50.29/6.80 | 39.60/5.2 | Cellular communication and signal transduction |
| B56 | 2-methylene-furan-3-one reductase (Solanum pennellii) | gi|970030197 | 81 | 9% | 40.92/8.80 | 36.78/5.2 | Stress response |
| B57 | Unknown protein 18 (Pseudotsuga menziesii) | gi|205830697 | 53 | 91% | 1.39/5.80 | 35.65/5.7 | Unknown |
| B58 | Isoflavone reductase-like protein (Olea europaea) | gi|218963723 | 36 | 3% | 59.54/8.70 | 33.39/5.8 | Stress response |
| B59 | Hypothetical protein OsI 007339 (Oryza sativa indica cultivar group) | gi|125539711 | 33 | 2% | 73.55/5.50 | 33.39/5.5 | Unknown |
| B60 | Triosephosphate isomerase (Petunia x hybrida) | gi|1351279 | 70 | 12% | 27.11/5.54 | 30.00/5.5 | Glycolysis and gluconeogenesis |
| B61 | Triosephosphate isomerase (Petunia x hybrida) | gi|1351279 | 62 | 10% | 27.11/5.54 | 30.00/5.3 | Glycolysis and gluconeogenesis |
| B62 | Syntaxin-52-like (Camelina sativa) | gi|727483504 | 31 | 4% | 26.07/9.07 | 28.11/5.6 | Cellular communication and signal transduction |
| B63 | Superoxide dismutase (Cu–Zn) (Panax ginseng) | SODCP_PANGI | 34 | 8% | 15.25/5.45 | 23.09/5.3 | Stress response |
| B65 | Superoxide dismutase (Cu–Zn) (Panax ginseng) | SODCP_PANGI | 146 | 22% | 15.25/5.45 | 21.41/5.6 | Stress response |
| B66 | Initiation factor eIF4A-15 (Helianthus annuus) | Q6T8C6_HELAN | 71 | 5% | 46.58/5.30 | 19.88/5.8 | Protein biosynthesis |
| B67 | Intracellular pathogenesis-related protein PR-107 (Lilium longiflorum) | gi|4325333 | 59 | 6% | 16.64/5.40 | 18.76/5.8 | Stress response |
| B68 | 60S ribosomal export protein NMD3 (Solanum pennellii) | gi|970037034 | 31 | 1% | 59.19/6.00 | 15.96/5.7 | Stress response |
| B69 | Glutathione-S-transferase (Avena sterilis subsp. ludoviciana) | gi|17384331 | 28 | 15% | 5.79/4.70 | 18.76/6.4 | Stress response |
| B70 | Hypothetical protein CHLREDRAFT 173629 (Chlamydomonas reinhardtii) | gi|159472713 | 34 | 1% | 94.32/6.70 | 18.76/6.8 | Unknown |
| B71 | Phytoene synthase (Oncidium Gower Ramsey) | gi|40557193 | 56 | 1% | 46.96/7.90 | 15.68/6.5 | Stress response |
| B73 | Os03g0365200 (Oryza sativa japonica) | gi|115453147 | 79 | 5% | 24.40/10.00 | 15.68/5.0 | Unknown |
| B74 | Multidrug Resistance associated Protein 1 (Catharanthus roseus) | gi|156556172 | 36 | 1% | 162.66/7.50 | 14.84/4.8 | Stress response |
| B75 | ABC transporter C family member 9 (Glycine max) | gi|356504494 | 49 | 1% | 17.01/7.33 | 14.84/4.6 | Stress response |
| B76 | RNA polymerase beta' subunit (Mesostigma viride) | gi|11466381 | 57 | 1% | 76.73/9.15 | 14.84/4.4 | Cellular communication and signal transduction |
| B77 | RNA polymerase beta' subunit (Mesostigma viride) | gi|11466381 | 57 | 1% | 76.73/9.15 | 15.68/4.0 | Cellular communication and signal transduction |
| B79 | RNA polymerase beta' subunit (Mesostigma viride) | gi|11466381 | 48 | 1% | 76.73/9.15 | 25.81/4.6 | Cellular communication and signal transduction |
| B80 | Hypothetical protein (Oryza sativa Japonica Group) | gi|12313682 | 64 | 5% | 10.78/13.00 | 25.81/4.8 | Unknown |
| Dendrobium Sonia EarSakul's leaves | |||||||
| C1 | Pyruvate orthophosphate dikinase (Eleocharis vivipara) | gi|2285879 | 394 | 11% | 95.97/5.21 | 98.00/5.7 | Photosynthesis and photorespiration |
| C2 | Heat shock protein 70 (Spinacia oleracea) | gi|2654208 | 409 | 18% | 76.09/5.19 | 86.66/4.7 | Stress response |
| C3 | Heat shock protein 70 (Dendrobium catenatum) | gi|525330265 | 342 | 15% | 71.46/5.13 | 81.50/5.2 | Stress response |
| C4 | Putative rubisco subunit binding-protein alpha subunit precursor (Oryza sativa Japonica group) | gi|31193919 | 139 | 6% | 61.36/5.21 | 66.00/4.9 | Photosynthesis and photorespiration |
| C5 | ATP synthase beta subunit (Coriaria ruscifolia) | gi|66276267 | 1077 | 43% | 50.97/5.20 | 60.25/5.2 | Photosynthesis and photorespiration |
| C6 | ATP synthase CF1 alpha subunit subunit precursor (Phalaenopsis aphrodite subsp. formosana) | gi|78103238 | 482 | 22% | 55.20/5.34 | 60.25/5.5 | Photosynthesis and photorespiration |
| C7 | Enolase (Elaeis guineensis) | gi|192910834 | 360 | 20% | 47.73/5.98 | 57.37/5.8 | Glycolysis and gluconeogenesis |
| C8 | Enolase 1 (Guzmania wittmackii x Guzmania lingulata) | gi|365200115 | 425 | 24% | 47.86/5.70 | 57.37/6.0 | Glycolysis and gluconeogenesis |
| C9 | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (Niedenzuella stannea) | gi|331690309 | 335 | 19% | 49.40/6.19 | 56.41/6.4 | Photosynthesis and photorespiration |
| C10 | 3-Phosphoglycerate kinase (Kengyilia hirsuta) | gi|351735496 | 354 | 35% | 31.44/4.84 | 50.66/6.0 | Glycolysis and gluconeogenesis |
| C11 | Phosphoglycerate kinase, cytosolic (Glycine max) | gi|356525744 | 212 | 13% | 42.37/6.28 | 46.83/5.7 | Glycolysis and gluconeogenesis |
| C12 | Glyceraldehyde-3-phosphate dehydrogenase, cytosolic (Petunia x hybrida) | gi|120673 | 418 | 27% | 36.50/6.68 | 40.83/6.8 | Glycolysis and gluconeogenesis |
| C13 | Probable long-chain-alcohol O-fatty-acyltransferase 3 (Brassica rapa) | gi|685282948 | 36 | 2% | 39.20/9.06 | 37.04/5.4 | Fatty acid metabolism |
| C14 | Oxygen-evolving enhancer protein 1, chloroplastic (Vitis vinifera) | gi|147791852 | 200 | 18% | 33.21/5.87 | 35.41/5.2 | Photosynthesis and photorespiration |
| C15 | Putative Nuclear inhibitor of protein phosphatase-1 (Zostera marina) | gi|901808822 | 38 | 1% | 84.48/5.36 | 31.08/5.6 | Fatty acid metabolism |
| C16 | F-box family protein (Theobroma cacao) | gi|590728568 | 33 | 2% | 47.82/4.60 | 31.08/6.1 | Stress response |
| C17 | Carbonic anhydrase 2 (Fragment) (Flaveria linearis) | gi|882244 | 55 | 5% | 20.57/6.21 | 28.20/5.8 | Carbohydrate metabolism |
| C18 | Photosystem II oxygen-evolving complex protein 2 (Arabidopsis thaliana (fragment)) | gi|1076373 | 90 | 92% | 1.43/9.71 | 25.50/5.8 | Photosynthesis and photorespiration |
| C20 | Mannose-binding protein, partial (Listera ovata) | gi|431099 | 86 | 11% | 17.66/9.39 | 15.59/4.2 | Stress response |
| C21 | Early nodulin-like protein 2 (Setaria italica) | gi|835974449 | 49 | 8% | 14.77/6.49 | 14.47/4.3 | Cellular communication and signal transduction |
| C22 | Mannose-binding protein, partial (Listera ovata) | gi|431099 | 92 | 11% | 17.66/9.39 | 15.27/4.5 | Stress response |
| C23 | Lectin, partial (Listera ovata) | gi|431097 | 49 | 6.81% | 18.65/5.52 | 14.10/4.8 | Stress response |
| C24 | Thioredoxin H3 (Ipomoea batatas) | gi|33621084 | 104 | 12% | 13.70/6.06 | 18.13/5.0 | Stress response |
| C25 | Pyruvate orthophosphate dikinase (Eleocharis vivipara) | gi|2285879 | 269 | 12% | 95.97/5.21 | 98.00/5.7 | Photosynthesis and photorespiration |
| C26 | UTP-glucose-1-phosphate uridylyltransferase (Hordeun vulgare) | gi|6136111 | 63 | 2% | 51.78/5.20 | 53.54/5.1 | Stress response |
| C27 | Ribulose-1,5-bisphosphate carboxylase|oxygenase (Haworthia vittata) | gi|33635955 | 197 | 14% | 49.17/6.43 | 57.37/6.2 | Photosynthesis and photorespiration |
| C28 | ATP synthase subunit beta-3 (Arabidopsis thaliana) | gi|22326673 | 415 | 21% | 59.82/6.06 | 57.37/5.3 | Photosynthesis and photorespiration |
| C29 | Sedoheptulose-1,7-bisphosphatase, chloroplast putative (Ricinus communis) | gi|255579134 | 148 | 9% | 41.97/5.95 | 43.95/4.9 | Carbohydrate metabolism |
| C30 | Phosphoribulokinase (Spinacia oleracea) | gi|125579 | 72 | 9% | 44.98/5.82 | 43.00/5.2 | Stress response |
| C31 | Actin (Gossypium hirsutum) | gi|32186894 | 249 | 26% | 41.67/5.31 | 49.70/5.4 | Stress response |
| C32 | Ribulose bisphosphate carboxylase|oxygenase activase (Solanum pennellii) | gi|10720247 | 59 | 13% | 50.67/8.61 | 44.92/5.5 | Photosynthesis and photorespiration |
| C33 | Monodehydroascorbate reductase (Malus x domestica) | gi|225380882 | 53 | 3% | 46.88/6.51 | 48.75/6.2 | Stress response |
| C34 | Fructose-bisphosphate aldolase (Codonopsis lanceolata) | gi|82941449 | 62 | 7% | 38.14/6.47 | 41.37/6.4 | Glycolysis and gluconeogenesis |
| C35 | NAD-dependent malate dehydrogenase (Prunus persica) | gi|15982948 | 41 | 7% | 35.82/6.60 | 39.21/6.2 | Carbohydrate metabolism |
| C36 | Glyceraldehyde-3-phosphate dehydrogenase, cytosolic (Petunia x hybrida) | gi|120673 | 362 | 22% | 36.50/6.68 | 38.66/6.5 | Glycolysis and gluconeogenesis |
| C37 | Probable adenylate kinase 6 (Tarenaya hassleriana) | gi|729401807 | 46 | 3% | 33.44/6.26 | 37.58/6.1 | Amino acid metabolism |
| C38 | Probable disease resistance protein RXW24L (Arabidopsis thaliana) | gi|6566297 | 35 | 1% | 104.27/6.62 | 34.87/6.3 | Stress response |
| C39 | Triosephosphate isomerase (Coptis japonica) | gi|136057 | 59 | 5% | 27.07/5.54 | 31.35/5.2 | Glycolysis and gluconeogenesis |
| C40 | Triosephosphate isomerase (Coptis japonica) | gi|136057 | 85 | 8% | 27.07/5.54 | 29.55/5.6 | Glycolysis and gluconeogenesis |
| C41 | Putative cytochrome c oxidase subunit II PS17 (Pinus strobus) | gi|109892850 | 26 | 50% | 1.71/9.62 | 25.05/5.1 | Photosynthesis and photorespiration |
| C42 | Carbonic anhydrase (Arabidopsis thaliana) | gi|15220853 | 65 | 4% | 28.81/6.59 | 28.20/6.2 | Carbohydrate metabolism |
| C43 | Probable adenylate kinase 6 (Tarenaya hassleriana) | gi|729401807 | 42 | 3% | 33.44/6.26 | 27.07/6.4 | Amino acid metabolism |
| C44 | PsbP domain-containing protein 4, chloroplastic (Arabidopsis thaliana) | gi|2829916 | 49 | 6% | 28.48/7.02 | 24.93/6.2 | Photosynthesis and photorespiration |
| Dendrobium Sonia EarSakul's pseudobulbs | |||||||
| D2 | Hypothetical protein CISIN_1g037404mg (Citrus sinensis) | gi|641853885 | 68 | 10% | 68.46/8.19 | 81.50/5.2 | Unknown |
| D3 | Phosphoglycerate mutase (Arabidopsis thaliana) | gi|2160168 | 67 | 2% | 62.63/5.36 | 79.28/5.4 | Glycolysis and gluconeogenesis |
| D4 | Heat shock protein 70 (Capsicum annuum) | gi|163311872 | 153 | 16% | 7.40/4.76 | 70.42/5.8 | Stress response |
| D5 | NADP-dependent malic enzyme 1 (Arabidopsis thaliana) | gi|15225262 | 41 | 6% | 64.24/6.32 | 70.42/5.9 | Glycolysis and gluconeogenesis |
| D6 | Phosphoribulokinase (Monoraphidium neglectum) | gi|926792189 | 36 | 5% | 26.15/8.91 | 574.85/.9 | Stress response |
| D7 | Hsp70-Hsp 90 organizing protein 2 (Arabidopsis thaliana) | gi|58331773 | 46 | 2% | 64.48/5.85 | 79.28/5.9 | Stress response |
| D8 | Malate dehydrogenase (Cicer arietinum) | gi|4586606 | 86 | 4% | 17.74/5.09 | 66.00/6.8 | Carbohydrate metabolism |
| D9 | NADP-dependent malic enzyme 1 (Arabidopsis thaliana) | gi|15225262 | 41 | 6% | 64.24/6.32 | 62.32/6.2 | Glycolysis and gluconeogenesis |
| D10 | Aldehyde dehydrogenase family 2 member B7, mitochondrial (Morus notabilis) | gi|21410404 | 84 | 2% | 58.01/6.16 | 58.64/6.3 | Stress response |
| D11 | Aldehyde dehydrogenase family 2 member (Morus notabilis) | gi|703113828 | 85 | 2% | 58.40/6.16 | 59.56/6.2 | Stress response |
| D12 | F1-ATPase alpha subunit (Calamus usitatus) | gi|1381685 | 38 | 5% | 45.79/7.89 | 59.56/5.9 | Photosynthesis and photorespiration |
| D13 | D-3-phosphoglycerate dehydrogenase (Phoenix dactylifera) | gi|672132227 | 53 | 4% | 66.01/6.36 | 59.56/5.8 | Fatty acid metabolism |
| D14 | ATP synthase subunit alpha (Phalaenopsis aphrodite subsp. formosana) | gi|78103238 | 162 | 14% | 55.20/5.43 | 59.56/5.7 | Photosynthesis and photorespiration |
| D15 | Enolase 1 (Zea mays) | gi|162458207 | 64 | 3% | 48.26/5.20 | 59.56/5.4 | Glycolysis and gluconeogenesis |
| D16 | Phosphoribulokinase (Monoraphidium neglectum) | gi|926792189 | 36 | 5% | 26.15/8.91 | 61.40/5.4 | Stress Response |
| D17 | Mitochondrial F1 ATP synthase beta subunit (Arabidopsis thaliana) | gi|17939849 | 200 | 17% | 63.33/6.52 | 64.62/5.4 | Photosynthesis and photorespiration |
| D18 | Enolase 2 (Hevea brasiliensis) | gi|14423687 | 73 | 100% | 48.11/5.92 | 60.48/5.2 | Glycolysis and gluconeogenesis |
| D19 | ABC transporter C family member 9 (Glycine max) | gi|356504494 | 46 | 1% | 17.01/7.33 | 61.40/5.1 | Stress response |
| D20 | D-3-phosphoglycerate dehydrogenase (Phoenix dactylifera) | gi|672132227 | 53 | 4% | 66.01/6.36 | 52.20/5.4 | Fatty acid metabolism |
| D21 | Actin-3 (Oryza sativa subsp. Indica) | gi|20331 | 70 | 15% | 41.68/5.31 | 51.28/5.7 | Stress response |
| D22 | ATP synthase CF1 alpha subunit (Phalaenopsis aphrodite subsp. formosana) | gi|78103238 | 127 | 12% | 55.20/5.34 | 53.12/5.8 | Photosynthesis and photorespiration |
| D23 | Unknown protein 18 (Vitis rotundifolia) | gi|205830697 | 78 | 100% | 1.39/5.80 | 51.28/6.1 | Unknown |
| D24 | ATPase alpha subunit (Thalassia testudinum) | gi|114509234 | 165 | 9% | 13.53/5.61 | 53.12/6.3 | Photosynthesis and photorespiration |
| D25 | Glyceraldehyde-3-phosphate dehydrogenase (Xerocladia viridiramis) | gi|158421228 | 243 | 29% | 5.08/10.20 | 43.92/6.3 | Glycolysis and gluconeogenesis |
| D26 | Alcohol dehydrogenase 1 (Solanum tuberosum) | gi|113365 | 91 | 12% | 41.07/5.92 | 43.00/6.4 | Stress response |
| D27 | Glyceraldehyde-3-phosphate dehydrogenase, cytosolic (Craterostigma plantagineum) | gi|460979 | 38 | 11% | 36.45/7.06 | 42.35/6.5 | Glycolysis and gluconeogenesis |
| D28 | Cytochrome c reductase 53 kDa subunit P1 peptide | gi|633898 | 231 | 31% | 0.21/9.87 | 43.00/6.7 | Photosynthesis and photorespiration |
| D29 | Glyceraldehyde-3-phosphate dehydrogenase (Mallotus nesophilus) | gi|156617106 | 83 | 12% | 6.50/10.70 | 41.70/6.8 | Glycolysis and gluconeogenesis |
| D30 | 2-alkenal reductase (NADP(+)-dependent) (Nicotiana tabacum) | gi|444302249 | 56 | 10% | 38.06/6.56 | 35.20/6.5 | Unknown |
| D33 | MORC family CW-type zinc finger 3a (Zostera marina) | gi|901809830 | 39 | 1% | 66.92/6.05 | 39.36/6.0 | Fatty acid metabolism |
| D34 | Glyoxalase I homolog 1 (Allium cepa) | gi|332629595 | 61 | 14% | 33.32/5.55 | 43.00/5.5 | Stress response |
| D35 | 20 kDa chaperonin, chloroplastic-like (Oryza brachyantha) | gi|573923036 | 41 | 3% | 38.64/5.88 | 39.75/5.2 | Glycolysis and gluconeogenesis |
| D36 | Triosephosphate isomerase TPI (Lactuca sativa) | gi|256124 | 212 | 27% | 4.67/4.43 | 36.50/5.2 | Glycolysis and gluconeogenesis |
| D37 | Serine/threonine-protein kinase (Vitis vinifera) | gi|225462205 | 34 | 2% | 43.06/6.35 | 35.85/4.8 | Fatty acid metabolism |
| D38 | Quinone oxidoreductase like protein (Arabidopsis thaliana) | gi|21553644 | 87 | 8% | 32.71/5.78 | 31.95/5.3 | Photosynthesis and photorespiration |
| D39 | Oxygen-evolving enhancer protein 1, chloroplastic (Fritillaria agrestis) | gi|11133881 | 77 | 10% | 34.85/6.26 | 30.00/5.4 | Photosynthesis and photorespiration |
| D40 | 2-methylene-furan-3-one reductase (Solanum lycopersicum) | gi|743758187 | 273 | 13% | 41.85/8.97 | 29.34/5.5 | Stress response |
| D41 | 2-methylene-furan-3-one reductase (Solanum lycopersicum) | gi|743758187 | 30 | 6% | 40.98/7.74 | 31.30/5.6 | Stress response |
| D42 | Chloroplast photosynthetic water oxidation complex 33 kDa subunit precursor (Morus nigra) | gi|152143640 | 121 | 10% | 28.25/5.30 | 24.95/5.6 | Photosynthesis and photorespiration |
| D43 | Triosephosphate isomerase (Zea mays) | gi|195605636 | 174 | 16% | 27.28/5.53 | 29.34/5.8 | Glycolysis and gluconeogenesis |
| D44 | Nuclear inhibitor of protein phosphatase-1 (Zostera marina) | gi|901808822 | 34 | 1% | 84.48/5.36 | 36.50/6.0 | Fatty acid metabolism |
| D45 | Triosephosphate isomerase (Petunia x hybrid) | gi|1351279 | 99 | 12% | 27.11/5.54 | 33.12/6.1 | Glycolysis and gluconeogenesis |
| D47 | Proteasome subunit alpha type-3 (Arabidopsis thaliana) | gi|51970040 | 30 | 11% | 27.36/5.93 | 32.60/6.4 | Amino acid metabolism |
| D48 | Glyceraldehyde-3-phosphate dehydrogenase C1 (Pyrus x bretschneideri) | gi|381393064 | 64 | 9% | 36.92/8.24 | 29.12/6.5 | Glycolysis and gluconeogenesis |
| D49 | Glyceraldehyde-3-phosphate dehydrogenase (Xerocladia viridiramis) | gi|158421228 | 187 | 9% | 5.08/10.20 | 29.34/6.9 | Glycolysis and gluconeogenesis |
| D50 | Glyceraldehyde-3-phosphate dehydrogenase (Lilium longiflorum) | gi|83839213 | 87 | 8% | 35.06/6.43 | 26.70/6.8 | Glycolysis and gluconeogenesis |
| D51 | Monodehydroascorbate reductase (Acanthus ebracteatus) | gi|117067068 | 96 | 10% | 46.55/5.15 | 26.92/6.3 | Stress response |
| D52 | Triosephosphate isomerase (Zea mays) | TPIS_MAIZE | 132 | 13% | 27.01/5.37 | 27.58/6.0 | Glycolysis and gluconeogenesis |
| D53 | Syntaxin-52-like (Camelina sativa) | gi|727483504 | 31 | 4% | 26.07/9.07 | 26.92/5.8 | Cellular communication and signal transduction |
| D54 | Adenylate kinase 6 (Tarenaya hassleriana) | gi|729401807 | 40 | 3% | 33.44/6.26 | 25.61/5.7 | Amino acid metabolism |
| D55 | Triosephosphate isomerase (Fragaria vesca subsp. vesca) | gi|470143704 | 214 | 16% | 27.40/6.34 | 25.17/5.5 | Glycolysis and gluconeogenesis |
| D56 | LRR repeats and ubiquitin-like (Pyrus x bretschneideri) | gi|694387665 | 45 | 10% | 14.66/6.82 | 26.27/5.5 | Stress response |
| D57 | Cytokinesis related Sec1 protein like (Oryza sativa Japonica Group) | gi|47497438 | 77 | 5% | 27.33/5.45 | 27.15/4.9 | Cellular communication and signal transduction |
| D58 | Predicted protein (Physcomitrella patens subsp patens) | gi|168062920 | 56 | 1% | 170.77/6.11 | 21.65/5.3 | Unknown |
| D59 | Phosphoinositide 4-kinase (Theobroma cacao) | gi|590679345 | 36 | 1% | 66.09/5.85 | 22.31/5.5 | Stress response |
| D61 | BnaC07g10230D (Brassica napus) | gi|674938758 | 40 | 2% | 4.29/4.66 | 22.31/5.7 | Unknown |
| D62 | Maturase K (Parkinsonia aculeate) | gi|68052508 | 57 | 1% | 60.21/9.30 | 22.31/5.9 | Cellular communication and signal transduction |
| D63 | Pathogenesis related protein (Asparagus officinalis) | gi|510940 | 51 | 6% | 16.47/7.19 | 18.20/6.7 | Stress response |
| D64 | LRR receptor-like serine/threonine-protein kinase GSO2 (Aegilops tauschii) | gi|475555744 | 35 | 10% | 131.06/6.21 | 17.92/6.8 | Stress response |
| D65 | Phosphoethanolamine N- methyltransferase 1 (Cucumis sativus) | gi|449439453 | 36 | 2% | 57.15/5.35 | 14.56/6.9 | Fatty acid metabolism |
| D66 | Mediator of RNA polymerase II transcription subunit 17 (Jatropha curcas) | gi|802640310 | 31 | 1% | 74.57/5.74 | 14.56/6.2 | Photosynthesis and photorespiration |
| D67 | ABC transporter C family member 9 (Glycine max) | gi|356504494 | 46 | 1% | 17.01/7.33 | 15.40/5.5 | Cellular communication and signal transduction |
| D68 | Dihydroflavonol 4-reductase (Rosa hybrid cultivar) | gi|1332411 | 61 | 3% | 39.00/5.94 | 17.92/5.2 | Stress response |
| D69 | DNA-directed RNA polymerase subunit beta' (Mesostigma viride) | gi|13878754 | 42 | 1% | 76.73/9.15 | 14.84/4.6 | Cellular communication and signal transduction |
| D70 | RNA polymerase beta' subunit (Mesostigma viride) | gi|11466381 | 57 | 1% | 76.73/9.15 | 14.84/4.2 | Cellular communication and signal transduction |
Note: All spots in this table are statistically significant at p < 0.05. a Theoretical molecular weight and pI were from MASCOT database, b Experimental molecular weight and pI were from our gels.
Fig. 2.
Functional annotation of highly expressed proteins from leaves and pseudobulbs of Bulbophyllum morphologlorum Kraenzl. and Dendrobium Sonia Earsakul are shown as bar graphs.
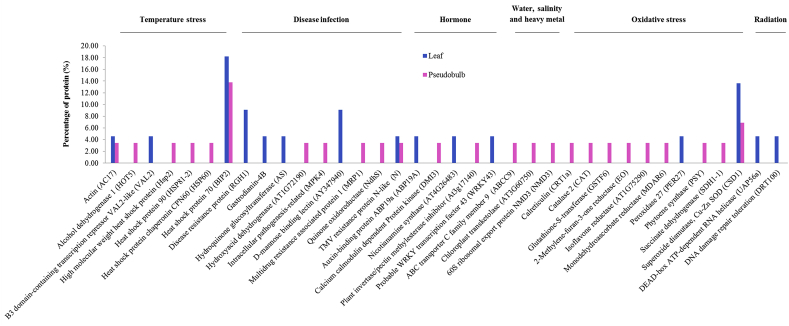
The thirty-six differentially expressed proteins from leaves and pseudobulbs of Bulbophyllum were mainly involved in stress activities and defense mechanisms and were classified into six sub-groups based on their role in responding to stress conditions as shown in Fig. 3, including temperature stress, disease infection, hormone, water, salinity and heavy metal, oxidative stress (enzymatic and non-enzymatic) and radiation. The stress response proteins associated with temperature stress and oxidative stress were most involved with heat shock protein 70 and superoxide dismutase (Cu–Zn), respectively. The gene names are also shown in addition to the protein names.
Fig. 3.
Percentage of the stress proteins associated with biotic stress (infection) and abiotic stress (temperature, hormone, water, salt, metal) in orchid leaves and pseudobulbs of Bulbophyllum morphologlorum Kraenzl.
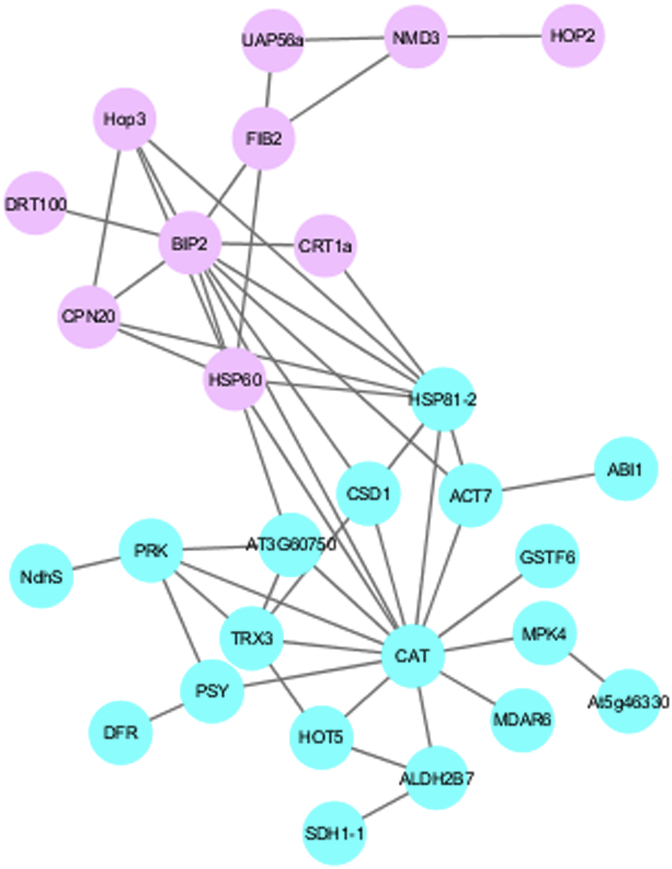
3.3. Protein-protein interaction network of stress response proteins from Bulbophyllum morphologlorum Kraenzl
The interaction network with the confidence score for the 36 stress response proteins from Bulbophyllum orchids was obtained by using the STRING database. The STRING was able to help predict the related functions of proteins obtained by accessing many free databases. Visualization of the network was performed by Cytoscape software. The clustering of biological processes was represented by different colors according to the related functions. The results for the network interaction (Fig. 4) indicate two clusters of high expression proteins, including proteins involved in response to temperature stress (ACT7, HOT5, CPN20, HSP81-2, HSP60, HOP2, HOP3 AND BIP2) and in oxidative stress (ALDH2B7, CRT1A, CAT, DFR. GSTF6, MDAR6, PSY, SDH1-1, CSD1 AND TRX3), respectively. Heat shock protein 70 (BIP2) and catalase 2 (CAT) were 2 proteins that showed core interaction with other proteins.
Fig. 4.
The interaction network of proteins involved in stress response of leaves and pseudobulbs of Bulbophyllum morphologlorum Kraenzl. The 2 major clusters are shown in pink and blue, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Validation of superoxide dismutase (Cu–Zn) by Native-PAGE and confirmed by LC/MS/MS
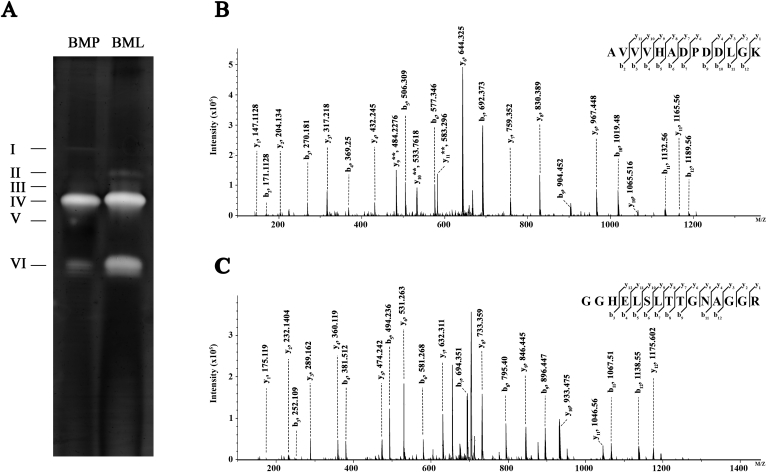
Extracted proteins from orchid, after ammonium sulfate precipitation, were subjected to native-PAGE, and incubated in riboflavin-NBT solution and treated with 25 W light exposure to induce superoxide synthesis. Six bands (band I, II, III, IV, V and VI) of superoxide dismutase activity were obtained from leaves (BML) and pseudobulbs (BMP) of Bulbophyllum orchid (Fig. 5A), All six bands were cut, digested by trypsin and analyzed by LC/MS/MS. Based on SWISSPROT database, Cu/Zn-SOD isoenzymes were only identified in band IV and VI as Cu/Zn-SOD 1 and Cu/Zn-SOD 2, respectively (Table 2). There were no significant differences in the activity of Cu/Zn-SOD 1 between BML and BMP. In contrast, the elevated Cu/Zn-SOD 2 activity was obviously detected in BML as compared to BMP. Representative MS/MS spectra of the sequence specific peptides for Cu/Zn-SOD 1 and Cu/Zn-SOD 2 were shown as AVVVHADPDDLGK and GGHELSLTTGNAGGR, respectively (Fig. 5B and C).
Fig. 5.
The Native PAGE of Superoxide dismutase isoenzyme activities in leaves and pseudobulbs of Bulbophyllum morphologlorum Kraenzl were shown (A). Representative MS/MS spectra of identified peptides from band IV (B) and VI (C) were AVVVHADPDDLGK of Cu/Zn-SOD 1 and GGHELSLTTGNAGGR of Cu/Zn-SOD 2, respectively.
Table 2.
Identification of protein bands (I-VI) from SOD activity native gels.
| Gel band | Identified protein (species) | Accession no. | Score | Peptide match | Unique seq. | pI/MW | Peptides |
|---|---|---|---|---|---|---|---|
| I | Enolase 1 (Zea mays) | ENO1_MAIZE | 548 | 1 | 1 | 5.20/48.03 | R.IEEELGDAAVYAGAK.F |
| II | Enolase 1 (Zea mays) | ENO1_MAIZE | 462 | 6 | 1 | 5.20/48.03 | K.IPLYQHIANLAGNK.T |
| K.EGLELLK.A | |||||||
| K.TCNALLLK.V | |||||||
| K.YNQLLR.I | |||||||
| R.IEEELGDAAVYAGAK.F | |||||||
| K.FRAPVEPY | |||||||
| III | Enolase 1 (Zea mays) | ENO1_MAIZE | 1542 | 8 | 1 | 5.20/48.03 | K.KIPLYQHIANLAGNK.T |
| K.IPLYQHIANLAGNK.T | |||||||
| K.EGLELLK.A | |||||||
| K.DKTYDLNFK.E | |||||||
| K.TCNALLLK.V | |||||||
| K.YNQLLR.I | |||||||
| R.IEEELGDAAVYAGAK.F | |||||||
| K.FRAPVEPY | |||||||
| IV | Superoxide dismutase [Cu–Zn] 1 | SODC1_ARATH | 126 | 2 | 2 | 5.54/15.25 | QIPLIGSGSIIGR.A |
| (Arabidopsis Thaliana) | R.AVVVHADPDDLGK.G | ||||||
| V | Enolase 1 (Zea mays) | ENO1_MAIZE | 136 | 3 | 1 | 5.20/48.03 | K.TCNALLLK.V |
| K.YNQLLR.I | |||||||
| R.IEEELGDAAVYAGAK.F | |||||||
| VI | Superoxide dismutase [Cu–Zn] 2 | SODC2_ARATH | 170 | 2 | 2 | 6.48/22.23 | R.AFVVHELKDDLGK.G |
| (Arabidopsis Thaliana) | K.GGHELSLTTGNAGGR.L |
4. Discussion
Antioxidant defenses are used to neutralize reactive oxygen and nitrogen species (RONS) which occur from both endogeneous and exogeneous processes to produce negative effects. When there is an imbalance between RONS and antioxidant defenses, oxidative stress occurs. During aging, the organ and tissue functions are progressively lost and involve oxidative stress related to many diseases such as cardiovascular disease, cancer, chronic kidney disease, neurodegenerative disease and etc [53] Natural antioxidants from plants have received much attention and have proven to be useful for preventing related oxidative stress diseases, thereby slowing ageing processes. Our results showed the Bulbophyllum ethanol crude extract had stronger exogenous antioxidant activities against free radical molecules than other orchid extracts. Usually, tolerant plants are reported to contain high antioxidants in order to protect from oxidative stress and keep maintaining a high amount under stress conditions.
The differential protein expression of phenol extracted proteins from leaves and pseudobulbs of Bulbophyllum morphologlorum Kraenzl. and Dendrobium Sonia Earsakul were compared by proteomic methods. A total of 233 proteins from selected spots were identified from Bulbophyllum and Dendrobium leaves and pseudobulbs. The predominant protein groups found in both orchids, particularly proteins in leaves and pseudobulbs of Bulbophyllum orchid, were involved in stress response. Interestingly, more than half of the annotated stress proteins highly expressed in Bulbophyllum were associated with temperature stress and oxidative stress response function. The protein-protein interaction network also showed clusters of antioxidant defense and heat shock proteins, respectively. Proteins from both leaves and pseudobulbs of Bulbophyllum that are involved in temperature stress are actin, alcohol dehydrogenase 1, B3 domain-containing transcription repressor, high molecular weight heat shock protein, heat shock protein 90, heat shock protein chaperonin CPN60 and heat shock protein 70 (HSP70). The most abundant protein identified in pseudobulbs of Bulbophyllum was HSP70. HSP70 proteins from leaf tissue play essential roles in various mechanisms, such as refolding protein conformations and protecting against harmful effects of abiotic stress [54,55]. Generally, a number of plant HSPs were detected in leaf and green tissues [56]. However, the expression of HSP70 was shown to be up-regulated in the mycorrhizal Bipinnula fimbriata roots cultured in heavy metal-polluted soil [43]. In addition, HSP90 has been reported to act as a co-chaperone, forming a chaperone complex with HSP70, which regulates a resistance gene in wheat [57] and Arabidopsis [58].
Proteins highly involved in oxidative stress response include calreticulin, catalase 2, glutathione-S-transferase, 2-methylene-furan-3-one reductase, isoflavone reductase, monodehydroascorbate reductase, peroxidase 27, phytoene synthase, succinate dehydrogenase and superoxide dismutase (Cu–Zn). The expression of enzymatic antioxidants from our work includes catalase 2, glutathione-S-transferase and Cu/Zn-SOD. One of the most important enzymatic antioxidants is SOD which showed high expression in both leaves and pseudobulbs of Bulbophyllum orchids, also detected by SOD activity staining on native-PAGE. LC/MS/MS was used to identify the type of SOD isoenzymes from activity bands, confirming the presence of Cu/Zn-SOD 1 and Cu/Zn-SOD 2. This is the first report on the Cu/Zn-SOD in the Bulbophyllum orchids. Our finding suggests that Cu/Zn-SOD 2 activity was highly elevated on Bulbophyllum leaves, as compared to Bulbophyllum pseudobulbs, whereas there were no differences in Cu/Zn-SOD 1 activity. In agreement with previous studies [59], Cu/Zn-SOD 2 is mainly localized in the plant chloroplast.
Antioxidants from natural sources have been shown to be good potential medicines for maintaining health, preventing oxidative stress related diseases and delaying the process of aging [60]. Antioxidants may also be used in cosmetics and food supplements [[61]]. Potato, legumes, berries, spinach, tomatoes, cherries, prunes, olives and citrus were identified to be non-enzymatic antioxidant sources [[62], [63]], as well as some orchids [64]. Studies on searching for new and safe endogenous antioxidants, of both enzymatic and non-enzymatic nature, from natural sources, is still of interest for use as supplements for antioxidant defense to prevent and manage oxidative stress related diseases. Our results suggest that Bulbophyllum orchid has the higher activity of Cu/Zn-SOD than of Dendrobium and can be a potential plant source for medicines and natural antioxidant supplements.
5. Conclusions
Proteomic study of the phenol extracted proteins of Bulbophyllum and Dendrobium led to distinctive and intense protein spots on 2-DE gel, allowing 233 proteins to be identified using LC-MS/MS analysis. Search for protein functions showed that the predominant annotated proteins in both orchids were stress response proteins, mostly associated with antioxidant and temperature which showed more variability in the Bulbophyllum than Dendrobium. Proteins related to stress conditions, such as heat shock proteins and Cu/Zn-SOD, showed particularly high expression in Bulbophyllum. The high expression of this antioxidant enzyme from Bulbophyllum morphologlorum Kraenzl was confirmed using superoxide dismutase activity staining on native-PAGE coupled with LC/MS/MS. The activity of Cu/Zn-SOD 2 was highly elevated on Bulbophyllum leaves as compared to Bulbophyllum pseudobulbs whereas there were no differences in Cu/Zn-SOD 1 activity. The results suggest that Bulbophyllum orchid can be a potential plant source for medicines and natural antioxidant supplements.
Author contributions
KB conducted the experiments. JS, CS and DC provided analytical tools and supervised 2DE and Image Master analysis. DC and CS identified proteins using LC-MS/MS. CW analyzed data using STRING, Cytoscape software and part of the mass spectrometry. PSH and SM conceived and designed experiment. PSH and CS analyzed data and wrote the manuscript. JS read and corrected the manuscript. All authors read and approved the final manuscript.
Ethical standards
Compliance with ethical standards.
Declaration of competing interest
The authors declared that they have no conflict of interest.
Acknowledgements
This work was supported by grants from the Chulabhorn Research Institute grant (BT 2011-01). The authors thank the Laboratory of Biotechnology and Laboratory of Biochemistry, Chulabhorn Research Institute for providing facilities.
Contributor Information
Pattana S. Huehne, Email: pattana@cri.or.th.
Kisana Bhinija, Email: kisana@cri.or.th.
Chantragan Srisomsap, Email: chantragan@cri.or.th.
Daranee Chokchaichamnankit, Email: daranee@cri.or.th.
Churat Weeraphan, Email: cweeraphan@gmail.com.
Jisnuson Svasti, Email: jisnuson@cri.or.th.
Skorn Mongkolsuk, Email: skorn@cri.or.th.
References
- 1.Sikora E., Cieślik E., Leszczyńska T., Filipiak-Florkiewicz A., Pisulewski P.M. The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chem. 2008;107(1):55–59. [Google Scholar]
- 2.Brewer M.S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011;10(4):221–247. [Google Scholar]
- 3.Beta T., Nam S., Dexter J.E., Sapirstein H.D. Phenolic content and antioxidant activity of pearled whet and roller-milled fractions. LWT- Food Sci. Tech. 2017;78:151–159. [Google Scholar]
- 4.Chen X., Liu S., Rao P., Bradshaw J., Weller R. Topical application of superoxide dismutase mediated by HIV-TAT peptide attenuates UVB-induced damages in human skin. Eur. J. Pharm. Biopharm. 2016;107:286–294. doi: 10.1016/j.ejpb.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Papa L., Manfredi G., Germain D. SOD1, an unexpected novel target for cancer therapy. Genes & Cancer. 2014;5(1–2):15–21. doi: 10.18632/genesandcancer.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defense grid. Alexandria J. Med. 2018;54:287–293. [Google Scholar]
- 7.Bridges S.M., Salin M.L. Distribution of iron-containing superoxide dismutase in vascular plants. Plant Physiol. 1981;68(2):275–278. doi: 10.1104/pp.68.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowler C., Van C.W., Van M.M., Izne D., Asada K. Superoxide dismutase in plants. Crit. Rev. Plant Sci. 1994;13(3):199–218. [Google Scholar]
- 9.Alscher R.G., Erturk N., Heath L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002;53(372):1331–1341. [PubMed] [Google Scholar]
- 10.Corpas F.J., Fernández-Ocaña A., Carreras A., Valderrama R., Luque F., Esteban F.J., Rodríguez-Serrano M., Chaki M., Pedrajas J.R., Sandalio L.M., del Río L.A., Barroso J.B. Plant Cell Physiol. 2006;47(7):984–994. doi: 10.1093/pcp/pcj071. [DOI] [PubMed] [Google Scholar]
- 11.Kliebenstein D.J., Monde R.A., Last R.L. Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998;118(2):637–650. doi: 10.1104/pp.118.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baek K.H., Skinner D.Z. Alteration of antioxidant enzyme gene expression during cold acclimation of near isogenic wheat lines. Plant Sci. 2003;165:1221–1227. doi: 10.1016/S0168-9452(03)00329-7. [DOI] [Google Scholar]
- 13.Moran J.F., James E.K., Rubio M.C., Sarath G., Klucas R.V., Becana M. Plant Physiol. 2003;133:773–782. doi: 10.1104/pp.103.023010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller A. Superoxide dismutases: ancient enzymes and new insights. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2012;586:585–595. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Río L.A., Sandalio L.M., Altomare D.A., Zilinskas B.A. Mitochondrial and peroxisomal manganese superoxide dismutase: differential expression during leaf senescence. J. Exp. Bot. 2003;54(384):923–933. doi: 10.1093/jxb/erg091. [DOI] [PubMed] [Google Scholar]
- 16.Lin C.T., Lin M.T., Chen Y.T., Shaw J.F. Subunit interaction enhances enzyme activity and stability of sweet potato cytosolic Cu/Zn superoxide dismutase purified by a His-tagged recombinant protein method. Plant Mol. Biol. 1995;28:303–311. doi: 10.1007/BF00020249. [DOI] [PubMed] [Google Scholar]
- 17.Kanematsu S., Asada K. Chloroplast and cytosol isozymes of Cu, Zn-superoxide dismutase: their characteristic amino acid sequences. Free Radic. Res. Commun. 1991;12–13:383–390. doi: 10.3109/10715769109145808. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa K., Kanematsu S., Asada K. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol. 1996;37(6):790–799. doi: 10.1093/oxfordjournals.pcp.a029096. [DOI] [PubMed] [Google Scholar]
- 19.Zafra A., Jiménez-Quesada M.J., Traverso J.A., Corpas F.J., Alché J.D. Peroxisomal localization of CuZn superoxide dismutase in the male reproductive tissues of the olive tree. Microsc. Microanal. 2012;18(S5):33–34. [Google Scholar]
- 20.Asensio A.C., Gil-Monreal M., Pires L., Gogorcena Y., Pedro María A., Moran J.F. Two Fe-superoxide dismutase families respond differently to stress and senescence in legumes. Plant Physiol. 2012;169:1253–1260. doi: 10.1016/j.jplph.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Yi Y., Xing F., Huang X., Chen H., Wang F. Medicinal plants of Bulbophyllum species in China. J. Trop. Subtropical Bot. 2005;13:65–69. [Google Scholar]
- 22.Boonkorkaew P. Public organization; Bangkok: 2010. List of Biodiversity Resource: Thai Orchid Database Biodiversity-Based Economic Development Office. [Google Scholar]
- 23.Gutiérrez R.M.P. Orchids: a review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res. 2010;4(8):592–638. [Google Scholar]
- 24.Hossain M.M. Therapeutic orchids: traditional uses and recent advances--an overview. Fitoterapia. 2011;82(2):102–140. doi: 10.1016/j.fitote.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Kong J.M., Goh N.K., Chia L.S., Chia T.F. Recent advances in traditional plant drugs and orchids. Acta Pharmacol. Sin. 2003;24(1):7–21. [PubMed] [Google Scholar]
- 26.Wu B., He S., Pan Y.J. New dihydrodibenzopin from Bulbophyllum kwangtungense. Planta Med. 2006;72(13):1244–1247. doi: 10.1055/s-2006-947200. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y.G., Xu J., Hong Y., Qing C., Zhang Y., Wang L., Liu Y., Wang J. Cytotoxic phenolics from Bulbophyllum odoratissimum. Food Chem. 2008;107(1):169–173. [Google Scholar]
- 28.Chand M.B., Paudel M.R., Pant B. The antioxidant activity of selected wild orchids of Nepal. J. Coast. Life Med. 2016;4(9):731–736. [Google Scholar]
- 29.Kalaiarasam A. Antioxidant properties of medicinal orchid in Indian Vegetation Flora. Biomed. Nurs. 2016;2(4):1–14. [Google Scholar]
- 30.Bhattachacharyya P., Kumaria S., Tandon P. High frequency regeneration protocol for Dendobrium nobile: a model tissue culture approach for propagation of medicinally important orchid species. South Afr. J. Bot. 2016;104:232–243. [Google Scholar]
- 31.Palama T.L., Fock I., Choi Y.H., Verpoorte R., Kodja H. Biological variation of Vanilla planifolia leaf metabolome. Phytochemistry. 2010;71(5–6):567–573. doi: 10.1016/j.phytochem.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Tan B.C., Chin C.F., Liddell S., Alderson P. Proteomic analysis of callus development in Vanilla planifolia Andrews. Plant Mol. Biol. Rep. 2013;31:1220–1229. [Google Scholar]
- 33.Chen G., Chen D., Wang T., Xu C., Li L. Analysis of the proteins related to browning in leaf culture of Phalaenopsis. Sci. Hortic. 2012;141:17–22. [Google Scholar]
- 34.Sedeek K.E.M., Qi W., Schauer M.A., Gupta A.K., Poveda L., Xu S., Liu Z.J., Grossniklaus U., Schiest F.P., Schluter P.M. Transcriptome and proteome data reveal candidate genes for pollinator attraction in sexually deceptive orchids. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0064621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Xu W., Chowdhury M.R., Jin F. Comparative proteomic analysis of labellum and inner lateral petals in Cymbidium ensifolium flowers. Int. J. Mol. Sci. 2014;15(11):19877–19897. doi: 10.3390/ijms151119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W., Yu H., Li T., Li L., Zhang G., Liu Z., Huang T., Zhang Y. Comparative proteomics analyses of pollination response in endangered orchid species Dendrobium chrysanthum. Int. J. Mol. Sci. 2017;18(12):2496. doi: 10.3390/ijms18122496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valadares R.B.S., Perotto S., Santos E., Lambais M. Proteome changes in Oncidium sphacelatum (Orchidaceae) at different trophic stages of symbiotic germination. Mycorrhiza. 2014;24(5):349–360. doi: 10.1007/s00572-013-0547-2. [DOI] [PubMed] [Google Scholar]
- 38.López-Chávez M.Y., Guillén-Navarro K., Bertolini V., Encarnación S., Hernández-Ortiz M., Guillén-Navarro K., Bertolini V., Magdalena S., Sánchez-Moreno I., Damon A. Proteomic and morphometric study of the in vitro interaction between Oncidium sphacelatum Lindl. (Orchidaceae) and thanatephorus sp. (ceratobasidiaceae) Mycorrhiza. 2016;26(5):353–365. doi: 10.1007/s00572-015-0676-x. [DOI] [PubMed] [Google Scholar]
- 39.Xu X.B., Ma X.Y., Lei H.H., Song H.M., Ying Q.C., Xu M.J., Liu S.B., Wang H.Z. Proteomic analysis reveals the mechanisms of Mycena dendrobii promoting transplantation survival and growth of tissue culture seedlings of Dendrobium officinale. J. Appl. Microbiol. 2015;18:1444–1455. doi: 10.1111/jam.12781. [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Liu S.S., Kohler A., Yan B., Luo H.M., Chen X.M., Guo S.X. iTRAQ and RNA-seq analyses provide new insights into regulation mechanism of symbiotic germination of Dendrobium officinale seeds (Orchidaceae) J. Proteome Res. 2017;16(6):2174–2187. doi: 10.1021/acs.jproteome.6b00999. [DOI] [PubMed] [Google Scholar]
- 41.Feng S., Jiao K., Guo H., Jiang M., Hao J., Wang H., Shen C. Succinyl-proteome profiling of Dendrobium officinale, an important traditional Chinese orchid herb, revealed involvement of succinylation in the glycolysis pathway. BMC Genom. 2017;18:598. doi: 10.1186/s12864-017-3978-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J.W., Chen X.D., Hu X.Y., Ma L., Zhang S.B. Comparative physiological and proteomic analyses reveal different adaptive strategies by Cymbidium sinense and C. tracyanum to drought. Planta. 2018;247:69–97. doi: 10.1007/s00425-017-2768-7. [DOI] [PubMed] [Google Scholar]
- 43.Herrera H., Valadares R., Oliveira G., Fuentes A., Almonacid L., do Nascimento S.V., Bashan Y., Arriagada C. Adaptation and tolerance mechanisms developed by mycorrhizal Bipinnula fimbriata plantlets (Orchidaceae) in a heavy metal-polluted ecosystem. Mycorrhiza. 2018;28(7):651–663. doi: 10.1007/s00572-018-0858-4. [DOI] [PubMed] [Google Scholar]
- 44.van Amsterdam F.T., Roveri A., Maiorino M., Ratti E., Ursini F. Lacidipine: a dihydropyridine calcium antagonist with antioxidant activity. Free Radic. Biol. Med. 1992;12(3):183–187. doi: 10.1016/0891-5849(92)90025-c. [DOI] [PubMed] [Google Scholar]
- 45.Hurkman W.J., Tanaka C.K. Solubilization of plant membrane proteins for analysis by two dimension gel electrophoresis. Plant Physiol. 1986;81:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpentier S.B., Witters E., Laukens K., Deckers P., Swennen R., Panis B. Preparation of protein extracts from recalcitrant plant tissues: an evaluation of different methods for two-dimensional gel electrophoresis analysis. Proteomics. 2005;5(10):2497–2507. doi: 10.1002/pmic.200401222. [DOI] [PubMed] [Google Scholar]
- 47.Koenig T., Menze B.H., Kirchner M., Monigatti F., Parker K.C., Patterson T., Steen J.J., Hamprecht F.A., Steen H. Robust prediction of the MASCOT score for an improved quality assessment in mass spectrometric proteomics. J. Proteome Res. 2008;7:3708–3717. doi: 10.1021/pr700859x. [DOI] [PubMed] [Google Scholar]
- 48.Kristiansen T.Z., Bunkenborg J., Gronborg M., Molina H., Thuluvath P.J., Argani P., Goggins M.G., Maitra A., Pandey A. A proteomic analysis of human bile. Mol. Cell. Proteomics. 2004;3(7):715–728. doi: 10.1074/mcp.M400015-MCP200. [DOI] [PubMed] [Google Scholar]
- 49.Huntley R.P., Sawford T., Mutowo-Muellenet P., Shypitsyna A., Bonilla C., Martin M.J., O'Donovan C. The Goa database: gene Ontology annotation updates for 2015. Nucleic Acids Res. 2014;34(D1):D1057–D1063. doi: 10.1093/nar/gku1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinkelmann K., Kempthorne O. second ed. John Wiley and sons; New Jersy: 2008. Design and Analysis of Experiments: Volume I, Introduction to Experimental Design. [Google Scholar]
- 51.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 52.Khanna-Chopra R., Sabarinath S. Heat-stable chloroplastic Cu/Zn superoxide dismutase in Chenopodium murale. Biochem. Biophys. Res. Commun. 2004;320(4):1187–1192. doi: 10.1016/j.bbrc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 53.Chandrasekaran A., Idelchick M.D.P.S., Melendez J.A. Redox control of senescence and age-related disease. Redox Biology. 2017;11:91–102. doi: 10.1016/j.redox.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnan M., Nguyen H.T., Burke J.J. Heat shock protein synthesis and thermal tolerance in wheat. Plant Physiol. 1989;90:140–145. doi: 10.1104/pp.90.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W., Vinocur B., Shoseyov O., Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9(5):244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Chen J., Gao T., Wan S., Zhang Y., Yang J., Yu Y., Wang W. Genome-wide identification, classification and expression analysis of the HSP gene superfamily in tea plant (Camellia sinensis) Int. J. Mol. Sci. 2018;19(9):2633. doi: 10.3390/ijms19092633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang G.F., Wei X., Fan R., Zhou H., Wang X., Yu C., Dong L., Dong Z., Wang X., Kang Z., Ling H., Shen Q.H., Wang D., Zhang X. Molecular analysis of common wheat genes encoding three types of cytosolic heat shock protein 90 (Hsp 90): functional involvement of cytosolic Hsp90s in the control of wheat seedling growth and disease resistance. New Phytol. 2011;191:418–431. doi: 10.1111/j.1469-8137.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 58.Noel L.D., Cagna G., Stuttmann J., Wirthmueller L., Betsuyaku S., Witte C.P., Bhat R., Pochon N., Colby T., Parker J.E. Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones. Plant Cell. 2007;19:4061–4076. doi: 10.1105/tpc.107.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zafra A., Castro A.J., Alché J. Identification of novel superoxide dismutase isoenzymes in the olive (Olea europaea L.) pollen. BMC Plant Biol. 2018;18:114. doi: 10.1186/s12870-018-1328-z. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caleja C., Barros L., Antonio A.L., Oliveira M.B.P., Ferreira I.C. A comparative study between natural and synthetic antioxidants: evaluation of their performance after incorporation into biscuits. Food Chem. 2017;216:342–346. doi: 10.1016/j.foodchem.2016.08.075. [DOI] [PubMed] [Google Scholar]
- 61.Hübner A.A., Neto A.V., Sobreira F., Pinto C., Dario M.F., Lourenço F.R., Baby A.R., Bacchi E.M. Phytochemistry, antioxidant activity, and sunscreen efficacy of hydroethanolic extract of Cabernet Sauvignon grape pomace (Vitis vinifera L.) Planta Med. 2016;82:S1–S381. S 01. [Google Scholar]
- 62.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Phcog. Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furata S., Nishiba Y., Suda I. Fluorometric assay for screening anti-oxidative activity of vegetables. J. Food Sci. 1997;62(3):526–528. [Google Scholar]
- 64.Chinsamy M., Finnie J.F., Van Staden J. Anti-inflammatory, antioxidant, anti-cholinesterase activity and mutagenicity of South African medicinal orchids. South Africa of Botany. 2014;91:88–98. [Google Scholar]