Abstract
Mechanisms leading to age-related reductions in bone formation and subsequent osteoporosis are still incompletely understood. We recently demonstrated that kynurenine (KYN), a tryptophan metabolite, accumulates in serum of aged mice and induces bone loss. Here, we report on novel mechanisms underlying KYN's detrimental effect on bone aging.
We show that KYN is increased with aging in murine bone marrow mesenchymal stem cells (BMSCs). KYN reduces bone formation via modulating levels of CXCL12 and its receptors as well as histone deacetylase 3 (Hdac3). BMSCs responded to KYN by significantly decreasing mRNA expression levels of CXCL12 and its cognate receptors, CXCR4 and ACKR3, as well as downregulating osteogenic gene RUNX2 expression, resulting in a significant inhibition in BMSCs osteogenic differentiation. KYN's effects on these targets occur by increasing regulatory miRNAs that target osteogenesis, specifically miR29b-1-5p.
Thus, KYN significantly upregulated the anti-osteogenic miRNA miR29b-1-5p in BMSCs, mimicking the up-regulation of miR-29b-1-5p in human and murine BMSCs with age. Direct inhibition of miR29b-1-5p by antagomirs rescued CXCL12 protein levels downregulated by KYN, while a miR29b-1-5p mimic further decreased CXCL12 levels. KYN also significantly downregulated mRNA levels of Hdac3, a target of miR-29b-1-5p, as well as its cofactor NCoR1. KYN is a ligand for the aryl hydrocarbon receptor (AhR). We hypothesized that AhR mediates KYN's effects in BMSCs. Indeed, AhR inhibitors (CH-223191 and 3′,4′-dimethoxyflavone [DMF]) partially rescued secreted CXCL12 protein levels in BMSCs treated with KYN. Importantly, we found that treatment with CXCL12, or transfection with an miR29b-1-5p antagomir, downregulated the AhR mRNA level, while transfection with miR29b-1-5p mimic significantly upregulated its level. Further, CXCL12 treatment downregulated IDO, an enzyme responsible for generating KYN. Our findings reveal novel molecular pathways involved in KYN's age-associated effects in the bone microenvironment that may be useful translational targets for treating osteoporosis.
Keywords: Kynurenine, AhR, CXCL12, Hdac3, miR-29b-1-5p
1. Introduction
Aging is accompanied by a universal, gradual decline in the integrity and functionality of organs and tissues. While every organism, organ, tissue, or cell population is affected by aging differently, the outcome is universally accompanied by a deterioration in well-being (López-Otín et al., 2013). The skeletal system, which is an active, responsive and dynamic tissue, is no exception (Lee et al., 2017). In fact, aging is the major risk factor for osteoporosis development (Infante and Rodriguez, 2018; Coipeau et al., 2009). The specific mechanisms underlying age-related bone loss remain incompletely defined, but are crucial to understand. Bone is a heterogeneous tissue made up of extracellular matrix (ECM) and different cell populations including mesenchymal stem cells (MSCs), pre-osteoblasts, osteoblasts, osteocytes, bone lining cells, osteoclasts, a complex vasculature system, and the hematopoietic system (Schönherr and Hausser, 2000; Manolagas, 2000; Manolagas and Parfitt, 2010; Schaffler et al., 2013; Matic et al., 2016; Kim et al., 2017). MSCs make up a small percentage of bone cells, but play a vital role in bone modeling and remodeling, fracture repair, and bone aging (da Silva Meirelles, 2006; Granero-Moltó et al., 2009). The balance between osteogenic and adipogenic MSC differentiation in bone marrow is tightly regulated and imperative for bone health (Coipeau et al., 2009; Stenderup, 2003; Tzeng et al., 2018). The literature reports a skewed differentiation pattern of bone marrow mesenchymal stem cells (BMSCs) with age, with both an overall decline in numbers and a shifting from osteogenic to more adipogenic differentiation (Lefterova et al., 2008; Kim and Ko, 2014; Chen et al., 2016). According to Infante et al. (Infante and Rodriguez, 2018), this shift from osteogenesis to adipogenesis in the aging bone marrow MSC population is orchestrated by a number of factors including transcription factors, miRNAs, autophagy levels, cell-extrinsic factors, and epigenetic modifications of DNA.
One such factor of interest is kynurenine (KYN), a primary active metabolite of tryptophan (TRP) that is produced by ROS-mediated oxidation and the action of indoleamine 2,3 dioxygenase-1 or -2 (IDO1 or IDO2) (Metz et al., 2014; Reyes Ocampo et al., 2014; Brooks et al., 2016; Lob et al., 2009; Merlo and Mandik-Nayak, 2016). A high KYN/TRP ratio is correlated with low bone mineral density (BMD) (Apalset et al., 2014). The essential amino acid TRP is crucial for anabolic BMSCs pathways, including those supporting cell proliferation and differentiation, but KYN inhibits these pathways (El Refaey et al., 2015). KYN is thought to mainly act through the aryl hydrocarbon receptor (AhR) (Mezrich et al., 2010; Kurz et al., 2011). Once activated, cytoplasmic AhR is transported into the nucleus where it binds the aryl hydrocarbon receptor nuclear translocator (ARNT) and acts as a transcription factor for a wide array of genes (Murray et al., 2014; Beischlag et al., 2008). We recently tested the hypothesis that a high KYN/TRP ratio mimics the aged bone environment and leads to decreased bone mass in mice, showing that a low TRP/high KYN diet leads to significant bone loss and high marrow adiposity in mice, resembling an aged bone phenotype (Hamrick et al., 2006; Refaey et al., 2017). We also recently reported elevated KYN concentrations in the bone marrow of aged humans (Kim et al., 2019).
KYN's negative effects on the skeletal system may be mediated by cytokine and epigenetic factors that impact BMSCs. Stromal cell-derived factor 1 (SDF-1 or CXCL12) (Carbone et al., 2017; Herberg et al., 2013; Mortensen and Hill, 2015) is implicated in myriad stem cells functions including cellular proliferation, differentiation, migration, and homing to niche sites (Cheng et al., 2017; Bromage et al., 2014; Yang et al., 2018a; Li et al., 2007; Broxmeyer et al., 2005). CXCL12 has multiple splice variants, the most common of which are CXCL12α and CXCL12β (Yu et al., 2006). Both isoforms act mainly through CXCR4 and CXCR7 (i.e., atypical chemokine receptor 3 [ACKR3]) receptors to achieve various downstream effects (Reid et al., 2018; Quinn et al., 2018; Sanchez-Martin et al., 2012). We previously reported that CXCL12 levels decline in bone marrow interstitial fluid with aging, while increasing in the peripheral circulation, which correlates with deteriorating bone health in older subjects (Carbone et al., 2017; Periyasamy-Thandavan et al., 2018). Our group and others have reported that CXCL12 is important for osteogenic differentiation primarally by augmenting the pro-osteogenic effects of BMP-2. CXCR4 signaling is required for the activation of the BMP-2R and increases BMP-2 mediated osteogenesis (Yang et al., 2018a; Herberg et al., 2014a; Herberg et al., 2015). Additionally, targeted deletion of CXCL12 BMSCs results in reduced trabecular bone content and increased bone marrow adiposity (Tzeng et al., 2018). Regarding epigenetic factors, histone deacetylases (Hdacs) are integrally involved in bone homeostasis. Hdacs remove acetyl groups from lysine residues in histones, which changes chromatin structure and in turn affects gene expression altering many signal transduction pathways. Histone deacetylase 3 (Hdac3) is expressed in osteoblasts and plays a critical role in bone development by binding to the osteogenic transcription factor Runx2 to regulate osteoblastic gene expression (Bradley et al., 2011). Conditional deletion of Hdac3 in osteoprogenitor cells (Hdac3-CKO) causes a reduction in osteoblastic activity and produces a low bone mass phenotype with increased BM adipogenesis (Trivedi et al., 2007). Another epigenetic factor that influences BMSC behavior is microRNAs (miRNAs) via effects on cell cycle, apoptosis, proliferation, stem cell maintenance, and differentiation (Khordadmehr et al., 2019; Cheng et al., 2005; Goldar et al., 2015) (Farina et al., 2014) (Wang et al., 2010; Skog et al., 2008; Valadi et al., 2007). MiRNAs are ~20 nucleotide RNAs that bind to mRNAs and post-transcriptionally regulate gene expression of the targeted mRNAs. Pre-miRNAs are processed to generate two partially complementary strands, the normally active guide strand and the passenger strand that is typically degraded and inactive. Several miRNAs play an important role in controlling osteogenic and osteoclastogenic differentiation in the bone marrow, making them valuable new diagnostic biomarkers for bone health (Kureel et al., 2014; Vimalraj et al., 2014; Lee et al., 2013). We have identified one of these as the passenger strand of the key osteogenic microRNA-29b-1 (miR-29b-1-5p). This novel passenger strand increases with age in human and murine BMSCs (Hill et al., 2016; Periyasamy-Thandavan et al., 2013; Baglio et al., 2013; Lee et al., 2016; Suh et al., 2013a; Shi et al., 2016; Li et al., 2009; William and Hill, 2016). The guide strand (miR-29b-1-3p) has been extensively documented to be critical in different stages of bone formation and fracture healing (Lee et al., 2016; Li et al., 2009; Suh et al., 2013b). Of interest miR-29b-1-3p (and other miR-29 family guide strands) target and suppress the CXCL12 regulator dipeptidyl peptidase-4 (DPP4) (Shi et al., 2016). Therefore, this miRNA is predicted to be central to local bone marrow DPP4 activity and CXCL12 ligand and proteolytic isoform levels. Current studies on miR-29b have focused on the guide strand miR-29b-1-3p (previously miR-29b-1-1) (Li et al., 2009). In contrast, the passenger strand miR-29b-1-5p (previously miR-29b-1-1*) has largely been considered to be a non-functional byproduct of the miR biogenesis process. However, our group has found miR-29b-1-5p to accumulate with aging in human and murine BMSCs (Betel et al., 2010). Further, when it is expressed in BMSCs it can induce aging-like actions targeting osteogenic genes like the CXCL12 axis, and based on in silico analysis, also Hdac3 (Betel et al., 2010).
In this paper, we describe three inter-related mechanisms (CXCL12, Hdac3, miR-29b-1-5p) through which KYN affects BMSCs in aging. These pathways are interconnected, and the crosstalk between them appears to be complex and highly regulated. It also appears that KYN's effect on CXCL12, miR-29b-1, and Hdac3 are all mediated through binding and nuclear translocation of the xenogeneic AhR.
2. Materials and methods
2.1. Animals
C57BL/6J mice were either provided by the National Institute on Aging (Bethesda, MD, USA) aged rodent colony or purchased from Jackson Laboratories (Bar Harbor, ME, USA). Animals were maintained at Augusta University in the Division of Laboratory Animal Services Facility. All aspects of the animal research were conducted in accordance with the guidelines set by Augusta University Institutional Animal Care and Use Committee (AU-IACUC) under AU-IACUC approved Animal Use Protocols. Mice were maintained on a standard 12-h light – 12 h dark protocol and permitted water and food ad libitum.
2.2. Isolation and culture of murine BMSCs
Murine BMSCs were derived from 3, 6, 11, 18, and 27-month-old male C57BL/6J mice at the Augusta University Stem Cell Core Facility. The BMSC isolation process, as well as the MSC characterization and multi-lineage potential (osteogenic, adipogenic and myogenic) have been described previously (Herberg et al., 2013; Zhang et al., 2008). In brief, six to eight mice of each age were euthanized by CO2 overdose followed by thoracotomy. Whole bone marrow aspirates were flushed from femora and tibiae and BMSCs isolated by negative immunodepletion using magnetic microbeads conjugated to anti-mouse CD11b (#558013) and CD45R/B220 (#551513) (BD Biosciences Pharmingen, San Diego, CA, USA), CD11c, and plasmacytoid dendritic cell antigen (PDCA)-1 (#130-092-283 Miltenyi Biotec, Auburn, CA) followed by positive immunoselection using anti-stem cell antigen (Sca)-1 microbeads (#130-092-529 Miltenyi Biotec, Auburn, CA), according to the manufacturer's recommendations. The enriched single-age pooled BMSC populations were maintained in Dulbecco's Modified Eagle Medium (#10-014-CM DMEM; Cellgro, Mediatech, Manassas, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (#S11150 Atlanta Biologicals, Lawrenceville, GA, USA) and used at 60–70% confluency.
To measure mRNA levels of some markers without culturing or plastic adherence, directly isolated BMSCs were derived from young adult (6–8 months) and geriatric (22–24 month-old) female C57BL/6J mice. Long bones (i.e., humeri, femora, tibiae) were dissected from mice and placed into mBMSC medium consisting of alpha MEM (#41061 Gibco), 20% FBS, 1% antibiotic/antimycotic, and 1% non-essential amino acids [NEAA] (#11140050 Gibco), on ice. BMSCs were then extracted by flushing marrow and filtering through 70 μm cell strainers.
2.3. Isolation and culture of human BMSCs
A direct-isolation procedure was used to rapidly capture human BMSCs directly from bone. Bone marrow aspirates from the proximal tibia (knee replacement surgery), proximal femur (hip replacement surgery) or iliac crest (spinal fusion surgery) were collected as orthopaedic surgical waste under IRB approval in EDTA blood collection tubes. The bone marrow aspirates were then run over a Ficoll gradient to collect the buffy coat within 30 min of collection. CD271 positive (+) hMSCs were then isolated from the nucleated cell layer using CD271 MicroBead Kits (#130-092-283 Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol to obtain a highly enriched BMSC population within 2 h of bone marrow aspiration. The cells were counted and frozen, or cultured for 1–2 passages prior to cryopreservation for in vitro cell culture studies. The isolated cells were first confirmed by FACS analysis to be positive for CD73, CD90 and CD105 and negative for CD34, CD45 and CD11b. Corresponding isotype control antibodies were used when cells were sorted according to criteria set by the International Society for Cellular Therapy (ISCT) to define hMSCs (Cao et al., 2015).
Isolating CD271+ cells allows for the direct isolation of BMSCs from orthopaedic patient's bone marrow aspirates within 2 h of collection. This is in contrast to the standard BMSCs isolation process that includes 1–2 day plastic adhesion followed by negative and positive selection and an additional 2–3 week passaging. This is now a standard rapid MSCs isolation method that both reduces the complexity of isolation and maintains cell expression profiles as close to in vivo as possible (Kuçi et al., 2019; Álvarez-Viejo, 2015; Cuthbert et al., 2015; Cox et al., 2012; Poloni et al., 2009).
The isolated cells were tested for multilineage properties, i.e., their capacities for osteogenic, adipogenic and chondrogenic differentiation and the expression levels of key genes (qRT-PCR analysis) regulating these differentiation pathways, including Runx2, Osx and ALP (osteogenic), PPARγ, and C/EBPβ and -α (adipogenic), and Sox-9, type I and type II collagen (chondrogenic). Colony-forming unit-fibroblast (CFU-f) assays were performed to calculate the population doubling capacity. CD271+ MSCs were isolated directly from bone marrow aspirates, washed with standard culture medium composed of DMEM medium (#10-014-CM Corning), 1% antibiotics antimycotics (#15240-062 AA; Invitrogen) and 15% FBS, with low glucose (1 g/l), transferred to 100 mm culture dish and incubated at 37 °C in a humidified atmosphere in 5% carbon dioxide (CO2). After 24 h, the medium with non-adherent cells is removed, and the adherent cells were carefully washed in DPBS (Dulbecco's phosphate-buffered saline) (#SH30028 HyClone) and further expanded in fresh culture medium. Culture-expanded CD271+ MSCs of passage 1–2 were used for in vitro studies.
2.4. KYN preparation and doses
Human and murine BMSCs were treated with different doses (10, 50, and 200 μM) of L-kynurenine (#K8625 Sigma-Aldrich). Different KYN doses were freshly prepared before administration. Previous data from the literature shows endogenously present KYN levels in the culture media of different cell types is highly variable, ranging from 5 to 60 μM and typically increases with time in culture (Opitz et al., 2011; Yamamoto et al., 2019). Indeed, utilizing an ELISA kit (#E4629 Biovision, Milpitas, CA) we identified that commercial DMEM media (#10-014-CM Corning) itself contains approximately 3–5 μM of KYN (unpublished data). Other researchers have consistently reported various specific dose-dependent effects using wide ranges of KYN doses (10–300 μM) (Kawasaki et al., 2014; Xiang et al., 2019). Moreover, a recently published paper reported that different doses of KYN (30–500 μM) significantly increased migration in 95D lung cancer cells (Duan et al., 2019). Taking all these different caveats in consideration, we wanted our data to represent three levels of Kyn: 10 μM (low dose close to that of cell-free media and serum levels), 50 μM (medium dose close to the previously reported conditioned media levels secreted by various cell types in culture), and 200 μM (high dose that exceeds the previously reported in vitro levels, falls within the range of KYN doses previously used in literature, and is similar to the in vivo dose used in our previous Kyn studies (Refaey et al., 2017).
2.5. Osteogenic differentiation assay
The ability of culture-expanded MSCs to differentiate into the osteogenic lineage was validated according to earlier described methods (Herberg et al., 2013; Gregory et al., 2004). In brief, cells were plated in 12-well plates at 50000 cells/cm2 and cultured in DMEM for 24 h. Culture medium was then aspirated and replaced with StemXVivo Osteogenic/Adipogenic Base Media (#CCM007 R&D Systems) supplemented with StemXVivo Human Osteogenic Supplement (#CCM008 R&D Systems). Treatment-containing medium was replaced 2 times per week. The early osteogenic differentiation marker, Alkaline Phosphatase, was assessed in cell culture media after 7 days using an Alkaline Phosphatase Assay Kit (#ab83369 Abcam). After 3 weeks, osteogenic differentiation was assessed by staining with Alizarin-Red Staining Solution; (#TMS-008-C Millipore Sigma). The cells were fixed with 10% formalin for 20 min at room temperature (RT) and stained with Alizarin-Red Staining Solution for 20 min at RT. Stained monolayers were visualized by phase-contrast microscopy using an inverted microscope (Nikon, Melville, NY). Differentiation was quantified as previously described (Ripoll and Bunnell, 2009). In brief, cells were destained using 10% cetylpyridinium chloride (#855561 Sigma-Aldrich) and collected samples analyzed using a microplate reader at 570 nm.
2.6. Cell density assay
To determine whether KYN affected BMSCs density, we utilized a Crystal violet Assay Kit (#ab232855 Abcam) according to the manufacturer's protocol. In brief, BMSCs were plated in 96 well plates at 5000 cells/well and cultured in DMEM for 24 h. Culture medium was then aspirated and replaced with StemXVivo Osteogenic/Adipogenic Base Media (#CCM007 R&D Systems) supplemented with StemXVivo Human Osteogenic Supplement (#CCM008 R&D Systems) with or without different doses of KYN (10, 50, 200 μM). After 3 days, the culture media was removed and the cells were washed and stained with the Crystal Violet Staining solution for 20 min at RT. Then, the staining solution was removed and the remaining stain was solubilized for 20 min with the Solubilization Solution. Finally, the Crystal Violet stain was quantified using a microplate reader at 595 nm.
2.7. Western blotting
Whole cell lysates of BMSCs were prepared in RIPA lysis and extraction buffer (#89901 ThermoFisher Scientific) containing protease and phosphatase inhibitor cocktail (Millipore Sigma). Protein concentration was determined using Pierce BCA Protein Assay Kit (#23225 ThermoFisher Scientific) and equal amounts (30 μg) of protein lysates were subjected to SDS-PAGE using gradient 4–12% NuPAGE Bis-Tris gels (#NP0321 Invitrogen) and transferred to 0.2 mm nitrocellulose membranes using Power Blotter Select Transfer Stacks (#PB3310 ThermoFisher Scientific).
Membranes were blocked with 5% Bovine Serum Albumin (#A2153 Sigma-Aldrich) in TBST. Osteogenic and Histone deacetylation markers were detected using specific primary antibodies (Table 1). Bound antibodies were visualized with Pierce ECL detection system (#32106 ThermoFisher Scientific) on Amersham Imager 600 (GE Healthcare, Pittsburgh, PA). The intensity of immunoreactive bands was quantified using Image Lab (Bio-Rad, Hercules, CA).
Table 1.
Specific antibodies used for WB protein analysis.
| Protein | Antibody company | Catalog no. |
|---|---|---|
| β-Actin | Sigma-Aldrich | A2228 |
| Hdac3 | Abcam | Ab7030 |
| H4 | Cell Signaling | 2935P |
| Acetyl-H4 | Cell Signaling | 8647P |
| Runx2 | Abcam | ab76956 |
2.8. Quantitative real time polymerase chain reaction (qRT-PCR)
The levels of 18S, CXCL12, CXCR4, ACKR3, IDO-1 and AhR mRNA in human cells undergoing various treatments were assessed using quantitative real-time PCR and TaqMan reagents. Total RNA was isolated from BMSCs and then purified using the RNAeasy kit (#74106 Qiagen, SantaClarita, CA). Purified total RNA was reverse transcribed with High-Capacity cDNA Kits (#4368814 USA – Applied Biosystems). PCR reactions were performed using TaqMan Fast Advanced Master Mix (#4444556 USA – Applied Biosystems). The pre-formulated assay primers used in this study were individual gene-expression assays (#A25576 Applied Biosystems) (Table 2). 18S was used as the endogenous control for mRNA. The target gene was normalized using the endogenous control to calculate ∆∆Ct values. For real-time PCR, 2 μL of the cDNA sample (100 ng/reaction), 10 μL TaqMan Fast Advanced Master Mix, and 1 μL primer assay were mixed with 7 μL nuclease-free water. All PCR reactions were performed in duplicate.
Table 2.
TaqMan mRNA and miRNA pre-formulated gene specific primer assay IDs.
| Gene | Assay ID/cat. no. |
|---|---|
| 18S | Mm03928990 |
| CXCL12 | Mm00445553_m1 |
| CXCR4 | Mm1996749 |
| ACKR3 | Mm02619632_s1 |
| IDO-1 | Mm00492590_m1 |
| AhR | Mm00478932_m1 |
| miR-29b-1-5p | Mmu-482721_mir |
| miR-29b-1-3p | Mmu481300_mir |
| U6 snRNA | Cat. no. 4427975 |
| Hs-RNU6 | Cat. no. MS00033740 |
Gapdh, Hdac3, Hsd11b1, NcoR1, and RUNX2 mRNA levels were assessed using quantitative real-time PCR and SYBR Green reagents. Mouse cells cultured in 6-well plates for 7 days were lysed using TRIzol reagent (#15596-018 Invitrogen), and total RNA was extracted using protocols as previously described. Purified mRNA extracts were reverse transcribed (RT; Bio-Rad C1000 Thermal Cycler, California, USA) to cDNA using commercially available SuperScript III First-Strand Synthesis RT reagents (#11752-050 Invitrogen). Gene expression was determined by real-time semi-quantitative PCR analysis (qPCR; Bio-Rad CFX Connect PCR System, California, USA) using 37.5 ng of cDNA per 15 μL well volume with QuantaBio PerfeCTa SYBR Green Supermix (#95054-500 VWR) and SYBR Green gene specific primers (Table 3). Gene expression levels were quantified using the comparative threshold cycle (2-∆∆Ct) method. Transcript levels were normalized to the reference gene Gapdh.
Table 3.
SYBR Green mRNA gene specific primer sequences.
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| Gapdh | GGGAAGCCCATCACCATCTT | GCCTCACCCCATTTGATGTT |
| Hdac3 | GCATTCGAGGACATGGGGAA | TTTCGGACAGTGTAGCCACC |
| Hsd11b1 | ACTCAGACCTCGCTGTCTCT | TGGGTCATTTTCCCAGCCAA |
| NcoR1 | TTATCGGAGCCACCTACCCA | CAGGTAAGCAGCAGCAGGAT |
| RUNX2 | GGCACAGACAGAAGCTTGATGA | GAATGCGCCCTAAATCACTGA |
Murine miR-29b-1-5p and miR-29b-1-3p levels were assayed using the TaqMan reagents mentioned above and the results were normalized to U6 snRNA (USA – Applied Biosystems). For human miRNA analysis, the expression levels of miR-29b-1-5p and miR-29b-1-3p were measured by miScript qPCR and were normalized to RNU-6 expression (Qiagen, SantaClarita, CA). The catalog numbers for different TaqMan and SYBR green primers are listed in Table 2, Table 3, respectively.
2.9. KYN enzyme linked Immunosorbent assay (ELISA)
Murine bone marrow derived mesenchymal stem cells (BMSCs) of 6 and 18 month old were seeded separately in 6-well flat bottom plates (100,000 cells/well). The cells were grown in DMEM media supplemented with 10% fetal bovine serum and 1× of Antibiotic/Antimycotic Solution (#15240062 Invitrogen) at 37 °C, 5% CO2 incubator. After 24 h and 48 h of incubation, a media sample was collected from each cell line, centrifuged for 20 min at 1000 ×g at 4 °C, transferred to new tube and stored in −80 °C until used. Kynurenine (KYN) ELISA Kit (#E4629 Biovision, Milpitas, CA) was used to measure kynurenine concentration in media samples by following the manufacturer's instructions.
2.10. CXCL12α enzyme linked Immunosorbent assay (ELISA)
CXCL12α was measured as described previously (Carbone et al., 2017; Herberg et al., 2013). Briefly, the CXCL12 capture antibody (R&D Systems, Minneapolis, MN) was incubated in sodium bicarbonate buffer overnight. Plates were blocked for 2 h with 1% bovine serum albumin (BSA) in PBS next day. Murine CXCL12α standards and samples were incubated for 2 h before incubating with the biotinylated anti-CXCL12α detection antibody (#MAB350 R&D Systems). Streptavidin-horseradish peroxidase (HRP) (#DY998 R&D Systems) was incubated for 20 min followed by the substrate reagent (#DY999 R&D Systems) for 20 min. 2 N sulfuric acid was added to stop the enzymatic color reaction and absorbance was read at 450 nm. CXCL12α protein expression was calculated using standard curves and normalized to total protein, which was quantified using the Pierce BCA Protein Assay Kit. For age-related CXCL12 plasma and bone marrow interstitial fluid levels one set of male C57BL/6 mice from six different age groups (3, 6, 12, 18, 24, and 29 months of age), 10 mice per age group, were obtained from the aged rodent colony at the National Institute on Aging. Our sample is therefore a cross-sectional as opposed to a longitudinal one. Mice were housed individually and all were fed ad libitum on NIH31 diet. Blood was collected via cardiac puncture once mice were euthanized, as per the IACUC protocol, in EDTA tubes which were centrifuged as previously described to obtain plasma, and stored frozen at −80 °C. Humeri and tibiae were collected to isolate bone marrow interstitial fluid via flushing of the bone marrow space as previously described (Herberg et al., 2013; Herberg et al., 2014b; Ding et al., 2007).
2.11. Mimic and inhibitor miRNA transfection and AhR inhibition
BMSCs were transfected with either a mimic or inhibitor (antagomir) for miR-29b-1-5p (Cat #YM00471910 and #YI04101505 Qiagen, SantaClarita, CA) according to the manufacturer's protocol. The recommended controls for miR-29b-1-5p mimic and inhibitor were used (Cat #YM00479902 and Cat #YI00199006 In brief, cells were seeded at 25,000–30,000 cells/well in a 24-well plate in 500 μl of an appropriate culture medium containing 10% serum and no antibiotics. For 1–3 h until transfection, cells were incubated under normal growth conditions (typically 37 °C and 5% CO2). miRNA mimics or inhibitors were resuspended prior to transfection in RNase-free water to achieve the recommended concentration of 20 μM. The miRNA inhibitor and mimic were diluted in Opti-MEM media to give a final miRNA inhibitor concentration of 100 nM and final miRNA mimic concentration of 1 nM for normal transfection experiments and 5 nM for 3′-UTR luciferase assays. HiPerFect transfection reagent (#301705 Qiagen, SantaClarita, CA) was added to the diluted miRNA mimic/inhibitor and mixed by vortexing. Then, the samples were incubated for 5–10 min at room temperature (15–25 °C) to allow the formation of transfection complexes. The complexes were added drop-wise onto the cells. BMSCs were incubated with the transfection complexes under their normal growth conditions for 6 h, and then the transfection media was removed and replaced with serum-free media containing the desired treatment. Cell culture media samples were collected for analysis, and cells were lysed and mRNA collected at the end of the incubation period. Negative control miR inhibitor was used as a negative control for miR-29b-1-5p inhibitor, and AllStars Hs Cell Death Control siRNA was used as a negative control for miR-29b-1-5p mimic. For AhR inhibition, AhR antagonist, CH-223191 (#C8124 Sigma-Aldrich) was dissolved in DMSO to make a 2 mg/mL stock solution, and a final concentration of 2 μg/mL was added to BMSCs culture medium (Asai et al., 2018; Yang et al., 2018b). A second AhR antagonist, 3′,4′-dimethoxyflavone (DMF) (#D6571 Sigma-Aldrich), was also used (10 μM).
2.12. Luciferase assay
Culture-expanded human BMSCs were co-transfected with a either wild type or mutated miTarget miRNA 3′-UTR luciferase functional reporter plasmid for CXCL12 or Hdac3 (#HmiT088617-MT06 and #HmiT115117-MT06 GeneCopeia, Rockville, MD) and either miR-29b-1-5p mimic or mimic control (#YM00471910 and #YM00479902 Qiagen, SantaClarita, CA) using Lipofectamine 3000 reagent (#L3000015 ThermoFisher Scientific, Waltham, MA). Mutated plasmids for CXCL12 (at 3 predicted target sites) and Hdac3 (at one predicted target site) were custom made and purchased from Genecopeia (Rockville, MD). The predicted binding sites for miR-29b-1-5p were determined using the prediction software and database MiRanda (Betel et al., 2010). Dual luciferase activity of the CXCL12 and Hdac3 reporter plasmids were measured 24 h after transfection using Luc-Pair™ Duo-Luciferase High Sensitivity Assay Kit (#LF004 GeneCopeia, Rockville, MD) according to the manufacturer's protocol, and compared to non-targeting miR-transfected controls. The Duo-Luciferase HS Assay Kit was used because it eliminates the need for separate controls to determine if differences are due to differential vector uptake and to normalize the outcomes, e.g. the use of beta-galactosidase which is typically used for normalization to luciferase counts. This is done by including Firefly luciferase (FLuc) and Renilla luciferase (RLuc), where the RLuc signal is used as a control for how much construct was taken into the cell while FLuc signal is used for measuring transcriptional activation. The final signal is expressed as FLuc to RLuc ration to compensate for different transfection efficiencies.
2.13. Statistical analysis
Experiments were performed at least three independent times. Data are expressed as means ± SD unless stated otherwise. Data were analyzed using GraphPad Prism 7.0 software (GraphPad Software Inc., La Jolla, CA, USA). Student t-test was used for comparisons between two groups and analysis of variance (ANOVA) followed by Tukey's multiple comparison test was used for comparisons between 3 or more groups. Null hypotheses were rejected at the 0.05 level. Statistical significance was determined and shown in figures and figure legends.
3. Results
3.1. CXCL12 axis is downregulated with both aging and KYN treatment in murine and human BMSCs
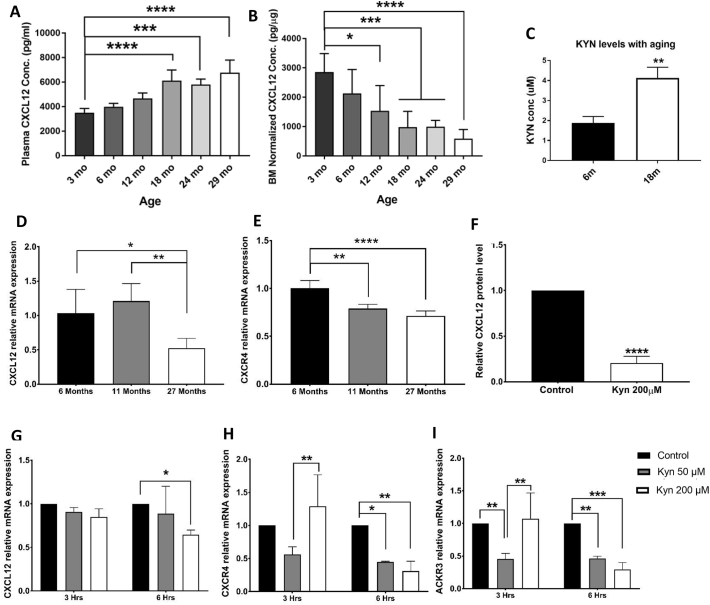
We previously showed that CXCL12 is essential in BMSC osteogenesis (Herberg et al., 2013; Herberg et al., 2015). We also established that KYN contributes to skeletal aging mechanisms and affects the balance between osteogenic and adipogenic differentiation in BMSCs (Refaey et al., 2017). For our initial experiment, we compared the effects of both aging and KYN on the CXCL12 axis. First, we utilized an established ELISA to measure CXCL12 protein levels in murine samples from bone marrow interstitial fluid and plasma at different ages of C57BL/6J mice (n = 10 per group). Levels of CXCL12 significantly declined with aging in bone marrow interstitial fluid starting around 12 months of age, while significantly rising in the peripheral circulation after that same age (Figs. 1A, B). The rise in plasma CXCL12 was similar to what we previously observed in human plasma (Carbone et al., 2017), while the decrease in CXCL12 levels in BM interstitial fluid in aged subjects was similar to our previous BM results in mice (Periyasamy-Thandavan et al., 2018). Interestingly, in our in vitro system, BMSCs isolated from 18 months old mice showed significantly increased levels of KYN in their cell culture media compared to BMSCs isolated from 6 months old mice after 48 h of incubation (Fig. 1C).
Fig. 1.
Aging and kynurenine downregulate CXCL12 axis. (A) CXCl12 protein levels in murine plasma from 3, 6, 12, 18, 24, and 29 months-old mice (n = 10). (B) CXCl12 protein levels in murine bone marrow interstitial fluid from 3, 6, 12, 18, 24, and 29 months-old mice (n = 10 mice per group). (C) Kynurenine level (μM) in cell culture media of BMSCs isolated from 6 and 18 months old mice (n = 3). (D,E) mRNA levels of CXCL12 (D) and CXCR4 (E) in BMSCs isolated from 6, 11, and 27 months-old mice (n = 4 for 6 and 11 months old mice and n = 7 for 27 months old mice). (F) CXCL12 protein levels in BMSCs isolated from pooled 6 months-old mice after treatment with 200 μM kynurenine for 48 h (n = 4). (G-I) mRNA levels of CXCL12 (F), and CXCR4 (G) and ACKR3 (H) after treatment with 50 and 200 μM kynurenine for 6 h (n = 3). Data presented as mean ± SD. Data analysis was done using one way ANOVA for panels A, B, D, E, F, G, H, and I, and using unpaired t-test for panel C. Data presented as mean ± SD. *P < 0.05, **P < 0.01, ***p < 0.001, ****p < 0.0001.
We also assessed how mRNA expression of CXCL12 and its main receptor, CXCR4, changed in BMSCs with aging. We used BMSCs isolated from mice at the ages of 6, 11, and 27 months. These ages correspond to adult (6 months), adult near the peak accumulation of bone mass prior to bone loss (11 months), which is prior to significant changes in CXCL12 protein levels in the BM interstitial fluid, and elderly (27 months), at which time there is significant age-associated bone loss and a significant reduction in BM interstitial fluid CXCL12 (Hamrick et al., 2006; Refaey et al., 2017). CXCL12 mRNA levels significantly declined to 50% at 27 months, while CXCR4 mRNA levels were significantly reduced earlier at both 11 and 27 months of age compared to 6 month-old mice (Figs. 1D, E).
Since KYN levels are linked to aging, we then tested if KYN treatment of BMSCs had a similar effect. BMSCs isolated from 3-, 6-, and 18-month-old mice showed significantly lower CXCL12 protein levels in the cell culture media upon treatment with 200 μM KYN compared to control. CXCL12 protein secreted in culture media from BMSCs isolated from young mice (6 months) was significantly reduced, almost 80%, when treated for 48 h with 200 μM KYN (Fig. 1F). Secretion of CXCL12 from BMSCs of 3 and 18 months old mice was also decreased with KYN (Supp. Figs. 1A,B). Consistently, there was a significant decrease in CXCL12 mRNA expression in the BMSCs as early as 6 h after treatment. Concomitant with reduction in CXCL12 expression, mRNA expression of the receptors, CXCR4 and CXCR7, was also decreased by almost 30–50% (Fig. 1G–I). BMSCs isolated from 6-month-old mice showed similar changes in CXCL12 mRNA expression at later time points with a significant decrease at 48 h post-treatment (Supp. Fig. 1C).
3.2. KYN attenuated osteogenesis and osteogenic gene expression in BMSCs
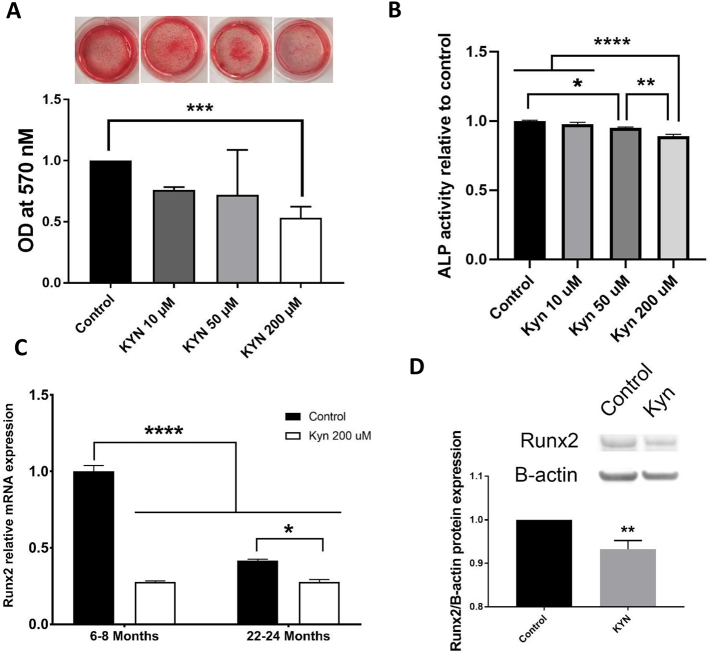
In vivo experiments have shown that KYN accumulates with aging and induces bone loss (Refaey et al., 2017). To establish if, at least in part, KYN achieves this function via inhibition of BMSC osteogenesis, we treated BMSCs isolated from 6- and 18-month-old mice with different doses of KYN (10, 50, 200 μM). Using osteogenic differentiation media for 21 days and Alizarin Red staining, KYN doses inhibited osteogenic differentiation in BMSCs from 6-month-old mice in a dose dependent manner, with the high KYN dose (200 μM) showing a significant 50% decrease (Fig. 2A). After 14 days, the high KYN dose also showed significant inhibition of minerlization with Alizarin Red staining (Supp. Fig. 2A), as well as intermittent doses (Supp. Fig. 2D). The early effects of KYN on osteogenic differentiation of BMSCs isolated from 6 months old mice were confirmed using alkaline phosphatase activity assay after 7 days of incubation in osteogenic differentiation media (Fig. 2B). To determine whether this KYN effect was in fact due to inhibition of osteogenesis and not an effect on cell density, a crystal violet assay assessed KYN cytotoxicity and showed that none of the doses of KYN (10. 50, and 200 μM) affected cell proliferation as determined by the number of BMSCs when cultured under the same conditions as the osteogenic differentiation experiments (Supp. Fig. 2C).
Fig. 2.
Kynurenine inhibits BMSCs osteogenic differentiation. (A) Optical density of alizarin red osteogenic assay staining and corresponding wells of BMSCs isolated from pooled 6 months-old mice after treatment with different doses of kynurenine (10, 50, and 200 μM) for 21 days (n = 6). (B) Alkaline Phosphatase (ALP) activity relative to control in BMSCs isolated from pooled 6 months-old mice treated with different doses of kynurenine (10, 50, and 200 μM) for 7 days (n = 6) (C) mRNA levels of Runx2 in BMSCs isolated from young (6–8 months-old) and old (22–24 months-old) mice, in osteogenic differentiation medium with or without 200 μM kynurenine for 7 days (n = 3 mice per group). (D) Protein levels of Runx2 in BMSCs isolated from 6 months mice after incubation in osteogenic differentiation medium with or without 200 μM kynurenine for 7 days. Data presented as mean ± SD. Data analysis was done using one way ANOVA for panels A, B, and C, and using unpaired t-test for panel D. *P < 0.05, **P < 0.01, ***p < 0.001, ****p < 0.0001.
Next, directly isolated BMSCs (i.e., without in vitro expansion/passaging prior to experimentation) from young and old mice (6–8 months versus 22–24 months) were used to assess how KYN affects their osteogenic differentiation marker expression. Basal expression level of the osteogenic marker, Runx2 was significantly lower in osteogenically-cultured BMSCs isolated from old mice compared to similarly cultured BMSCs isolated from young mice. We also found that treating BMSCs from either younger or older mice with KYN significantly reduced their mRNA levels of Runx2, resembling the effect of aging. Runx2 protein expression was also significantly downregulated after 7 days of KYN treatment in osteogenic differentiation media (Fig. 2C, D).
3.3. The KYN treatment phenotype resembles aging in upregulating CXCL12-targeting miR-29b-1-5p
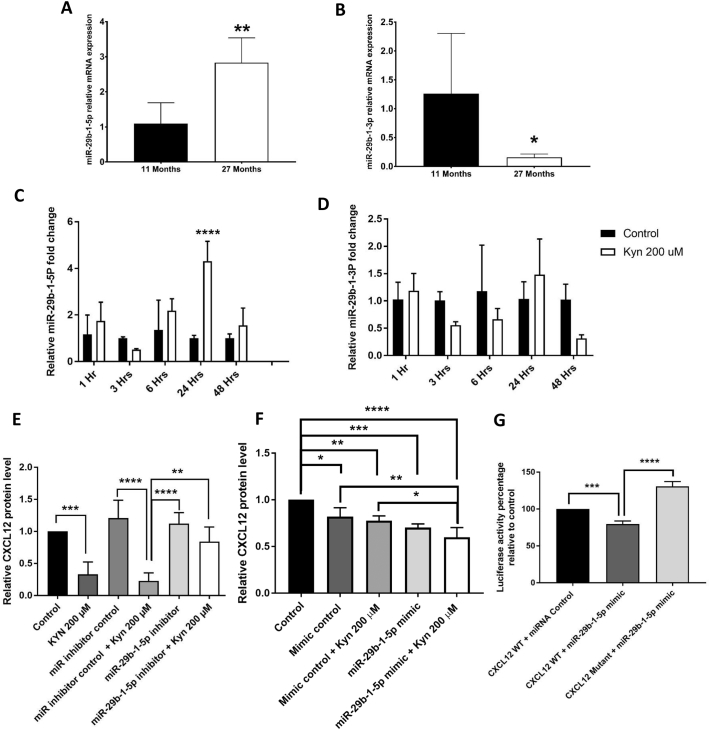
To determine how in vitro administration of KYN induced a phenotype reminiscent of organismal aging in terms of miR-29b-1 expression, we measured miR-29b-1-5p levels in BMSCs from young versus old mice. BMSCs isolated from aged mice had significantly higher levels of miR-29b-1-5p and significantly lower levels of miR-29b-1-3p compared to BMSCs from younger mice (Fig. 3A, B). As in the mice we also found that miR-29b-1-5p levels in aged human MSCs were significantly upregulated in aged vs. adult MSCs, but different from the murine the miR-29b-1-3p levels were not significantly changed in humans with age (Supp. Fig. 3A).
Fig. 3.
Kynurenine upregulates pro-aging and CXCL12-targeting miR-29b-1-5p. (A,B) miR-29b-1-5p (A) and miR-29b-1-3p (B) levels in BMSCs isolated from 11 versus 27 months old (n = 4 for 11 months old mice and n = 7 for 27 months old mice). (C,D) miR-29b-1-5p levels (C) and miR-29b-1-3p levels (D) in BMSCs isolated from pooled 6 months-old mice after treatment with 200 μM kynurenine for 1, 3, 6, 24 and 48 h (n = 3). (E,F) CXCL12 levels in BMSCs isolated from 6 months old mice after treatment with 200 μM kynurenine with or without transfection with miR-29b-1-5p inhibitor (E) or miR-29b-1-5p mimic (F) (n = 8 for inhibitor and n = 4 for mimic). (G) Luciferase activity of CXCL12 reporter plasmids (wild type and mutant) after transfection with miR-29b-1-5p mimic relative to luciferase activity of wild type CXCL12 reporter plasmid after transfection with miRNA mimic control for 24 h (n = 3). Data presented as mean ± SD. Data analysis was done using one way ANOVA for panels E, F amd G, using two way ANOVA for panels C and D, and using unpaired t-test for panels A and B. *P < 0.05, **P < 0.01, ***p < 0.001, ****p < 0.0001.
We then measured miR-29b-1-5p levels in murine BMSCs from 6-month-old mice with or without KYN treatment for 1, 3, 6, 24, and 48 h. KYN transiently, upregulated miR-29b-1-5p beginning at 24 h (Fig. 3C). The levels of miR-29b-1-5p expression in response to KYN was almost back to normal by 48 h.
To confirm that increased levels of miR-29b-1-5p mediated KYN's effect on the CXCL12 axis, rather than being an independent downstream effect of KYN, we transfected BMSCs isolated from 6-month-old mice with a miR-29b-1-5p inhibitor, and treated them with KYN for 24 h. The miR-29b-1-5p inhibitor alone did not significantly upregulate CXCL12 protein level when there was low basal levels of miR-29b-1-5p to start with. However, it restored CXCL12 protein levels when combined with 200 μM KYN, which by itself increased miR-29b-1-5p and significantly downregulated CXCL12 protein levels (Fig. 3E). We further confirmed this result by treating the same BMSCs with 200 μM KYN in the presence or absence of the miR-29b-1-5p mimic. Consistently, both KYN and the miR-29b-1-5p mimic significantly downregulated CXCL12 protein levels by almost 30%. Further, combining both miR-29b-1-5p and KYN together lead to a significant almost a 40% inhibition of the CXCL12 protein level (Fig. 3F).
To determine whether miR-29b-1-5p's effect on CXCL12 was direct or indirect, we utilized a 3′-UTR Duo-Luciferase assay, which controls for vector uptake and transcriptional regulation in the same assay. A reporter plasmid for wild type CXCL12 3′-UTR and another reporter plasmid for CXCL12 3′-UTR mutated at all three potential binding sites for miR-29b-1-5p according to the target prediction database Miranda (Miranda et al., 2006) (Table 4) were used to assess whether the miR-29b-1-5p directly targets the predicted binding sites. Transfection of murine BMSCs with the miR-29b-1-5p mimic resulted in a significant reduction (almost 30%) in the luciferase activity of the CXCL12 reporter plasmid. When the wild type CXCL12 3′-UTR was replaced with the mutated CXCL12 3′-UTR, the luciferase activity was significantly increased indicating that miR-29b-1-5p could not bind and inhibit CXCL12 luciferase activity (Fig. 3F).
Table 4.
Sequences of predicted binding sites between miR-29b-1-5p and the 3′-UTR of Hdac3 and CXCL12 according to MiRanda database. The miR-29b-1-5p sequence is the top of each pairing.
| Target accession | Score | miRNA start-end | 3′utr start-end | 3′-miRNA-5′-alignment-5′-utr3-3′ |
|---|---|---|---|---|
| Hdac3 (NM_001355039) | 122.00 | 2–23 | 83–106 | |
| CXCL12 (NM_000609) | 163.00 | 2–22 | 32–54 | |
| CXCL12 (NM_000609) | 151.00 | 3–22 | 2858–2880 | |
| CXCL12 (NM_000609) | 141.00 | 2–23 | 558–582 |
3.4. KYN and miR-29b-1-5p differentially modulated Hdac3 levels
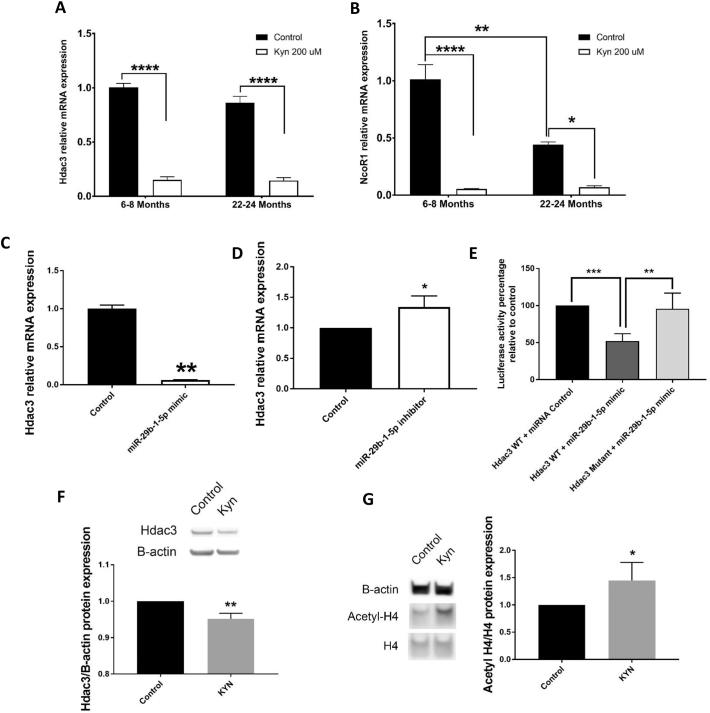
We previously demonstrated that administration of KYN in vivo downregulates the levels of Hdac3 as well as its cofactor NCoR1 (Refaey et al., 2017). We further showed that Hdac3 plays an important role in the regulation of osteogenic and adipogenic differentiation in BMSCs in aging (Refaey et al., 2017; Razidlo et al., 2010). Here, we measured the basal mRNA levels of Hdac3 and its cofactor NcoR1 in BMSCs isolated from young adult mice versus old mice. When BMSCs were grown in osteogenic differentiation medium for 7 days, the mRNA levels of these two markers were downregulated in murine BMSCs with aging. In addition, treatment with KYN significantly downregulated mRNA levels of both markers in young and old mice (Fig. 4A, B).
Fig. 4.
Kynurenine downregulates Hdac3 and its cofactors. (A,B) mRNA expression levels of (A) Hdac3 and (B) NcoR1 in BMSCs isolated directly from young and old mice and cultured in osteogenic differentiation medium with or without 200 μM kynurenine for 7 days (n = 3). (C,D) mRNA expression levels of Hdac3 in BMSCs isolated from pooled 6 months-old mice and transfected with either (C) miR-29b-1-5p mimic or (D) miR-29b-1-5p inhibitor compared to their respective controls (n = 3). (E) Luciferase activity of Hdac3 reporter plasmids (wild type and mutant) after transfection with miR-29b-1-5p mimic relative to luciferase activity of wild type Hdac3 reporter plasmid after transfection with miRNA mimic control for 24 h (n = 3). (F,G) Protein levels of Hdac3 (F) and H4 acetylation (G) in BMSCs isolated from pooled 6 months-old mice after incubation in osteogenic differentiation medium with or without 200 μM kynurenine for 7 days. Data presented as mean ± SD. Data analysis was done using one way ANOVA for panel E, using two way ANOVA for panels A and B, and using unpaired t-test for panels C, D, F, and G. *P < 0.05, **P < 0.01, ***p < 0.001, ****p < 0.0001.
To establish the relationship between miR-29b-1-5p levels and Hdac3 levels, we measured Hdac3 mRNA expression in cells transfected with either the miR-29b-1-5p mimic or the miR-29b-1-5p inhibitor. Consistently, miR-29b-1-5p mimic significantly downregulated, while miR-29b-1-5p inhibitor significantly upregulated, Hdac3 mRNA levels (Fig. 4C, D).
To determine whether the effect of miR-29b-1-5p on Hdac3 is direct or indirect, we designed another 3′-UTR Duo-Luciferase assay using wild type and mutated Hdac3 3′-UTR vector plasmids. We found that miR-29b-1-5p significantly reduced luciferase activity for the wild type Hdac3 3′-UTR, and had no effect on the mutated Hdac3 3′-UTR, confirming that miR-29b-1-5p acts directly on Hdac3 via the predicted binding site (Miranda database) (Miranda et al., 2006) (Fig. 4E) (Table 4).
We also measured Hdac3 protein expression and H4 acetylation levels in response to 200 KYN μM or miR-29b-1-5p mimic to confirm our previous results and found that consistent with our mRNA data, both KYN and miR-29b-1-5p inhibited Hdac3 protein levels and we demonstrated the expected downstream functional effect of Hdac3 suppression as evidenced by the significant increase in H4 acetylation (Fig. 4G, H).
3.5. AhR mediates the effects of KYN on CXCL12
KYN has been shown to act through binding to the aryl hydrocarbon receptor (AhR), which then translocates to the nucleus (Beischlag et al., 2008). We tested if the AhR receptor was mediating KYN's effects on CXCL12 protein levels, and on CXCL12, CXCR4, and ACKR3 mRNA levels. We utilized the AhR antagonist 3′,4′-dimethoxyflavone (DMF), and found that after 48 h of treatment, KYN significantly reduced secreted CXCL12 in the cell culture media of BMSCs from 6-month-old mice, and that co-treatment with DMF was able to restore CXCL12 protein level (Fig. 5). Of translational importance, KYN treatment (50 and 200 μM) downregulated secreted CXCL12 from human BMSCs level and the AhR antagonist CH-223191 restored it within 24 h (Supp. Fig. 2B).
Fig. 5.
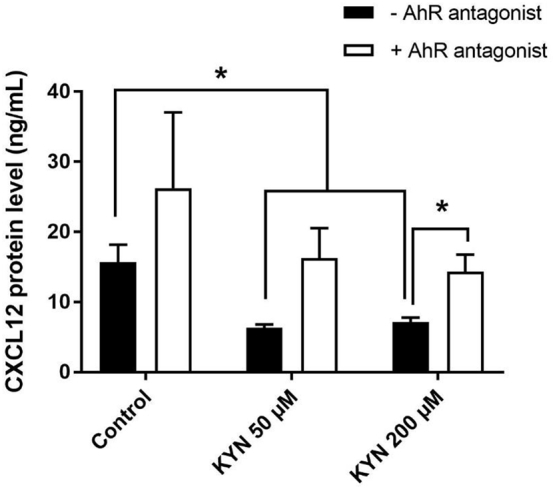
AhR antagonist inhibits kynurenine-induced CXCL12 downregulation. CXCL12 protein levels in BMSCs isolated from pooled 6-month-old mice after treatment for 48 h with 100 or 200 μM kynurenine with or without 10 μM of AhR-antagonist 3′,4′-Dimethoxyflavone (DMF) (n = 3). Data presented as mean ± SD. Data analysis was done using unpaired t-test. **P < 0.01.
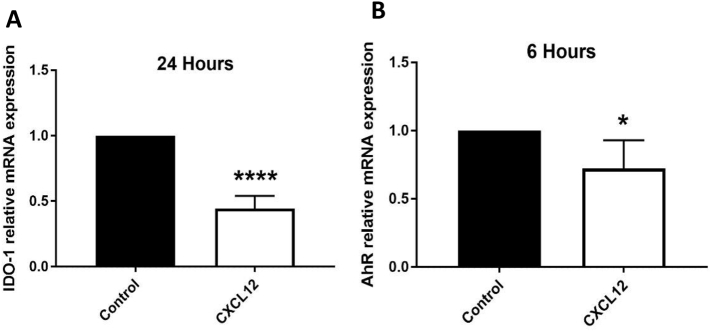
3.6. CXCL12 regulates IDO-1 and AhR mRNA levels
To test whether there was cross-talk between CXCL12 and KYN whereby CXCL12 could suppress KYN-related pathways, we treated BMSCs isolated from 6-month-old mice with 200 ng/mL CXCL12. Ttreatment with CXCL12 significantly downregulated the mRNA levels of AhR after 6 h and IDO-1, the gene encoding an enzyme that can generate KYN, after 24 h suggesting that cross-talk exists (Fig. 6A, B).
Fig. 6.
CXCL12 regulates mRNA levels of IDO-1 and AhR. (A,B) mRNA expression levels of (A) IDO-1, and (B) AhR after treatment with CXCL12 (200 ng/mL) for 24 and 6 h respectively (n = 4). Data presented as mean ± SD. Data analysis was done using one way ANOVA for panel C, and using unpaired t-test for panels A and B. *P < 0.05, ***p < 0.001, ****p < 0.0001.
4. Discussion
Tryptophan metabolism directly through the KYN pathway is involved in the regulation of key physiological functions such as immunity, CNS/neuronal function, gastrointestinal homeostasis and we now recognize in bone metabolism. Metabolic dysregulation in disorders affecting these areas including aging, cancer, and neurodegenerative disease have led to significant clinical-translational interest and even clinical trials targeting the KYNP pathway, specifically KYN, its downstream metabolites and the AhR signaling pathway that responds to KYN and its metabolites (Platten et al., 2019). Based on our work, and that of others, it is now clear that this system is also critically involved in osteoporosis and age-related bone loss. Here we help delineate the mechanisms linking KYN and bone loss.
Osteoporosis is a bone disease characterized by a decrease in the ratio of bone formation to bone resorption with aging. Osteoporosis is mostly asymptomatic during the first few years which makes early diagnosis more difficult (Phetfong et al., 2016). Currently available treatments for osteoporosis can be associated with a wide range of side effects (Antebi et al., 2014). A key barrier to preventing osteoporosis or developing efficient new treatments is understanding the mechanisms underlying the aging-related deterioration in bone health and subsequent progression of osteoporosis. Our group and others have demonstrated that osteoporosis is - at least in part - a stem cell disease (Infante and Rodriguez, 2018; Herberg et al., 2015; Antebi et al., 2014). This study aimed to investigate molecular pathways triggering age-associated changes in the bone marrow stem cell populations that could lead to osteoporosis and thus may represent a new therapeutic target.
Osteogenic differentiation is critical in bone aging; as we age, there is a shift in BMSCs from osteogenic to adipogenic differentiation (Infante and Rodriguez, 2018). Our group and other groups have reported the beneficial role of CXCL12α and β in the osteogenic differentiation of BMSCs (Yang et al., 2018a; Herberg et al., 2014a; Herberg et al., 2015). We have found that increased KYN levels, as well as an elevated KYN to TRP ratio, contribute to reductions in BMSCs osteogenesis and subsequent decreased bone formation in association with aging (El Refaey et al., 2015; Kim et al., 2019). We have previously reported increased serum and bone marrow levels of KYN with aging in humans (Refaey et al., 2017) (Kim et al., 2019). While the specific mechanism of age-related increases in KYN levels in different tissue compartments is still not fully understood, the elevated KYN levels may be due to upregulated local IDO activity with aging, as well as increased ROS mediated conversion of tryptophan to KYN. These, or potentially other mechamisms can lead to higher KYN levels and an increase in the KYN/TRP ratio (Souza et al., 2018; Sas et al., 2018). KYN also downregulates Hdac3 and its cofactor NcoR1 which increases the degree of adiposity in murine bone marrow together with reducing bone mass (Refaey et al., 2017). We also recently assessed an array of miRNAs that are upregulated in human BMSCs with aging, identifying miR-29b-1-5p as a key miRNA involved in inhibiting osteogenesis with age (Hill et al., 2016; Periyasamy-Thandavan et al., 2013; Baglio et al., 2013; Lee et al., 2016; Suh et al., 2013a; Shi et al., 2016; Li et al., 2009; William and Hill, 2016; Periyasamy-Thandavan et al., 2012). Here, we investigate how these different anti-osteogenic pathways interact, underscoring new biomarkers that can be targeted for clinical intervention to prevent or treat osteoporosis at the stem cell level.
Our results here confirm that KYN inhibits BMSC osteogenesis and downregulates osteogenic gene expression. This is in agreement with work from Dinçel et al., who showed a significant correlation between a high KYN/TRP ratio and the incidence of osteoporotic hip fracture (Dincel et al., 2017). We have also shown that KYN levels and the KYN/TRP ratio in the human bone marrow microenvironment are increased with age (Kim et al., 2019). More importantly, we showed that subjects with increased bone fragility, marked by decreased total femur BMD and increased markers of bone resorption, have significantly higher KYN levels and KYN/TRP ratios than those without (Kim et al., 2019). Another group demonstrated that high levels of peripheral KYN and AhR are significantly correlated with decreased bone strength, in contrast high central KYN levels in the frontal cortex of the brain are correlated with increased bone strength (Kalaska et al., 2017a; Kalaska et al., 2017b). These investigators believe that similar to serotonin, another TRP metabolite, KYN can exert opposing effects on bone formation depending on its site of action (Kalaska et al., 2017b; Ducy and Karsenty, 2010).
The identification of mechanisms underlying KYN's anti-osteogenic effects has been limited. Here, we demonstrate potential novel mechanisms based on downregulation of CXCL12 axis mRNA expression and protein levels, via KYN ligand signaling through the AhR and mediated by epigenetic changes in miRNAs and Hdac3. The decrease in CXCL12 protein levels in response to KYN treatment and our previous findings indicating that CXCL12 is important for osteogenesis, in turn support the theory that KYN inhibits BMSCs osteogenesis via downregulation of the CXCL12 axis. We also found that KYN-mediated downregulation of CXCL12 mimics the decline in CXCL12 protein levels in human and murine bone marrow interstitial fluid with aging. The reason for the corresponding age-related increase in CXCL12 levels in murine and human plasma is not clear, but it could be due to summing of inflammatory responses throughout the body in the plasma, or to regulated compartmentalized changes in CXCL12 expression that are different in the bone marrow versus plasma, and/or to an accumulation of DPP4-cleaved non-CXCR4-activating CXCL12 in plasma subsequent to feedback (Carbone et al., 2017; Ding et al., 2007; Elmansi et al., 2019). Recently, links between AhR signaling and both CXCR4 and ACKR3 levels have been suggested, where high AhR expression was correlated with high expression of CXCR4 in breast tumors (Vacher et al., 2018), and hepatic toxicity in response to AhR activation was mediated, at least in part, by changes in ACKR3 mRNA levels (Watson et al., 2017). Another potential link between the KYN pathway and the CXCL12 axis, is the reported IDO- and tryptophan-mediated regulation of CXCR4 expression and responsiveness to chemokines in dendritic cells (Hwang et al., 2005).
KYN may achieve its anti-osteogenic effects in part by upregulating miR-29b-1-5p, which targets CXCL12 and other osteogenic genes. We noticed that changes in miR-29b-1-5p in response to KYN treatment of 200 μM peaked around 24 h and was almost back to basal level by 48 h, which is expected due to the transient on/off nature of miRNA regulation (Bail et al., 2010; Zhang et al., 2012; Rüegger and Großhans, 2012). This miRNA upregulation supports the idea that increased KYN levels with age may be a key regulator of the age-associated decrease in osteogenesis. In this study, we focused mainly on miR-29b-1-5p since, it targeted both CXCL12 and Hdacs, a second distinct epigenetic regulatory system. We had in silico evidence that miR-29b-1-5p targets position 278–302 of the Hdac3 3′ UTR (Miranda et al., 2006). We confirmed here, for the first time that this miRNA directly targets and down-regulates both Hdac3 and CXCL12. Whether KYN can downregulate CXCL12 independently from miR-29b-1-5p is yet to be determined. We found that KYN's effect on the CXCL12 protein level is attenuated by the use of a miR-29b-1-5p (antagomir) inhibitor and increased by the use of the miR-29b-1-5p mimic. This is further evidence that miR-29b-1-5p is involved in KYN's downregulation of CXCL12. This age-associated miRNA upregulation supports the idea that increased KYN levels with age may be a key regulator of the age-associated decrease in osteogenesis and may be linked to aging in other systems.
The effect of KYN on miR29b-1-5p supports results from other groups that miR-29b may play a role in osteoarthritis development through inhibiting the expression of collagen I by chondrocytes and collagen III by BMSCs (Mayer et al., 2017). We also found that miR-29b-1-5p and miR-29b-1-3p levels change in opposite directions in mice with aging. This is interesting, because Feichtinger et al. (Feichtinger et al., 2018) found that high levels of miR-29b-1-3p are associated with healthy bone microstructure and histomorphometry. In previous work on human BMSCs, we only observed an increase in the miR-29b-1-5p passenger strand with aging without a concomitant decrease in the miR-29b-1-3p guide strand (Supp. Fig. 3C) (Periyasamy-Thandavan et al., 2012); thus, there may be a difference between human and murine age-associated expression of the guide and passenger strand levels with the regulation of the 5p arm being more critical in aging humans. miR-29b is not the only miRNA bone marker that shows opposing effects of its guide and passenger strands on bone health, as miR-500a-3p was shown to have a positive correlation with bone surface, as well as mineral apposition rate, while miR-500a-5p was shown to play an inhibitory role in osteogenic differentiation and was reported as a novel biomarker for fracture risk in postmenopausal women (Feichtinger et al., 2018; Heilmeier et al., 2016). These observations support the idea that in pathologies, including aging, there may be a dysregulation of the microRNA processing leading to significant changes in the targeting of mRNAs.
Importantly, KYN also downregulates Hdac3 and its cofactor NcoR1; an effect that we previously linked to decreased osteogenesis and increased adipogenesis in murine bone marrow (Refaey et al., 2017; Razidlo et al., 2010). Hdac3 plays a critical role in the process of endochondral ossification and bone matrix remodeling. Loss of Hdac3 in osteochondral progenitors leads to an osteopenic phenotype from impaired bone formation and increasing marrow adiposity, and postnatal inducible conditional knockout of chondrocyte Hdac3 leads to several skeletal abnormalities as well as increased osteoclastogenesis (Razidlo et al., 2010; Carpio et al., 2016; Gordon et al., 2015; Feigenson et al., 2017; McGee-Lawrence et al., 2016). These studies all implicate Hdac3 as a crucial mediator of endochondral ossification, particulary in the context of aging. It is also important to note that Hdac3 plays an important role in adipogenesis, where lower Hdac3 levels correlated with an increase in bone marrow fat (Razidlo et al., 2010). This effect could explain the increase in bone marrow adipose tissue we previously reported in mice that received a high KYN diet (Refaey et al., 2017).
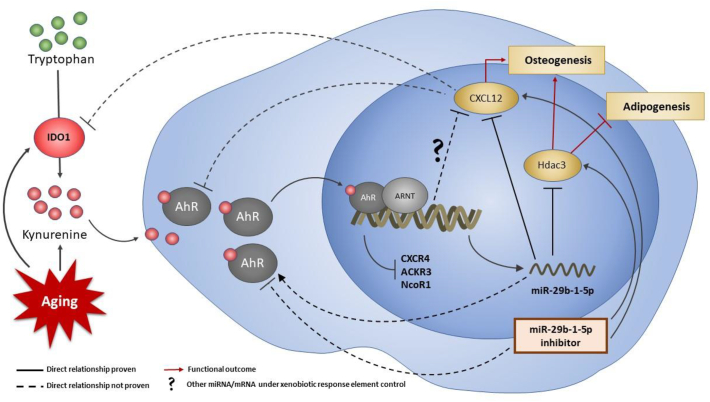
We believe that the effects of KYN reported here are primarily, but not necessarily exclusively, mediated through AhR mediated signaling. In other work, it was demonstrated that AhR activation can affect bone health through downstream effects including reduced ossification as well as inhibition of osteogenic differentiation and osteoblast functions (Yun et al., 2018; Jamsa et al., 2001; Milbrath et al., 2009). Here, we found that the use of the AhR antagonists DMF and CH-223192 in combination with KYN partially rescued CXCL12 gene and protein expression. The anti-osteogenic action of KYN may be mediated through other pathways as well. Indeed, it was recently reported that KYN can regulate Wnt signaling and inhibit β-catenin expression, which is a key regulatory pathway for osteogenesis and bone formation (Park et al., 2018), and miR-29b-1-5p is predicted to potentially target Wnt1. Furthermore, the miR-29b-1-5p mimic significantly upregulated AhR mRNA expression levels, while both the miR-29b-1-5p inhibitor and CXCL12 significantly downregulated those levels and CXCL12 also downregulated IDO-1 expression. This result suggests that either miR-29b-1-5p is upstream of AhR activation, or a feedback regulation mechanism exists between the two, as well as cross-talk between CXCL12 with IDO-1 and AhR, as well as being potentially able to target multiple osteogenic genes. (Fig. 7).
Fig. 7.
Proposed pathway of kynurenine effects on BMSCs. Aging upregulates activity of IDO1 which converts tryptophan to kynurenine. Kynurenine is then taken up by BMSCs and binds to cytoplasmic AhR. Upon binding, ligand-bound AhR is transported into the nucleus where it forms a complex with Aryl hydrocarbon receptor nuclear translocator (ARNT). The newly formed complex acts as a transcription factor and downregulates Hdac3 and CXCL12 transcription while upregulating miR-29b-1-5p. Hence, the overall effect of kynurenine and AhR complex is shifting from osteogenic to adipogenic differentiation in BMSCs. CXCL12 acts via a feedback regulation mechanism to downregulate IDO1 and AhR mRNA levels, while miR-29b-1-5p upregulates AhR mRNA level.
In summary, we show that KYN contributes to bone aging via AhR activation, which leads to upregulation of miR-29b-1-5p with downregulation of both Hdac3 and CXCL12. These combined effects lead to a reduced osteogenic differentiation capacity in BMSCs, and contributes to the aging-induced decline in bone formation. Whether the effects of KYN are solely mediated via AhR is yet to be determined. We believe that KYN pathway mediators including IDO-1 and AhR, as well as the downstream molecules investigated in this work (CXCL12, Hdac3, and miR29b-1-5p), offer new potential targets for clinical intervention to prevent or treat osteoporosis.
Declaration of competing interest
Drs. William Hill, Sergio Mas Herrero, and Sudharsan Periyasamy-Thandavanis are inventors on U.S. Patent No. 9,267,139, “Compositions and Methods for Treating Musculoskeletal Disorders” issued. Ahmed Elmansi, Galina Kondrikova, Jessica Pierce, Helen Kaiser, Drs. Khaled Hussein, Xue Jiang, Alexandra Aguilar-Pérez, Dmitry Kondrikov, Nada H. Eisa, Ke-Hong Ding, Aisha Walker, Sadanand Fulzele, Wendy B. Bollag, Mohammed Elsalanty, Qing Zhong, Xing-ming Shi, Yun Su, Maribeth Johnson, Monte Hunter, Charles Reitman, Brian Volkman, Mark Hamrick, Carlos Isales, Meghan McGee-Lawrence have no conflicts of interest or financial ties to disclose.
Acknowledgments
Acknowledgments
This publication is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration Office of Research and Development, Clinical Science Research and Development Program (VA Merit Award 1I01CX000930-01, WDH) and the National Institutes of Health (NIA-AG036675 SF, MWH, CMI, MM-L, and WDH). The contents of this publication do not represent the views of the Department of Veterans Affairs, or the United States Government.
Authors' roles
Study design: KAH, AME, MM-L, and WDH. Study conduct: AME, KAH, SMH, SP-T, NHE, XJ, AA-P, ALW, X-MS, YS, and HK. Data collection: AME, SMH, NHE, GK, JP, and ALW. Data analysis: SMH, KAH, AME, WDH and MM-L. Statistical Analysis: AME, KAH, and MJ. Data interpretation: AME, SMH, SF, WBB, WDH, MM-L. Drafting manuscript: AME, KAH and WDH. Revising manuscript content: K-HD, ME, QZ, X-MS, MH, MWH, CR, BFV, SF, DK, CMI, MM-L, and WDH. Approving final version of manuscript: MWH, CMI, SF, MM-L, and WDH. MM-L and WDH take responsibility for the integrity of the data analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2020.100270.
Contributor Information
Meghan E. McGee-Lawrence, Email: mmcgeelawrence@augusta.edu.
William D. Hill, Email: hillwi@musc.edu.
Appendix A. Supplementary data
Supplementary Fig. 1. (A,B) Kynurenine at 200 μM significantly downregulates secreted CXCL12 protein level in cell culture media of BMSCs isolated from (A) pooled 3 months-old and (B) pooled 18 months-old mice (n = 4). (C) Kynurenine at 200 μM significantly downregulates CXCL12 mRNA level in BMSCs isolated from pooled 6 months-old mice after 24 and 48 h of treatment (n = 4). Data presented as mean ± SD. Data analysis was done using two way ANOVA for panel C, and using unpaired t-test for panels A and B. *P < 0.05, **P < 0.01, ***p < 0.001, ****p < 0.0001.
Supplementary Fig. 2. (A) Kynurenine at 200 μM significantly inhibits early osteogenic differentiation of BMSCs isolated from pooled 6 months-old mice as shown by Alizarin Red staining after 14 days of treatment (n = 6). (B) Kynurenine (50, 100, 200 μM) downregulates CXCL12 level in cell culture media of human BMSCs (74 year-old female) after 24 h of treatment, and AhR antagonist, CH-223191 (2 μg/mL), significantly rescues CXCL12 level with the 50 μM dose (n = 3). (C) Effect of kynurenine at different doses (10, 50, 200 μM) on cell density of BMSCs isolated from pooled 6 months-old mice as shown by crystal violet assay shows no changes in cell density. (D) Optical density of alizarin red osteogenic assay staining and corresponding wells of BMSCs isolated from 6 months-old mice after treatment with different doses of kynurenine (10, 25, 50, 100 μM) for 21 days shows inhibition of osteogenesis at all KYN doses (n = 6). Data presented as mean ± SD. Data analysis was done using one way ANOVA for panel C, using two way ANOVA for panels B and D, and using unpaired t-test for panel A. *P < 0.05, **P < 0.01. ***p < 0.001, ****p < 0.0001.
Supplementary Fig. 3. miR-29b-1-5p is upregulated in culture-expanded human CD271+ BMSCs (passage one) with aging or in response to KYN treatment. Transcript analysis using cultured MSCs and specific miRNA primers showed that miR-29b-1-5p was significantly upregulated in Aged (>60 year-old) relative to adult (26-59 year-old) human MSCs, while no changes in the mature miR-29b-1-3p were observed. Data are shown as means ± SEM. (n = 7 subjects per age group). *P < 0.05.
References
- Álvarez-Viejo M. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World Journal of Stem Cells. 2015;7(2):470. doi: 10.4252/wjsc.v7.i2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antebi B., Pelled G., Gazit D. Stem cell therapy for osteoporosis. Current osteoporosis reports. 2014;12(1):41–47. doi: 10.1007/s11914-013-0184-x. [DOI] [PubMed] [Google Scholar]
- Apalset E.M., Gjesdal C.G., Ueland P.M., Midttun Ø., Ulvik A., Eide G.E. Interferon (IFN)-γ-mediated inflammation and the kynurenine pathway in relation to bone mineral density: the Hordaland Health Study. Clinical & Experimental Immunology. 2014;176(3):452–460. doi: 10.1111/cei.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H., Hirata J., Watanabe-Akanuma M. Indoxyl glucuronide, a protein-bound uremic toxin, inhibits hypoxia-inducible factor–dependent erythropoietin expression through activation of aryl hydrocarbon receptor. Biochem. Biophys. Res. Commun. 2018;504(2):538–544. doi: 10.1016/j.bbrc.2018.09.018. [DOI] [PubMed] [Google Scholar]
- Baglio S.R., Devescovi V., Granchi D., Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527(1):321–331. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Bail S., Swerdel M., Liu H., Jiao X., Goff L.A., Hart R.P. Differential regulation of microRNA stability. RNA (New York, NY) 2010;16(5):1032–1039. doi: 10.1261/rna.1851510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag T.V., Luis Morales J., Hollingshead B.D., Perdew G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008;18(3):207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betel D., Koppal A., Agius P., Sander C., Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11(8):R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E.W., McGee-Lawrence M.E., Westendorf J.J. Hdac-mediated control of endochondral and intramembranous ossification. Crit. Rev. Eukaryot. Gene Expr. 2011;21(2):101–113. doi: 10.1615/critreveukargeneexpr.v21.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromage D.I., Davidson S.M., Yellon D.M. Stromal derived factor 1alpha: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol. Ther. 2014;143(3):305–315. doi: 10.1016/j.pharmthera.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A.K., Lawson M.A., Smith R.A., Janda T.M., Kelley K.W., McCusker R.H. Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the kynurenine pathway in the mouse hippocampus. J. Neuroinflammation. 2016;13(1) doi: 10.1186/s12974-016-0563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H.E., Orschell C.M., Clapp D.W., Hangoc G., Cooper S., Plett P.A. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201(8):1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Ou G., Yang N., Ding K., Kream B.E., Hamrick M.W. Impact of targeted PPARγ disruption on bone remodeling. Mol. Cell. Endocrinol. 2015;410:27–34. doi: 10.1016/j.mce.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone L., Buzkova P., Fink H., Robbins J., Bethel M., Hamrick M.W. Association of plasma SDF-1 with bone mineral density, body composition and hip fractures in older adults: the cardiovascular health study. Calcif. Tissue Int. 2017;100:599–608. doi: 10.1007/s00223-017-0245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpio L.R., Bradley E.W., McGee-Lawrence M.E., Weivoda M.M., Poston D.D., Dudakovic A. Histone deacetylase 3 supports endochondral bone formation by controlling cytokine signaling and matrix remodeling. Sci. Signal. 2016;9(440):ra79. doi: 10.1126/scisignal.aaf3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Shou P., Zheng C., Jiang M., Cao G., Yang Q. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death & Differentiation. 2016;23(7):1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A.M., Byrom M.W., Shelton J., Ford L.P. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33(4):1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Wang H., Zhang X., Zhao S., Zhou Z., Mu X. The role of SDF-1/CXCR4/CXCR7 in neuronal regeneration after cerebral ischemia. Front. Neurosci. 2017;11:590. doi: 10.3389/fnins.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coipeau P., Rosset P., Langonné A., Gaillard J., Delorme B., Rico A. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11(5):584–594. doi: 10.1080/14653240903079385. [DOI] [PubMed] [Google Scholar]
- Cox G., Boxall S.A., Giannoudis P.V., Buckley C.T., Roshdy T., Churchman S.M. High abundance of CD271+ multipotential stromal cells (MSCs) in intramedullary cavities of long bones. Bone. 2012;50(2):510–517. doi: 10.1016/j.bone.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert R.J., Giannoudis P.V., Wang X.N., Nicholson L., Pawson D., Lubenko A. Examining the feasibility of clinical grade CD271+ enrichment of mesenchymal stromal cells for bone regeneration. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0117855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincel E., Ozkan Y., Sukuroglu M., Ozsoy H., Sepici Dincel A. Evaluation of tryptophan/kynurenine pathway relevance with immune system biomarkers of low energy trauma hip fractures in osteoporotic patients. Archives of rheumatology. 2017;32(3):203–208. doi: 10.5606/ArchRheumatol.2017.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K.-H., Shi X.-M., Zhong Q., Kang B., Xie D., Bollag W.B. Impact of glucose-dependent insulinotropic peptide on age-induced bone loss. J. Bone Miner. Res. 2007;23(4):536–543. doi: 10.1359/JBMR.071202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z., Li L., Li Y. Involvement of miR-30b in kynurenine-mediated lysyl oxidase expression. J. Physiol. Biochem. 2019;75(2):135–142. doi: 10.1007/s13105-019-00686-4. [DOI] [PubMed] [Google Scholar]
- Ducy P., Karsenty G. The two faces of serotonin in bone biology. J. Cell Biol. 2010;191(1):7–13. doi: 10.1083/jcb.201006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Refaey M., Watkins C.P., Kennedy E.J., Chang A., Zhong Q., Ding K.-H. Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol. Cell. Endocrinol. 2015;410:87–96. doi: 10.1016/j.mce.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmansi A.M., Awad M.E., Eisa N.H., Kondrikov D., Hussein K.A., Aguilar-Pérez A. What doesn’t kill you makes you stranger: Dipeptidyl peptidase-4 (CD26) proteolysis differentially modulates the activity of many peptide hormones and cytokines generating novel cryptic bioactive ligands. Pharmacol. Ther. 2019;198:90–108. doi: 10.1016/j.pharmthera.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina N.H., Wood M.E., Perrapato S.D., Francklyn C.S., Stein G.S., Stein J.L. Standardizing analysis of circulating microRNA: clinical and biological relevance. J. Cell. Biochem. 2014;115(5):805–811. doi: 10.1002/jcb.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feichtinger X., Muschitz C., Heimel P., Baierl A., Fahrleitner-Pammer A., Redl H. Bone-related circulating microRNAs miR-29b-3p, miR-550a-3p, and miR-324-3p and their association to bone microstructure and histomorphometry. Sci. Rep. 2018;8(1):4867. doi: 10.1038/s41598-018-22844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson M., Shull L.C., Taylor E.L., Camilleri E.T., Riester S.M., van Wijnen A.J. Histone deacetylase 3 deletion in mesenchymal progenitor cells hinders long bone development. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017;32(12):2453–2465. doi: 10.1002/jbmr.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldar S., Khaniani M.S., Derakhshan S.M., Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pacific journal of cancer prevention : APJCP. 2015;16(6):2129–2144. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- Gordon J.A.R., Stein J.L., Westendorf J.J., van Wijnen A.J. Chromatin modifiers and histone modifications in bone formation, regeneration, and therapeutic intervention for bone-related disease. Bone. 2015;81:739–745. doi: 10.1016/j.bone.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granero-Moltó F., Weis J.A., Miga M.I., Landis B., Myers T.J., O’Rear L. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C.A., Gunn W.G., Peister A., Prockop D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Hamrick M.W., Ding K.-H., Pennington C., Chao Y.J., Wu Y.-D., Howard B. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39(4):845–853. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Heilmeier U., Hackl M., Skalicky S., Weilner S., Schroeder F., Vierlinger K. Serum miRNA signatures are indicative of skeletal fractures in postmenopausal women with and without type 2 diabetes and influence osteogenic and adipogenic differentiation of adipose tissue-derived mesenchymal stem cells in vitro. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31(12):2173–2192. doi: 10.1002/jbmr.2897. [DOI] [PubMed] [Google Scholar]
- Herberg S., Fulzele S., Yang N.L., Shi X.M., Hess M., Periyasamy-Thandavan S. Stromal cell-derived Factor-1 beta potentiates bone morphogenetic Protein-2-stimulated osteoinduction of genetically engineered bone marrow-derived mesenchymal stem cells in vitro. Tissue Eng. A. 2013;19(1–2):1–13. doi: 10.1089/ten.tea.2012.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S., Susin C., Pelaez M., Howie R.N., Moreno de Freitas R., Lee J. Low-dose bone morphogenetic protein-2/stromal cell-derived factor-1beta cotherapy induces bone regeneration in critical-size rat calvarial defects. Tissue Eng Part A. 2014;20(9–10):1444–1453. doi: 10.1089/ten.tea.2013.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S., Kondrikova G., Hussein K.A., Periyasamy-Thandavan S., Johnson M.H., Elsalanty M.E. Total body irradiation is permissive for mesenchymal stem cell-mediated new bone formation following local transplantation. Tissue Eng. A. 2014;20(23–24):3212–3227. doi: 10.1089/ten.tea.2013.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S., Kondrikova G., Hussein K.A., Johnson M.H., Elsalanty M.E., Shi X. Mesenchymal stem cell expression of stromal cell-derived factor-1β augments bone formation in a model of local regenerative therapy. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2015;33(2):174–184. doi: 10.1002/jor.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W.D., Periyasami-Thandavan S., Samuel H., Inventors; Augusta University, Assignee . 2016. Compositions and Methods for Treating Musculoskeletal Disorders. USA. [Google Scholar]
- Hwang S.L., Chung N.P., Chan J.K., Lin C.L. Indoleamine 2, 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res. 2005;15(3):167–175. doi: 10.1038/sj.cr.7290282. [DOI] [PubMed] [Google Scholar]
- Infante A., Rodriguez C.I. Osteogenesis and aging: lessons from mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):244. doi: 10.1186/s13287-018-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamsa T., Viluksela M., Tuomisto J.T., Tuomisto J., Tuukkanen J. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on bone in two rat strains with different aryl hydrocarbon receptor structures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(10):1812–1820. doi: 10.1359/jbmr.2001.16.10.1812. [DOI] [PubMed] [Google Scholar]
- Kalaska B., Pawlak K., Domaniewski T., Oksztulska-Kolanek E., Znorko B., Roszczenko A. Elevated levels of peripheral kynurenine decrease bone strength in rats with chronic kidney disease. Front. Physiol. 2017;8:836. doi: 10.3389/fphys.2017.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska B., Pawlak K., Oksztulska-Kolanek E., Domaniewski T., Znorko B., Karbowska M. A link between central kynurenine metabolism and bone strength in rats with chronic kidney disease. PeerJ. 2017;5 doi: 10.7717/peerj.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Chang H.W., Tseng H.C., Hsu S.C., Yang S.J., Hung C.H. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy. 2014;69(4):445–452. doi: 10.1111/all.12346. [DOI] [PubMed] [Google Scholar]
- Khordadmehr M., Shahbazi R., Ezzati H., Jigari-Asl F., Sadreddini S., Baradaran B. Key microRNAs in the biology of breast cancer; emerging evidence in the last decade. J. Cell. Physiol. 2019;234(6):8316–8326. doi: 10.1002/jcp.27716. [DOI] [PubMed] [Google Scholar]
- Kim J., Ko J. A novel PPARγ2 modulator sLZIP controls the balance between adipogenesis and osteogenesis during mesenchymal stem cell differentiation. Cell Death & Differentiation. 2014;21(10):1642–1655. doi: 10.1038/cdd.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Lu Y., Williams E.A., Lai F., Lee J.Y., Enishi T. Sclerostin antibody administration converts bone lining cells into active osteoblasts. J. Bone Miner. Res. 2017;32(5):892–901. doi: 10.1002/jbmr.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Hamrick M.W., Yoo H.J., Lee S.H., Kim S.J., Koh J.M. The detrimental effects of Kynurenine, a tryptophan metabolite, on human bone metabolism. J. Clin. Endocrinol. Metab. 2019;104:2334–2342. doi: 10.1210/jc.2018-02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuçi S., Kuçi Z., Schäfer R., Spohn G., Winter S., Schwab M. Molecular signature of human bone marrow-derived mesenchymal stromal cell subsets. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-38517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureel J., Dixit M., Tyagi A.M., Mansoori M.N., Srivastava K., Raghuvanshi A. miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation. Cell Death Dis. 2014;5:e1050. doi: 10.1038/cddis.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz K., Herold M., Winkler C., Klotz W., Russe E., Fuchs D. Effects of adalimumab therapy on disease activity and interferon-gamma-mediated biochemical pathways in patients with rheumatoid arthritis. Autoimmunity. 2011;44(3):235–242. doi: 10.3109/08916934.2010.528476. [DOI] [PubMed] [Google Scholar]
- Lee Y., Kim H.J., Park C.K., Kim Y.G., Lee H.J., Kim J.Y. MicroRNA-124 regulates osteoclast differentiation. Bone. 2013;56(2):383–389. doi: 10.1016/j.bone.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Lee W.Y., Li N., Lin S., Wang B., Lan H.Y., Li G. miRNA-29b improves bone healing in mouse fracture model. Mol. Cell. Endocrinol. 2016;430:97–107. doi: 10.1016/j.mce.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Lee W.-C., Guntur A.R., Long F., Rosen C.J. Energy metabolism of the osteoblast: implications for osteoporosis. Endocr. Rev. 2017;38(3):255–266. doi: 10.1210/er.2017-00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterova M.I., Zhang Y., Steger D.J., Schupp M., Schug J., Cristancho A. PPAR and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22(21):2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Basu S., Han M.-K., Kim Y.-J., Broxmeyer H.E. Influence of ERK activation on decreased chemotaxis of mature human cord blood monocyte-derived dendritic cells to CCL19 and CXCL12. Blood. 2007;109(8):3173–3176. doi: 10.1182/blood-2006-04-014753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Hassan M.Q., Jafferji M., Aqeilan R.I., Garzon R., Croce C.M. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J. Biol. Chem. 2009;284(23):15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lob S., Konigsrainer A., Zieker D., Brucher B.L., Rammensee H.G., Opelz G. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer immunology, immunotherapy : CII. 2009;58(1):153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas S.C. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21(2):115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- Manolagas S.C., Parfitt A.M. What old means to bone. Trends in Endocrinology & Metabolism. 2010;21(6):369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matic I., Matthews B.G., Wang X., Dyment N.A., Worthley D.L., Rowe D.W. Quiescent bone lining cells are a major source of osteoblasts during adulthood. Stem Cells. 2016;34(12):2930–2942. doi: 10.1002/stem.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U., Benditz A., Grassel S. miR-29b regulates expression of collagens I and III in chondrogenically differentiating BMSC in an osteoarthritic environment. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-13567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence M.E., Carpio L.R., Schulze R.J., Pierce J.L., McNiven M.A., Farr J.N. Hdac3 deficiency increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31(1):116–128. doi: 10.1002/jbmr.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo L.M.F., Mandik-Nayak L. IDO2: a pathogenic mediator of inflammatory autoimmunity. Clinical medicine insights Pathology. 2016;9(Suppl. 1):21–28. doi: 10.4137/CPath.S39930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R., Smith C., DuHadaway J.B., Chandler P., Baban B., Merlo L.M.F. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int. Immunol. 2014;26(7):357–367. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. Journal of immunology (Baltimore, Md : 1950) 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrath M.O., Wenger Y., Chang C.W., Emond C., Garabrant D., Gillespie B.W. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ. Health Perspect. 2009;117(3):417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K.C., Huynh T., Tay Y., Ang Y.-S., Tam W.-L., Thomson A.M. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Mortensen L.J., Hill W.D. Skeletal stem cells for bone development, homeostasis and repair: one or many? Bonekey Rep. 2015;4:769. doi: 10.1038/bonekey.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray I.A., Patterson A.D., Perdew G.H. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat. Rev. Cancer. 2014;14(12):801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz C.A., Litzenburger U.M., Sahm F., Ott M., Tritschler I., Trump S. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- Park J.H., Lee J.M., Lee E.J., Kim D.J., Hwang W.B. Kynurenine promotes the goblet cell differentiation of HT-29 colon carcinoma cells by modulating Wnt, Notch and AhR signals. Oncol. Rep. 2018;39(4):1930–1938. doi: 10.3892/or.2018.6266. [DOI] [PubMed] [Google Scholar]
- Periyasamy-Thandavan S.M., Fulzele S., Hamrick M.W., Shi X.-M., Isales C.M., Chutkan N., Ruark R., Hinson J., Hunter M., Corpe R., Xu H., Hill W.D. ASBMR; Minneapolis, USA: 2012. Differential Expression of MicroRNAs in Human Mesenchymal Stem Cells With Age May Be Related to Musculoskeletal Disorders. [Google Scholar]
- Periyasamy-Thandavan S., Mas S., Fulzele S., Shi X.M., Chutkan N., Ruark R. Age-associated changes in MicroRNA expression affects differentiation potential in human mesenchymal stem cells. JBMR. 2013;28:SA0002. Sup 1. [Google Scholar]
- Periyasamy-Thandavan S., Burke J., Mendhe B., Kondrikova G., Kolhe R., Hunter M. MicroRNA-141-3p negatively modulates SDF-1 expression in age-dependent pathophysiology of human and murine bone marrow stromal cells. The Journals of Gerontology: Series A. 2018;74:1368–1374. doi: 10.1093/gerona/gly186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phetfong J., Sanvoranart T., Nartprayut K., Nimsanor N., Seenprachawong K., Prachayasittikul V. Osteoporosis: the current status of mesenchymal stem cell-based therapy. Cellular & molecular biology letters. 2016;21:12. doi: 10.1186/s11658-016-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten M., Nollen E.A.A., Röhrig U.F., Fallarino F., Opitz C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019;18(5):379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- Poloni A., Maurizi G., Rosini V., Mondini E., Mancini S., Discepoli G. Selection of CD271+ cells and human AB serum allows a large expansion of mesenchymal stromal cells from human bone marrow. Cytotherapy. 2009;11(2):153–162. doi: 10.1080/14653240802582125. [DOI] [PubMed] [Google Scholar]
- Quinn K.E., Mackie D.I., Caron K.M. Emerging roles of atypical chemokine receptor 3 (ACKR3) in normal development and physiology. Cytokine. 2018;109:17–23. doi: 10.1016/j.cyto.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razidlo D.F., Whitney T.J., Casper M.E., McGee-Lawrence M.E., Stensgard B.A., Li X. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaey M.E., McGee-Lawrence M.E., Fulzele S., Kennedy E.J., Bollag W.B., Elsalanty M. Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss. J. Bone Miner. Res. 2017;32(11):2182–2193. doi: 10.1002/jbmr.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.C., Tanasijevic B., Golubeva D., Boyd A.L., Porras D.P., Collins T.J. CXCL12/CXCR4 signaling enhances human PSC-derived hematopoietic progenitor function and overcomes early in vivo transplantation failure. Stem Cell Reports. 2018;10(5):1625–1641. doi: 10.1016/j.stemcr.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes Ocampo J., Lugo Huitrón R., González-Esquivel D., Ugalde-Muñiz P., Jiménez-Anguiano A., Pineda B. Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxidative Med. Cell. Longev. 2014;2014:1–22. doi: 10.1155/2014/646909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll C.B., Bunnell B.A. Comparative characterization of mesenchymal stem cells from eGFP transgenic and non-transgenic mice. BMC Cell Biol. 2009;10:3. doi: 10.1186/1471-2121-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegger S., Großhans H. MicroRNA turnover: when, how, and why. Trends Biochem. Sci. 2012;37(10):436–446. doi: 10.1016/j.tibs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Sanchez-Martin L., Sanchez-Mateos P., Cabanas C. CXCR7 impact on CXCL12 biology and disease. Trends Mol. Med. 2012;19(1):12–22. doi: 10.1016/j.molmed.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Sas K., Szabo E., Vecsei L. Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Molecules (Basel, Switzerland) 2018;23(1) doi: 10.3390/molecules23010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler M.B., Cheung W.-Y., Majeska R., Kennedy O. Osteocytes: master orchestrators of bone. Calcif. Tissue Int. 2013;94(1):5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr E., Hausser H.-J. Extracellular matrix and cytokines: a functional unit. Dev. Immunol. 2000;7(2–4):89–101. doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Kanasaki K., Koya D. Linagliptin but not Sitagliptin inhibited transforming growth factor-beta2-induced endothelial DPP-4 activity and the endothelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2016;471(1):184–190. doi: 10.1016/j.bbrc.2016.01.154. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L.C., Jesse C.R., Del Fabbro L., de Gomes M.G., Gomes N.S., Filho C.B. Aging exacerbates cognitive and anxiety alterations induced by an intracerebroventricular injection of amyloid-beta1-42 peptide in mice. Mol. Cell. Neurosci. 2018;88:93–106. doi: 10.1016/j.mcn.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Stenderup K. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Suh J.S., Lee J.Y., Choi Y.S., Chung C.P., Park Y.J. Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation. Biomaterials. 2013;34(17):4347–4359. doi: 10.1016/j.biomaterials.2013.02.039. [DOI] [PubMed] [Google Scholar]
- Suh J.S., Lee J.Y., Choi Y.S., Chong P.C., Park Y.J. Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation. Biomaterials. 2013;34(17):4347–4359. doi: 10.1016/j.biomaterials.2013.02.039. [DOI] [PubMed] [Google Scholar]
- Trivedi C.M., Luo Y., Yin Z., Zhang M., Zhu W., Wang T. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat. Med. 2007;13(3):324–331. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- Tzeng Y.S., Chung N.C., Chen Y.R., Huang H.Y., Chuang W.P., Lai D.M. Imbalanced osteogenesis and adipogenesis in mice deficient in the chemokine Cxcl12/Sdf1 in the bone mesenchymal stem/progenitor cells. J. Bone Miner. Res. 2018;33(4):679–690. doi: 10.1002/jbmr.3340. [DOI] [PubMed] [Google Scholar]
- Vacher S., Castagnet P., Chemlali W., Lallemand F., Meseure D., Pocard M. High AHR expression in breast tumors correlates with expression of genes from several signaling pathways namely inflammation and endogenous tryptophan metabolism. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Vimalraj S., Partridge N.C., Selvamurugan N. A positive role of microRNA-15b on regulation of osteoblast differentiation. J. Cell. Physiol. 2014;229(9):1236–1244. doi: 10.1002/jcp.24557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Zhang S., Weber J., Baxter D., Galas D.J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38(20):7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J.D., Prokopec S.D., Smith A.B., Okey A.B., Pohjanvirta R., Boutros P.C. 2,3,7,8 Tetrachlorodibenzo-p-dioxin-induced RNA abundance changes identify Ackr3, Col18a1, Cyb5a and Glud1 as candidate mediators of toxicity. Arch. Toxicol. 2017;91(1):325–338. doi: 10.1007/s00204-016-1720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William D., Hill S.H. 2016. Sudharsan Periyasamy-Thandavan Inventor Compositions and Methods for Treating Musculoskeletal Diseases and Disorders. US. [Google Scholar]
- Xiang Z., Li J., Song S., Wang J., Cai W., Hu W. A positive feedback between IDO1 metabolite and COL12A1 via MAPK pathway to promote gastric cancer metastasis. J. Exp. Clin. Cancer Res. 2019;38(1):314. doi: 10.1186/s13046-019-1318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Hatabayashi K., Arita M., Yajima N., Takenaka C., Suzuki T. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal. 2019;12(587):eaaw3306. doi: 10.1126/scisignal.aaw3306. [DOI] [PubMed] [Google Scholar]
- Yang F., Xue F., Guan J., Zhang Z., Yin J., Kang Q. Stromal-cell-derived factor (SDF) 1-alpha overexpression promotes bone regeneration by osteogenesis and angiogenesis in osteonecrosis of the femoral head. Cell. Physiol. Biochem. 2018;46(6):2561–2575. doi: 10.1159/000489684. [DOI] [PubMed] [Google Scholar]
- Yang S.-C., Wu C.-H., Tu Y.-K., Huang S.-Y., Chou P.-C. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin increases the activation of aryl hydrocarbon receptor and is associated with the aggressiveness of osteosarcoma MG-63 osteoblast-like cells. Oncol. Lett. 2018;16(3):3849–3857. doi: 10.3892/ol.2018.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Cecil J., Peng S.B., Schrementi J., Kovacevic S., Paul D. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–179. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Yun C., Katchko K.M., Schallmo M.S., Jeong S., Yun J., Chen C.H. Aryl hydrocarbon receptor antagonists mitigate the effects of dioxin on critical cellular functions in differentiating human osteoblast-like cells. Int. J. Mol. Sci. 2018;19(1):225. doi: 10.3390/ijms19010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Ou G., Hamrick M., Hill W., Borke J., Wenger K. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J. Bone Miner. Res. 2008;23(7):1118–1128. doi: 10.1359/JBMR.080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Qin Y.W., Brewer G., Jing Q. MicroRNA degradation and turnover: regulating the regulators. Wiley interdisciplinary reviews RNA. 2012;3(4):593–600. doi: 10.1002/wrna.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. (A,B) Kynurenine at 200 μM significantly downregulates secreted CXCL12 protein level in cell culture media of BMSCs isolated from (A) pooled 3 months-old and (B) pooled 18 months-old mice (n = 4). (C) Kynurenine at 200 μM significantly downregulates CXCL12 mRNA level in BMSCs isolated from pooled 6 months-old mice after 24 and 48 h of treatment (n = 4). Data presented as mean ± SD. Data analysis was done using two way ANOVA for panel C, and using unpaired t-test for panels A and B. *P < 0.05, **P < 0.01, ***p < 0.001, ****p < 0.0001.
Supplementary Fig. 2. (A) Kynurenine at 200 μM significantly inhibits early osteogenic differentiation of BMSCs isolated from pooled 6 months-old mice as shown by Alizarin Red staining after 14 days of treatment (n = 6). (B) Kynurenine (50, 100, 200 μM) downregulates CXCL12 level in cell culture media of human BMSCs (74 year-old female) after 24 h of treatment, and AhR antagonist, CH-223191 (2 μg/mL), significantly rescues CXCL12 level with the 50 μM dose (n = 3). (C) Effect of kynurenine at different doses (10, 50, 200 μM) on cell density of BMSCs isolated from pooled 6 months-old mice as shown by crystal violet assay shows no changes in cell density. (D) Optical density of alizarin red osteogenic assay staining and corresponding wells of BMSCs isolated from 6 months-old mice after treatment with different doses of kynurenine (10, 25, 50, 100 μM) for 21 days shows inhibition of osteogenesis at all KYN doses (n = 6). Data presented as mean ± SD. Data analysis was done using one way ANOVA for panel C, using two way ANOVA for panels B and D, and using unpaired t-test for panel A. *P < 0.05, **P < 0.01. ***p < 0.001, ****p < 0.0001.
Supplementary Fig. 3. miR-29b-1-5p is upregulated in culture-expanded human CD271+ BMSCs (passage one) with aging or in response to KYN treatment. Transcript analysis using cultured MSCs and specific miRNA primers showed that miR-29b-1-5p was significantly upregulated in Aged (>60 year-old) relative to adult (26-59 year-old) human MSCs, while no changes in the mature miR-29b-1-3p were observed. Data are shown as means ± SEM. (n = 7 subjects per age group). *P < 0.05.