Abstract
Background
Free abdominal fat transfer is commonly used to restore facial volume and improve cosmesis after parotidectomy for pleomorphic adenomas. We describe the radiographic characteristics of these grafts on follow-up imaging.
Methods
Medical records of four patients who underwent parotidectomy with abdominal fat graft in 2016 and had follow up imaging available were retrospectively analyzed. An otolaryngologist and neuroradiologist reviewed imaging studies, evaluated the fat grafts, and monitored for residual or recurrent disease.
Results
The abdominal fat was successfully grafted in all four patients. Post-operative baseline magnetic resonance imaging and additional surveillance imaging showed fat grafts with minimal volume loss. However, there was development of irregular enhancement consistent with fat necrosis in two of the four patients.
Conclusions
Radiographic surveillance of free fat graft reconstruction after pleomorphic adenoma resection shows minimal contraction in size but development of fat necrosis. Recognition of expected changes should help avoid confusion with residual or recurrent disease, reassuring both patient and treating physician.
Keywords: Surgery, Oncology, Eye-ear-nose-throat, Medical imaging, Clinical research, Abdominal fat graft, Radiograph, Surveillance, Parotid, Pleomorphic adenoma
Surgery; Oncology; Eye-ear-nose-throat; Medical imaging; Clinical research; Abdominal fat graft; Radiograph; Surveillance; Parotid; Pleomorphic adenoma.
1. Introduction
Free fat grafts have been used since the 1970s to restore volume defects after parotidectomy in both benign and malignant disease for improved cosmesis and to prevent Frey syndrome [1, 2, 3, 4, 5]. Initially, fat grafts were not used in the immediate reconstruction due to concerns of complicating disease monitoring. Curry et al. and Conger et al. have shown, however, that free fat grafts can be effectively used in reconstruction after resection of malignant tumor without interfering with tumor surveillance [6, 7]. Free fat grafts have also been shown to be viable post-radiation and to be distinguishable from other structures on magnetic resonance imaging (MRI) [8].
We hypothesize that placing fat grafts in the parapharyngeal space after deep parotid lobe tumor resection is advantageous during radiographic surveillance for potential recurrence. Given their radiographically distinct characteristics, fat grafts may provide guidance on relative location and extent of recurrence, including whether the tumor is adjacent to the main trunk of the facial nerve or the great vessels. This information has the potential to be particularly useful in patient education and planning the proper surgical approach, especially for pleomorphic adenomas, which are the most common parotid tumors and the most common diagnosis in our clinic. Prior studies have not considered this additional non-cosmetic use of free fat grafts to aid in surveillance and surgical planning in recurrent disease.
We report four adult patients who underwent deep parotid lobe pleomorphic adenoma resection with placement of fat grafts in the parapharyngeal space. All cases were staffed by the senior author, and all radiographs were reviewed by an otolaryngologist and a neuroradiologist. The MRI appearance of the free fat grafts at post-operative baseline imaging and additional follow-up imaging is reported. This study met criteria for exemption from the Stanford University Institutional Review Board.
2. Case report
2.1. Case 1
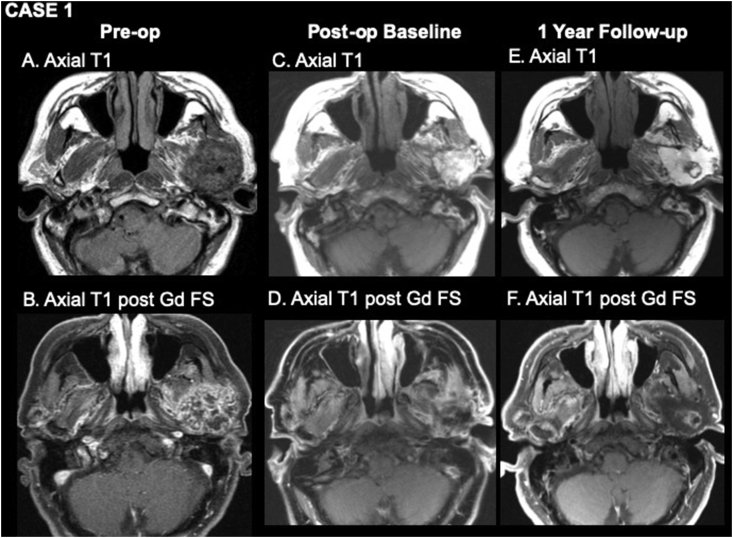
A 66-year-old male presented with a long-standing parotid mass. He had undergone parotid surgery with incomplete excision 20 years earlier. Fine needle aspiration (FNA) cytology of the residual lesion was indeterminate. Pre-operative MRI showed a left parotid mass measuring 27 × 36 × 41 mm with heterogeneous enhancement involving both superficial and deep lobes (Figure 1A,B). The patient underwent a left total parotidectomy with dissection of the parapharyngeal space and infratemporal fossa. A partial mandibulectomy was performed to improve access and ensure adequate margins as the mandibular condyle and subcondyle were enveloped by the tumor. An abdominal fat graft was placed in the parapharyngeal space, and a local rotational flap using the temporal-parietal fascia was used to secure and vascularize the fat graft. Final histopathologic diagnosis was consistent with pleomorphic adenoma. The one-month post-operative baseline MRI showed a viable fat graft measuring 34 × 31 × 29 mm and expected post-surgical changes with no evidence of gross residual disease (Figure 1C,D). The one-year post-operative MRI revealed a fat graft measuring 40 × 29 × 32 mm with no significant change in volume, but there was an irregular ring of central enhancement consistent with fat necrosis in the middle of the graft (Figure 1E,F). The three-year post-operative MRI (not shown) revealed a stable size of the fat graft measuring 42 × 29 × 32 mm with slightly decreasing, irregular central enhancement consistent with evolving fat necrosis.
Figure 1.
Pre-operative (A, B), one-month post-operative (C, D), and one year post-operative (E, F) MR imaging. A, axial T1WI shows a rounded mass of left parotid gland consistent with pleomorphic adenoma. B, axial T1WI post-gadolinium with fat suppression shows heterogeneous enhancement of the mass. C, axial T1WI from baseline post-operative study shows a homogeneously bright fat graft. D, axial T1WI post-gadolinium with fat suppression shows low signal where fat has been suppressed and mild enhancement around the periphery of the graft due to post-surgical change. E, axial T1WI one year after surgery shows a rounded lesion with intermediate signal in the center of the fat graft. F, axial T1WI post-gadolinium with fat suppression shows enhancement of the central lesion consistent with fat necrosis. MR = magnetic resonance; WI = weighted image.
2.2. Case 2
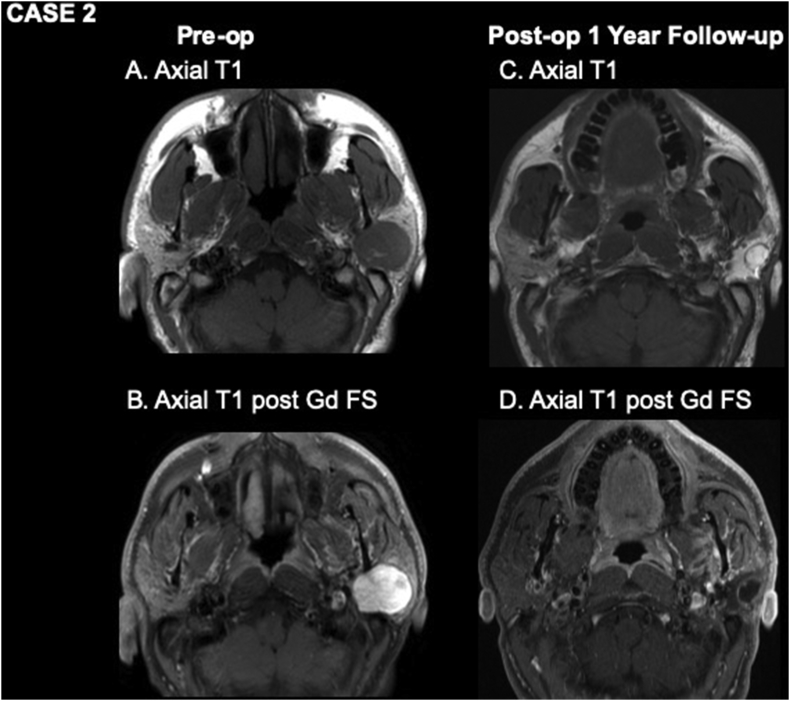
A 39-year-old male presented with a two-year history of left parotid mass. FNA cytology was consistent with pleomorphic adenoma, and pre-operative MRI showed a left 33 × 27 × 25 mm solid mass involving the superficial and deep lobes of the parotid (Figure 2A,B). The patient underwent left total parotidectomy with complete excision of the tumor and an abdominal fat graft was placed lateral to the facial nerve. Final histopathologic diagnosis was consistent with pleomorphic adenoma with negative margins. The patient did not comply with initial post-operative MRI. His one-year post-operative MRI (Figure 2C,D), however, showed a viable fat graft measuring 22 × 24 × 40 mm with a rounded area of irregular enhancement surrounding the central fat, consistent with fat necrosis and no evidence of residual or recurrent disease.
Figure 2.
Pre-operative (A, B) and one-year post-operative (C, D) MR imaging. A and B show a rounded, well circumscribed, homogeneously enhancing left parotid mass consistent with pleomorphic adenoma. C, axial T1WI one year after surgery shows a rounded lesion with intermediate signal around its margin and fat signal in its center centered on the lateral aspect of the fat graft. D, axial T1WI post-gadolinium with fat suppression shows enhancement of the rim of the lesion, consistent with fat necrosis.
2.3. Case 3
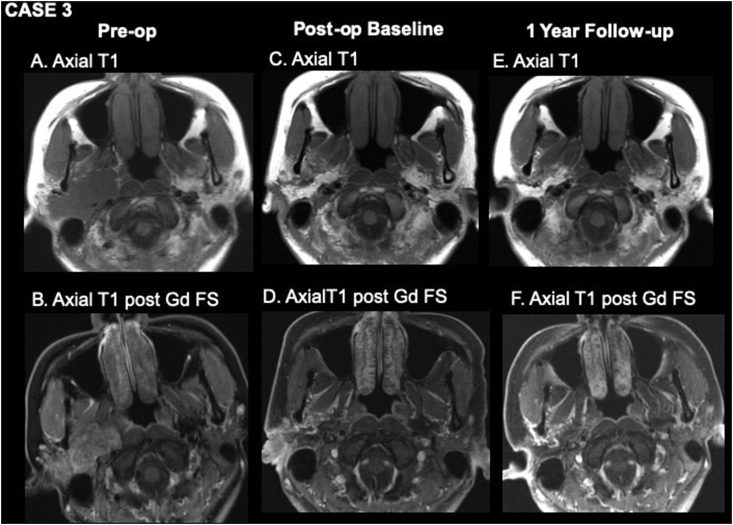
A 31-year-old female presented with a two-year history of right parotid mass. FNA was consistent with pleomorphic adenoma, and the pre-operative MRI showed a 53 × 25 × 46 mm lobulated lesion involving both superficial and deep lobes of the parotid (Figure 3A,B). The patient underwent right total parotidectomy with extensive parapharyngeal and infratemporal fossa dissection. An abdominal fat graft was placed overlying the carotid artery and jugular vein in the parapharyngeal space as well as the preauricular space covering the facial nerve. The three-month post-operative baseline MRI showed expected surgical changes with the fat graft measuring 41 × 14 × 35 mm (Figure 3C,D). At her one-year post-operative MRI, the fat graft was stable, measuring 36 × 12 × 31 mm; some irregular enhancement was present along the peripheral margin of the graft, attributed to fat necrosis versus post-operative change. No residual or recurrent disease was seen on imaging (Figure 3E,F).
Figure 3.
Pre-operative (A, B), three-month post-operative (C, D), and one-year post-operative (E, F) MR imaging. A, axial T1WI shows a large lobulated mass arising from the right parotid gland consistent with pleomorphic adenoma. B, axial T1WI post-gadolinium with fat suppression shows moderate homogeneous enhancement of the mass. C, axial T1WI from baseline post-operative study shows a homogeneously bright fat graft oriented obliquely along the resection bed. D, axial T1WI post-gadolinium with fat suppression shows low signal where fat has been suppressed and mild enhancement around the periphery of the graft due to post-surgical change. E, axial T1WI one year after surgery shows a stable appearance of the fat graft. F, axial T1WI post-gadolinium with fat suppression shows suppression of the fat signal of the graft and no evidence of fat necrosis.
2.4. Case 4
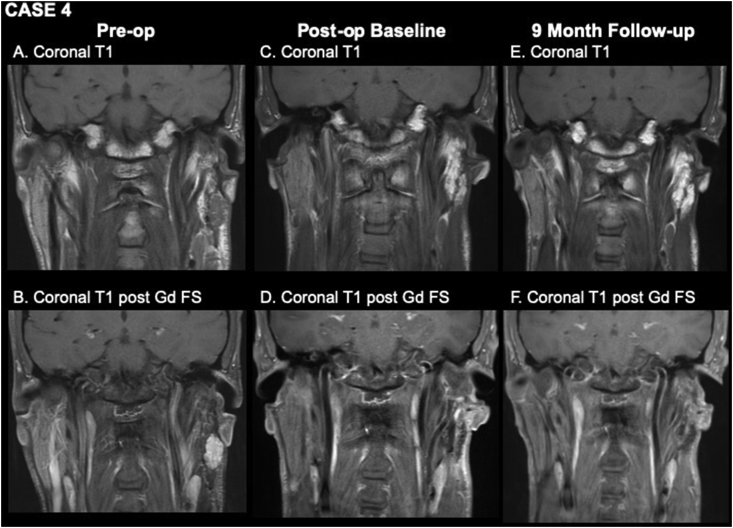
A 33-year-old male with history of prior left parotidectomy at another center for pleomorphic adenoma presented with a slowly enlarging nodule in his left parotid gland, and follow-up MRI and subsequent FNA were consistent with recurrent pleomorphic adenoma, measuring 26 × 25 × 20 mm (Figure 4A,B). The patient underwent left revision parotidectomy with abdominal fat graft. The three-month post-operative baseline MRI showed the expected appearance of the fat graft (Figure 4C,D). Follow-up imaging at nine months post-revision showed a stable fat graft without clear evidence of fat necrosis and no evidence of residual or recurrent tumor (Figure 4E,F).
Figure 4.
Pre-operative (A, B), three-month post-operative (C, D), and nine-month post-operative (E, F) MR imaging. A, coronal T1WI shows a lobulated mass arising from the residual left parotid gland consistent with recurrent pleomorphic adenoma. B, coronal T1WI post-gadolinium with fat suppression shows moderate homogeneous enhancement of the mass. C, coronal T1WI from baseline post-operative study shows a homogeneously bright fat graft in the resection bed. D, coronal T1WI post-gadolinium with fat suppression shows low signal where fat has been suppressed. E, coronal T1WI one year after surgery shows a grossly stable appearance of the fat graft. F, coronal T1WI post-gadolinium with fat suppression shows suppression of the fat signal of the graft and no evidence of fat necrosis.
3. Discussion
We present four cases of pleormorphic adenoma who underwent parotidectomy and abdominal fat graft for volume reconstruction and review graft appearance on post-operative radiographic surveillance (Table 1). All cases were at significant risk for recurrence due to tumor location, size, or prior history of recurrence. Median age among these patients was 32 years. All four patients were diagnosed with pleomorphic adenoma and underwent parotidectomy and reconstruction with abdominal fat graft, and two of the four cases were revision cases. No patients, including revision cases, received post-operative radiation therapy. The free fat graft was harvested by making a curvilinear incision inferiorly to the umbilicus and in one large piece without the dermis using bovie cautery. The volume of fat that was placed within the parotidectomy defect was roughly equivalent or slightly larger than the size of the resection specimen. The fat graft was placed in the parotidectomy defect and secured with sutures to limit mobility. In cases where superficial and deep lobes of the parotid are involved, the fat graft is placed in the deep lobe and carried laterally to completely encircle the facial nerve. One patient (Case 1) experienced temporary facial nerve weakness of only the temporal branch but had full recovery at the one-year post-operative visit. There were no other complications, including wound infections, dehiscence, or Frey syndrome. All patients had a Jackson-Pratt drain at the primary site, and two patients (Case 2 and 3) also had a drain in the abdominal fat donor site; these were removed within one week after surgery.
Table 1.
Patient characteristics.
| ID | Age/Sex | Diagnosis | Pre-operative tumor size∗ | Tumor location | Baseline post-operative fat graft size∗ | One-year post-operative fat graft size∗ | Fat necrosis |
|---|---|---|---|---|---|---|---|
| 1 | 66/M | Recurrent pleomorphic adenoma | 27 × 36 × 41 mm | Deep lobe | 34 × 31 × 29 mm | 40 × 29 × 32 mm | Central |
| 2 | 39/M | Pleomorphic adenoma | 33 × 27 × 25 mm | Deep lobe | n/a | 22 × 24 × 40 mm | Central |
| 3 | 31/F | Pleomorphic adenoma | 53 × 25 × 46 mm | Deep lobe | 41 × 14 × 35 mm | 36 × 12 × 31 mm | Peripheral |
| 4 | 33/M | Recurrent pleomorphic adenoma | 26 × 25 × 20 mm | Deep lobe | 15 × 14 × 43 cm | 14 × 17 × 42 mm | Central |
Measurements: transverse x anterior-posterior x cranio-caudal.
We reviewed all available imaging from these patients and evaluated for tumor recurrence and the appearance of the abdominal fat graft. On MRI, fat has high signal intensity on spin-echo T1-weighted images, and its high signal intensity can be suppressed using fat suppression pulses. Using this distinct characterization of fat on MRI, the fat grafts were easily identified on all post-operative imaging studies and showed no or minimal resorption during our follow-up period. Two fat grafts, however, showed areas of irregular ring enhancement within the central aspect of the fat pad, surrounding an island of central fat, a finding that is consistent with fat necrosis.
The presence of fat necrosis is an important radiographic finding has not been previously characterized in this setting but potentially impacts post-operative radiographic surveillance for residual or recurrent tumor, as areas of irregular enhancement can be confused with persistent or recurrent disease. Prior studies support the use of fat grafts to restore volume and prevent Frey's syndrome. Our hypothesis-generating case series suggests an additional use of fat grafts as a spacer in the parapharyngeal space: the graft's T1 high signal enables better identification of intermediate-signal recurrences and also reduces the number of vital structures potentially in contact with future recurrences. Although there were no recurrences or re-operation in this case series, we hypothesize that the innate radiographic signal of the free fat grafts have the potential to provide spatial landmarks for segregating recurrent disease into compartments, thereby aiding in surgical access during re-operations. For example, a fat graft may help prevent a recurrent tumor in the parapharyngeal space from becoming adherent to both the great vessels and/or the main trunk of the facial nerve. This potential physical separation and facilitated imaging evaluation could potentially enable safer revision surgery and possible avoidance of adjuvant radiotherapy at a younger age. Revision parotidectomies have a high risk of facial nerve paralysis due to dense scar tissue that encases the nerve, leading to difficulty in identifying and isolating the nerve from scar tissue. The autologous fat grafts, unlike dermal allografts, likely stimulate less foreign body reaction in the wound bed and may provide better protection of the facial nerve during reoperation. This hypothesis requires further longitudinal studies with longer follow-up given recurrence after initial parotidectomy can take years until presentation.
Ambro et al. showed that fat grafts were visible on MRI three months after radiation therapy [8]. While Loyo et al. demonstrated minimal resorption of fat after 6 months, Herly et al. showed the average time to reach steady state was about 2.2 years, with an average of 50.6% of patients with graft retention [2, 9]. Although the steady state timing and retention of graft may vary, we believe the fat grafts can serve as an important spacer and marker for radiographic surveillance for years following surgery. Fat grafts in breast reconstruction have the potential for hindering cancer surveillance, as the graft can lead to calcifications that require more frequent mammography and breast biopsies [10]. This is unlikely to be relevant for parotid pleomorphic adenomas, as radiographic characteristics are unique among these tissues.
One limitation of this study is only 3 of the 4 patients underwent post-operative baseline imaging, making it difficult to establish a baseline for comparison with future imaging. In order to further evaluate the potential of abdominal fat grafts as a spacer and marker for radiographic surveillance, a larger cohort with longer follow-up is needed to determine the impact of fat grafts on surveillance and revision surgeries for recurrent disease.
4. Conclusion
In patients who undergo parotidectomy involving the parapharyngeal space/infratemporal fossa and who are reconstructed with an abdominal fat graft, radiographic surveillance of the fat pad may show early post-operative irregular enhancement within the fat pad due to fat necrosis, a finding that should not be misinterpreted as a post-operative complication or as tumor recurrence. Additionally, as the T1 bright signal of the fat on MRI stands in contrast to the typically intermediate signal intensity of residual or recurrent tumor, and the presence of fat grafts may facilitate earlier detection of residual/recurrent disease.
Declarations
Author contribution statement
Y. Lee: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
N. Fischbein: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
U. Megwalu, F. Baik, V. Divi and M. Kaplan: Analyzed and interpreted the data; Wrote the paper.
D. Sirjani: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nosan D.K., Ochi J.W., Davidson T.M. Preservation of facial contour during parotidectomy. Otolaryngol. Head Neck Surg. 1991;104(3):293–298. doi: 10.1177/019459989110400302. [DOI] [PubMed] [Google Scholar]
- 2.Loyo M., Gourin C.G. Free abdominal fat transfer for partial and total parotidectomy defect reconstruction. Laryngoscope. 2016;126(12):2694–2698. doi: 10.1002/lary.26025. [DOI] [PubMed] [Google Scholar]
- 3.Honeybrook A., Athavale S.M., Rangarajan S.V., Rohde S.L., Netterville J.L. Free dermal fat graft reconstruction of the head and neck: an alternate reconstructive option. Am. J. Otolaryngol. 2017;38(3):291–296. doi: 10.1016/j.amjoto.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Balasundaram I., Nasser N.A. Paraumbilical fat graft for the correction of contour deformity following parotidectomy and prevention of Frey syndrome. Int. J. Oral Maxillofac. Surg. 2016;45(3):380–382. doi: 10.1016/j.ijom.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Chan L.S., Barakate M.S., Havas T.E. Free fat grafting in superficial parotid surgery to prevent Frey's syndrome and improve aesthetic outcome. J. Laryngol. Otol. 2014;128(Suppl 1):S44–S49. doi: 10.1017/S0022215113001394. [DOI] [PubMed] [Google Scholar]
- 6.Conger B.T., Gourin C.G. Free abdominal fat transfer for reconstruction of the total parotidectomy defect. Laryngoscope. 2008;118(7):1186–1190. doi: 10.1097/MLG.0b013e31816dd2e9. [DOI] [PubMed] [Google Scholar]
- 7.Curury J.M., Fisher K.W., Heffelfinger R.N., Rosen M.R., Keane W.M., Pribitkin E.A. Superficial musculoaponeurotic system elevation and fat graft reconstruction after superficial parotidectomy. Laryngoscope. 2008;118(2):210–215. doi: 10.1097/MLG.0b013e3181581f94. [DOI] [PubMed] [Google Scholar]
- 8.Ambro B.T., Goodstein L.A., Morales R.E., Taylor R.J. Evaluation of superficial musculoaponeurotic system flap and fat graft outcomes for benign and malignant parotid disease. Otolaryngol. Head Neck Surg. 2013;148(6):949–954. doi: 10.1177/0194599812474969. [DOI] [PubMed] [Google Scholar]
- 9.Herly M., Orholt M., Glovinski P.V. Quantifying long-term retention of excised fat grafts: a longitudinal, retrospective cohort study of 108 patients followed for up to 8.4 years. Plast. Reconstr. Surg. 2017;139(5):1223–1232. doi: 10.1097/PRS.0000000000003237. [DOI] [PubMed] [Google Scholar]
- 10.Pinell-White X.A., Etra J., Newell M., Tuscano D., Shin K., Losken A. Radiographic implications of fat grafting to the reconstructed breast. Breast J. 2015;21(5):520–525. doi: 10.1111/tbj.12450. [DOI] [PubMed] [Google Scholar]