Abstract
Purpose
The study sought to determine whether the onset of canonical vocalizations in children with cochlear implants (CIs) is related to speech perception skills and spoken vocabulary size at 24 months postactivation.
Method
The vocal development in 13 young CI recipients (implanted by their third birthdays; mean age at activation = 20.62 months, SD = 8.92 months) was examined at every 3-month interval during the first 2 years of CI use. All children were enrolled in auditory–oral intervention programs. Families of these children used spoken English only. To determine the onset of canonical syllables, the first 50 utterances from 20-min adult–child interactions were analyzed during each session. The onset timing was determined when at least 20% of utterances included canonical syllables. As children's outcomes, we examined their Lexical Neighborhood Test scores and vocabulary size at 24 months postactivation.
Results
Pearson correlation analysis showed that the onset timing of canonical syllables is significantly correlated with phonemic recognition skills and spoken vocabulary size at 24 months postactivation. Regression analyses also indicated that the onset timing of canonical syllables predicted phonemic recognition skills and spoken vocabulary size at 24 months postactivation.
Conclusion
Monitoring vocal advancement during the earliest periods following cochlear implantation could be valuable as an early indicator of auditory-driven language development in young children with CIs. It remains to be studied which factors improve vocal development for young CI recipients.
Cochlear implants (CIs) provide deaf children robust access to sound, but even after consistent use, children continue to exhibit variability in spoken language skills (Niparko et al., 2010). Therefore, identifying early predictors for spoken language outcomes in children with CIs has been an area of great interest to many researchers and clinicians (Bavin et al., 2018; Boons et al., 2012; Castellanos et al., 2014; Connor et al., 2006; Percy-Smith et al., 2013). Early predictors indicate which factors are important and thus can inform intervention approaches for children with CIs.
For instance, an important predictor of spoken language outcomes is speech perception (i.e., the ability to segment, discriminate, categorize, and recognize the sounds that comprise spoken language). These skills are highly dependent on children's hearing experience and form the foundation of spoken language development (Houston & Warner-Czyz, 2018). Indeed, speech perception has been found to predict spoken language skills in children with CIs (Hunter et al., 2017). However, it is challenging to assess speech perception skills in infants with CIs because established tests of speech perception require children to follow instructions—something infants cannot do (Eisenberg et al., 2006). For infants, new tools are being developed to assess speech perception (Houston et al., 2007; Jusczyk & Aslin, 1995; Kemler Nelson et al., 1995; McMurray & Aslin, 2005; Uhler et al., 2015; Werker et al., 1997). However, they are still in experimental stages and have significant challenges to overcome in terms of their reliability and cognitive demands (Cristia et al., 2016; Uhler et al., 2017 , 2011). Thus, there is still a need to explore methods of estimating speech perception skills during infancy.
The study reported here investigates the possibility that the onset of the production of canonical syllables may serve as an estimate of infants' speech perception abilities. Speech production skills can be reliably assessed at very young ages (Ertmer & Jung, 2012b; Ertmer et al., 2007; Nathani et al., 2006; Oller & Eilers, 1988; Walker & Bass-Ringdahl, 2008), and as reviewed below, perception and production skills have been found to be closely coupled in children with normal hearing (NH; Bruderder et al., 2015; D'Ausilio et al., 2012; DePaolis et al., 2011; Imada et al., 2006; Skipper et al., 2017), raising the possibility that assessment of early speech productions skills may serve as an estimate of speech perception and a predictor for later language outcomes (Walker & Bass-Ringdahl, 2008). However, little is known about the relationship between speech perception and speech production skills in children with CIs. We investigated the relationship between early speech production (i.e., vocalizations) and later perception skills to determine the extent to which these processes are tightly coupled in young children with CIs. If they are tightly coupled, then speech production measures may be a reliable indicator of CI children's speech perception skills and language development.
The Link Between Speech Perception and Speech Production in Children With NH and CIs
Many studies suggest that speech perception and speech production are integrally related with each other (Bruderer et al., 2015; D'Ausilio et al., 2012; DePaolis et al., 2011; Fernald et al., 2006; Imada et al., 2006; Kuhl et al., 2005; Marchman & Fernald, 2008; McCathren et al., 1999; McGillion et al., 2016; Pulvermüller et al., 2006; Skipper et al., 2017; Vihman et al., 2014; Whitehurst et al., 1991). One study by Imada et al. (2006) found that, when 6- and 12-month-olds with NH heard speech sounds, they showed a coupled activation in the auditory cortex and motor cortex (i.e., speech production areas). This coupled activation was not found either in newborns or with nonspeech stimuli, suggesting that it was not innate but instead acquired through the experience of hearing speech.
Infants accumulate speech perception experience not only by hearing the speech of others but also through their own vocalizations. Most NH infants begin producing canonical syllables (i.e., the combination(s) of adultlike consonant [C] and vowel [V] with rapid transition between the two segments, e.g., CV, CVCV; Fagan, 2015; Nathani et al., 2006; Oller & Eilers, 1988) at around 6 months of age (Nathani et al., 2006; Oller & Eiler, 1988). A particular feature of the vocalizations at this age is that infants frequently produce canonical syllables spontaneously (Fagan & Doveikis, 2017; Iyer et al., 2016; Majorano et al., 2014;). Fagan (2015) argued that the voluntary productions of canonical vocalization played a significant role in auditory skill development. Among canonical syllable types, Fagan focused on reduplicated syllables (e.g., /baba/, /dada/) and asserted that they are self-generated auditory stimuli that allow the infant to explore the link between perception and production. The study examined vocal development in young CI recipients (i.e., implanted before 16 months of age, M = 12.9 months, SD = 2.3 months, range: 8–16 months) at pre-implant and approximately 4.2 months postimplantation (SD = 2.6 months). Consistent with the previous finding that the production of canonical syllable is affected by children's hearing (Ertmer et al., 2007; Kishon-Rabin et al., 2005; Moeller et al., 2007; Oller & Eilers, 1988; Välimaa et al., 2019), Fagan found that canonical vocalizations increased after cochlear implantation. In addition, during a relatively short period after implantation, the young CI recipients increased their production of reduplicated syllables and matched the production frequency of their NH peers. Fagan argued that the rapid increase in production of this particular vocal type reflected children's interest in auditory feedback using self-generated stimuli. Thus, it is important to monitor the onset of canonical syllables, of which the main subtype is repetitive syllables, to understand auditory skill development. If the onset of canonical vocalizations represents infants' interest in exploring the auditory feedback of speech sounds, those who had the onset of canonical syllables relatively soon after CI would have more experience of actively listening to speech than those with a later onset. This indicator of active listening may explain the variability in spoken language outcomes better than simply the amount experience of using CIs.
The Link Between Prelinguistic Vocalizations and Spoken Language Outcomes
Vocal development has important clinical implications because it is strongly associated with language outcomes (Lohmander et al., 2017; Oller, 2000). Children's first words often incorporate syllable structures and sounds (e.g., vowels, consonants) they produced in prelinguistic vocalizations. Thus, limited production skills during prelinguistic vocalizations can result in the delay of lexical development (Oller et al., 1999; Stoel-Gammon, 1989; Vihman, 1993; Vihman et al., 1985). For example, a recent study found that children who produced two supraglottal consonants consistently early showed better vocabulary development than those who produced them later (McGillion et al., 2016). Similarly, other researchers have found that children with expressive language delays produce less diverse phonetic inventories in their prelinguistic vocalizations than children without language delays (Fasolo et al., 2008; Whitehurst et al., 1991).
Connection between prelinguistic vocalizations and later language outcomes has also been found in young CI recipients. Walker and Bass-Ringdahl (2008) examined the first year of vocal development in young CI recipients (mean age at activation = 18.21 months, range: 11–27 months) using a vocal assessment system, the Mean Babbling Level (Stoel-Gammon, 1989). The system assigns high scores to more advanced vocalizations. The sum is then divided by the total occasions of vocalizations to provide a measure of vocal complexity. The study showed that vocal complexity during and after the interval of 6–9 months postactivation predicted the speech-language outcomes at 4 years old. These findings indicated that advanced prelinguistic vocalizations during the first year of cochlear implantation are associated with better language outcomes. The Walker and Bass-Ringdahl findings were the first to show a positive relationship between a measure of vocal production and a measure of language outcomes in children with CIs. This study provides direct evidence that early measures of speech production may serve as a predictor of later outcomes. Given that it is important to determine whether children with CIs are on track to acquire speech and language after implantation as young as possible, a next step would be to investigate the possibility that even earlier measures of speech production (e.g., before 6 months postactivation) may predict later outcomes.
As the early predictor for later speech-language outcomes in children with CIs, there is another potential milestone in vocal development—the onset timing of canonical syllables. As mentioned in the previous section, the typical onset timing of canonical syllables is around 5–10 months of age for infants with NH, and it is quite robust across infant populations. The timing is unaffected by social economic status, prematurity, or exposure to multiple languages (Eilers et al., 1993; Nathani et al., 2006; Oller, 2000; Törölä et al., 2012). Typically hearing infants with delayed onset of canonical syllables are at high risk for speech-language–related disorders (Oller et al., 1998, 1999; Stoel-Gammon, 1989).
In contrast to the reliable timing of canonical syllable onset in children with NH, children with hearing loss frequently show delayed onset (Lynch, 1989; Oller & Eiler, 1988). If children use amplification (i.e., hearing aids or CIs), the age of canonical syllable onset is reduced but variable (Bass-Ringdahl, 2010). This variability is correlated with children's degree of aided hearing acuity. To show onset of canonical syllables, Bass-Ringdahl (2010) argues that children with hearing loss need a certain level of audibility (i.e., Speech Intelligibility Index ≥ .35). Notably, in Bass-Ringdahl's study, the Speech Intelligibility Index for all participants with CI was greater than .35 (range: .65–.93), which suggests that all of the CI recipients' hearing was sensitive enough to develop the canonical syllables.
However, even after receiving a CI, the onset timing of canonical syllables still shows enormous variability (Ertmer & Jung, 2012b). Schauwers et al. (2004) found that the onset timing (i.e., ≥ 20% of reduplicated CV or VC combinations) in 10 children who were implanted between 6 and 20 months of age ranged from pre-implant to 7 months postactivation. Kishon-Robin et al. (2005) found similar variability when assessing vocal development using parental reports. The large variability has been also found in a recent study using a vocal assessment tool specifying canonical syllables into two levels. Ertmer et al. (2012b) followed young CI recipients' vocal development during the first year after CI activation using the Consolidated Stark Assessment of Early Vocal Development–Revised (Consolidated SAEVD-R; Ertmer et al., 2007, 2013; Ertmer & Jung, 2012b; see Table 1). The Consolidated SAEVD-R differentiates canonical syllables into Basic Canonical Syllables (BCS) and Advanced Forms (AF). These two levels of vocalizations include rapid formant transition between segments as adult forms. However, they represent different development statuses; the syllable complexity in these two levels is different, and in typically developing children, the onset timing is 5–10 months for BCS and 9–18 months for AF. In fact, many other systems do not fully cover the AF level of vocalizations (Oller, 2000; Schauwers et al., 2004). Therefore, the BCS level is comparable to canonical syllables in previous studies using different classification systems (e.g., Oller & Eilers, 1988; Schauwers et al., 2004). Ertmer and Jung (2012b) demonstrated that there was a large variability in young CI recipients' onset timing of BCS (i.e., 1–12 months postactivation). As mentioned in the previous section, if the onset of canonical vocalizations represents the timing that infants enjoy self-exploration of the auditory feedback of speech sounds, the variability in the onset timing may explain why young CI recipients have variable speech perception ability with generally sufficient hearing sensitivity (Bass-Ringdahl, 2010). In addition, examining the onset timing of canonical syllables and its relation to later perception skill outcomes may reveal an important predictor for language outcomes that is available during the very early phase of CI use.
Table 1.
Consolidated Stark Assessment of Early Vocal Development–Revised (SAEVD-R).
| Consolidated SAEVD-R level (Ertmer et al., 2013) | SAEVD-R level (age of onset in typically developing children in months; Nathani et al., 2006) | Examples |
|---|---|---|
| Precanonical Syllables | Reflexive (0–2) | Vegetative sounds, crying, quasiresonant nuclei |
| Control of phonation (1–4) | Fully resonant nuclei, closant (consonant-like segment), isolated consonant, combination of vowel-like and closant segments | |
| Expansion (3–8) | Single or series of vowels, ingressive sounds, squeal, vowel glides | |
| Basic Canonical Syllables | Basic Canonical Syllables (5–10) | Single, or a series of consonant–vowel combination with rapid transition, whispered vocalizations |
| Advanced Forms | Advanced Forms (9–18) | Complex syllables, diphthongs, and jargons with varied intonation pattern |
Study Purpose and Research Questions
This study aimed to examine the underlying association between the onset timing of canonical syllables and speech perception to determine the potential of early speech production as a predictor for language outcomes in children with CIs. We hypothesized that children who demonstrated early onset of BCS would have better speech perception skills and better spoken language outcomes (i.e., spoken vocabulary). To address this hypothesis, three research questions were asked: (a) Is the onset timing of canonical syllables correlated with later speech recognition scores? (b) Is the onset timing of canonical syllables correlated with later spoken language outcomes measured by vocabulary size? (c) Does the onset timing of canonical syllables predict speech recognition and vocabulary size when controlling for other important factors known to contribute to outcomes (e.g., age at activation)?
Method
Participants
The current study included 13 young CI recipients who were implanted by their third birthday (mean age at activation = 21 months, SD = 8.92 months, range: 9–36 months). They were part of a 2-year longitudinal investigation of speech development after activation (see Ertmer & Jung, 2012b; Ertmer et al., 2013). All CI users had bilateral severe-to-profound or profound hearing loss. Six children were unilateral CI users: One child had bilateral CIs simultaneously, and five children received their second CIs sequentially during the 2 years. Parents, interventionists, and/or medical reports confirmed that these children had no additional disabilities.
To evaluate children's auditory accessibility during the study participation, we examined their audiometry results collected across 2 years. Each child provided, on average, 2.85 audiometric reports (SD = 1.21). To approximate aided hearing thresholds across the years, we averaged them (mean CI-aided hearing thresholds = 26.0 dB HL, SD = 5.74, range: 18–41.1 dB HL). See Table 2 for individual information. All children came from families that used spoken English only. In addition, these children were all enrolled in auditory–oral intervention programs. This study was approved by the institutional review board of Purdue University.
Table 2.
Audiological information and outcomes.
| Child | Gender | Age at activation in months (2nd CI) | Mean CI-aided hearing thresholds across 2 years (SF or better ear; dB HL) | Device model, processing strategy | BCS onset session | LNT Word (%) | LNT Phoneme (%) | CDI |
|---|---|---|---|---|---|---|---|---|
| CI01 | M | 12 | 24 | Freedom, ACE | 6 | 10.5 | 63.5 | 389 |
| CI02 | M | 21 | 22.3 | Freedom, ACE | 6 | 76.0 | 88.0 | 675 |
| CI03 | F | 25 | 32.5 | Freedom, ACE | 1 | 64.0 | NA | 680 |
| CI04 | F | 27 | 23 | Harmony, HiRes-P | 3 | 78.0 | 90.3 | 666 |
| CI05 | M | 26 | 22.3 | Freedom, ACE | 9 | 30.0 | 66.5 | 313 |
| CI06 | F | 36 | 18 | Freedom, ACE | 1 | 80.0 | 90.9 | 678 |
| CI07 | F | 36 | 24.7 | Freedom, ACE | 6 | 72.0 | 68.5 | 283 |
| CI08 | M | 9 (22) | 22.4 | Freedom, ACE | 6 | 80.0 | 89.5 | 542 |
| CI09 | M | 13 (13) | 41.1 | Freedom, ACE | 12 | 54.0 | 64.5 | 190 |
| CI10 | F | 13 (19) | 27.5 | Freedom, ACE | 3 | NA | NA | 631 |
| CI11 | F | 13 (27) | 27 | Freedom, ACE | 12 | 30.0 | 54.0 | 340 |
| CI12 | F | 18 (20) | 26.7 | Freedom, ACE | 3 | 64 | 83.5 | 533 |
| CI13 | F | 19 (38) | 27.7 | Harmony, HiRes-P | 9 | 60 | 82 | 383 |
|
M |
|
20.62 (23.17) |
26.08 |
|
|
58.21 |
76.51 |
484.85 |
| SD | 8.92 (8.57) | 5.74 | 22.97 | 13.31 | 175.37 |
Note. CI = cochlear implant; SF = sound field; BCS = Basic Canonical Syllables; LNT = Lexical Neighborhood Test (Kirk et al., 1999); CDI = MacArthur–Bates Communicative Development Inventories (Fenson et al., 2007); M = male; ACE = advanced combination encoder; F = female; NA = not available; HiRes-P = HiResolution-Paired.
Data Collection Procedure
Vocalization Samples
To collect children's vocalization samples, 20-min adult–child interactions were audio- and video-recorded every 3-month intervals after CI. Some children were available for pre-implant sessions (n =3) and early sessions (i.e., within a month after activation; n = 7). That is, the children who contributed to early sessions had their next sessions (i.e., 3 months postactivation) in 2 months, but after the 3-month postactivation session, the intervals were consistent. The current study included samples from the early sessions to 24 months postactivation (a total of 107 sessions).
The recordings were made using Sony mini-DVD camcorders (Model No. DCR-DVD504) and a wireless microphone (SONY; ECM-HW1). The children wore a specially designed vest with a pocket at chest holding the microphone (SONY; ECM-HW1), enabling the recording of children's vocal production at a consistently close distance. A standardized set of toys was provided to every child, including food items, books, a baby doll, soft blocks, toy animals, vehicles, and puzzles. Most sessions involved interactions between children and their parents (74.7%) across 2 years of CI use. When the parents were unavailable, the children interacted with their interventionists who were familiar to them. Only two children interacted with interventionists more than two sessions during the 1-year monitoring period.
The Onset of the BCS Level
From each session, all available utterances were parsed up to a maximum of 50 utterances for analyses. A total of 97 sessions (90.65%) contained more than 50 utterances. Ten sessions from five children contained fewer than 50 (range: 8–49). The utterances were separated by either audible breath or a silence of ≥ 1 s (Ertmer et al., 2013). Each utterance was then classified into three levels (Precanonical Syllables, BCS, and AF; see Table 1) following the Consolidated SAEVD-R (Ertmer et al., 2007, 2013). The proportion of BCS was our primary interest because this level contains various types of well-shaped CV combinations, including reduplicated syllables, and its emergence represents the effect of audition (Fagan, 2015; Oller & Eilers, 1988). Ertmer et al. (2012b, 2013) defined the onset of BCS to be when the child produced it at least 20% of utterances within an individual session. To confirm the reliability of the onset timing, we adapted the criteria so that this proportion (≥ 20%) was required to be achieved also in the subsequent session. 1 The total number of utterances included in the current study was 5,183. Five graduate research assistants, who had completed a Phonetic course and had been trained to use the Consolidated SAEVD-R, reviewed the DVDs of the adult–child interactions using headphones. Intra- and intercoder reliability were examined in approximately 8% of the randomly selected samples, using Cohen's kappa analysis. The κ value was .97 for intracoder reliability and .87 for intercoder reliability. According to McHugh (2012), a κ value between .8 and .9 represents strong agreement, and a value above .90 is almost perfect.
Lexical Neighborhood Tests
Children's speech recognition ability at 24 months postactivation was evaluated using the Lexical Neighborhood Test (LNT; Kirk et al., 1999, 1995). The LNT contains two 50 monosyllable word lists: easy and hard words. Each list provides two kinds of scores: the percentage of accurately identified phonemes and words. The test was conducted as a sound field test in a quiet audiometric booth with a monitored live voice (50 dB HL) provided by the children's school audiologists. Children were asked to repeat what they heard. One child missed the test appointment, and another had a reported score for words only; as a result, 11 children's scores were available for phonemes, and 12 were available for words.
MacArthur–Bates Communicative Development Inventories
Expressive vocabulary sizes of CI recipients were assessed using the MacArthur–Bates Communicative Development Inventories (CDI): Words and Sentences version (Fenson et al., 2007) at every 6-month interval after CI activation. This checklist contains 680 vocabulary items to be checked by parents, who marked CDI word items with different colored markers on one form at different intervals so that they did not have to repetitively fill in the same words at each interval. The current analyses included the vocabulary size at 24 months postactivation as a dependent variable. Thal et al. (2007) demonstrated that there were high correlations (range of r: .71–.88) between the scores measured by the CDI: Words and Sentences version and those by a standardized language assessment (i.e., the Reynell Developmental Language Scales; Reynell & Gruber, 1990), suggesting that it is a valid measure of language ability in young children with CIs.
Data Analysis
Pearson correlation analysis was conducted to test the relationship between BCS onset timing and both later speech recognition ability (LNT scores) and expressive vocabulary size at 24 months postactivation. Shapiro–Wilk test results indicated that the four variables were normally distributed. We have conducted paired t tests of the LNT scores of the easy word list and the hard word list to examine whether there was any difference in children's scores of the two lists. The results indicated that there was no significant difference between the two lists for words, t(11) = 1.448, p = .176, and for phonemes, t(10) = 0.864, p = .408. Therefore, the scores from the easy and hard lists were collapsed for word and phoneme scores. After examining the relationship between BCS onset timing and outcomes, regression models were conducted to determine whether BCS onset timing predicts outcomes independently from other factors that may affect both (i.e., age at activation, pre-CI hearing thresholds, bilateral implantation).
Results
Descriptive Analyses
Children's onset timing of BCS varied from 1 to 12 months postactivation (M = 5.92 months, SD = 3.73 months). Because the BCS level requires consonantal components (in CV combinations), we also examined children's consonant inventory at their BCS onset. The consonants were identified when they were produced greater than or equal to two times in a session. Bilabial voiced stop /b/ and nasal /m/ were the most commonly produced consonants (both produced by 12 of 13 children; see Iyer et al., 2017, for more information on phonetic inventories in young CI recipients). At BCS onset, children's accumulative consonant inventory size was, on average, 6.46 (SD = 3.18, range: 2–12). The LNT word scores (average of easy and hard lists) was 58.21 (SD = 22.97, range: 10.5–80). The mean score for LNT phoneme score (average of easy and hard lists) was 76.51 (SD = 13.31, range: 54.00–90.90). The mean vocabulary size at 24 months postactivation was 484.85 words (SD = 175.37, range: 190–680 words). The following section will present the correlation analyses and regression analyses in the order of our research questions.
The Correlation Analysis of the Onset Timing of BCS and Outcomes at 24 Months Postactivation
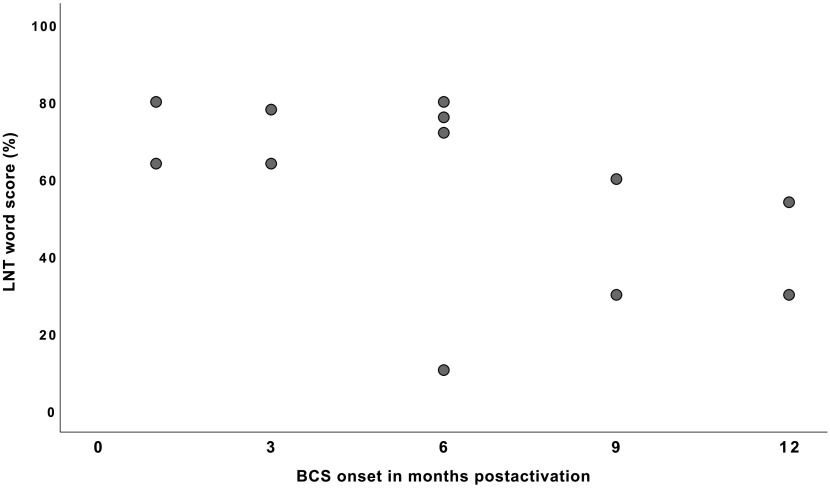
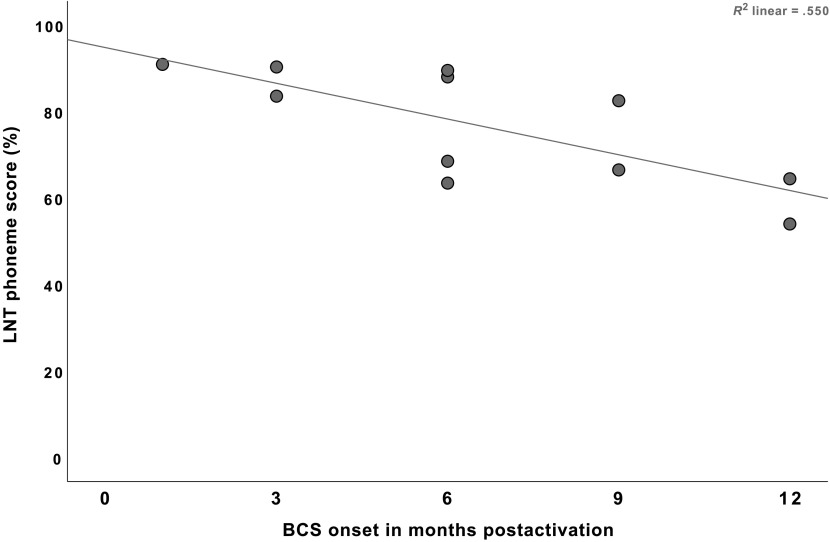
Since three correlation coefficients were calculated, we adopted an adjusted p value at .016. The results indicated that the LNT “word” scores were not correlated with the BCS onset, r = −.511, p = .090. Additional analyses were conducted for each LNT word list (i.e., “easy” and “hard” word lists) to examine whether the lack of correlation resulted from the fact that the scores were combined. No significant correlations were found. In contrast, the LNT “phoneme” recognition scores were significantly correlated to the onset of BCS, r = −.742, p = .009. That is, children who demonstrated BCS onsets earlier attained better phonemic recognition abilities at 24 months postactivation than those who demonstrated BCS onsets later. Figures 1 and 2 present the relationship between the onset timing of BCS and LNT scores (words and phonemes, respectively).
Figure 1.
Relationship between the onset timing of Basic Canonical Syllables (BCS) level and speech recognition scores measured by the Lexical Neighborhood Test (LNT) Words. Each dot represents individual scores. Note that the regression line was not marked because there was no significant relationship.
Figure 2.
Relationship between the onset timing of Basic Canonical Syllables (BCS) level and speech recognition scores measured by the Lexical Neighborhood Test (LNT) phonemes. Each dot represents individual scores.
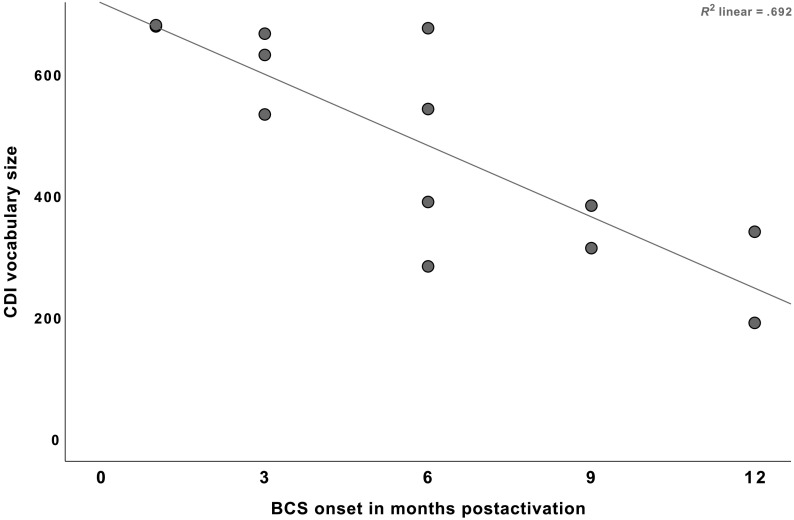
The onset of BCS was also significantly correlated with the vocabulary size at 24 months postactivation, r = −.832, p < .001. Children who demonstrated BCS onsets earlier showed larger vocabulary size at 24 months postactivation than those who showed the onset of the level later. Table 3 contains the results of Pearson correlation analysis of the relationship between the onset of BCS level and later outcomes. Figure 3 also presents the vocabulary size measured by the CDI at 24 months postactivation as the function of BCS onset timing.
Table 3.
Pearson correlations between Basic Canonical Syllable (BCS) onset timing and outcome measures at 24 months postactivation.
| Variable | BCS onset timing | LNT a Word scores | LNT Phoneme scores | CDI b |
|---|---|---|---|---|
| BCS onset timing | 1 | –.511 | –.742* | –.832* |
| LNT Word scores | 1 | .816* | .572 | |
| LNT Phoneme scores | 1 | .841* | ||
| CDI | 1 |
Lexical Neighborhood Test (Kirk et al., 1999).
MacArthur–Bates Communicative Development Inventories (Fenson et al., 2007).
p < .01.
Figure 3.
Relationship between the onset timing of Basic Canonical Syllables (BCS) level and vocabulary size measured by MacArthur–Bates Communicative Development Inventories (CDI). Each dot represents individual scores.
Multiple Regression Analyses: The Timing of BCS Onset as a Predictor for Outcomes at 24 Months Postactivation
We conducted a regression analysis to further understand the impact of the BCS onset timing on later outcomes. Children's vocal development and speech-language outcomes could be influenced by children's age or hearing sensitivity (Niparko et al., 2010; Oller & Eilers, 1988). Therefore, prior to conducting regression analyses, we explored the relationships of BCS onset timing with three independent variables—age at activation, the CI-aided hearing thresholds, and the use of bilateral CIs. Correlation analyses indicated that the BCS onset timing did not correlate significantly with age at activation or with the average of CI-aided hearing thresholds across 2 years (all ps > .156). With respect to a possible effect of bilateral CIs, four out of six bilateral CI users showed the onset of BCS before their second implantation (one child received both CIs simultaneously, and five were implanted sequentially). Therefore, it was not possible to identify the effect of bilateral CI use on the onset of BCS using the current data set. We also tested the effect of the three variables on the later outcomes (LNT and CDI) but did not find any significant correlations. However, given the fundamental importance of age at activation on speech-language outcomes (Connor et al., 2006; Houston & Miyamoto, 2010; Houston et al., 2012; Svirsky et al., 2009; Tomblin et al., 2005), we included the age at activation with the onset timing of BCS as predictors. Regression analyses were not conducted for LNT word scores because of the lack of correlation with BCS onset.
The results of regression analyses are displayed in Table 4. As can be seen in the table, BCS onset timing predicted a significant amount of variance in both LNT phoneme scores and CDI scores at 24 months postactivation independently from any effects of age at activation. In summary, the onset timing of BCS showed clear correlations with later phonemic recognition skills and spoken vocabulary size. The children who showed the early onset developed the perception skill and vocabulary better than those who showed the onset of the BCS level later. The regression analyses demonstrated that, controlling for the effect of age at activation, the onset timing of BCS predicted speech recognition skills and spoken vocabulary skills at 24 months postactivation.
Table 4.
Coefficients of the independent variables.
| Dependent variables | Independent variables | B | SE B | β | t | p |
|---|---|---|---|---|---|---|
| LNT a Phonemes | Age at activation | –0.211 | 0.384 | –.148 | –0.551 | .597 |
| BCS onset | –3.031 | 1.000 | –.817 | –3.032 | .016* | |
| CDI b | Age at activation | –3.975 | 3.597 | –.202 | –1.105 | .295 |
| BCS onset | –43.054 | 8.577 | –.916 | –5.020 | .002** |
Note. BCS = Basic Canonical Syllables.
Lexical Neighborhood Test (Kirk et al., 1999).
MacArthur–Bates Communicative Development Inventories (Fenson et al., 2007).
p < .025.
p < .01.
Discussion
The Relationship Between the Canonical Syllable Onset Timing and Speech Recognition
This study revealed a significant relationship between the onset timing of BCS and phoneme recognition scores in LNT at 24 months postactivation. CI recipients who showed the BCS onset relatively soon after activation showed better speech recognition skills after 2 years of CI use than those who demonstrated BCS onset relatively later. This finding supports the possibility that children who actively produced canonical syllables attend to the auditory feedback that they create to explore the auditory–motor link (Fagan, 2015). For instance, one child may show the BCS onset at 3 months postactivation; and another child, at 12 months postactivation. When both of them have 24 months of CI experience, the former child would have longer experience of actively using auditory feedback (i.e., 21 months) than the latter child (i.e., 12 months), and the increased experience may contribute to better phonemic perception skills. It was not clear why the onset timing of BCS was not related to LNT word scores. It is possible that these young children may still explore various phonemic representations and not necessarily focus on word levels. This aspect should be studied further. We also noted that young CI recipients showed individual variability not only in the BCS onset timing but also in the size of consonant inventory at the onset timing. Although the impact of consonant inventory on speech recognition skills was not within the scope of this study, we acknowledge that the relationship needs to be examined in future studies.
The Relationship Between the Onset Timing of Canonical Syllables and Spoken Language Outcomes Measured by Vocabulary Size
The findings of the current study also indicated that children who showed the onset of the BCS soon after CI activation demonstrated larger productive vocabularies than those who showed it later. This finding is in line with the Walker and Bass-Ringdahl (2008) finding that babbling complexity predicts later language outcomes. Given that parents respond more frequently to speech-related vocalizations (Warlaumont et al., 2014) and the quantity of child-directed input is correlated with children's spoken language ability (Weisleder & Fernald, 2013), producing vocalizations resembling speech sounds could be advantageous for the children to have a supportive environment for vocabulary learning. Studies on typically developing infants show that parents' contingent response improve infants' vocalizations (i.e., number and structure of vocalizations; Bloom et al., 1987; Goldstein et al., 2003; Goldstein & Schwade, 2008). In addition, a recent study using an eye-tracking paradigm by Goldstein et al. (2010) found that children who were provided contingent labels when they vocalized to the objects showed better novel word learning than those who were provided the labels when they did not produce vocalizations. They argued that children's vocalization may indicate an enhanced attention state. As the study did not specify vocalization types (i.e., canonical or precanonical), it was not clear how the quality of vocalization is related to novel word learning. However, the study does point to parental response as a possible mechanism by which children's prelinguistic vocal production is related to their perceptual learning. To our knowledge, there are no studies on the effect of parent–child interaction to facilitate vocal development in young children with CIs. The strategies to promote child's vocalizations toward speechlike forms should be studied further because there is potential that vocal production could accompany auditory skill development.
Additionally, we did not find that age at activation correlated with the onset of canonical syllables. This may be because all of our children were implanted at an early age (< 3 years). Given the relationship between the onset timing of BCS and speech recognition test scores, the finding is in line with previous studies showing that speech perception skills were relatively unaffected by age at CI in children implanted at early ages (Holt & Svirsky, 2008; Houston & Miyamoto, 2010; Leigh et al., 2013; Phan et al., 2016). We also did not find a relationship between CI-aided hearing thresholds and onset of canonical syllables. However, this is probably due to the small variance in hearing thresholds that CI provided (M = 26.09, SD = 5.74), which is in line with Bass-Ringdahl's (2010) finding that overall young CI recipients' hearing sensitivity was sufficient to develop canonical syllables. Future works with a larger sample of children could shed light on the role of audibility on the prelinguistic vocal development and speech-language outcomes.
Clinical Implications
Early vocal development was significantly related to later speech perception skills and spoken vocabulary size. The individual variability in the onset timing indicated that the emergence of well-shaped CV combinations is an important target to be monitored by clinicians. There are various commercially available assessment tools, but often, they incorporate quite extensive definitions for vocalization. Instead, some research-based measures, such as the Consolidated SAEVD-R or Mean Babbling Level (Stoel-Gammon, 1989), may be clinically applicable given that they have few levels to categorize. We acknowledge that, in clinical settings, time could be too limited to record and analyze additional speech samples. As an alternative, a short structured test such as the Conditioned Assessment of Speech Production (Ertmer & Jung, 2012a; Ertmer & Stoel-Gammon, 2008), which can be administered in about 10 min, may be feasible. Regardless of the measures, development of canonical syllables should be one of the early intervention targets once children have robust auditory access through CIs.
Future Directions and Limitations of the Study
Given our findings, it would be worth examining which factors contribute to the onset of BCS. Overall, the relationship between maternal speech input and children's language development is well established (DesJardin & Eisenberg, 2007; Gilkerson et al., 2018; Goldstein et al., 2010; Hart & Risley, 1995; Ramírez-Esparza et al., 2014; Romeo et al., 2018; Weisleder & Fernald, 2013). Moreover, adults respond more frequently to children's speechlike vocalizations than nonspeech-related vocalizations, and their feedback promotes children's following vocalizations to be speech related (i.e., “social feedback loop”; Warlaumont et al., 2014). Therefore, there might be a link between more responsive parents and rapid BCS onset timing in children with CIs, which is in line with better language outcomes. However, in the pediatric CI population, to our knowledge, no study has examined the effect of parental input on vocal development. Further investigation is warranted to understand the factors to promote rapid BCS onset after cochlear implantation.
Another possibility is that children's vocabulary ability at activation may affect vocal development: Children with larger vocabulary sizes at activation may have enhanced parent–child interactions, which may lead to earlier onset of BCS. Controlling for the effect of spoken vocabulary size at activation will remove the potential of the cascading effect of early language ability on later outcomes. The vocabulary size at activation may also influence children's speech recognition skills. Thus, controlling for the effect of early vocabulary skills will help us clearly understand the effects of the BCS onset timing as the indicator that children use auditory feedback from self-generated speech stimuli.
This study had a few limitations. First, the post-CI intervals at which we collected samples were every 3 months. Collecting samples more often, such as at monthly intervals, would enable us to more precisely examine onset timing of BCS. Second, we used expressive language outcome measures that require children's speech production (CDI spoken vocabulary and LNT). We were not able to include children who had additional disabilities (e.g., speech–motor challenges) because of the generic feature of the measures. By including receptive vocabulary and a speech perception testing that does not require children's motor skills, such as adopting tasks measuring outcomes through eye gaze behavior (e.g., Houston et al., 2007, 2012), we could extend our understanding to young CI recipients with additional disabilities.
Conclusion
Children who demonstrated earlier onset of BCS had better outcomes in phoneme recognition tasks and vocabulary skills than those who had later onsets of BCS. Thus, vocal development may be tracked as an early indicator of auditory-driven language development in young children with CIs. It remains to be studied which factors improve vocal development for young CI recipients.
Acknowledgments
This study was supported by a grant from the National Institutes of Health and the National Institute on Deafness and Other Communication Disorders (R01DC007863) awarded to David J. Ertmer. We give special thanks to Ertmer for the generous support for this study. We also appreciate Irina Castellanos for the insightful feedback on the article. This study was possible with the invaluable support from the staff of Child's Voice School (Wood Dale, IL), the St. Joseph Institute for the Deaf (Chesterfield, MO), the St. Joseph Institute for the Deaf (Indianapolis, IN), the Moog Center (Chesterfield, MO), and Ohio Valley Voice (Loveland, OH). We are also grateful for the effort of the research assistants who helped to collect, code, and analyze the data. Sincere appreciation is given to the children and parents who participated in the study.
Funding Statement
This study was supported by a grant from the National Institutes of Health and the National Institute on Deafness and Other Communication Disorders (R01DC007863) awarded to David J. Ertmer.
Footnote
One child produced the exact 20% of BCS within the first month post-activation; however, during the following 8 months, the proportions of BCS in this child were below 20%. Therefore, the onset was determined at 9 months postactivation session when the criteria were reached again and maintained at the following session.
References
- Bass-Ringdahl S. M. (2010). The relationship of audibility and the development of canonical babbling in young children with hearing impairment. Journal of Deaf Studies and Deaf Education, 15(3), 287–310. https://doi.org/10.1093/deafed/enq013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavin E. L., Sarant J., Leigh G., Prendergast L., Busby P., & Peterson C. (2018). Children with cochlear implants in infancy: Predictors of early vocabulary. International Journal of Language & Communication Disorders, 53(4), 788–798. https://doi.org/10.1111/1460-6984.12383 [DOI] [PubMed] [Google Scholar]
- Bloom K., Russell A., & Wassenberg K. (1987). Turn taking affects the quality of infant vocalizations. Journal of Child Language, 14(2), 211–227. [DOI] [PubMed] [Google Scholar]
- Boons T., Brokx J. P. L., Dhooge I., Frijns J. H. M., Peeraer L., Vermeulen A., Wouters J., & van Wieringen A. (2012). Predictors of spoken language development following pediatric cochlear implantation. Ear and Hearing, 33(5), 617–639. https://doi.org/10.1097/AUD.0b013e3182503e47 [DOI] [PubMed] [Google Scholar]
- Bruderer A. G., Danielson D. K., Kandhadai P., & Werker J. F. (2015). Sensorimotor influences on speech perception in infancy. Proceedings of the National Academy of Sciences of the United States of America, 112(44), 13531–13536. https://doi.org/10.1073/pnas.1508631112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos I., Kronenberger W. G., Beer J., Henning S. C., Colson B. G., & Pisoni D. B. (2014). Preschool speech intelligibility and vocabulary skills predict long-term speech and language outcomes following cochlear implantation in early childhood. Cochlear Implants International, 15(4), 200–210. https://doi.org/10.1179/1754762813Y.0000000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor C. M., Craig H. K., Raudenbush S. W., Heavner K., & Zwolan T. A. (2006). The age at which young deaf children receive cochlear implants and their vocabulary and speech-production growth: Is there an added value for early implantation? Ear and Hearing, 27(6), 628–644. https://doi.org/10.1097/01.aud.0000240640.59205.42 [DOI] [PubMed] [Google Scholar]
- Cristia A., Seidl A., Singh L., & Houston D. (2016). Test–retest reliability in infant speech perception tasks. Infancy, 21(5), 648–667. https://doi.org/10.1111/infa.12127 [Google Scholar]
- D'Ausilio A., Craighero L., & Fadiga L. (2012). The contribution of the frontal lobe to the perception of speech. Journal of Neurolinguistics, 25(5), 328–335. https://doi.org/10.1016/j.jneuroling.2010.02.003 [Google Scholar]
- DePaolis R. A., Vihman M. M., & Keren-Portnoy T. (2011). Do production patterns influence the processing of speech in prelinguistic infants? Infant Behavior & Development, 34(4), 590–601. https://doi.org/10.1016/j.infbeh.2011.06.005 [DOI] [PubMed] [Google Scholar]
- DesJardin J. L., & Eisenberg L. S. (2007). Maternal contributions: Supporting language development in young children with cochlear implants. Ear and Hearing, 28(4), 456–469. https://doi.org/10.1097/AUD.0b013e31806dc1ab [DOI] [PubMed] [Google Scholar]
- Eilers R. E., Oller K. D., Levine S., Basinger D., Lynch M. P., & Urbano R. (1993). The role of prematurity and socioeconomic status in the onset of canonical babbling in infants. Infant Behavior & Development, 16(3), 297–315. [Google Scholar]
- Eisenberg L. S., Johnson K. C., Martinez A. S., Cokely C. G., Tobey E. A., Quittner A. L. Fink N. E., Wang N.-Y., & Niparko J. K.(2006). Speech recognition at 1-year follow-up in the childhood development after cochlear implantation study: Methods and preliminary findings. Audiology & Neuro-Otology, 11(4), 259–268. https://doi.org/10.1159/000093302 [DOI] [PubMed] [Google Scholar]
- Ertmer D. J., & Jung J. (2012a). Monitoring progress in vocal development in young cochlear implant recipients: Relationships between speech samples and scores from the Conditioned Assessment of Speech Production (CASP). American Journal of Speech-Language Pathology, 21(4), 313–328. https://doi.org/10.1044/1058-0360(2012/11-0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertmer D. J., & Jung J. (2012b). Prelinguistic vocal development in young cochlear implant recipients and typically developing infants: Year 1 of robust hearing experience. Journal of Deaf Studies and Deaf Education, 17(1), 116–132. https://doi.org/10.1093/deafed/enr021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertmer D. J., Jung J., & Kloiber D. T. (2013). Beginning to talk like an adult: Increases in speech-like utterances in young cochlear implant recipients and typically developing children. American Journal of Speech-Language Pathology, 22(4), 591–603. https://doi.org/10.1044/1058-0360(2013/12-0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertmer D. J., & Stoel-Gammon C. (2008). The Conditioned Assessment of Speech Production (CASP): A tool for evaluating auditory-guided speech development in young children with hearing loss. The Volta Review, 108(1), 59–80. [Google Scholar]
- Ertmer D. J., Young N. M., & Nathani S. (2007). Profiles of vocal development in young cochlear implant recipients. Journal of Speech, Language, and Hearing Research, 50(2), 393–407. https://doi.org/10.1044/1092-4388(2007/028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan M. K. (2015). Why repetition? Repetitive babbling, auditory feedback, and cochlear implantation. Journal of Experimental Child Psychology, 137, 125–136. https://doi.org/10.1016/j.jecp.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan M. K., & Doveikis K. N. (2017). Ordinary interactions challenge proposals that maternal verbal responses shape infant vocal development. Journal of Speech, Language, and Hearing Research, 60(10), 2819–2817. https://doi.org/10.1044/2017_JSLHR-S-16-0005 [DOI] [PubMed] [Google Scholar]
- Fasolo M., Majorano M., & D'Odorico L. (2008). Babbling and first words in children with slow expressive development. Clinical Linguistics & Phonetics, 22(2), 83–94. https://doi.org/10.1080/02699200701600015 [DOI] [PubMed] [Google Scholar]
- Fenson L., Marchman V. A., Thal D. J., Dale P. S., & Reznick J. S. (2007). MacArthur–Bates Communicative Development Inventories: User's guide and technical manual. Brookes. [Google Scholar]
- Fernald A., Perfors A., & Marchman V. A. (2006). Picking up speed in understanding: Speech processing efficiency and vocabulary growth across the 2nd year. Developmental Psychology, 42(1), 98–116. https://doi.org/10.1037/0012-1649.42.1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkerson J., Richards J. A., Warren S. F., Oller D. K., Russo R., & Vohr B. (2018). Language experience in the second year of life and language outcomes in late childhood. Pediatrics, 142(4), e20174276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. H., King A. P., & West M. J. (2003). Social interaction shapes babbling: Testing parallels between birdsong and speech. Proceedings of the National Academy of Sciences of the United States of America, 100(13), 8030–8035. https://doi.org/10.1073/pnas.1332441100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. H., & Schwade J. A. (2008). Social feedback to infants' babbling facilitates rapid phonological learning. Psychological Science, 19(5), 515–523. https://doi.org/10.1111/j.1467-9280.2008.02117.x [DOI] [PubMed] [Google Scholar]
- Goldstein M. H., Schwade J., Briesch J., & Syal S. (2010). Learning while babbling: Prelinguistic object-directed vocalizations indicate a readiness to learn. Infancy, 15(4), 362–391. https://doi.org/10.1111/j.1532-7078.2009.00020.x [DOI] [PubMed] [Google Scholar]
- Hart B., & Risley T. R. (1995). Meaningful differences in the everyday experience of young American children. Brookes. [Google Scholar]
- Holt R. F., & Svirsky M. A. (2008). An exploratory look at pediatric cochlear implantation: Is earliest always best? Ear and Hearing, 29(4), 492–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. M., Horn D. L., Qi R., Ting J. Y., & Gao S. (2007). Assessing speech discrimination in individual infants. Infancy, 12(2), 119–145. https://doi.org/10.1111/j.1532-7078.2007.tb00237.x [DOI] [PubMed] [Google Scholar]
- Houston D. M., & Miyamoto R. T. (2010). Effects of early auditory experience on word learning and speech perception in deaf children with cochlear implants. Otology & Neurotology, 31(8), 1248–1253. https://doi.org/10.1097/MAO.0b013e3181f1cc6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. M., Stewart J., Moberly A., Hollich G., & Miyamoto R. T. (2012). Word learning in deaf children with cochlear implants: Effects of early auditory experience. Developmental Science, 15(3), 448–461. https://doi.org/10.1111/j.1467-7687.2012.01140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. M., & Warner-Czyz A. (2018). Speech perception and auditory development in infants with and without hearing loss. In Bar-On A. & Dorit R. (Eds.), Handbook of communication disorders. Theoretical, empirical, and applied linguistics perspectives (pp. 43–62). De Gruyter Mouton. [Google Scholar]
- Hunter C. R., Kronenberger W. G., Castellanos I., & Pisoni D. B. (2017). Early postimplant speech perception and language skills predict long-term language and neurocognitive outcomes following pediatric cochlear implantation. Journal of Speech, Language, and Hearing Research, 60(8), 2321–2336. https://doi.org/10.1044/2017_JSLHR-H-16-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada T., Zhang Y., Cheour M., Taulu S., Ahonen A., & Kuhl P. K. (2006). Infant speech perception activates Broca's area: A developmental magnetoencephalography study. NeuroReport, 17(10), 957–962. https://doi.org/10.1097/01.wnr.0000223387.51704.89 [DOI] [PubMed] [Google Scholar]
- Iyer S. N., Denson H., Lazar N., & Oller D. K. (2016). Volubility of the human infant: Effects of parental interaction (or lack of it). Clinical Linguistics & Phonetics, 30(6), 470–488. https://doi.org/10.3109/02699206.2016.1147082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S. N., Jung J., & Ertmer D. J. (2017). Consonant acquisition in young cochlear implant recipients and their typically developing peers. American Journal of Speech-Language Pathology, 26(2), 413–427. https://doi.org/10.1044/2016_AJSLP-16-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusczyk P. W., & Aslin R. N. (1995). Infants' detection of the sound patterns of words in fluent speech. Cognitive Psychology, 29(1), 1–23. https://doi.org/10.1006/cogp.1995.1010 [DOI] [PubMed] [Google Scholar]
- Kemler Nelson D. G., Jusczyk P. W., Mandel D. R., Myers J., Turk A., & Gerken L. (1995). The head-turn preference procedure for testing auditory perception. Infant Behavior & Development, 18(1), 111–116. https://doi.org/10.1016/0163-6383(95)90012-8 [Google Scholar]
- Kirk K. I., Eisenberg L. S., Martinez A. S., & Hay-McCutcheon M. (1999). Lexical Neighborhood Test: Test–retest reliability and interlist equivalency. Journal of the American Academy of Audiology, 10(3), 113–123. [Google Scholar]
- Kirk K. I., Pisoni D. B., & Osberger M. J. (1995). Lexical effects on spoken word recognition by pediatric cochlear implant users. Ear and Hearing, 16(5), 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishon-Rabin L., Taitelbaum-Swead R., Ezrati-Vinacour R., & Hildesheimer M. (2005). Prelexical vocalization in normal hearing and hearing-impaired infants before and after cochlear implantation and its relation to early auditory skills. Ear and Hearing, 26(4), 17S–29S. [DOI] [PubMed] [Google Scholar]
- Kuhl P. K., Conboy B. T., Padden D., Nelson T., & Pruitt J. (2005). Early speech perception and later language development: Implications for the “critical period.” Language Learning and Development, 1(3–4), 237–264. https://doi.org/10.1080/15475441.2005.9671948 [Google Scholar]
- Leigh J., Dettman S., Dowell R., & Briggs R. (2013). Communication development in children who receive a cochlear implant by 12 months of age. Otology & Neurotology, 34(3), 443–450. [DOI] [PubMed] [Google Scholar]
- Lohmander A., Holm K., Eriksson S., & Lieberman M. (2017). Observation method identifies that a lack of canonical babbling can indicate future speech and language problems. Acta Paediatrica, 106(6), 935–943. https://doi.org/10.1111/apa.13816 [DOI] [PubMed] [Google Scholar]
- Lynch M. P., Oller D. K., & Steffens M. (1989). Development of speech-like vocalizations in a child with congenital absence of cochleas: The case of total deafness. Applied Psycholinguistics, 10(3), 315–333. https://doi.org/10.1017/S0142716400008651 [Google Scholar]
- Majorano M., Vihman M. M., & DePaolis R. A. (2014). The relationship between infants' production experience and their processing of speech. Language Learning and Development, 10(2), 179–204. https://doi.org/10.1080/15475441.2013.829740 [Google Scholar]
- Marchman V. A., & Fernald A. (2008). Speed of word recognition and vocabulary knowledge in infancy predict cognitive and language outcomes in later childhood. Developmental Science, 11(3), F9–F16. https://doi.org/10.1111/j.1467-7687.2008.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCathren R. B., Yoder P. J., & Warren S. F. (1999). The relationship between prelinguistic vocalization and later expressive vocabulary in young children with developmental delay. Journal of Speech, Language, and Hearing Research, 42(4), 915–924. https://doi.org/10.1044/jslhr.4204.915 [DOI] [PubMed] [Google Scholar]
- McGillion M., Herbert J. S., Pine J., Vihman M., dePaolis R., Portnoy T. K., & Matthews D. (2016). What paves the way to conventional language? The predictive value of babble, pointing, and socioeconomic status. Child Development, 88(1), 156–166. https://doi.org/10.1111/cdev.12671 [DOI] [PubMed] [Google Scholar]
- McHugh M. L. (2012). Interrater reliability: The kappa statistic. Biochemia Medica, 22(3), 276–282. [PMC free article] [PubMed] [Google Scholar]
- McMurray B., & Aslin R. N. (2005). Infants are sensitive to within-category variation in speech perception. Cognition, 95(2), B15–B26. https://doi.org/10.1016/j.cognition.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Moeller M. P., Hoover B., Putman C., Arbataitis K., Bohnenkamp G., Peterson B., Wood S., Lewis D., Pittman A., & Stelmachowicz P. (2007). Vocalizations of infants with hearing loss compared with infants with normal hearing: Part I—Phonetic development. Ear and Hearing, 28(5), 605–627. https://doi.org/10.1097/AUD.0b013e31812564ab [DOI] [PubMed] [Google Scholar]
- Nathani S., Ertmer D. J., & Stark R. E. (2006). Assessing vocal development in infants and toddlers. Clinical Linguistics & Phonetics, 20(5), 351–369. https://doi.org/10.1080/02699200500211451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko J. K., Tobey E. A., Thal D. J., Eisenberg L. S., Wang N.-Y., Quittner A. L.. Fink N. E., & for the CDaCI Investigative Team. (2010). Spoken language development in children following cochlear implantation. Journal of the American Medical Association, 303(15), 1498–1506. https://doi.org/10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller D. K. (2000). The emergence of the speech capacity. Erlbaum. [Google Scholar]
- Oller D. K., & Eilers R. E. (1988). The role of audition in infant babbling. Child Development, 59(2), 441–449. https://doi.org/10.2307/1130323 [PubMed] [Google Scholar]
- Oller D. K., Eilers R. E., Neal A. R., & Cobo-Lewis A. B. (1998). Late onset canonical babblings: A possible early marker of abnormal development. American Journal on Mental, 103(3), 249–263. [DOI] [PubMed] [Google Scholar]
- Oller D. K., Eilers R. E., Neal A. R., & Schwartz H. K. (1999). Precursors to speech in infancy: The prediction of speech and language disorders. Journal of Communication Disorders, 32(4), 223–245. https://doi.org/10.1016/S0021-9924(99)00013-1 [DOI] [PubMed] [Google Scholar]
- Percy-Smith L., Busch G., Sandahl M., Nissen L., Josvassen J. L., Lange T., Rusch E., & Cayé-Thomasen P. (2013). Language understanding and vocabulary of early cochlear implanted children. International Journal of Pediatric Otorhinolaryngology, 77(2), 184–188. https://doi.org/10.1016/j.ijporl.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Phan J., Houston D. M., Ruffin C., Ting J., & Holt R. F. (2016). Factors affecting speech discrimination in children with cochlear implants: Evidence from early-implanted infants. Journal of the American Academy of Audiology, 27(6), 480–488. https://doi.org/10.3766/jaaa.15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F., Huss M., Kherif F., Moscoso del Prado Martin F., Hauk O., & Shtyrov Y. (2006). Motor cortex maps articulatory features of speech sounds. Proceedings of the National Academy of Sciences of the United States of America, 103(20), 7865–7870. https://doi.org/10.1073/pnas.0509989103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Esparza N., Sierra A. G., & Kuhl P. K. (2014). Look who's talking: Speech style and social context in language input to infants are linked to concurrent and future speech development. Developmental Science, 17(6), 880–891. https://doi.org/10.1111/desc.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynell J. K., & Gruber C. P. (1990). Reynell Developmental Language Scales. Western Psychological Services. [Google Scholar]
- Romeo R. R., Leonard J. A., Robinson S. T., West M. R., Mackey A. P., Rowe M. L., & Gabrieli J. D. E. (2018). Beyond the 30-Million-Word Gap: Children's conversational exposure is associated with language-related brain function. Psychological Science, 29(5), 700–710. https://doi.org/10.1177/0956797617742725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwers K., Gillis S., Daemers K., De Beukelaer C., & Govaerts P. J. (2004). Cochlear implantation between 5 and 20 months of age: The onset of babbling and the audiologic outcome. Otology & Neurotology, 25(3), 263. [DOI] [PubMed] [Google Scholar]
- Skipper J. I., Devlin J. T., & Lametti D. R. (2017). The hearing ear is always found close to the speaking tongue: Review of the role of the motor system in speech perception. Brain and Language, 164, 77–105. https://doi.org/10.1016/j.bandl.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Stoel-Gammon C. (1989). Prespeech and early speech development of two late talkers. First Language, 9(6), 207–223. https://doi.org/10.1177/014272378900900607 [Google Scholar]
- Svirsky M. A., Chin S. B., & Jester A. (2009). The effects of age at implantation on speech intelligibility in pediatric cochlear implant users: Clinical outcomes and sensitive periods. Audiological Medicine, 5(4), 293–306. https://doi.org/10.1080/16513860701727847 [Google Scholar]
- Thal D., DesJardin J. L., & Eisenberg L. S. (2007). Validity of the MacArthur–Bates Communicative Development Inventories for measuring language abilities in children with cochlear implants. American Journal of Speech-Language Pathology, 16(1), 54–64. https://doi.org/10.1044/1058-0360(2007/007) [DOI] [PubMed] [Google Scholar]
- Tomblin J. B., Barker B. A., Spencer L. J., Zhang X., & Gantz B. J. (2005). The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. Journal of Speech, Language, and Hearing Research, 48(4), 853–867. https://doi.org/10.1044/1092-4388(2005/059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törölä H., Lehtihalmes M., Heikkinen H., Olsén P., & Yliherva A. (2012). Early vocalization of preterm infants with extremely low birth weight (ELBW), part I: From birth to expansion stage. Clinical Linguistics & Phonetics, 26(4), 330–344. https://doi.org/10.3109/02699206.2011.636499 [DOI] [PubMed] [Google Scholar]
- Uhler K. M., Baca R., Dudas E., & Fredrickson T. (2015). Refining stimulus parameters in assessing infant speech perception using visual reinforcement infant speech discrimination: Sensation level. Journal of the American Academy of Audiology, 26(10), 807–814. https://doi.org/10.3766/jaaa.14093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler K., Warner-Czyz A., Gifford R., & . Working Group, P. (2017). Pediatric Minimum Speech Test Battery. Journal of the American Academy of Audiology, 28(3), 232–247. https://doi.org/10.3766/jaaa.15123 [DOI] [PubMed] [Google Scholar]
- Uhler K., Yoshinaga-Itano C., Gabbard S. A., Rothpletz A. M., & Jenkins H. (2011). Longitudinal infant speech perception in young cochlear implant users. Journal of the American Academy of Audiology, 22(3), 129–142. https://doi.org/10.3766/jaaa.22.3.2 [DOI] [PubMed] [Google Scholar]
- Välimaa T. T., Kunnari S. M., Laukkanen-Nevala P., & Ertmer D. J. (2019). Vocal Development in infants and toddlers with bilateral cochlear implants and infants with normal hearing. Journal of Speech, Language, and Hearing Research, 62(5), 1296–1308. [DOI] [PubMed] [Google Scholar]
- Vihman M. M. (1993). Variable paths to early word production. Journal of Phonetics, 21(1–2), 61–82. [Google Scholar]
- Vihman M. M., DePaolis R. A., & Keren-Portnoy T. (2014). The role of production in infant word learning, 64(s2), 121–140. https://doi.org/10.1111/lang.12058 [Google Scholar]
- Vihman M. M., Macken M. A., Miller R., Simmons H., & Miller J. (1985). From babbling to speech: A re-assessment of the continuity issue. Language, 61(2), 397–445. https://doi.org/10.2307/414151 [Google Scholar]
- Walker E. A., & Bass-Ringdahl S. (2008). Babbling complexity and its relationship to speech and language outcomes in children with cochlear implants. Otology & Neurotology, 29(2), 225–229. https://doi.org/10.1097/mao.0b013e31815f6673 [DOI] [PubMed] [Google Scholar]
- Warlaumont A. S., Richards J. A., Gilkerson J., & Oller D. K. (2014). A social feedback loop for speech development and its reduction in autism. Psychological Science, 25(7), 1314–1324. https://doi.org/10.1177/0956797614531023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder A., & Fernald A. (2013). Talking to children matters: Early language experience strengthens processing and builds vocabulary. Psychological Science, 24(11), 2143–2152. https://doi.org/10.1177/0956797613488145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker J. F., Polka L., & Pegg J. E. (1997). The conditioned head turn procedure as a method for testing infant speech perception. Early Development and Parenting, 6(3–4), 171–178. [Google Scholar]
- Whitehurst G. J., Smith M., Fischel J. E., Arnold D. S., & Lonigan C. J. (1991). The continuity of babble and speech in children with specific expressive language delay. Journal of Speech and Hearing Research, 34(5), 1121–1129. https://doi.org/10.1044/jshr.3405.1121 [DOI] [PubMed] [Google Scholar]