Abstract
Background
Dysmenorrhea affects approximately 80% of women in Japan and has a negative impact on health-related quality of life (HRQoL). Low-dose estrogen/progestin combined oral contraceptives have been shown to reduce the severity of dysmenorrhea symptoms. This study characterized HRQoL in Japanese women with dysmenorrhea before and after ethinylestradiol/drospirenone (EE/DRSP) treatment.
Methods
This prospective, observational study recruited 531 patients, of which 186 were evaluated after treatment with EE 20 μg/DRSP 3 mg for dysmenorrhea in a 24/4 cyclic regimen. The primary endpoints were mean baseline and post-treatment 36-Item Short-Form Health Survey version 2.0 (SF-36v2) scores for study patients compared with the general female population of Japan (calculated using norm-based scoring), and mean changes in study patient SF-36v2 scores between baseline and 6 to 8 treatment cycles.
Results
Compared with Japanese norms, women with dysmenorrhea had lower pre-treatment SF-36v2 scores, except for the physical functioning domain. After 6–8 cycles of EE/DRSP treatment, all 8 SF-36v2 domain scores were significantly higher than baseline. The greatest improvements were observed in bodily pain and social functioning (mean change [standard deviation (SD)]: physical functioning: 1.4 [5.7], role physical: 3.2 [8.1], bodily pain: 7.8 [10.0], general health: 3.0 [7.0], vitality: 2.7 [8.1], social functioning: 3.5 [9.8], role emotional: 3.3 [9.2], and mental health: 3.0 [7.3]; p< 0.001 for all). Compared with the Japanese general population, study patients’ post-treatment scores were significantly higher for the general health domain (p= 0.008) and physical summary scores (p= 0.033).
Conclusion
Dysmenorrhea has a profound impact on all aspects of functioning and well-being. This study, conducted in a real-world setting, found that physical, social, and mental HRQoL improved significantly after a cyclic regimen of EE/DRSP in Japanese patients with dysmenorrhea. This regimen may have the potential to provide an effective option to improve patient HRQoL.
Trial Registration
Study sample was drawn from patients enrolled in a post-marketing surveillance study, registered June 20, 2011 (NCT 01375998).
Keywords: patient-reported outcomes, SF-36, dysmenorrhea, quality of life, ethinylestradiol/drospirenone, Japan
Introduction
Dysmenorrhea, which is the most prevalent gynecological condition worldwide, affects approximately 45–95% of women globally1 and 80% of women in Japan.2 Primary dysmenorrhea is characterized by spasmodic, cramping menstrual pain and discomfort in the absence of pelvic pathology,1 while secondary dysmenorrhea is associated with specific pelvic pathology, such as endometriosis or uterine fibroids.3 Dysmenorrhea is most severe during the initial 24–48 hours and can last up to 72 hours. Onset typically occurs on the first day of menstruation or just before; pain is typically focused in the abdominal region and can also extend to the thighs and back. Other symptoms include diarrhea, fatigue, and insomnia.1 In a survey of Japanese women (N=3,941), one-third characterized their dysmenorrhea as severe and generally required analgesic use.2
Women with dysmenorrhea may experience impaired functioning and a substantial negative impact on health-related quality of life (HRQoL); this burden exists worldwide. In research from the US, China, and Turkey, women and adolescents with dysmenorrhea scored significantly lower than those without dysmenorrhea on the following quality of life (QoL) domains in the 36-Item Short-Form Health Survey (SF-36): bodily pain, general health perceptions, role functioning due to physical limitations, physical functioning, and social functioning.4–6 Women and adolescents in Brazil, Malaysia, Mexico, and Turkey have also reported a negative impact of dysmenorrhea on friendships and family relationships, work/school performance and absenteeism, and social and recreational activities, with increased severity of dysmenorrhea further exacerbating these HRQoL effects.7–10 Indeed, for some women, the impact of dysmenorrhea on daily life is comparable to that of other major medical conditions. In a study among menstruating female veterans (N=1,744) in the US, the difference in SF-36 domain scores between women who reported menstrual symptoms and those who reported none was similar to the difference found between patients with and without angina and arthritis.4
Additionally, dysmenorrhea poses a considerable economic burden due to its impact on work productivity and health-care resource utilization.1,11 Results from a large survey of Japanese women (N=19,254) showed that severe menstrual pain and heavy bleeding were associated with increased outpatient medical visits and decreased work productivity.11 Based on a retrospective analysis of health insurance data, mean total health-care costs among Japanese women with primary dysmenorrhea were 2.2 times higher than matched controls.12 In Japan, the annual economic burden due to menstrual symptoms has been estimated at 683 billion Japanese Yen (¥) ($8.6 billion US; 2010 data). Work productivity loss accounts for 72% of this (¥491.1 billion), and outpatient medical care and drug therapy cost ¥93.0 and ¥98.7 billion per year, respectively.11
Low-dose estrogen/progestin (LEP) combined oral contraceptives have been shown to reduce the severity of dysmenorrhea symptoms.3,12,13 In Japan, a 28-day cyclic regimen and a flexible extended regimen of ethinylestradiol/drospirenone (EE/DRSP) were approved for this indication in 2010 and 2016, respectively, and the combination ethinylestradiol/norethisterone was approved in 2013.3,14,15 The Japan Society of Obstetrics and Gynecology (JSOG) and the Japan Association of Obstetricians and Gynecologists (JAOG) guidelines for gynecologic practice recommend nonsteroidal anti-inflammatory drugs, LEPs, and/or levonorgestrel-releasing intrauterine systems as first-line treatment for primary dysmenorrhea; traditional Chinese medicines or anti-cramping medicines may also be used.12,16
While the safety and efficacy of LEPs for the treatment of dysmenorrhea in Japanese women have been verified,3,13 the effectiveness of these drugs to improve HRQoL in a real-world Japanese setting has yet to be evaluated. The objective of this study was to assess HRQoL in Japanese women with dysmenorrhea treated with EE 20 μg/DRSP 3 mg. Secondary objectives included exploratory analyses to assess whether patient characteristics (dysmenorrhea etiology and severity, patient age, and the presence of physical and/or mental discomfort) have an effect on mean changes in SF-36v2 domain scores.
Patients and Methods
Patient Population
The study included women in Japan who received a cyclic regimen of EE 20 μg/DRSP 3 mg (Bayer Yakuhin Ltd., Osaka, Japan) for the treatment of dysmenorrhea. The study sample was drawn from patients enrolled in a post-marketing surveillance (PMS) study (NCT01375998).17 The objective of the original PMS study was to assess the safety and efficacy of EE/DRSP in clinical practice settings. Women who had been prescribed EE/DRSP for dysmenorrhea were followed for 3 years subsequent to treatment initiation. Primary outcomes of the original PMS study included the incidence of adverse drug reactions, severe adverse events, and changes in dysmenorrhea severity between baseline and the sixth menstrual cycle. Secondary outcomes of the original study included the incidence of adverse events, unpleasant physical and/or psychological symptoms, use of analgesic drugs for dysmenorrhea, and HRQoL assessed using SF-36 version 2.0 (SF-36v2).
All treatment decisions were physician-directed and administered in the context of routine clinical practice. Women were advised to take one active tablet every day at the same time for 24 consecutive days, followed by 4 days of hormone-free tablets, for a 28-day treatment cycle. The next cycle began on the 29th day, irrespective of whether bleeding was finished. Patients were advised to adhere to Japanese prescribing information for EE/DRSP (YAZ [drospirenone 3 mg and ethinyl estradiol 20 μg]) and were excluded if they had been treated with an estrogen or estrogen-combination drug in the prior 6 months.
For the present study, 531 patients were recruited from 33 centers in Japan between May 2011 and May 2013. This analysis included the 186 PMS study patients who completed the SF-36v2 questionnaire before and after 6 to 8 cycles of EE/DRSP treatment in a cyclic regimen. Patients completed paper-based SF-36v2 questionnaires at the initial visit (before the start of drug administration [baseline]) and after 6 to 8 cycles of treatment. Physicians retrospectively abstracted medical charts to identify eligible patients and obtain their demographic and clinical characteristics, such as age, severity of dysmenorrhea (ie, the degree to which dysmenorrhea impairs daily activities), dysmenorrhea etiology (primary/secondary), and the presence of physical and/or mental discomfort at baseline, as well as baseline and post-treatment SF-36v2 scores.
This study was conducted in accordance with Good Post-Marketing Study Practice.18 Ethical approvals was obtained in accordance with the requirements of participating institutions. All included patients provided written informed consent.
HRQoL Assessment and Study Outcomes
HRQoL was measured using the Japanese language version of the SF-36v2, which has been translated and culturally adapted for use with Japanese patients.19 The SF-36v2 is a generic instrument designed to measure current health status, with a recall period of 4 weeks. It consists of 36 self-administered questions that measure physical and mental components of HRQoL using 8 domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. The 8 domains contribute to 2 resulting component summaries: a physical component summary and mental component summary. These summaries represent weighted aggregations of scores for the physical (physical functioning, role physical, bodily pain, general health) and mental (vitality, social functioning, role emotional, mental health) subdomains. Each domain is scored on a scale of 0–100, with lower scores indicating poorer HRQoL.20 Intrinsic to the SF-36v2 is the use of norm-based scoring to calculate domain and component summary scores. Using this method, rather than unadjusted scores of 0–100, each domain score is re-calculated based on a standardized mean and standard deviation (SD) relative to the general population (ie, norm-based). SF-36v2 domain and component summary scores were standardized to mean scores of 50 with SDs of 10.21
The primary study endpoints were mean baseline and post-treatment SF-36v2 domain scores for study patients compared with mean SF-36v2 scores from the general population of Japan in 2007,19,22 and mean changes in study patients’ SF-36v2 domain scores between baseline and 6 to 8 cycles of treatment. Additional study endpoints were evaluated by analyzing the effects of the following patient characteristics on mean changes in SF-36v2 domain scores: dysmenorrhea etiology (primary or secondary), degree of impairment of daily activities (none/mild, moderate, severe; defined in Table 1), the presence of physical and/or mental discomfort, and patient age.
Table 1.
Severity of Dysmenorrhea Definitions
| Severity of Dysmenorrhea (Degree of Overall Impairment of Work, including Schoolwork and Housework) | Description |
|---|---|
| None | No impairment |
| Mild | Slight impairment of work, including schoolwork and housework |
| Moderate | Impairs work, including schoolwork and housework to the degree one wants to lie down and take a rest |
| Severe | In bed for all of 1 day or more and unable to carry out work, including schoolwork and housework |
Statistical Analyses
SF-36v2 domain scores were compared with scores for the Japanese population according to the manual of the Japanese version of the SF-36v219,22,23 using independent sample t-tests.
Additionally, paired t-testing was used to compare mean change in the 2 component summary scores and 8 domain scores of the SF-36v2 in patients with dysmenorrhea at baseline and after 6 to 8 months of treatment with EE/DRSP. All statistical tests were 2-tailed, with statistical significance set at 0.05. Statistical tests were performed using Statistical Analysis Software: (SAS) Version 9.2.
Regression models were generated for all exploratory predictors and tested for parallelism; when parallelism was confirmed, a one-way analysis of covariance (ANCOVA) was conducted. Mean changes from baseline in each SF-36v2 domain score were used as dependent variables.
Results
Sample Characteristics
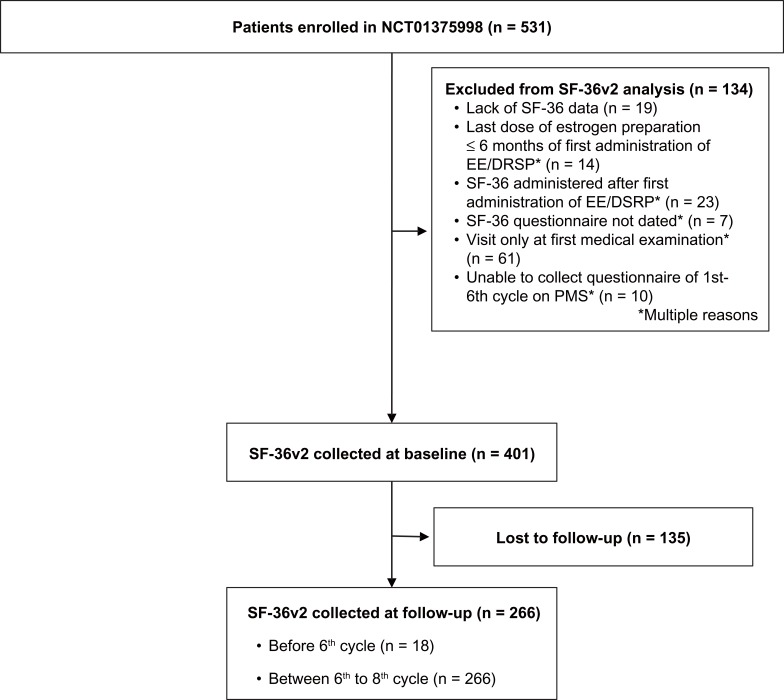
Patient enrollment data are shown in Figure 1. A total of 266 patients completed the SF-36v2 at baseline and follow up; 80 patients were excluded per protocol due to completing the survey before the 6th cycle of treatment (n=18) or at/after the 9th cycle (n=62). Characteristics of the 186 patients included in the current study are presented in Table 2. Most patients (79.0%) were between 20 and 40 years of age, with a mean age of 30.1 ± 8.0, and an age range of 16 to 57 years. The degree of impairment of daily activities experienced by women in this sample ranged from none/mild (26.4%) to moderate (53.2%) and severe (20.4%). Approximately one-half of patients (57.0%) had a diagnosis of primary dysmenorrhea, while 30.1% had been diagnosed with endometriosis, 7.5% had uterine fibroids, 8.1% had adenomyosis, and 2.7% had dysmenorrhea from another cause.
Figure 1.
Patient enrollment flowchart.
Abbreviations: EE/DSRP, ethinylestradiol/drospirenone; PMS, post-marketing surveillance; SF-36v2, 36-Item Short-Form Health Survey version 2.0.
Table 2.
Baseline Patient Characteristics
| Demographic and Clinical Variables | Total (n=186) |
|---|---|
| Age (years), mean ± SD | 30.1 ± 8.0 |
| Age (years), n (%) | |
| <20 | 15 (8.1) |
| 20–30 | 83 (44.6) |
| 30–40 | 64 (34.4) |
| 40–50 | 23 (12.4) |
| ≥50 | 1 (0.5) |
| Severity of dysmenorrhea (degree of impairment of daily activities), n (%) | |
| None | 5 (2.7) |
| Mild | 44 (23.7) |
| Moderate | 99 (53.2) |
| Severe | 38 (20.4) |
| Diagnosis, n (%) | |
| Primary dysmenorrhea | 106 (57.0) |
| Secondary dysmenorrhea | 80 (43.0) |
| Endometriosis | 56 (30.1) |
| Uterine fibroid | 14 (7.5) |
| Adenomyosis | 15 (8.1) |
| Other | 5 (2.7) |
| BMI (kg/m2), n (%) | |
| <18.5 | 36 (19.4) |
| 18.5–25 | 131 (70.4) |
| 25–30 | 12 (6.5) |
| ≥30 | 1 (0.5) |
| Unknown | 6 (3.2) |
| Physical discomfort, n (%) | |
| No | 36 (19.4) |
| Yes* | 150 (80.6) |
| Lower abdominal pain | 140 (75.3) |
| Lower back pain | 88 (47.3) |
| Fatigue | 52 (28.0) |
| Headache | 40 (21.5) |
| Lower back discomfort | 38 (20.4) |
| Abdominal bloating | 34 (18.3) |
| Breast pain | 34 (18.3) |
| Diarrhea | 24 (12.9) |
| Foot discomfort | 20 (10.8) |
| Acne vulgaris | 20 (10.8) |
| Nausea | 14 (7.5) |
| Decreased appetite | 10 (5.4) |
| Constipation | 10 (5.4) |
| Increased appetite | 8 (4.3) |
| Stiff neck | 5 (2.7) |
| Mental discomfort, n (%) | |
| No | 105 (56.5) |
| Yes* | 81 (43.5) |
| Irritability | 60 (32.3) |
| Depressed | 48 (25.8) |
| Drowsiness | 44 (23.7) |
| Easily offended | 42 (22.6) |
| Emotional lability | 37 (19.9) |
| Dysphoria | 36 (19.4) |
| Loss of concentration | 33 (17.7) |
| Getting emotional | 11 (5.9) |
| Decreased motivation | 9 (4.8) |
| Restless | 4 (2.2) |
Note: *Patients could select more than one response.
Abbreviations: BMI, body mass index; SD, standard deviation.
Impact of Dysmenorrhea on HRQoL
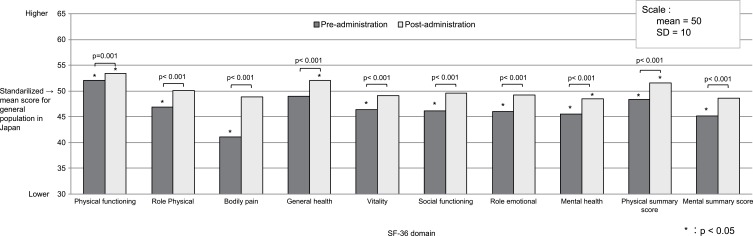
Compared with the general Japanese population, women with dysmenorrhea had significantly lower mean baseline scores on almost all SF-36v2 domains. The only exceptions were the domain measuring physical functioning (which was significantly higher in women with dysmenorrhea) and the general health score (which was similar for both populations) (Figure 2). Compared to the general Japanese population, women with dysmenorrhea were most affected by the bodily pain domain, followed by the mental health and role emotional domains; mental component summary scores for study patients were also low compared with the Japanese population.
Figure 2.
Standardized mean SF-36v2 scores for patients with dysmenorrhea before and after 6 to 8 treatment cycles with ethinylestradiol/drospirenone vs scores for the general population of Japan.
Abbreviation: SD, standard deviation.
Effect of EE/DRSP Treatment on HRQoL
Significant improvement was observed between pre- and post-treatment across all SF-36v2 domains (Table 3), with the greatest improvement observed in the bodily pain domain. Adjusted mean change and SD for evaluated parameters were as follows (all p< 0.001): physical functioning: 1.4 (5.7), role physical: 3.2 (8.1), bodily pain: 7.8 (10.0), general health: 3.0 (7.0), vitality: 2.7 (8.1), social functioning: 3.5 (9.8), role emotional: 3.3 (9.2), mental health: 3.0 (7.3), physical component summary: 3.3 (6.8), and mental component summary: 3.4 (6.9). Pearson testing confirmed a linear relationship between individual SF-36v2 domain scores and post-treatment score changes (p< 0.05 for all).
Table 3.
SF-36v2 Scores Before and After Ethinylestradiol/Drospirenone Treatment for Dysmenorrhea
| SF-36v2 Domain | Pre-administration (n = 186) | Post-administration (n = 186) | Change from Pre- to Post-administration |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Physical functioning | 52.0 (50.6–53.4) | 53.4 (52.1–54.6) | 1.4 (0.5–2.2) |
| Role physical | 46.9 (45.5–48.3) | 50.1 (49.0–51.3) | 3.2 (2.1–4.4) |
| Bodily pain | 41.1 (39.4–42.8) | 48.9 (47.4–50.4) | 7.8 (6.4–9.3) |
| General health† | 49.0 (47.6–50.3) | 52.0 (50.7–53.3) | 3.0 (2.0–4.0) |
| Vitality | 46.4 (44.9–47.9) | 49.1 (47.9–50.4) | 2.7 (1.5–3.9) |
| Social functioning | 46.1 (44.7–47.5) | 49.6 (48.3–51.0) | 3.5 (2.1–4.9) |
| Role emotional | 46.0 (44.4–47.5) | 49.2 (47.9–50.6) | 3.3 (2.0–4.6) |
| Mental health | 45.5 (44.1–46.9) | 48.5 (47.2–49.8) | 3.0 (1.9–4.0) |
| Physical component summary† | 48.4 (46.9–49.8) | 51.6 (50.3–52.9) | 3.3 (2.3–4.3) |
| Mental component summary† | 45.2 (43.9–46.5) | 48.6 (47.4–49.9) | 3.4 (2.4–4.4) |
Notes: Pre-administration results were collected prior to beginning treatment; post-administration results were collected between the first day of 6th cycle and the last day of 8th menstrual cycle. †The number of patients with pre-administration scores was 184.
Abbreviations: CI, confidence interval; SF-36v2, 36-Item Short-Form Health Survey version 2.0.
After 6 to 8 treatment cycles with EE/DRSP, standardized SF-36v2 domain scores for women with dysmenorrhea improved to a level that was equal to or better than standardized scores for the general population in Japan (Table 3; Figure 2). Mean (SD) post-administration scores were significantly higher than the adjusted threshold of 50 for general health (52.0 [9.0]) and the physical component summary (51.6 [9.1]) (p= 0.008 and p= 0.033, respectively).
Analysis of Covariance
One-way ANCOVA identified several statistically significant relationships between mean changes in SF-36v2 domain scores and potential predictors (Table 4). Women with primary compared with secondary dysmenorrhea had significantly greater improvement in the bodily pain domain (mean 2.53; 95% confidence interval [CI] 0.160–4.900, p= 0.037). Patients with none/mild impairment of daily activities at baseline had greater improvement than patients with severe dysmenorrhea in the general health domain (mean 3.02; 95% CI 0.306–5.743, p= 0.029) and social functioning domain (mean 3.76; 95% CI 0.326–7.186, p= 0.032), while patients with moderate dysmenorrhea had greater improvement in the role emotional domain (mean 2.83; 95% CI 0.019–5.637, p= 0.048). General health domain scores were also significantly improved in women who reported baseline mental discomfort (mean 3.13; 95% CI 1.296–4.969; p< 0.001) or physical discomfort (mean 2.64; 95% CI 0.323–4.960, p= 0.026). No significant relationships were observed between mean changes and predictors in the role physical, vitality, and mental health domains, although the direction of change in these domain scores was generally consistent with what was found in the other domains.
Table 4.
Covariance of Mean Changes in SF-36v2 Domain Scores and Potential Predictors
| SF-36v2 Domain | Patient Characteristics | Parallelism Test of Regression Line | Analysis of Covariance* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LSM | Variance | ||||||||
| Estimate | 95% CI | Estimate | 95% CI | t-Statistic | p-value | ||||
| Physical functioning | Diagnosis | Primary | p= 0.221 | 1.60 | 0.606–2.589 | 0.55 | −0.974–2.065 | 0.71 | p= 0.480 |
| Secondary | – | 1.05 | −0.090–2.195 | – | – | – | – | ||
| Role physical | Severity of dysmenorrhea† | None/mild | p= 0.218 | 4.17 | 2.320–6.010 | 1.33 | −1.469–4.121 | 0.94 | p= 0.351 |
| Moderate | – | 2.92 | 1.626–4.223 | 0.09 | −2.384–2.555 | 0.07 | p= 0.946 | ||
| Severe | – | 2.84 | 0.741–4.938 | – | – | – | – | ||
| Bodily pain | Diagnosis | Primary | p= 0.681 | 8.92 | 7.376–10.469 | 2.53 | 0.160–4.900 | 2.11 | p= 0.037 |
| Secondary | – | 6.39 | 4.611–8.175 | – | – | – | – | ||
| Severity of dysmenorrhea† | None/mild | p= 0.342 | 7.82 | 5.504–10.145 | 0.57 | −2.953–4.084 | 0.32 | p= 0.751 | |
| Moderate | – | 8.06 | 6.442–9.679 | 0.80 | −2.271–3.875 | 0.51 | p= 0.607 | ||
| Severe | – | 7.26 | 4.641–9.877 | – | – | – | – | ||
| Mental discomfort | No | p= 0.615 | 7.72 | 6.156–9.292 | – | – | – | – | |
| Yes | – | 7.98 | 6.192–9.763 | 0.25 | −2.126–2.632 | 0.21 | p= 0.834 | ||
| Age | <20 | p= 0.682 | 10.47 | 6.370–14.567 | 4.98 | −0.263–10.227 | 1.87 | p= 0.062 | |
| 20–30 | – | 8.98 | 7.241–10.728 | 3.50 | −0.201–7.197 | 1.87 | p= 0.064 | ||
| 30–40 | – | 6.61 | 4.624–8.590 | 1.12 | −2.679–4.922 | 0.58 | p= 0.561 | ||
| ≥40 | – | 5.49 | 2.236–8.737 | – | – | – | – | ||
| General health | Diagnosis | Primary | p= 0.426 | 3.64 | 2.414–4.859 | 1.55 | −0.320–3.415 | 1.63 | p= 0.104 |
| Secondary | – | 2.09 | 0.680–3.499 | – | – | – | – | ||
| Severity of dysmenorrhea† | None/mild | p= 0.715 | 4.63 | 2.811–6.447 | 3.02 | 0.306–5.743 | 2.19 | p= 0.029 | |
| Moderate | – | 2.71 | 1.458–3.963 | 1.11 | −1.272–3.485 | 0.92 | p= 0.360 | ||
| Severe | – | 1.60 | −0.417–3.626 | – | – | – | – | ||
| Physical discomfort | No | p= 0.203 | 0.85 | −1.231–2.926 | – | – | – | – | |
| Yes | – | 3.49 | 2.466–4.512 | 2.64 | 0.323–4.960 | 2.25 | p= 0.026 | ||
| Mental discomfort | No | p= 0.308 | 1.61 | 0.406–2.814 | – | – | – | – | |
| Yes | – | 4.74 | 3.368–6.117 | 3.13 | 1.296–4.969 | 3.37 | p< 0.001 | ||
| Age | <20 | p= 0.754 | 2.60 | −0.654–5.847 | 1.17 | −2.978–5.314 | 0.56 | p= 0.579 | |
| 20–30 | – | 3.71 | 2.306–5.110 | 2.28 | −0.672–5.231 | 1.52 | p= 0.129 | ||
| 30–40 | – | 2.69 | 1.101–4.283 | 1.26 | −1.758–4.284 | 0.83 | p= 0.410 | ||
| ≥40 | – | 1.43 | −1.152–4.009 | – | – | – | – | ||
| Vitality | Diagnosis | Primary | p= 0.090 | 3.09 | 1.821–4.362 | 0.94 | −1.020–2.897 | 0.95 | p= 0.346 |
| Secondary | – | 2.15 | 0.686–3.619 | – | – | – | – | ||
| Severity of dysmenorrhea† | None/mild | p= 0.557 | 3.97 | 2.112–5.821 | 2.77 | −0.028–5.566 | 1.95 | p= 0.052 | |
| Moderate | – | 2.63 | 1.326–3.927 | 1.43 | −1.036–3.893 | 1.14 | p= 0.254 | ||
| Severe | – | 1.20 | −0.896–3.291 | – | – | – | – | ||
| Mental discomfort | No | p= 0.051 | 2.86 | 1.574–4.145 | – | – | – | – | |
| Yes | – | 2.46 | 0.996–3.934 | −0.39 | −2.375–1.586 | −0.39 | p= 0.695 | ||
| Social functioning | Diagnosis | Primary | p= 0.394 | 4.29 | 2.740–5.834 | 1.75 | −0.605–4.113 | 1.47 | p= 0.144 |
| Secondary | – | 2.53 | 0.753–4.314 | – | – | – | – | ||
| Severity of dysmenorrhea† | None/mild | p= 0.630 | 5.16 | 2.895–7.428 | 3.76 | 0.326–7.186 | 2.16 | p= 0.032 | |
| Moderate | – | 3.54 | 1.949–5.137 | 2.14 | −0.888–5.165 | 1.39 | p= 0.165 | ||
| Severe | – | 1.40 | −1.168–3.978 | – | – | – | – | ||
| Age | <20 | p= 0.936 | 6.41 | 2.275–10.540 | 4.69 | −0.567–9.945 | 1.76 | p= 0.080 | |
| 20–30 | – | 3.50 | 1.729–5.267 | 1.78 | −1.956–5.515 | 0.94 | p= 0.348 | ||
| 30–40 | – | 3.58 | 1.583–5.585 | 1.87 | −1.958–5.690 | 0.96 | p= 0.337 | ||
| ≥40 | – | 1.72 | −1.552–4.989 | – | – | – | – | ||
| Role emotional | Severity of dysmenorrhea† | None/mild | p= 0.052 | 3.35 | 1.248–5.459 | 2.13 | −1.055–5.313 | 1.32 | p= 0.189 |
| Moderate | – | 4.05 | 2.572–5.533 | 2.83 | 0.019–5.637 | 1.99 | p= 0.048 | ||
| Severe | – | 1.22 | −1.163–3.612 | – | – | – | – | ||
| Age | <20 | p= 0.254 | 2.39 | −1.465–6.237 | 0.03 | −4.844–4.911 | 0.01 | p= 0.989 | |
| 20–30 | – | 2.74 | 1.106–4.366 | 0.38 | −3.065–3.832 | 0.22 | p= 0.827 | ||
| 30–40 | – | 4.57 | 2.723–6.424 | 2.22 | −1.331–5.772 | 1.23 | p= 0.219 | ||
| ≥40 | – | 2.35 | −0.677–5.382 | – | – | – | – | ||
| Mental health | Diagnosis | Primary | p= 0.947 | 3.05 | 1.804–4.293 | 0.16 | −1.752–2.073 | 0.17 | p= 0.869 |
| Secondary | – | 2.89 | 1.453–4.323 | – | – | – | – | ||
| Physical discomfort | No | p= 0.247 | 2.10 | −0.043–4.242 | – | – | – | – | |
| Yes | – | 3.19 | 2.151–4.231 | 1.09 | −1.301–3.483 | 0.90 | p= 0.369 | ||
| Mental discomfort | No | p= 0.641 | 2.39 | 1.144–3.642 | – | – | – | – | |
| Yes | – | 3.74 | 2.314–5.166 | 1.35 | −0.570–3.264 | 1.39 | p= 0.167 | ||
Notes: A standard ANCOVA model was used, which assumed a linear relationship between the response and covariate: [Score valuation] = [score pre-administration] + [category data] + [intercept]. *Results of analysis of covariance were validated from the linearity of regression and parallelism using t-test. †Severity of dysmenorrhea refers to the degree of impairment of daily activities.
Abbreviations: CI, confidence interval; LSM, least squares mean; SD, standard deviation; SF-36v2, 36-Item Short-Form Health Survey version 2.0.
Discussion
This study found that women with dysmenorrhea in Japan had poorer HRQoL compared with the general population of Japan, and that these women showed significant improvements in HRQoL, as measured by the SF-36v2, after 6–8 treatment cycles of EE/DRSP. In women with dysmenorrhea, mean baseline scores across 7 of the 8 SF-36v2 domains were below the standardized mean score for the general Japanese population, with the exception being the domain measuring physical functioning. Significant improvements in HRQoL, equal to or better than standardized scores for the general population in Japan, were also seen after EE/DRSP treatment.
The greatest improvement was observed in the domain measuring bodily pain; mean scores increased by 7.8 points (from 41.1 at baseline to 48.9 after 6–8 treatment cycles). This domain evaluates the intensity of bodily pain and the extent that pain interferes with normal work. In exploratory analyses, the bodily pain score improved to a similar level following LEP treatment, regardless of baseline dysmenorrhea severity. Since the primary symptom of dysmenorrhea is pain, and as clinical trial data have demonstrated pain reduction with EE/DRSP treatment,3 these findings are not surprising.1
In other domains, HRQoL after EE/DRSP treatment varied based on dysmenorrhea severity; patients with none/mild impairment of daily activities at baseline showed greater responsiveness in both general health and social functioning, while patients with moderate dysmenorrhea had a significant improvement in role emotional compared with patients with severe dysmenorrhea at baseline. Additionally, patients with primary dysmenorrhea were more likely to show an improved bodily pain score, and women who had mental and physical discomfort had greater improvement in general health following treatment.
This is the first real-world study to examine HRQoL among women receiving LEP for the treatment of dysmenorrhea in Japan. Previous research has evaluated the prevalence of dysmenorrhea and its effect on HRQoL in women in the US, China, and Turkey. Those prospective cross-sectional studies found that women with dysmenorrhea experience poorer HRQoL than those without dysmenorrhea, and that HRQoL worsens with increasing dysmenorrhea severity.4–6 Consistent with the current findings, showing that women with dysmenorrhea experience the greatest burden in the bodily pain domain, 2 of these prior studies (Turkey and China) found that SF-36 bodily pain scores had the largest subtracted difference for women with dysmenorrhea vs those without.5,6 The US study also found a significant impact of dysmenorrhea on the bodily pain score.4 Prior research also found minimal impact of dysmenorrhea on physical functioning scores.4–6
Women in the current study reported that mental health was the second-most affected domain. This finding differed from prior data from China and Turkey, in which dysmenorrhea had the second-greatest impact on role physical or bodily pain domains, and minimal impact on mental health.4–6 The US study found significantly lower mental health scores in women with dysmenorrhea and premenopausal symptoms; however, women with dysmenorrhea who reported only pain or abnormal periods did not experience any mental health impact.4
Because this study compared women with dysmenorrhea with the general population, rather than with women who did not report dysmenorrhea, the magnitude of difference reported by previous research cannot be directly compared to the current study. However, the current findings of a 8.9-point baseline difference in the bodily pain domain and a 4.5-point difference in baseline mental health in patients with dysmenorrhea compared with the general population suggest a comparable or greater effect on HRQoL than chronic conditions like angina, hypertension, and myocardial infarction.24
The majority of published research evaluating the impact of treatment on HRQoL in patients with dysmenorrhea has studied traditional and complementary medical approaches.25–27 HRQoL outcomes have also been evaluated in patients with dysmenorrhea who underwent hysterectomy.28 However, minimal research exists to evaluate the impact of LEP or any hormonal contraceptives on HRQoL in patients with dysmenorrhea. A 2015 prospective, observational study evaluated the impact of 2 contraceptive regimens (monophasic 24/4 estradiol/nomegestrol acetate and estradiol/chlormadinone acetate) on patient HRQoL using the SF-36. Researchers found that the monophasic regimen was associated with significant increases in overall scores, as well as the physical and mental domains.29
According to a study that established the minimal clinically important differences (MCID) for validated QoL instruments among patients with advanced cancer and painful bone metastases, the MCID (using a distribution-based method) was 0.3 SDs for improvement and 0.5 SDs for deterioration.30 An improvement of 0.3 SDs is equivalent to 3 points in the SF-36v2. As such, in the present study, patient scores for almost all SF-36v2 domains, except for physical functioning and vitality, exceeded the MCID threshold. Considering this interpretation, women with dysmenorrhea experienced a clinically meaningful improvement in HRQoL after EE/DRSP treatment.
This study had several limitations. Results were not adjusted for potential confounding variables within the sample population. This is relevant because age, smoking status, coffee consumption, depression, and sexual trauma have all been reported to impact HRQoL in women with dysmenorrhea.4 Specific to age, patient responses were compared directly with mean SF-36v2 subdomain scores from the general population in Japan. The age range differed substantially between the current study group (16 to 57 years) and the general Japanese population (20 to 79 years), which was stratified for representative sampling and included a higher proportion of older patients (24% were ≥60 years of age).22 It has been shown that older patients tend to score more poorly on physical symptoms in the SF-36.31 However, based on findings from a previous assessment of SF-36 scores in women with dysmenorrhea, it is likely that the direction of change in all SF-36 domain scores would persist after controlling for these factors, although the magnitude of difference between women with dysmenorrhea and the general population would be affected.4 Furthermore, although the baseline physical functioning score from the study sample (52.0) is higher than that of the general population (50.0), this difference diminishes when the study sample is compared with the general population aged 30–39 years (52.4). This group is closest to the mean age of the study population (30.1 ± 8.0). As there are no considerable differences in the standardized mean scores in other domains between the whole population and the female population aged 30–39 years, it is anticipated that results would not be affected, even after controlling for age.
In addition, due to the small sample size for this study (in particular, for the exploratory ANCOVA analyses), results may not be generalizable to all women in Japan. Additionally, the current study population was drawn from women who sought treatment for dysmenorrhea, and these women have been reported to have more severe symptoms than those who do not seek care.32 The study also did not account for the respondents’ menstrual cycle phase at the time they completed the survey. The SF-36v2 has a 1-month recall period and respondents may have underestimated the impact of dysmenorrhea on HRQoL if they completed the survey at a time when they were not experiencing symptoms. Moreover, the scale used to measure dysmenorrhea severity was not validated. Similarly, changes in bodily pain reflected by the SF-36v2 may or may not reflect changes in menstrual pain, as this instrument is not specific to menstrual pain. Study findings should also be interpreted with caution due to missing patient-reported outcome data. An overestimation of HRQoL improvement could result if a positive association existed between patient HRQoL and the questionnaire return rate. Finally, due to the nature of real-world evidence study design, some key variables (eg, severity and etiology of dysmenorrhea) were retrospectively abstracted from medical records rather than assessed at scheduled visits, as would occur in clinical trial design.
This study contributes to the existing literature by demonstrating the substantial impact of dysmenorrhea on women’s HRQoL, and the potential for EE/DRSP treatment administered in a cyclic regimen to improve associated physical and mental conditions. Additional research should assess the HRQoL differences between women with dysmenorrhea and those with no dysmenorrhea, the impact of dysmenorrhea severity on HRQoL, and how treatment effectiveness may be affected by different patient characteristics and concomitant medication use.
Conclusion
Dysmenorrhea has a profound impact on all aspects of functioning and well-being among women in Japan. Physical functioning, as well as social and mental components of HRQoL, showed significant signs of improvement after treatment with EE/DRSP in Japanese patients with dysmenorrhea. This EE/DRSP regimen may provide health-care professionals with an effective option to improve HRQoL in patients with dysmenorrhea.
Acknowledgments
The authors are thankful to all the study-clinic health-care professionals for their participation in this study. The authors would like to thank Caitlin Rothermel (McCANN HEALTH CMC) for medical writing assistance. The authors also wish to thank the Bayer PMS team for their contributions to the study operation.
Funding Statement
Conduct of this study was funded by Bayer Yakuhin, Ltd. Medical writing assistance was provided by McCann MDS Inc. and was funded by Bayer Yakuhin, Ltd.
Abbreviations
ANCOVA, analyses of covariance; CI, confidence interval; HRQoL, health-related quality of life; JAOG, Japan Association of Obstetricians and Gynecologists; JSOG, Japan Society of Obstetrics and Gynecology; LEP, low-dose estrogen/progestin; LSM, least squares mean; MCID, minimal clinically important differences; PMS, post-marketing surveillance; QOL, quality of life; SD, standard deviation; SF-36, 36-Item Short-Form Health Survey.
Data Sharing Statement
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing, upon request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the US and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 1, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to available datasets and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the “Study sponsors” section of the portal. Data access will be granted to available datasets, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Ethics and Consent Statement
Japan’s Ministry of Health, Labour, and Welfare requires post-marketing surveillance for all drugs, and the current study was conducted as part of a regulatory mandate. All study data were collected during actual clinical practice; patients provided prior, written informed consent for their data to be used in research, but no national ethical approvals were required to conduct this study.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Mikio Momoeda was a paid medical advisor to Bayer Yakuhin, Ltd. for the duration of the study. Sayako Akiyama was an employee of Bayer Yakuhin, Ltd. at that time of the study. Kota Tanaka is an employee of EPS, which received funding from Bayer for the statistical analysis and reporting of study data. Yoshimi Suzukamo was a paid advisor on patient-reported outcome research to Bayer Yakuhin, Ltd. for the duration of the study. The authors report no other conflicts of interest in this work.
References
- 1.Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. doi: 10.1093/humupd/dmv039 [DOI] [PubMed] [Google Scholar]
- 2.Osuga Y, Hayashi K, Kobayashi Y, et al. Dysmenorrhea in Japanese women. Int J Gynaecol Obstet. 2005;88(1):82–83. doi: 10.1016/j.ijgo.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 3.Momoeda M, Kondo M, Elliesen J, Yasuda M, Yamamoto S, Harada T. Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study. Int J Women's Health. 2017;9:295–305. doi: 10.2147/IJWH.S134576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard K, Frayne SM, Skinner KM, Sullivan LM. Health status among women with menstrual symptoms. J Women's Health (Larchmt). 2003;12(9):911–919. doi: 10.1089/154099903770948140 [DOI] [PubMed] [Google Scholar]
- 5.Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115(2):138–145. doi: 10.3109/03009730903457218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CL. Health-related quality of life among Chinese adolescent girls with dysmenorrhoea. Reprod Health. 2018;15(1):. doi: 10.1186/s12978-018-0540-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz MI, Rangel-Flores E, Carrillo-Alarcon LC, Veras-Godoy HA. Prevalence and impact of primary dysmenorrhea among Mexican high school students. Int J Gynaecol Obstet. 2009;107(3):240–243. doi: 10.1016/j.ijgo.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 8.Eryilmaz G, Ozdemir F, Pasinlioglu T. Dysmenorrhea prevalence among adolescents in eastern Turkey: its effects on school performance and relationships with family and friends. J Pediatr Adolesc Gynecol. 2010;23(5):267–272. doi: 10.1016/j.jpag.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 9.Wong LP, Khoo EM. Dysmenorrhea in a multiethnic population of adolescent Asian girls. Int J Gynaecol Obstet. 2010;108(2):139–142. doi: 10.1016/j.ijgo.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 10.Pitangui AC, Gomes MR, Lima AS, Schwingel PA, Albuquerque AP, de Araujo RC. Menstruation disturbances: prevalence, characteristics, and effects on the activities of daily living among adolescent girls from Brazil. J Pediatr Adolesc Gynecol. 2013;26(3):148–152. doi: 10.1016/j.jpag.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka E, Momoeda M, Osuga Y, et al. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J Med Econ. 2013;16(11):1255–1266. doi: 10.3111/13696998.2013.830974 [DOI] [PubMed] [Google Scholar]
- 12.Akiyama S, Tanaka E, Cristeau O, Onishi Y, Osuga Y. Evaluation of the treatment patterns and economic burden of dysmenorrhea in Japanese women, using a claims database. Clinicoecon Outcomes Res. 2017;9:295–306. doi: 10.2147/CEOR.S127760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada T, Momoeda M, Terakawa N, Taketani Y, Hoshiai H. Evaluation of a low-dose oral contraceptive pill for primary dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2011;95(6):1928–1931. doi: 10.1016/j.fertnstert.2011.02.045 [DOI] [PubMed] [Google Scholar]
- 14.YAZ approved in Japan for the treatment of dysmenorrhea. The Financial website. 2011. Available from: https://www.finchannel.com/business/161-pharmacy/27581. Accessed January2, 2019.
- 15.Ethinylestradiol/norethisterone ultra low dose - Nobelpharma. Adis Insight website. September 30, 2013. Available from: https://adisinsight.springer.com/drugs/800033007. Accessed January2, 2019.
- 16.Takeda T, Wong TF, Adachi T, et al. Guidelines for office gynecology in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2011 edition. J Obstet Gynaecol Res. 2012;38(4):615–631. doi: 10.1111/j.1447-0756.2012.01858.x [DOI] [PubMed] [Google Scholar]
- 17.Bayer. YAZ post-marketing surveillance in Japan. ClinicalTrials.gov website. June 20, 2011. [updated April 3, 2018] Available from: https://clinicaltrials.gov/ct2/show/NCT01375998. Accessed January2, 2019.
- 18.Kumano S. GPSP: good post-marketing study practice. Nihon Yakurigaku Zasshi. 2012;140(2):81–84. doi: 10.1254/fpj.140.81 [DOI] [PubMed] [Google Scholar]
- 19.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J Clin Epidemiol. 1998;51(11):1037–1044. doi: 10.1016/S0895-4356(98)00095-X [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Snow K, Kosinski M, Gandek B. SF36 health survey: manual and interpretation guide. The Health Institute, New England Medical Center; 1993. Available from: https://www.researchgate.net/publication/247503121_SF36_Health_Survey_Manual_and_Interpretation_Guide. Accessed January2, 2019. [Google Scholar]
- 21.Ware JE, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User’s Manual for the SF-36v2 Health Survey. Lincoln: QualityMetric Inc; 2007. [Google Scholar]
- 22.Fukuhara S, Ware JE Jr, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol. 1998;51(11):1045–1053. doi: 10.1016/S0895-4356(98)00096-1 [DOI] [PubMed] [Google Scholar]
- 23.Fukuhara S, Suzukamo Y. Manual of SF-36v2 Japanese Version. Vol. 2004 Kyoto: iHope International Inc; 2015. [Google Scholar]
- 24.Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions. Results from the medical outcomes study. JAMA. 1989;262(7):907–913. doi: 10.1001/jama.1989.03430070055030 [DOI] [PubMed] [Google Scholar]
- 25.McGovern CE, Cheung C. Yoga and quality of life in women with primary dysmenorrhea: a systematic review. J Midwifery Women's Health. 2018;63(4):470–482. doi: 10.1111/jmwh.12729 [DOI] [PubMed] [Google Scholar]
- 26.Bazarganipour F, Taghavi S-A, Allan H, et al. A randomized controlled clinical trial evaluating quality of life when using a simple acupressure protocol in women with primary dysmenorrhea. Complement Ther Med. 2017;34:10–15. doi: 10.1016/j.ctim.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 27.Abaraogu UO, Tabansi-Ochuogu CS. As acupressure decreases pain, acupuncture may improve some aspects of quality of life for women with primary dysmenorrhea: a systematic review with meta-analysis. J Acupunct Meridian Stud. 2015;8(5):220–228. doi: 10.1016/j.jams.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 28.Berner E, Qvigstad E, Myrvold AK, Lieng M. Pain reduction after total laparoscopic hysterectomy and laparoscopic supracervical hysterectomy among women with dysmenorrhoea: a randomised controlled trial. BJOG. 2015;122(8):1102–1111. doi: 10.1111/1471-0528.13362 [DOI] [PubMed] [Google Scholar]
- 29.Grandi G, Napolitano A, Xholli A, Tirelli A, Di Carlo C, Cagnacci A. Effect of oral contraceptives containing estradiol and nomegestrol acetate or ethinyl-estradiol and chlormadinone acetate on primary dysmenorrhea. Gynecol Endocrinol. 2015;31(10):774–778. doi: 10.3109/09513590.2015.1063118 [DOI] [PubMed] [Google Scholar]
- 30.Raman S, Ding K, Chow E, et al. Minimal clinically important differences in the EORTC QLQ-C30 and brief pain inventory in patients undergoing re-irradiation for painful bone metastases. Qual Life Res. 2018;27(4):1089–1098. doi: 10.1007/s11136-017-1745-8 [DOI] [PubMed] [Google Scholar]
- 31.Walters SJ, Munro JF, Brazier JE. Using the SF-36 with older adults: a cross-sectional community-based survey. Age Ageing. 2001;30(4):337–343. doi: 10.1093/ageing/30.4.337 [DOI] [PubMed] [Google Scholar]
- 32.Thompson ML, Gick ML. Medical care-seeking for menstrual symptoms. J Psychosom Res. 2000;49(2):137–140. doi: 10.1016/S0022-3999(00)00149-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- YAZ approved in Japan for the treatment of dysmenorrhea. The Financial website. 2011. Available from: https://www.finchannel.com/business/161-pharmacy/27581. Accessed January2, 2019.
- Ethinylestradiol/norethisterone ultra low dose - Nobelpharma. Adis Insight website. September 30, 2013. Available from: https://adisinsight.springer.com/drugs/800033007. Accessed January2, 2019.
- Bayer. YAZ post-marketing surveillance in Japan. ClinicalTrials.gov website. June 20, 2011. [updated April 3, 2018] Available from: https://clinicaltrials.gov/ct2/show/NCT01375998. Accessed January2, 2019.