Abstract
Bismuth is a water-insoluble non-toxic metallic element used in a wide array of pharmaceutical products, cosmetics, and catalysts, among others. Yet, the research regarding the use of bismuth nanoparticles (BiNPs) for antimicrobial treatments is scarce. Most of the current protocols for synthesizing BiNPs suitable for medical uses cannot be easily replicated in non-specialized laboratories. The objective of this work is to provide a fast, facile and economical method for synthesizing BiNPs. Bismuth nanoparticles were synthesized by a chemical reduction process, in less than 1 h, in a heated alkaline glycine solution; by the chelation and reduction of the bismuth (III) ions using dimercaptopropanol (BAL) and sodium borohydride respectively, and then coated and stabilized by polyvinylpyrrolidone (PVP). The resulting PVP-BiNPs were characterized by UV–Vis spectrophotometry and transmission electron microscopy (TEM).
• We describe a simple, rapid and inexpensive method for the synthesis of bismuth nanoparticles.
• This method allows synthesizing small nanoparticles with an aspect ratio close to one.
• Bismuth nanoparticles have antimicrobial properties, this easy-to-replicate protocol may further the research on bismuth nanoparticles for biomedical applications.
Keywords: Bismuth nanoparticles, Synthesis method, Nanoantibiotics
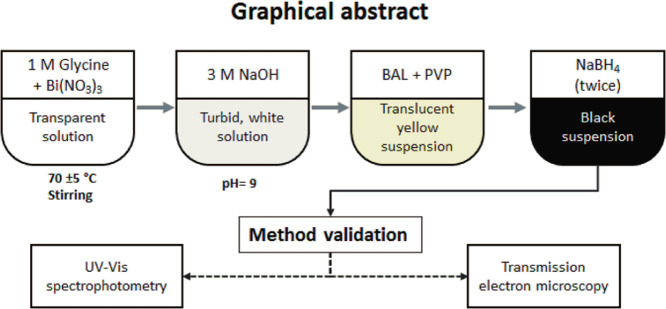
Graphical abstract
Specifications Table
| Subject Area: | Materials Science |
| More specific subject area: | Synthesis of metallic nanoparticles |
| Method name: | Synthesis of Biocompatible BAL-mediated PVP-Bismuth nanoparticles for biomedical applications |
| Name and reference of original method: | This is an optimized method based on different methods of bismuth nanoparticles available in the literature. |
| Resource availability: | N/A |
Background
Bismuth is a water-insoluble metallic element used in a wide array of medical applications, because it is considered non-toxic for humans (Lethal Intake >5–20 g/day/Kg, for years) [8], [14]. When bismuth is chelated with hydroxyl or sulfhydryl containing molecules, its water solubility and biocompatibility are both increased. The water solubility and lipophilicity of bismuth are substantially enhanced when bismuth ions (Bi3+) are complexed with small lipophilic molecules, such as dimercaptopropanol (BAL) [2]. BAL is an FDA approved drug [15] for treatments of metal poisoning [1].
Bismuth is used in the manufacture of pharmaceutical products, cosmetics, catalysts, pigments, electronics, and alloys. Water-soluble biocompatible bismuth complexes are used in health and cosmetics products and medicine. Also, bismuth and compounds present antimicrobial properties. It has been demonstrated that it exhibits high antibacterial activities against several bacterial species, including Clostridium difficile, Helicobacter pylori, Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, and Staphylococcus aureus [6], [9]. BAL-bismuth compounds display increased antibacterial activity [20]. Yet, it has been barely studied for synthesizing antimicrobial nanoparticles (nanoantibiotics).
Bismuth–based nanostructures have been used for different applications, such as photocatalytic oxidative desulfurization processes [10], [11]. There are only a handful of studies regarding the synthesis and evaluation of bismuth nanoparticles for antimicrobial treatments. Usually, the synthesis methods for BiNPs require specialized equipment [5], [13] or controlled conditions [3], [7], [18], [19]. As such, most current protocols for synthesizing biologically suitable BiNPs cannot be replicated in non-specialized laboratories without great difficulties [2], [4], [12]. Facilitated protocols for nanomaterials synthesis expand the research for biomedical applications [16]. Here we propose a fast, facile and economical method for synthesizing BiNPs that does not require the use of advanced equipment. The justification of this research is grounded on the potential antibiotic properties of BiNPs. These nanoparticles may be potent nanoantibiotics, but their antimicrobial activity remains vastly explored.
Method details
Required reagents and equipment
Reagents: bismuth nitrate [Bi(NO3)3*5H2O], sodium borohydride (NaBH4), 2,3-dimercapto-1-propanol (BAL), sodium hydroxide (NaOH), polyvinylpyrrolidone MW=10 KD (PVP-10 K), and glycine. All reagents were purchased from Sigma Aldrich (MO).
Equipment: 200 ml beaker, stirring hot plate, stir bar, thermometer, pH-meter, 12 ml plastic tubes, 1000 µl pipette, 200 µl pipette, 50 mL plastic tube, aluminum foil.
Procedure
Preparation
-
(1)
The beaker, thermometer, pH-meter, and the stir bar must be perfectly clean, washed with distilled water.
-
(2)
The following stock solutions were prepared using Milli Q water (or distilled water): 1 M glycine, 3 M NaOH, 3 mM PVP, and 1 M NaBH4.
NOTE 1: NaBH4 loses its activity very fast when diluted in water, it should be freshly prepared, immediately before using it.
NOTE 2: PVP-10 K molecular mass is 10,000. For a 3 mM solution, 0.3 g of PVP were diluted in 10 ml of Milli Q water.
NOTE 3: For a typical reaction, low volumes can be used: as an example, for a single-synthesis reaction we prepared (in Milli Q water or distilled water) the following: 1 M glycine (20 ml), 3 M NaOH (5 ml), 3 mM PVP (5 ml), and 1 M NaBH4 (10 ml).
-
(3)
Bismuth nitrate and BAL are used as received. BAL has a strong odor, and it should be opened and handled inside a chemical hood. The use of gloves and masks, and all other appropriate safety measures and pertinent protective equipment, is highly encouraged during the synthesis process.
Synthesis of the PVP-BiNPs
BAL-mediated PVP-BiNPs were synthesized by the chemical reduction of bismuth ions in an organic solution. The following is a full step-by-step description of the proposed synthesis method.
-
(1)
20 ml of 1 M glycine solution were heated up to 70 ± 5 °C, under continuous vigorous stirring. This temperature range must be kept through all the synthesis process.
NOTE: temperature is crucial for the proper synthesis. Lower temperatures result in a highly unstable suspension that precipitates within minutes after the synthesis.
-
(2)
146.2 µg of the Bi(NO3)3*5 H2O crystals were added to the pre-warmed glycine solution (for an initial 15 mM bismuth solution).
-
(3)
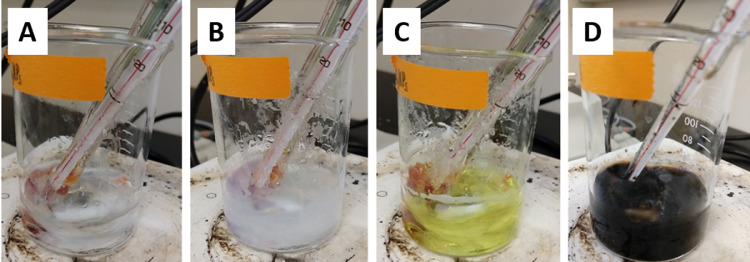
After ~2 min, enough volume of 3 M NaOH was added to raise the solution pH to 9. This turns the solution from transparent to a turbid white color Fig. 1A and B. This alkaline pH is kept during the whole synthesis process.
NOTE: pH is likely to drop during the synthesis process, more NaOH can be added to keep it at 9.
-
(4)
After 3 min, 75 µL of 8.1 M of 2,3-dimercaptopropanol (BAL) were added, rapidly turning the turbid whitish appearance to a translucent bright yellow color Fig. 1C.
-
(5)
Immediately, 3 mL of 3 mM PVP-K10 were added to the stirring suspension.
-
(6)
Finally, about 1 min later, 5 mL of 1 M NaBH4 were added dropwise. The suspension rapidly turns to a deep black color Fig. 1D.
NOTE: NaBH4 induces an exothermic reaction increasing the temperature of the solution, it is important to add it slowly.
-
(7)
Approximately 3 min later, another 2 ml of 1 M NaBH4 were added dropwise and it was left for vigorous stirring for ~10 additional minutes.
NOTE: If the protocol was followed as written, and the volume of NaOH was around 1.5–2 ml, the final concentration of the total bismuth should be around ~9.3–9.5 mM (~1254–1985 µg ml−1). There may be other bismuth species in the solution but those can be removed by washing the BiNPs (see below).
-
(8)
The BAL-mediated PVP-BiNPs black suspension was stored in a Falcon® plastic tube and cooled down to room temperature and posteriorly stored at 4 °C.
Fig. 1.
Synthesis process: from the initial colorless bismuth salt solution (A), it turned to a turbid white color after the addition of NaOH and PVP (B); when BAL was added it changed to a translucent yellow color (C). Finally, when NaBH4 is added, the solution immediately turned to pitch-black color (D). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Optional: washing the PVP-BiNPs*
The Bismuth nanoparticles can be washed to remove other bismuth species. For the washing process, we performed the following procedure:
-
(1)
BiNPs are centrifuged at 4000 rpm for 25 min and then washed with Milli-Q water, twice;
-
(2)
BiNPs were centrifuged again, then left to dry until they form a dry powder, then kept at 4 °C, in a light-protected container;
-
(3)
Dried BiNPs can be suspended in sterile Milli Q water.
*If BiNPs are washed, the concentration of bismuth can be adjusted as desired, by weighting the BiNPs in the desired volume of Milli Q water.
Method validation
After the method was standardized, the BAL-mediated BiNPs were synthesized in more than 10 rounds, on different days, to verify the reproducibility of the protocol. The measurement of the BiNPs size was performed on randomly selected different rounds of synthesis, for the TEM and the DLS analysis. The statistical analysis was performed on the Prism 8 (GraphPad Software Inc) software.
Characterization of the BAL mediated PVP-BiNPs
UV–Vis spectroscopy
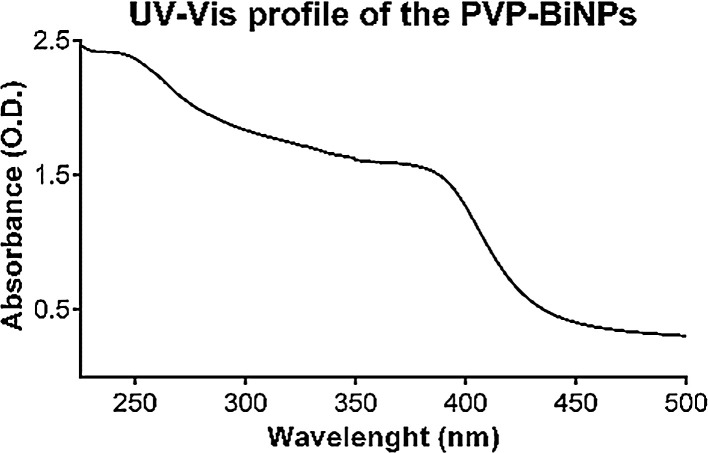
BAL-mediated PVP-BiNPs absorbance profile was collected in a UV–Vis-NIR Cary 500 spectrophotometer (Agilent Technologies), in a wavelength range from 225 to 500 nm, in 1 nm steps. Bismuth Nanoparticles showed a constant absorbance from 225 nm to 500 nm, then decreases at λ = 385 nm. Results from the UV–Vis spectrophotometry analysis suggest the transformation from bismuth (III) ions to bismuth nanoparticles Fig. 2. This UV–Vis profile is similar to the one reported by Wang & Kim for PVP-BiNPs [17].
Fig. 2.
The UV Vis absorbance profile of the BAL-mediated PVP-BiNPs reveals a peak around the 400 nm position.
High-resolution transmission electron microscopy
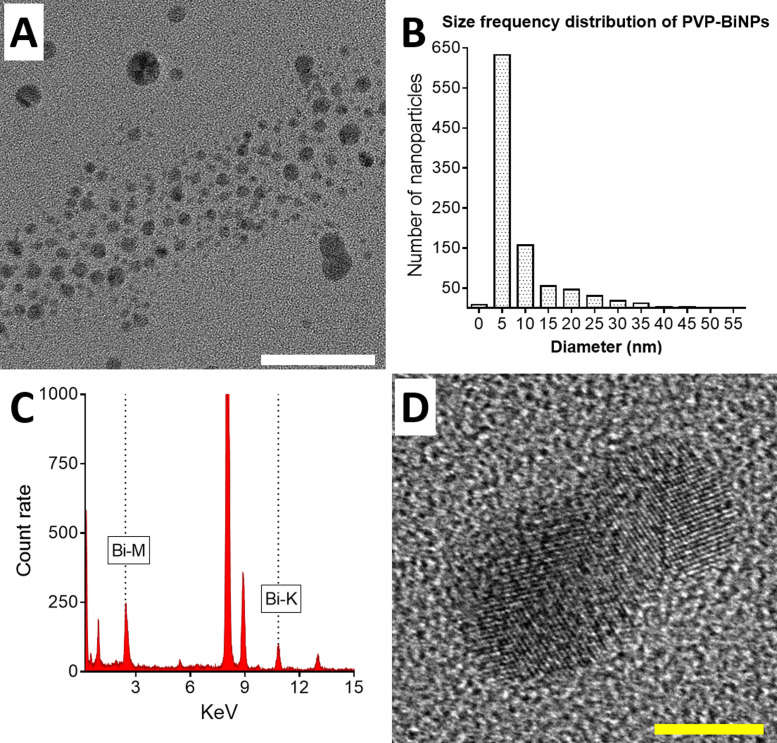
10 µL from the PVP-BiNPs suspension were deposited on Type-B Carbon-coated copper grids (Ted Pella Inc.) and left to dry overnight. The BiNPs were analyzed in a JEOL 2010-F HR-TEM (Jeol Ltd.), with an accelerating voltage of 200 kV. TEM images confirm the presence of small nanoparticles, with an aspect ratio of close to 1 Fig. 3A. The statistical analysis of the frequency distribution size (performed on Prism 8, GraphPad Software Inc.) reveals that the average diameter of the nanoparticles was 8.57 ± 7.52 nm (n = 964) Fig. 3B. An Energy Dispersive X-ray spectroscopy (EDS) analysis was performed to assess the chemical elemental composition of the nanoparticles. The AgNPs were deposit in a copper grid (Ted Pella) and analyzed using an EDAX collector Fig. 3C. The HR-TEM analysis of a single particle confirms the crystalline arrangement of the bismuth nanoparticles Fig. 3D.
Fig. 3.
Electron microscopy characterization. HR-TEM images reveal that the BAL-mediated PVP-BiNPs were small nanoparticles, most of them with an aspect ratio close to 1 (A). The size distribution for the statistical analysis is shown in panel B. An EDS Analysis confirms the presence of Bismuth on the nanoparticles (C). A closer view reveals the crystalline arrangement of the nanoparticles. Scale bar: white =50 nm (A), yellow=5 nm (D). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
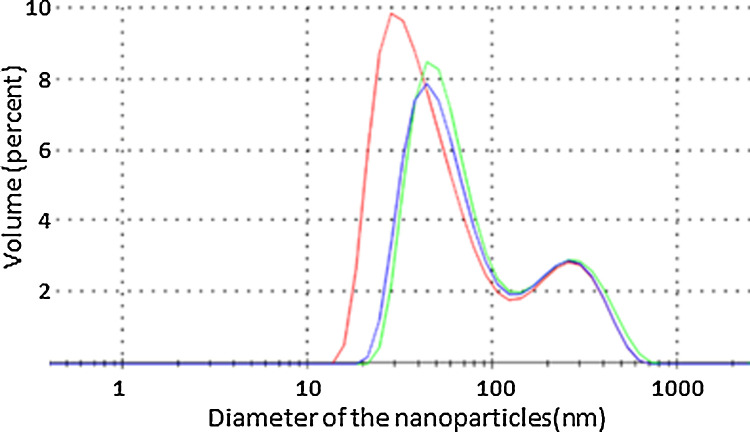
Dynamic light scattering (DLS) analysis
The Hydrodynamic size of the BAL-mediated PVP-BiNPs was determined by a DLS analysis. Briefly, the bismuth nanoparticles -diluted in Milli Q water- were transferred to a DTS1070 cell and analyzed in a Zetasizer Nano ZS (Malvern Panalytical), at room temperature, in triplicate. The BAL –mediated PVP-BiNPs hydrodynamic size is 22.5 ± 0.06 Fig. 4. The hydrodynamic size is greater than the metallic core observed on electron micrographs. This can be attributed to the extended PVP chain-like molecules from the coating, which hydrated under the aqueous environment. According to Wang et al., FTIR demonstrates that PVP interacts chemically with bismuth [17], resulting in PVP-coated bismuth nanoparticles.
Fig. 4.
The DLS analysis reveals that the bismuth nanoparticles size is around 20 nm, although some nanoparticles and clusters are larger than 100 nm.
Conclusion
In summary, we describe a facile, fast, and economical method for the synthesis of BAL-mediated PVP-BiNPs, using basic laboratory instruments and reagents readily available in most laboratories. This method can be easily replicated for research or educational purposes in nanotechnology-related fields, thus encouraging the research for BiNPs as nonantibiotics. These BAL-mediated PVP-BiNPs were small spheroids (<15 nm). The potential of BiNPs as promising nanoantibiotics remains relatively unexplored, despite multiple advantages, such as low cost, low toxicity, and potent antimicrobial activity.
Declaration of Competing Interest
The authors declare that this research study was performed in the absence of any commercial relationship that may be a potential conflict of interest. The funders had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
RV-M acknowledges the receipt of a postdoctoral scholarship from the Mexican Council of Science and Technology of Mexico (Conacyt). Support in the laboratory was provided by the Margaret Batts Tobin Foundation, San Antonio, TX, USA (to JLL-R). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript, and the content is solely the responsibility of the authors.
Contributor Information
Roberto Vazquez-Munoz, Email: roberto.vazquezmunoz@utsa.edu.
Jose L. Lopez-Ribot, Email: jose.lopezribot@utsa.edu.
References
- 1.Andersen O. Chemical and biological considerations in the treatment of metal intoxications by chelating agents. Mini Rev. Med. Chem. 2005;4(1):11–21. doi: 10.2174/1389557043487583. [DOI] [PubMed] [Google Scholar]
- 2.Badireddy A.R., Hernandez-Delgadillo R., Sánchez-Nájera R.I., Chellam S., Cabral-Romero C. Synthesis and characterization of lipophilic bismuth dimercaptopropanol nanoparticles and their effects on oral microorganisms growth and biofilm formation. J. Nanoparticle Res. 2014;16(6) doi: 10.1007/s11051-014-2456-5. [DOI] [Google Scholar]
- 3.Bi H., He F., Dong Y., Yang D., Dai Y., Xu L., Lv R., Gai S., Yang P., Lin J. Bismuth nanoparticles with “Light” property served as a multifunctional probe for X-ray computed tomography and fluorescence imaging. Chem. Mater. 2018;30(10):3301–3307. doi: 10.1021/acs.chemmater.8b00565. [DOI] [Google Scholar]
- 4.Brown A.L., Goforth A.M. pH-dependent synthesis and stability of aqueous, elemental bismuth glyconanoparticle colloids: potentially biocompatible X-ray contrast agents. Chem. Mater. 2012;24(9):1599–1605. doi: 10.1021/cm300083j. [DOI] [Google Scholar]
- 5.El-Batal A.I., El-Sayyad G.S., El-Ghamry A., Agaypi K.M., Elsayed M.A., Gobara M. Melanin-gamma rays assistants for bismuth oxide nanoparticles synthesis at room temperature for enhancing antimicrobial, and photocatalytic activity. J. Photochem. Photobiol. B Biol. 2017;173(April):120–139. doi: 10.1016/j.jphotobiol.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Folsom J.P., Baker B., Stewart P.S. In vitro efficacy of bismuth thiols against biofilms formed by bacteria isolated from human chronic wounds. J. Appl. Microbiol. 2011;111(4):989–996. doi: 10.1111/j.1365-2672.2011.05110.x. [DOI] [PubMed] [Google Scholar]
- 7.Gomez C., Hallot G., Pastor A., Laurent S., Brun E., Sicard-Roselli C., Port M. Metallic bismuth nanoparticles: towards a robust, productive and ultrasound assisted synthesis from batch to flow-continuous chemistry. Ultrason. Sonochem. 2019;56:167–173. doi: 10.1016/j.ultsonch.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Ma L., Wu J., Wang S., Yang H., Liang D., Lu Z. Synergistic antibacterial effect of Bi2S3nanospheres combined with ineffective antibiotic gentamicin against methicillin-resistant staphylococcus aureus. J. Inorg. Biochem. 2017;168:38–45. doi: 10.1016/j.jinorgbio.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Mahony D.E., Lim-Morrison S., Bryden L., Faulkner G., Hoffman P.S., Agocs L., Briand G.G., Burford N., Maguire H. Antimicrobial activities of synthetic bismuth compounds against clostridium difficile. Antimicrob. Agents Chemother. 1999;43(3):582–588. doi: 10.1128/aac.45.5.1417-1421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mousavi-Kamazani M. Facile hydrothermal synthesis of egg-like BiVO4 nanostructures for photocatalytic desulfurization of thiophene under visible light irradiation. J. Mater. Sci. Mater. Electron. 2019;30(19):17735–17740. doi: 10.1007/s10854-019-02123-0. [DOI] [Google Scholar]
- 11.Mousavi-Kamazani M. Cube-like Cu/Cu2O/BiVO4/Bi7VO13 composite nanoparticles: facile sol-gel synthesis for photocatalytic desulfurization of thiophene under visible light. J. Alloy. Compd. 2020;823 doi: 10.1016/j.jallcom.2020.153786. [DOI] [Google Scholar]
- 12.Petsom K., Kopwitthaya A., Horphathum M., Kaewkhao J., Sangwaranatee N. The effect of additive chemicals on synthesis of bismuth nanoparticles. Mater. Today Proc. 2018;5(6):14057–14062. doi: 10.1016/j.matpr.2018.02.061. [DOI] [Google Scholar]
- 13.Reus T.L., Machado T.N., Bezerra A.G., Marcon B.H., Paschoal A.C.C., Kuligovski C., de Aguiar A.M., Dallagiovanna B. Dose-dependent cytotoxicity of bismuth nanoparticles produced by LASiS in a reference mammalian cell line BALB/c 3T3. Toxicol. In Vitro. 2018;53:99–106. doi: 10.1016/J.TIV.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Sun H. Biological Chemistry of Arsenic, Antimony and Bismuth. 1st ed. Wiley; United Kingdom: 2011. Chapter 1. The Chemistry of Arsenic, Antimony and Bismuth. [Google Scholar]
- 15.U.S. Food and Drug Administration . U.S. Food and Drug Administration; 2019. Drugs@FDA: FDA-Approved Drugs.https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process [Google Scholar]
- 16.Vazquez-Muñoz R., Arellano-Jimenez M.J., Lopez F.D., Lopez-Ribot J.L. Protocol optimization for a fast, simple and economical chemical reduction synthesis of antimicrobial silver nanoparticles in non-specialized facilities. BMC Res. Notes. 2019;12(1):773. doi: 10.1186/s13104-019-4813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y.W., Hong B.H., Kim K.S. Size Control of Semimetal Bismuth Nanoparticles and the UV - Visible and IR Absorption Spectra. J. Phys. Chem. 2005;109(15):7067–7072. doi: 10.1021/jp046423v. [DOI] [PubMed] [Google Scholar]
- 18.Wei B., Zhang X., Zhang C., Jiang Y., Fu Y.Y., Yu C., Sun S.K., Yan X.P. Facile synthesis of uniform-sized bismuth nanoparticles for CT visualization of gastrointestinal tract in vivo. ACS Appl. Mater. Interfaces. 2016;8(20):12720–12726. doi: 10.1021/acsami.6b03640. [DOI] [PubMed] [Google Scholar]
- 19.Winter H., Christopher-Allison E., Brown A.L., Goforth A.M. Aerobic method for the synthesis of nearly size-monodisperse bismuth nanoparticles from a redox non-innocent precursor. Nanotechnology. 2018;29(15) doi: 10.1088/1361-6528/aaacb9. [DOI] [PubMed] [Google Scholar]
- 20.Domenico Philip. Enhancement of Bismuth Antibacterial Activity with Lipophilic Thiol Chelators. Antimicrobial Agents and Chemotherapy. 1997;41(8):1697–1703. doi: 10.1128/aac.41.8.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]