Abstract
The main aim of this research work was to isolate and characterize the antimicrobial compounds that can be extracted from the leaves of Dodonaea viscosa (D. viscosa) and to assess their antimicrobial potency by established in-vitro agar diffusion method. The methanol extract was liquefied and fractioned by using a separatory funnel and organic solvents having different polarities. The agar diffusion technique was used to assess the antimicrobial potency of extracts and pure compounds against 5 g (+and -) microbial strains. Antimicrobial potency results showed that all extracts and isolated pure compounds provided significant antimicrobial potency against the applied microbial strains. The highest activity chloroform extract was analysed by column chromatography (CC) for the isolation of antimicrobial compounds. The structure of the isolated compounds was characterized based on 1D, 2D NMR and MS spectra. In conclusion, purest compounds might be useful as a remedy for infectious diseases.
Keywords: Dodonaea viscosa, Soxhlet, Antimicrobial potency, Antimicrobial compounds, Chromatography, NMR, MS
Graphical abstract
The compound 3, 5, 7, 3′,4′-pentahydroxyflavone (1) and as 6-hydroxy-5, 8, 9-trimethyl-18-carboxylclerodane (2) were isolated from the leaves of Adenium ob Dodonaea viscosa. The isolated compounds were report first times from the active chloroform crude extract of D. viscosa.
Highlights
-
•
Dodonaea viscosa is used traditionally in Oman to treat rheumatism, gout, and aches.
-
•
The antimicrobial activity of extracts and compounds was evaluated by diffusion method.
-
•
The highest active extract was used to isolate antimicrobial compounds.
-
•
The compounds were isolated for the first time from the selected Omani species.
-
•
The extract and compound could be used as antibities for infectous diseases.
1. Introduction
Recently, the use of synthetic or natural antibiotics to treat infectious diseases is increased tremendously. In fact, nosocomial infections reported in Europe have resulted in more than 25,000 deaths annually, and productivity losses and health care costs of at least €1.5 billion annually [1].
Morbidity and mortality linked to infection in Oman have been increased tremendously due to the scarce availability of effective drugs. The sultanate of Oman is among the Gulf Countries exhibiting the highest biodiversity, having a wide variety of plant resources. Scientists are now focusing to find naturally occurring antibiotics as herbal remedy to treat different diseases instead of synthetic drugs, to address the increasingly severe issue of drug resistance. Therefore, it is mandatory to find new compounds from natural sources capable of exerting significant biological activity [1]. Subsequently, more effective antimicrobial drugs are being studied among plants and marine sources, to discover suitable biomarkers that can assist as an initial source and pattern for the synthesis of new drugs [2]. Comparable data are measured in Oman [1]. Currently, available synthetic drugs in the global market, including Oman, exhibit some drawbacks, linked to side effects, scarce availability (often connected to high costs) and/or development of resistance [1]. Therefore, drugs are needed for the treatment of diseases, that can mitigate these issues. Plants are considered to be valuable alternative natural antibiotics resources [5]. Therefore, now-a-days, plants are used globally for treating different diseases including infections. Dodonaea viscosa (D. viscosa), also known as Shahs or Zaitoon Alramal, is an indigenous medicinal plant belonging to the family of Sapindaceae [6]. In Oman, it three different species of Dodonaea viscosa are available, all showing significant biological activities [6]. Due to the geographic distribution of Oman, the selected plant is widely available in the Sultanate of Oman. It is mostly distributed in the mountains such as AL-Jabal AL-Akhdar, Jabal Shams and Salalah.
The selected plant contains various bioactive compounds, which are reported by several authors [5,7]. The main chemical constituents of D. viscose are as tannins, alkaloids, carbohydrates, terpenoids, steroids, flavonoids and saponins [6,7]. The plant has many medical properties and has been used by different communities since long time back [5]: (i) the whole plant is used traditionally as a remedy for fever, sore throats, and cold [4]; (ii) the paste of stems is used as fumigants to treat rheumatism and gout; (iii) the paste of the leaves is used to treat itching, fever, swellings, and aches and (v) as an antispasmodic agent; (v) the leaves and root paste is used to treat pain and headaches [[7], [8], [9], [10], [11], [12]].
The selected plant is also used as antimicrobial agents against Gram-positive microbial strains [11]. Very significant inhibition against Staphylococcus aureus, Streptococcus pyogenes, and Corynebacterium diphtheria have been reported, while no inhibition was found against Escherichia coli and Pseudomonas aeruginosa [[7], [8], [9], [10], [11], [12], [13], [14]]. To the authors’ best knowledge, isolation of bioactive compounds from the selected species has not yet been reported. Therefore, the aim of this paper is to describe isolation, separation, purification, and characterization of antimicrobial compounds from the leaves of D. viscosa, native of Oman.
2. Materials and methods
2.1. Chemicals and materials
Different polarity solvents such as CHCl3, CH3OH, C4H10O, DMSO (dimethyl sulfoxide, purity 99%) were collected from Shin-Etsu Chemical, Japan. n-Hexane, acetone, and ethyl acetate were collected from Bayer, Netherland. Levofloxacin, silica gel G254 260 mesh, and pre-coated aluminum and glass thin layer chromatography (TLC) plate were collected from E. Merck, Germany. Whatman filter paper was used as a disc (6 mm) for the detection of antimicrobial activity. All solvents were analytical reagent grade.
2.2. Microorganisms
Four Gram-positive microbial strains: Staphylococcus aureus (S. aureus, Code no. GPB-207), Enterococcus asini (E. asini, Code no. GPB-341), and Streptococcus HVS (S. HVS, Code no. GNB-257), Streptococcus gp B-HVS (S. B-HVS, Code no. GNB-340) and one Gram-negative microbial strains: Homophiles Influenza (H. Influenza, Code no. GNB-236), were obtained from the Microbiological Department of Nizwa Hospital, Oman.
2.3. Sample collection
The leaves of this plant species were collected from Nizwa, Oman. The leaves were separated from the stems and healthy leaves were separated for drying. The leaves were collected during flowering in the month of August 2016 and the plant was identified by a Taxonomist, University of Nizwa, Oman. A voucher specimen number of the selected plant with reference number NP/021 was deposited in the herbarium of Graduation Research Laboratory, University of Nizwa, Oman.
2.4. Extraction
The samples were washed and dried in the shade at ambient temperature for 5 days. Dried leaves samples were crushed into a coarse powder and the yield was 187.38 g. The samples were stored in airtight amber colour bottles. About 57 gm of powdered samples was extracted with 200 ml of methanol by using a Soxhlet method for 36 h. The methanol solvent from the extract was evaporated at 22 °C until the achievement of a paste residue.
The crude extract (16.5 g) was liquefied in 200 ml of distilled water and partitioned separately with 50 ml of n-hexane, chloroform, ethyl acetate, and n-butanol. The isolated different parts, including the water, were evaporated by an evaporator system at different temperatures to give n-hexane (1.9 g), chloroform (5.49 g), ethyl acetate (4.05 g), n-butanol (1.54 g) and water (2.41 g) crude extract. The prepared extracts were used to determine for their in vitro antimicrobial potency for the isolation of antimicrobial compounds [15].
2.5. Preparation of concentration for antimicrobial potency
Four different concentrations (2, 1, 0.5, 0.25 mg/ml) of various polarities extracts of D. viscosa and pure compounds were prepared by dissolving 10 mg of each extract and pure compounds with 5 ml of DMSO solvent. The same solvent (2.5 ml) was also used for dissolving the levofloxacin standard (3 mg).
2.6. Evaluation of antimicrobial potency
The antimicrobial potency of the selected plant extracts and isolated pure compounds were estimated using the adapted disc diffusion technique [15]. The antimicrobial potency of extracts, as well as an isolated compound at various concentrations, was estimated against both Gram (+and -) microbial strains. Levofloxacin and DMSO were used as positive and negative control. In this experiment, the prepared extracts and isolated pure compounds at four concentrations were used to determine antimicrobial potency. Briefly, all concentrations of the extracts were loaded onto 6 mm diameter paper discs. Each clinically isolated organism was placed on the agar plates and spread to form a layer of individual organisms by cotton buds. Filter paper discs were placed in each concentration of extract and positive and negative control. Then, the filter dices were placed on the plates. Agar plates were incubated for 24 h at 37 °C. Antimicrobial potency was expressed in terms of the diameter of the zone of inhibition. The inhibition zone of all discs was measured in millimeters and recorded. The experiment was repeated twice.
2.7. Isolation and characterization of antimicrobial compounds
Among the six extracts, the most active chloroform extract was used to isolate antimicrobial compounds based on the highest antimicrobial potency. The column was packed with silica gel particles (240–260 mesh) with a hexane solvent. After packing the column, the chloroform crude extract (11.49 g) was loaded into the column and eluted initially with hexane. As a mobile phase hexane: ethyl acetate (7:3) was used as eluent with gradually increasing the proportion of ethyl acetate.
The eluents from the column were collected in several test tubes with approximately 5 ml capacity. Rf values All the collected test tubes were examined by thin-layer chromatography (TLC). According to the same, similar elutes were combined together to give Fraction 1, Fraction 2, Fraction 3, Fraction 4, Fraction 5, Fraction 6, Fraction 7, Fraction 8, and Fraction 9. All of these nine fractions were evaporated at room temperature and examined by TLC. Among them, Fraction 6 showed the highest antimicrobial activity.
2.8. Fraction 6
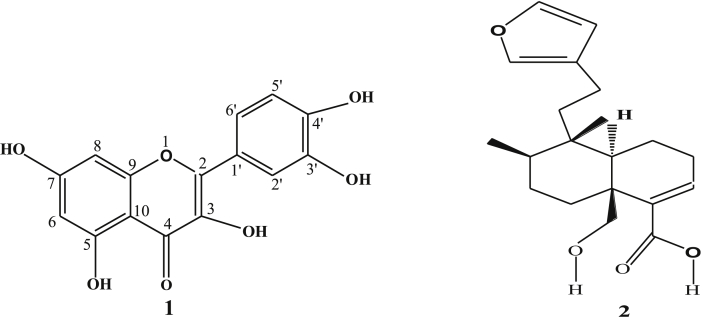
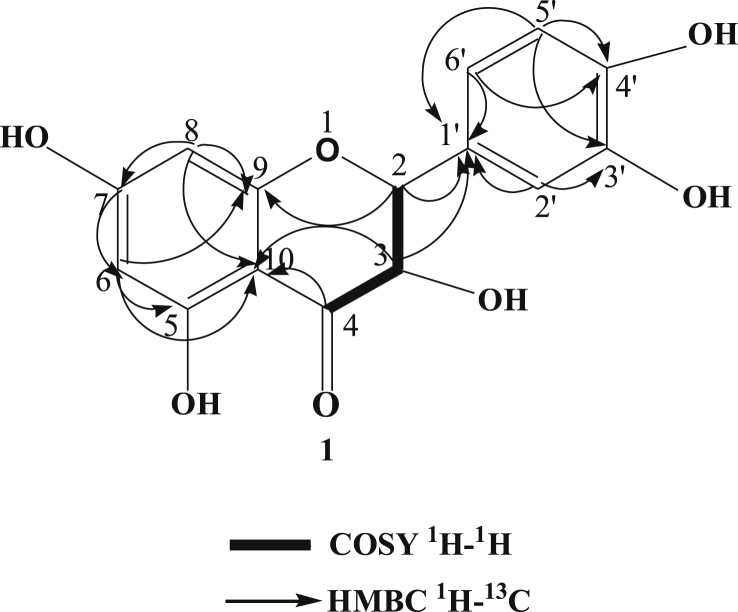
Fraction 6 obtained from the column chromatography was repeated obtain three minor fractions (A-C) based on the Rf values. Fraction A showed several spots and the main compound was purified by preparative thin-layer chromatography (PTLC) using ethyl acetate-dichloromethane (10:7) as a developing solvent. The major compound was further purified by PTLC to obtain two compounds. The highest percentage of the compound was obtained from the preparative TLC by spatula and the compounds were eluted by the acetone. The isolated and purified compound 1 was 9 mg. Finally, it was crystallized from a mixture of solvent hexane-methanol as yellow needle crystals (6 mg), m.p. 316 °C; (M+, 302, Waters Inc. USA, EI source). Based on the spectral data, the isolated compound 1 was characterized as 3, 5, 7, 3′,4′-pentahydroxyflavone (Fig. 1 and Table 1).
Fig. 1.
Chemical structure of compound 1 and compound 2.
Table 1.
1H, 13C, HMBC and COSY NMR spectroscopic data (600 MHz, DMSO-d6) of compounds 1.
| C | δH | HMQC |
HMBC | 1H–1H COSY |
|---|---|---|---|---|
| δC | ||||
| 1 | 175.85 | H-5, H-7, H-8 | ||
| 2 | 163.90 | H-9, H-1ʹ | ||
| 3 | 9.34 (s, 1H, 3-OH) | 160.74 | H-10, H-1ʹ | |
| 4 | 156.14 | H-10, H-5, H-6 | ||
| 5 | 12.47 (s, 1H, 5-OH) | 147.72 | H-6 | |
| 6 | 6.16 (d, 1H, J = 2.04 Hz, H-6) | 123.11 | H-5, H-7, H-8 | H-8 |
| 7 | 10.76 (s, 1H, 7-OH) | 146.81 | H-8, H-6 | |
| 8 | 6.38 (d, 1H, J = 2.04 Hz, H-8) | 93.36 | H-7, H-9, H-10 | H-6 |
| 9 | 156.14 | H-7, H-8, H-8ʹ | ||
| 10 | 103.02 | H-5ʹ, H-7ʹa, 7ʹb, H-8ʹ | ||
| 1ʹ | 121.96 | |||
| 2ʹ | 6.65 (d, 1H, J = 2.16 Hz, H-2′) | 115.61 | H-1ʹ, H-3ʹ | |
| 3ʹ | 9.28 (s, 1H, 3′-OH) | 146.14 | H-2ʹ, H-4ʹ | |
| 4ʹ | 9.56 (s, 1H, 4′-OH) | 145.21 | ||
| 5ʹ | 6.86 (d, 1H, J = 8.46 Hz, H-5′) | 115.61 | H-1ʹ, H-4ʹ, H-3ʹ | H-6ʹ |
| 6ʹ | 7.51 (dd, 1H, J = 2.22 &2.22 Hz, H-6′) | 119.98 | H-1ʹ, H-4ʹ | H-5ʹ |
The isolated and purified compound 2 obtained from fraction 6 by PTLC was solid compound (7 mg). It was a yellowish crystal (3.8 mg), 1H NMR (Bruker 600 MHz, chemical shifts in δ, ppm). DMSO-d6): 0.84 (d, 3H, J = 6.8 Hz, H-19), 0.83 (d, 3H, J = 6.8 Hz, H-20), 1.22 (s, 3H, H-17), 4.99 (dd, 2H, J = 8.5 and 2.5 Hz, H-1 and H-2), 6.86 (dd, 1H, J = 2.9 and 4.6 Hz, H-3), 6.30 (s, 1H, H-14), 7.38 (t, 1H, J = 0.8 Hz, H-16), 7.27 (s, 1H, H-15), 3.64 (dd, 1H, J = 10.8 Hz and 5.1 Hz, H-6),. 13C NMR: 15.2 (C-17), 16.1 (C-19), 17.4 (C-20), 17.5 (C-1), 18.0 (C-12), 27.4 (C-2), 34.4 (C-8), 36.4 (C-7), 39.0 (C-11), 39.3 (C-9), 45.1 (C-5), 75.5 (C-6), 143.2 (C-15), 111.1 (C-14), 141.9 (C-4), 140.6 (C-3), 139 (C-16) and 125.9 (C-13). On the basis of spectral data compound 2 was identified as hautriwaic acid (Fig. 1).
3. Results
The collected coarse samples were used to prepare the extract by using methanol. The methanol solvent was evaporated from the crude extract and it was defeated with water. Then it was successive separation with different polarities of solvents. All the isolated fractions were used to determine their antimicrobial potency for the selection of the highest potency crude extract.
3.1. Antimicrobial potency
The antimicrobial potency of crude extracts was determined by using a disc diffusion technique against different Gram (+and -) microbial strains that were collected from the local hospital. The highest potency was showed in ethyl acetate and chloroform extracts and the lowest activity was in the hexane extract (Table 2). In addition, the antimicrobial potency of the isolated pure compounds at different concentrations was also determined against 2-g positive microbial strains S. aureus (Code no. GPB-207) and Streptococcus gp B-HVS (S. B-HVS, Code no. GNB-340). Both the compounds which were isolated from the selected plant species showed moderate antimicrobial potency against S. aureus (Code no. GPB-207). However, compound 2 showed significant potency against Streptococcus gp B-HVS (S. B-HVS, Code no. GNB-340, Table 2).
Table 2.
Antimicrobial activity of different crude extracts and pure compounds of D. viscosa against the selected bacterial strain.
| Extracts | Conc. mg/ml |
S. HVS (mm) (GNB-257) |
S. aureus (mm) (GPB-207) |
E. anisi (mm) (GPB-341) |
S. B HVS (mm) (GNB-340) |
H. Influenza (mm) (GNB-236) |
|---|---|---|---|---|---|---|
| Methanol | 2 | 10.00 ± 0.23 | 13.00 ± 0.15 | nd | 18.00 ± 0.41 | nd |
| 1 | 8.00 ± 0.52 | 4 ± 0.88 | nd | 12.00 ± 0.23 | nd | |
| 0.5 | 9.00 ± 0.25 | 6.00 ± 0.56 | nd | 9.00 ± 0.33 | nd | |
| 0.25 | 7.00 ± 0.32 | 6.00 ± 0.71 | nd | 12.00 ± 0.55 | nd | |
| Control | 25.00 ± 0.14 | 15.00 ± 0.45 | nd | 31.00 ± 0.34 | 31.00 ± 0.26 | |
| Hexane | 2 | 10.00 ± 0.45 | nd | 8.00 ± 0.55 | 2.000 ± 0.47 | nd |
| 1 | nd | nd | nd | 18.00 ± 0.33 | nd | |
| 0.5 | nd | nd | nd | 12.00 ± 0.31 | nd | |
| 0.25 | nd | nd | nd | 11.00 ± 0.69 | nd | |
| Control | 30.00 ± 0.29 | 19.00 ± 0.23 | 46.00 ± 0.56 | 31.00 ± 0.09 | nd | |
| Chloroform | 2 | 10.00 ± 0.98 | nd | nd | 15.00 ± 0.29 | nd |
| 1 | 9.00 ± 0.56 | nd | nd | 13.00 ± 0.47 | nd | |
| 0.5 | nd | nd | nd | 12 ± 0.33 | nd | |
| 0.25 | nd | nd | nd | 13.00 ± 0.65 | nd | |
| Control | 28.00 ± 0.87 | 18.00 ± 0.51 | 53.00 ± 0.15 | 33.00 ± 0.52 | nd | |
| Ethyl acetate | 2 | 10.00 ± 0.23 | nd | nd | 16.00 ± 0.21 | nd |
| 1 | 7.00 ± 0.55 | nd | nd | 13.00 ± 0.24 | nd | |
| 0.5 | 9.00 ± 0.45 | nd | nd | 13.00 ± 0.27 | nd | |
| 0.25 | 6.00 ± 0.78 | nd | nd | 8.00 ± 0.11 | nd | |
| Control | 22.00 ± 0.34 | 16.00 ± 0.49 | 6.00 ± 0.34 | 26.00 ± 0.09 | nd | |
| Butanol | 2 | 11.00 ± 0.49 | nd | nd | 19.00 ± 0.14 | nd |
| 1 | 9.00 ± 0.34 | nd | nd | 15.00 ± 0.23 | nd | |
| 0.5 | nd | nd | nd | nd | nd | |
| 0.25 | nd | nd | nd | nd | nd | |
| Control | 28.00 ± 0.36 | 16.00 ± 0.29 | nd | 35.00 ± 0.77 | 30.00 ± 0.92 | |
| Water | 2 | 11.00 ± 0.67 | nd | nd | nd | nd |
| 1 | nd | nd | nd | nd | nd | |
| 0.5 | nd | nd | nd | nd | nd | |
| 0.25 | nd | nd | nd | nd | nd | |
| Control | 26.00 ± 0.85 | 16.00 ± 1.20 | 45.00 ± 0.66 | 34.00 ± 0.40 | nd | |
| 2 | nd | 11.00 ± 0.11 | nd | 9.00 ± 0.21 | nd | |
| 1 | nd | 15.00 ± 0.23 | nd | nd | nd | |
| 1 | 0.5 | nd | 9.00 ± 0.41 | nd | nd | nd |
| 0.25 | nd | 6.00 ± 0.94 | nd | nd | nd | |
| Control | 27.00 ± 0.12 | 25.00 ± 0.22 | nd | 30.00 ± 0.27 | nd | |
| DMSO | nd | nd | nd | nd | nd | |
| 2 | 10.00 ± 0.43 | 15.00 ± 1.52 | ||||
| 2 | 1 | nd | 8.00 ± 0.34 | nd | 9.00 ± 1.52 | nd |
| 0.5 | nd | 8.00 ± 0.77 | nd | 9.00 ± 1.52 | nd | |
| 0.25 | nd | nd | nd | 8.00 ± 2.51 | nd | |
| Control | 26.00 ± 0.85 | 30.00 ± 0.29 | 31.00 ± 0.61 | 25.00 ± 2.00 | 27.11 ± 0.10 | |
| DMSO | nd | nd | nd | nd | nd |
nd = not detected; Each value is a mean of three biological replicates.
3.2. Compound 1 and compound 2
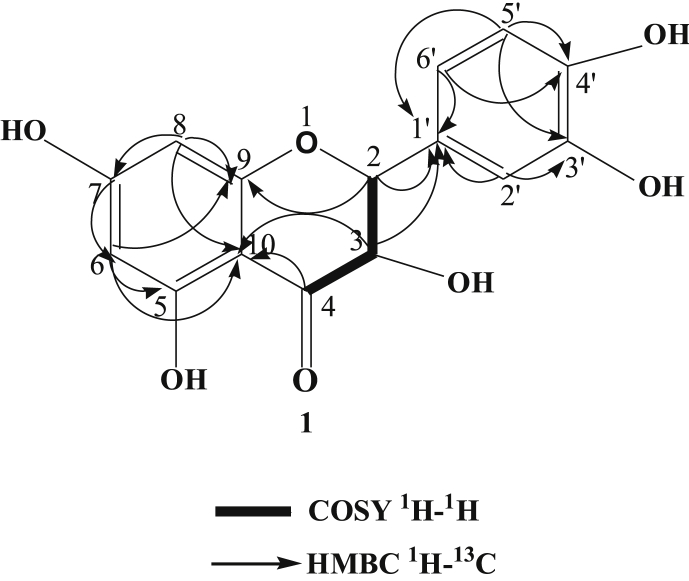
The highest antimicrobial potency directed fraction chloroform extract was used to separate the antimicrobial compounds from the locally grown D. viscosa. Repeated column chromatography followed by PTLC was used to obtain one flavonoid, 3, 5, 7, 3′,4′-pentahydroxyflavone (1) and one diterpene, hautriwaic acid (2). The isolated compound 1 was found from PTLC as yellow needle crystals and it had a melting point of 316 °C (lit. m.p. 315-17 °C) [16]. The molecular formula, as well as the molecular weight of the isolated biomarkers 1, were C15H10O7 and M+, 302 amu. The isolated compound 1 was characterized by 1D and 2D NMR (Fig. 2, Fig. 3, Fig. 4, Fig. 5). The isolated compound 2 obtained from the same fraction by PTLC was also characterized by using NMR (1D and 2D) and mass spectra, which was a comparison of NMR and MS data with values reported in the literature [11].
Fig. 2.
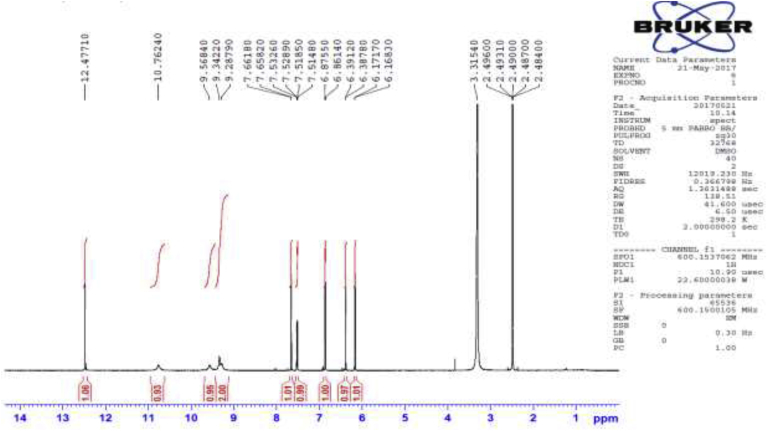
1H NMR spectra of Compound 1.
Fig. 3.
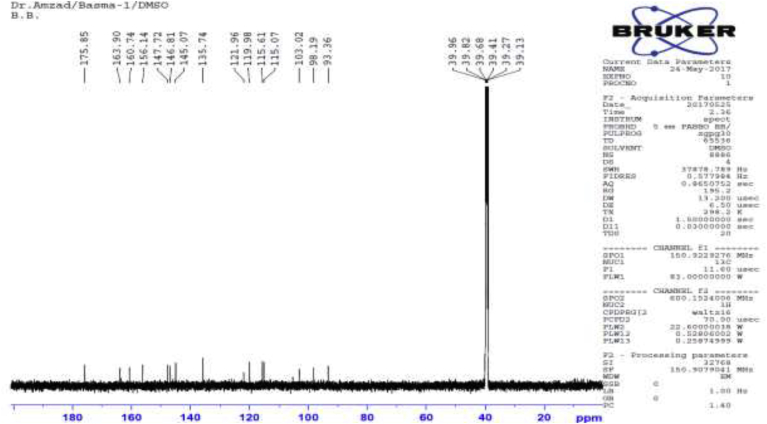
13C NMR spectra of Compound 1.
Fig. 4.
DEPT 90 and 135 spectrum of compound 1.
Fig. 5.
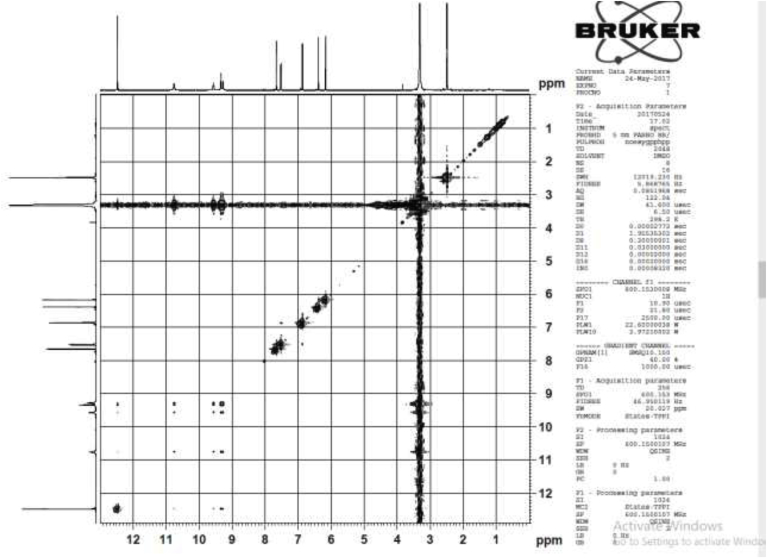
COSY spectrum of compound 1.
4. Discussion
Several antibacterial bioassays such as agar diffusion, micro-dilution, and bio autography are used for the determination of zone inhibition [18]. The disc diffusion bioassay is used widely and well accepted for its simplicity and ability to analyze a huge quantity of samples [18]. Although, the disc diffusion method has several limitations; e.g., this method is not suitable for non-polar samples. In addition, the diffusion method is not appropriate for testing non-polar samples or samples that do not easily diffuse into agar [19]. However, the disc diffusion method is widely used worldwide for the screening of antimicrobial potency. In the present study, the disc diffusion method was used to determine the antibacterial effect of the crude extracts and pure biomarkers of leaves of D. viscosa. All tested discs were incubated and the results were recorded after 24 h by measuring the diameter of inhibition zones. The ethyl acetate crude extract showed the highest zone of inhibition against S. HVS and S. BHVS microbial strains at 2 mg/ml concentration. The other three concentrations (1, 0.5 and 0.25 mg/ml) of ethyl acetate extract also showed significant potency against the S. HVS and S. BHVS microbial strains. Similarly, the chloroform crude extract showed potency against S. HVS at the concentrations of 2 and 1 mg/ml with a zone of inhibition of 10 and 9 mm, respectively. On the other hand, the chloroform extract also showed very good potency against S. BHVS with the inhibition zones of 0–15 mm. The methanol extract did not show activity against E. anisi. In addition, methanol extract also showed significant potency against gram-positive microbial S. aureus. The hexane extract showed good potency against S. BHVS at all applied concentrations. However, the extract gave moderate potency S. HVS and E. anisi only at the highest concentration (2 mg/ml), while other concentrations of hexane extract did not show any potency. The water crude extract did not show any potency at any selected microbial strains except 2 mg/ml against S. HVS. Phytochemical screening reported by Veerapur et al. [11], showed that the selected species have several bioactive compounds, i e., alkaloids, tannins, and saponin, flavonoids, terpenoids, and they are highly responsible for biological activities. Previously, it has been reported that D. viscose does not show inhibitory potency against gram-negative microbial strains [7,20]. However, other studies reported that the extracts showed promising potency against gram-negative microbial strains [3]. In our case, it was also observed that the leaves extract of D. viscose showed low potency against gram-negative microbial strains. Therefore, our result supports what has been reported by Khurram et al. [3] that gram-negative microbial were less sensitive than gram-positive microbial strains. Mehmood et al. [6] reported that S. aureus was showed high potency against methanol extract of D. viscose leaves. Results showed that the plant extracts showed more potency against Streptococcus gp B HVS (Table 2). They showed that the methanol extract shows good inhibitory action against S. aureus (Code no. GPB-207). The isolated pure compounds (1 and 2) also showed significant antimicrobial potency against S. aureus and S. BHVS at most of the concentrations. The compound 1 showed very high potency against S. aureus at all concentrations, but against S. BHVS it showed potency only the concentration of 2 mg/ml. However, compound 2 showed very good potency at all concentrations against both bacterial strains. Some of the prepared extracts did not show any potency, which could be due to bacterial resistance or insufficient concentration of the antimicrobial compound. Compound 1 (yellow needle crystals) has molecular formula C15H10O7 and molecular weight 302 amu. The structure was characterized based on different spectral data (1D, 2D and mass spectra). In proton NMR spectrum, four doublets at different positions δ 6.16, 6.38, 6.86 and 7.65 with comparatively low coupling constant which indicated the presence one proton each position at H-6, H-8, H-5′ and H-2'. One doublet of doublet at 7.51 also low coupling constant indicated the presence of one proton at position H-6' (Fig. 2). In addition, five sharp singlets in the proton-NMR spectrum at positions δ 9.28, 9.34, 9.56, 10.76 and 12.47 which indicated the presence –OH each position at the position C-3′, C-3, C-4′, C-7 and C-5. In the DEPT experiment data indicated that compound 1 has a flavonol skeleton with total 15 carbons; including five aromatic –CH–, ten quaternary carbons, suggesting that it is pentahydroxyflavone (Figs. 3 and 4). All the carbons and hydrogen atom of compound 1 were characterized by 1D and 2D NMR and confirmed on the basis of HSQC and HMBC correlations (Fig. 5 and Table 1). The structure of compound 1 was again supported by the COSY, NOSEY and HMBC correlations as shown in Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7. Although compound 1 had been previously isolated from several plant species, as reported earlier [8,9,17,21], to the authors’ best knowledge, the extraction from the selected plant and the evaluation of its antimicrobial potency have not yet been reported.
Fig. 6.
NOSEY spectrum of compound 1.
Fig. 7.
COSY and HMBC correlation of compound 1.
The diterpenoid type compound 2 was isolated from the leaves of D. viscosa and it was found in fractions 3 and sub-fraction 2. It was also purified by using several chromatographic techniques and its structure was determined by using 1D and 2D NMR spectroscopy including MS. The isolated compound 2 was as a yellowish solid and showed single absorption in UV at 217.7 nm. Its molecular formula, as well as molecular weight, was C20H28O4 and m/z 332.1879. The 1H NMR spectrum displayed two doublets at δ 0.84, and 0.83 indicated the presence of two –CH3 and one singlet at 1.22 indicating the presence of another –CH3 at position C-17. Furthermore, two sharp singlets at 6.30 and 7.27 indicated the presence of H-14 and H-15. In the DEPT experiment among 20 carbons, three methyls, five methylene, seven methine and five quaternary carbons were distinguished. However, compound 2 was previously isolated and characterized earlier from the selected plant species [[11], [21], [22], [23]].
5. Conclusion
The isolation and characterization of two antimicrobial compounds of the chloroform extract derived fraction of D. viscosa, whose structures have been confirmed as 3,5,7,3′,4′-pentahydroxyflavone (1) and hautriwaic acid (2) on the basis of extensive 1D (1H and 13C) and 2D-NMR (COSY, NOSEY and HMBC) as well as mass spectral data. To the authors' best knowledge, it is the first report on isolation and characterization of antimicrobial compounds from the locally grown D. viscosa. The attendance of antibacterial biomarkers might explain the present ethnomedical practices on this locally grown species. Therefore, further in vitro and vivo studies are needed to investigate possible toxicity of the compounds and to confirm the antimicrobial efficacy.
CRediT authorship contribution statement
Basma Mubarak Hamed Al Bimani: Investigation. Mohammad Amzad Hossain: Supervision, Conceptualization, Methodology, Software, Writing - original draft.
Acknowledgements
The authors are grateful to the Oman Research Council (TRC), Sultanate of Oman for providing funding through FURAP project (Call 4, 2016) to carry out the project. The authors would like to express our gratefulness to the University of Nizwa, Nizwa, Sultanate of Oman for providing all enriched learning facilities. Our thanks to the staff of Pharmaceutical Chemistry and Pharmacognosy Laboratories for their assistance to carry out the FURAP project successful. Finally yet importantly, we would like to thank the efforts provided by the Writing Center (TWC) for their professional assistance throughout the writing process of my research project.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.M Aaerestrup F., Kane A.A., Cars O O. World health organization; Geneva, Switzerland: 2012. The Evolving Threat of Antimicrobial Resistance Options for Action.http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf Available at: [Google Scholar]
- 2.Al-matani S.K., Al-Wahaibi R.N.S., Hossain M.A. In vitro evaluation of the total phenolic and flavonoid contents and the antimicrobial and cytotoxicity activities of crude fruit extracts with different polarities from Ficus sycomorus. Pac. Sci. Rev A Nat. Sci. Eng. 2015;17(3):103–108. [Google Scholar]
- 3.Pretorius J.C., Magama S., Zietsman P.C., Van Wyk BE B.E. Growth inhibition of plant pathogenic bacteria and fungi by extracts from selected South African plant species. South Afr. J. Bot. 2009;69(2):186–192. [Google Scholar]
- 4.Khurram K., Khan M.A., Hameed A. Antibacterial activities of Dodonaea viscosa using contact bioautography technique. Molecules. 2009;14(3):1332–1341. doi: 10.3390/molecules14031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aly M., Balkhy H.H. The prevalence of antimicrobial resistance in clinical isolates from Gulf Corporation Council countries. Antimicrob. Resist. Infect. Contr. 2012;1(1):1–5. doi: 10.1186/2047-2994-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehmood A., Murtaza G., Nasir M. Antibacterial and antifungal potency of D. viscosa (L.) Jacq., a wild plant of Azad Jammu and Kashmir. Int. J. Biosci. 2013;3(9):1–7. [Google Scholar]
- 7.Getie M., Gebre-Mariam T., Rietz R. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia. 2003;74(1):139–143. doi: 10.1016/s0367-326x(02)00315-5. [DOI] [PubMed] [Google Scholar]
- 8.Akhtar M., Gülsen T.C., Öztürk M. Biologically active flavonoids from Dodonaea viscosa and their structure-activity relationships. Ind. Crop. Prod. 2015;78:66–72. [Google Scholar]
- 9.Akhtar M., Gülsen T.C., Öztürk M. Phytochemicals from Dodonaea viscosa and their antioxidant and anticholinesterase activities with structure-activity relationships. Pharm. Biol. 2016;54(9):1649–1655. doi: 10.3109/13880209.2015.1113992. [DOI] [PubMed] [Google Scholar]
- 10.Rojas A., Cruz S., Ponce-Monter H., Mata R. Smooth muscle relaxing compounds from Dodonaea viscosa. Planta Med. 1996;62:154–159. doi: 10.1055/s-2006-957840. 02. [DOI] [PubMed] [Google Scholar]
- 11.Veerapur V.P., Badiger A.M., Joshi S.D., Nayak V.P., Shastry C.S. Antiulcerogenic potency of various extracts of Dodonaea viscosa (L) Jacq. Indian J. Pharmaceut. Sci. 2004;6(4):407–411. [Google Scholar]
- 12.Ramachandran N., Subramanian A.G., Sankara S. Isorhamnetin and quercetin glycosides from Dodonaea viscosa and Sapindus emarginatus. Indian J. Chem. 1975;13:639–640. [Google Scholar]
- 13.Al-Snafi A.E. A review on Dodonaea viscosa: a potential medicinal plant. IOSR J. Pharm. 2017;7(2):10–21. [Google Scholar]
- 14.Esmaeel Z.A., AL-Jobori K.M. Antimicrobial effect of Dodonea viscosa JACQ extracts against some pathogenic microorganisms. Iraqi J. Sci. 2011;52(4):425–439. [Google Scholar]
- 15.Al Jadidi H.S.K., Hossain M.A. Determination of the total phenols, flavonoids and antimicrobial potency of the crude extracts from locally grown neem stems. Asian Pac J Trop Dis. 2016;6(5):376–379. [Google Scholar]
- 16.Al Matani S.K., Al Wahaibi R.N.S., Hossain M.A. In vitro evaluation of the total phenolic and flavonoid contents and the antimicrobial and cytotoxicity activities of crude fruit extracts with different polarities from Ficus sycomorus. Pac. Sci. Rev A Nat. Sci. Eng. 2015;17(3):103–108. [Google Scholar]
- 17.Leena P.N., Aleykutty N.A. Isolation and spectral identification of flavonoids from the alcoholic root extract of Cclerodendrum paniculatum linn. Int. J. Pharmaceut. Sci. Res. 2016;7(1):47–50. [Google Scholar]
- 18.Rasoanaivo P., Ratsimamanga-Urverg S. Biological evaluation of plants with reference of Malagasy flora. Toxicology. 1993;39:603–613. [Google Scholar]
- 19.Gould I.M. Towards a common susceptibility testing method. J. Antimicrob. Chemother. 2000;45:757–762. doi: 10.1093/jac/45.6.757. [DOI] [PubMed] [Google Scholar]
- 20.Forestrania R.C., Munoz-Acuña U., Freitas N.F., Carcache de Blanco E.J. Biological potency of compounds from Dodonaea viscosa (L.) Jacq. Var. Angustifolia. Planta Med. 2016;82:210–220. [Google Scholar]
- 21.Sathyadevi M., Subramanian S. Extraction, isolation and characterization of bioactive flavonoids from the fruits of Physalis peruviana linn extract. Asian J. Pharmaceut. Clin. Res. 2015;8(1):152–157. [Google Scholar]
- 22.Ali H., Kabir N., Muhammad A. Hautriwaic acid as one of the hepatoprotective constituent of Dodonaea viscosa. Phytomedicine. 2014;21(2):131–140. doi: 10.1016/j.phymed.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 23.AL Oraimi A.A., Hossain M.A. In vitro total flavonoids content and antimicrobial capacity of different organic crude extracts of Dodonaea viscosa. J. Biol. Act. Prod. Nat. 2016;6(2):150–165. [Google Scholar]