Highlights
-
•
Endophytic activity of pre-conditioned and encapsulated cells in amidated pectin beads
-
•
Hydroxyectoine-added cells within pectin amidated beads increase endophytismus
-
•
Radish yields increased through the application of encapsulated K. radicincitans cells
-
•
Entrapped cells chemoattraction towards radish visualized by multispectral imaging
Keywords: Bacterial aggregates, hydroxyectoine, encapsulation, PGPB endophytes, salt stress
Abstract
Despite the benefits of bacterial endophytes, recent studies on the mostly Gram-negative bacteria lack of regard for formulation strategies. The encapsulation into biopolymeric materials such as amidated pectins hydrogels is a suitable alternative. Here, this research aimed at supporting the capability of the plant growth-promoting bacteria Kosakonia radicincitans DSM16656T to endophytically colonize plant seedlings. In this approach, the pre-conditioned cells through osmoadaptation and hydroxyectoine accumulation were used. In general, pre-osmoadapted and hydroxyectoine-supplemented bacteria cells formulated in amidated pectin dried beads increased the endophytic activity by 10-fold. Moreover, plant promotion in radish plants enhanced by 18.9% and 20.7% for a dry matter of tuber and leaves. Confocal microscopy studies with GFP-tagged bacteria revealed that bacterial aggregates formed during the activation of beads play an essential role in early colonization stages. This research encourages the integration of fermentation and formulation strategies in a bioprocess engineering approach for exploiting endophytic bacteria.
1. Introduction
Plant growth-promoting endophytic bacteria (PGPEB)-based formulations are considered a suitable alternative to overcome issues caused by chemical fertilizer usage [1]. These bacteria offer substantial advantages for their interaction with hosts [2,3], in comparison to native superficially occurring bacteria that persist mainly in soil and root plants neighborhood. Bacterial endophytes are isolated from surface-disinfested plant tissue or extracted from the plant endosphere [4,5]. Since bacterial endophytes can enter and persist into plant tissue, giving to the host physiological advantages against biotic and abiotic stresses, research attention has surged during the last years [2,6].
Regular application targets for PGPEB such as soil or phyllosphere, represent harsh environments, where conditions can fluctuate significantly at nanometer and micrometer levels [7,8]. These alterations include microbial interaction, temperature, UV radiation, free moisture, pH, organic matter content and nutrients supply. Gram-negative bacteria are generally less tolerant facing disturbing surroundings than Gram-positive bacteria, yeasts or filamentous fungi. The lower sensitivity to environmental changes owing to mainly the chemical composition of outer cellular layers [[9], [10], [11]]. Thus, shortly after bacteria inoculation into the soil without a proper carrier, the population declines rapidly for most PGPB species [12]. For Gram-negative bacteria, the ability to withstand abiotic stresses during technical formulation processing is a central problem that requires research efforts. More challenging is to conserve the biological activity and further ability to colonize the plant endosphere.
The potential of PGPBE has primarily been acknowledged and well documented across the globe in the last few decades [1,13]. Hence, plant growth stimulation and yield improvements by endophytic bacteria were evident in laboratory, greenhouse and field levels in several host species [14,15]. Some of these studies carried out even under drought stress, nitrogen deficiency and excessive salinity [16].
A well-studied PGPBE is Kosakonia radicincitans (syn. Enterobacter radicincitans) [17], which endophytic preferences in plant tissue were demonstrated [18]. K. radicincitans can stimulate growth in a range of plant hosts [[18], [19], [20]], solubilize inorganic phosphate [21], fix atmospheric nitrogen and to produce phytohormones such as auxins and cytokinins [22,23]. However, despite the reported benefits, most of the investigations regarding PGPBE, including K. radicincitans, used fresh cultivated cells previously to reveal beneficial effects on plants, without formulation proceedings, which could guarantee a long shelf life of the product and further reproducibility [19,24,25].
Previous studies with promising bacteria in agriculture suggested that salt-stressed cells and compatible solutes inclusion could potentiate their biological activity. For Staphylococcus saprophyticus (ST1), the biofilm formation and exopolysaccharides (EPS) production increased along with NaCl concentration, enhancing its plant growth-promoting abilities [26]. A similar strategy was extended to endophytic bacterial, which studies revealed that pre-conditioning at high salinities and the accumulation of selected compatible solutes could drive the strengthening of bacterial phenotypes [27]. Recently, K. radicincitans upon osmoadaptation and hydroxyectoine accumulation increased its phosphorous solubilization ability, and interestingly plant tissue colonization [28]. Hydroxyectoine also provided benefits on drying survival and endogenous metabolome shifting [29]. As outlined above, salt stress pre-conditioning mechanism along with an intracellular compatible solutes accumulation is a feasible alternative to strengthen endophytic bacteria cells, previously encapsulation processing and application.
Encapsulation/immobilization within hydrogels is a sizeable emerging field in the pharmaceutics, nanotechnology, medicine, aquaculture, and cosmetics industries [12]. The strategy focuses on increasing the microorganism tolerance against unfavorable surroundings caused by biotic and abiotic factors, such as antagonists or dryness [[30], [31], [32]]. Indeed, the immobilization of microorganisms in polymeric materials provides them with several significant advantages over free-living suspensions. Thereby, this approach may offer an uninterrupted supply of nutrients without competing with other microbes, protection against environmental stress and longer shelf life in storage [[33], [34], [35]].
Pectins are a diverse family of biopolymers extracted from the plant cell wall, with an anionic polysaccharide backbone of α-1,4-linked ᴅ-galacturonic acids. Uronic acids integrate into the backbone carboxyl groups arepartially substituted by methyl esters and/or carboxamide groups. Pectins differ according to their degree of esterification (DE) and/or degree of amidation (DA). Both parameters represent the percentage of carboxyl groups esterified and/or amidated, respectively [36]. Due to differences in DE and DA, the viscosity, gelling, mechanical properties, and stability of pectin are affected [37,38]. Pectins operate widely as emulsifiers, gelling agents, glazing agents, stabilizers, and/or thickeners in food, pharmaceutical and personal care products [39].
Amidated pectins can serve as scaffolding drug delivery systems, owing to their versatility targeting specific sites and releasing rates [[40], [41], [42]]. Nevertheless, considering the entrapment of microbes, the amidated pectin-based hydrogels as a biologicals carrier remain relatively unexplored. However, some studies suggested the advantages of this biopolymer for entrapping microbial cells. Indeed, amidated pectins provided favorable characteristics for encapsulating live bacteria, such as Lactobacillus casei supporting protection to gastric acids [43,44]. This biopolymer is also cytocompatible with B-16 melanoma cells and human blood [45]. Krell et al, (2018) co-encapsulated the fungal endophyte Metarhizium brunneum CB15 and cellulase in amidated pectin beads, increasing the endophytic capacity in potato plants. For bacteria immobilization, the incorporation of calcium salts to pectin matrices can enhance polysaccharides reactivity to the bacterial enzymatic arsenal, since many pectinases require calcium ions or are stimulated by their presence [46,47]. Regardless of these benefits, a detailed investigation on amidated pectin as an advantageous carrier for PGPBE is so far missing.
This research aimed at determining the capability of amidated pectin dried beads as a delivery system for the bacterium endophyte K. radicincitans. Moreover, the ability of encapsulated pre-osmoadapted and hydroxyectoine-added cells to colonize the endosphere in radish plants was assessed.
2. Materials and methods
Hydroxyectoine was acquired from Sigma Aldrich (Cat: 70709, Sigma Aldrich Corporation, Germany). Amidated pectin references were provided by Herbstreith & Fox KG (Neuenbuerg/Wuertt, Germany). Details are given in Table 1. All other materials corresponded to analytical reagent grade and were used as received.
Table 1.
Psychochemical properties of selected pectin references used for screening in microfermentation at BioLector®
| Pectin Reference | DE | DA | Galacturonic acid content |
|---|---|---|---|
| Pectin Classic CU 902 | 5.7% | - | 75% |
| Pectin Classic AU-L 061/10 | 40% | - | 85% |
| Pectin Classic AU-L 062/10 | 30% | - | 84% |
| Pectin Amid AU-L 063/10 | 33% | 15% | 83% |
| Pectin Amid CU-L 065/10 | 44% | 11% | 89% |
| Pectin Amid CU-L 066/10 | 24% | 24% | 91% |
Degree of esterification (DE); Degree of amidation (DA)
2.1. Bacterial endophyte and growth conditions
Bacterial endophyte Kosakonia radicincitansDSM16656T[Ref: 6554: Leibnitz-Institut DSMZ] was provided by the Leibnitz Institute of Vegetable and Ornamental Crops in Grossbeeren, Germany. Bacterial cells were maintained on glycerol (50% w/v) and ENDO agar (Merck, Darmstadt, Germany) stocks at −80 °C. Liquid starter cultures were produced in standard nutrient broth (Merck, Darmstadt, Germany) at 30 °C and 190 rpm for 24 h.
Chemically growth medium (DM) was used composed by (g L-1): glycerol (15), yeast extract (8), K2HPO4 (2.74), KH2PO4 (1.31), MgSO4.7H2O (0.5), FeSO4H20 (0.06), MnSO4 (0.01) at pH 7.4. Pre-conditioning of bacteria before the formulation step was ensured by amending DM with NaCl [1, 4%] to obtain water activities (aw) at 0.96 and 0.95, respectively (LabMaster-aw, Novasina AG, Lachen, Switzerland) [29,48].
Bacterial suspensions for encapsulation procedures were prepared as follows: DM (100 ml) was poured into 250 ml baffled Erlenmeyer flasks that were autoclaved at 121 °C, 1.5 atm, for 30 min. The initial inoculum concentration in the media was adjusted at 106 cells ml-1. Cultivation was carried out at 190 rpm in a rotary incubator at 30 °C (IKA KS 4000 ic control, Staufen, Germany). Hydroxyectoine was sterilized separately by filtration through a 0.2 μm membrane filter (Durapore® 0.2 μm PVDF, Millipore, Ireland). Afterward, cells harvesting was conducted at the exponential phase after 21 h (OD600 0.7-0.9) by centrifugation at 2352 g for 15 min (Mikro HT 200R, Hettich GmbH & Co. KG, Tuttlingen, Germany). To prevent osmotic imbalance, the obtained pellet of bacteria was washed and centrifuged twice with a corresponded NaCl solution [1, 4%]. The bacterial cells were stored in the same NaCl solution adjusted at OD600 at 1.5 until use in the formulation assays. Previously to encapsulate the bacteria cells, the intracellular uptake of hydroxyectoine was confirmed. Briefly, 50 μl of concentrated biomass was extracted for quantitative evaluations with 570 μl of an extraction solution (methanol/chloroform/water 10:4:4, v/v) by intense shaking for 5 min followed by the inclusion of equal volumes (170 μl) of chloroform and water [29]. The hydrophilic top layer was analyzed by HPLC, using an EC 150/4.6 NUCLEODUR® 100-5 NH2-RP column and a UV-detector at 215 nm. The chromatographic separation was performed at a flow rate of 1 ml min-1 at 30 °C, accompanied by a column heater and using a solvent gradient established between eluents A and B (80% ACN in HPLC water) [29,49].
2.2. Screening of pectin materials references as a nutrient source
Several pectin references with a range of galacturonic acid contents, esterification and amidation degrees were selected (Table 1). The pectins were screened by their compatibility and potential for serving as a nutrient source for K. radicincitans cells. Briefly, 1 ml of 2% pectin material supplemented with 1% yeast extract were placed in a microtiter plate (MTP) for conducting cultivation (RoboLector-BioLector system, m2p-labs, Baesweiler, Germany). The dissolved oxygen tension and GFP signal intensity as parameters for detecting bacterial growth activity were selected. All BioLector tests were monitored online with a pO2-optode (filter DO [Pst3] Ex (nm) = 520; Em = 600) and GFP filter (filter GFP Gemini [Pst3], Gain = 5, Ex (nm) = 470; Em = 525). The experiments were performed at 30 °C under constant agitation (1200 rpm, shaking diameter = 3 mm, orbital) in 48-well MTP-48-BO flower- plates, Lot No: 1711 (mp2-labs, Baesweiler, Germany) with a working volume of 1000 μl DM. Each treatment was replicated three times.
2.3. Encapsulation
Calcium amidated pectin hydrogel beads as immobilization support were investigated. Briefly, an encapsulation suspension was obtained by mixing ALM pectin solution [4% w/v Amid CU-L 066/10 (DE 24% and DA 24%)] in ultrapure water (Elix Advantage Water Purification System, Merck Millipore, Darmstadt, Germany) at 50% w/w. The pectin solution was supplemented with 14% w/w maltodextrin, 1% w/w sorbitol and 1% w/w monosodium glutamate. Amyloglucosidase (Panzym® HT 300, Novozymes A/S, Bagsværd, Denmark) was used as amylolytic enzyme for maltodextrin degradation at 0.5 AGU. g-1 of the matrix [50]. K. radicincitans cells suspension for the osmoadaptation and hydroxyectoine treatments were added into the matrix to a final concentration of 15% w/w (∼ 8 × 109 viable cells ml−1), and after gently stirred for 5 min. For bead formation, the suspension was dripped into a sterile calcium gluconate cross-linking solution (0.1 M) by using a syringe with a cannula (diameter 2.1 x 0.8 mm, Sterican, B. Braun Melsungen AG, Melsungen, Germany) [51]. The gelled beads cured in the calcium gluconate cross-linking solution for at least 10 min. Beads were separated by filtration and washed with the corresponding NaCl solution [1%, 4%] to remove residual calcium gluconate. Beads were dried to low water content in a two-step drying process [51]. Briefly, beads were put in an oven at 30 °C for 24 h, and later in a desiccator filled with silica gel for another 24 h at room temperature to reach aw <0.3 (LabMASTER-aw at 25 °C, Novasina AG, Lagen, Switzerland). 1 ml of free-living cells at 1.0 × 107 CFU ml-1 was used as drying process control. Four replicates composed each treatment. Bead’s diameter before and after drying with a digital image analyzer was assessed (Digimizer image, MedCalc Software, Ostend, Belgium).
2.4. Encapsulation efficiency
The encapsulation efficiency or survival after entrapping K. radicincitans cells in amidated pectin beads was carried out as followed: 10 beads were disintegrated in a solution containing 0.03 M citric acid and 0.05 M sodium carbonate (pH 7 ± 2) for 1 h in a rotatory shaker at 150 rpm [52]. After complete dissolution, the entrapped viable bacteria were counted by diluted samples and plated on standard nutrient agar media (Merck, Darmstadt, Germany), and incubated at 30 °C for 24 hours. Bacterial cells encapsulation efficiency (BEE) was calculated (Eq. (1)).
| (1) |
Where N is the number of viable entrapped bacterial cells and N0 displays the free viable bacterial cells before encapsulation [53].
Similarly, encapsulation efficiency for sorbitol as a chemical parameter was determined. Here, the amount of sorbitol in the remaining calcium gluconate cross-linking solution was quantified. Briefly, after 10 min of hardening time, beads were separated and 1 ml of calcium gluconate solution was recovered, centrifuged at 21130 x g for 5 min (Mikro HT 200R, Hettich GmbH & Co. KG, Tuttlingen, Germany), and filtered through a 0.45 μm membrane filter. The concentration of sorbitol by HPLC (EC 150/4.6 NUCLEODUR® 100-5 NH2-RP column, RI detector) was determined. The chromatographic separation was performed at a flow rate of 1 ml min-1 at 30 °C, controlled with a column heater, and using a solvent gradient established between eluents A and B (80% acetonitrile in HPLC water). The peak areas were integrated and compared with calibration curves constructed with sorbitol [0.2-20 mg ml-1]. The entrapment efficiency (EE) for sorbitol was calculated (Eq. (2)):
| (2) |
2.5. Plant growth promotion in radish by osmoadapted and encapsulated K. radicincitans cells
The efficacy of amidated pectin beads as formulation alternative for the endophyte K. radicincitans under glasshouse conditions was assessed. Moreover, the extended cross-effect of pre-conditioning by osmoadaptation and the inclusion of hydroxyectoine was also addressed. Radish (R. sativus L. var. sativus) seeds of cultivar Rondar (F1 Hybrid; S & G GmbH, Kleve, Germany) were used as plant systems. Ten radish seeds were placed per pot, with ten pots per treatment, filled with 1.5 L of a 1:1 (v/v) quartz-sand soil mixture (Fruhstorfer Erde type T25: P2O5: 200-300 mg L-1, Hawita Gruppe GmbH Vechta, Germany). Afterward, pots were randomly placed on trivets to avoid the transfer of bacteria [19]. The viable cells concentration in dried beads was adjusted by considering the higher desiccation tolerance encountered previously in cells upon salt stress and hydroxyectoine addition (Fig. S1) [29]. Thus, K. radicincitans inoculation with osmoadapted as well as bacteria cells charged with hydroxyectoine was conducted by locating two dried beads with the same log unit of cells concentration (∼ 2.0 × 106 CFU/bead) under every single seed. The treatments were beads with osmoadapted bacteria cells at 1% NaCl, 4% NaCl and at 4% NaCl with hydroxyectoine [1 mM]. Besides, amidated pectin dried beads without the endophyte as a control for the formulation components were established. Native seed without any treatment as the absolute control was used. Free-living cells as traditional endophyte seed application treatment was also tested [19]. Seedlings were irrigated and fertilized manually with Hoagland solution (50 ml per day) [54]. Plants under natural light conditions were maintained. Temperature and humidity were recorded over the growth period, with an average temperature of 18 ± 2 °C and with an air humidity > 45% [28].
Plant sampling was conducted one-week post-planting from three different locations per plot. The seedlings were rinsed thoroughly with sterile water for removing soil with beads adhering to the roots. Further, samples were flash frozen prior to the isolation of nucleic acid procedures. At this plant age, the root length with a digital image analyzer (Digimizer image, MedCalc Software, Ostend, Belgium) was measured. The plants were equally thinned to five plants per pot, avoiding space limitation during growing and tuber maturation stage. Final sampling at five weeks post-planting was carried out. Five radish plants from the center of each pot were harvested. The total fresh mass of tuber and leaves material and the tuber diameter of each plant were measured. The leaves were separated from roots, oven-dried at 60 °C for 4 days until constant weight, and dry weight of tubers and leaves were determined. The whole experiment was repeated twice.
2.6. Nucleic acid extraction and quantification of K. radicincitans in planta using qPCR
Bacterial DNA was extracted from approx. 50 mg lyophilized plant material using DNeasy plant mini kits (Qiagen, Hilden GmbH, Germany) according to the manufacturer’s instructions. The lysis of bacterial cells was ensured through adding 5 mm sterile metal beads and using a Retsch MM200 mechanical disrupter (Haan, Germany) at 30 rpm for 5 min. The quality and purity of DNA were determined with a NanoDrop (Thermo Fischer Scientific, Darmstadt, Germany). Quantitative real-time PCR (qPCR) measurements were carried out using an Advanced TM Universal SYBR® Green I dye Supermix system (Bio-Rad Laboratories, Hercules, CA, USA). K. radicincitans species-specific primer and plant TEF reference gene for in planta bacterial quantification were used [55]. The fold colonization of K. radicincitans entrapped cells in treated plants concerning to the reference gene and to the control plants was calculated and represented with the 2−ΔΔcq method [56].
2.7. Encapsulated bacteria and radish seedlings interaction: GFP-tagged bacteria approach
An in vitro study was conducted to visualize the endophytic mode of action and the chemotactic performance of encapsulated bacteria cells within radish seedlings. As described in Witzel et al. (2017), electrocompetent bacterial cells were transformed with plasmid pMP4655 [57]. Single colonies of eGFP mutants of K. radicincitans grown on Luria-Bertani agar plus gentamycin (150 μg ml-1) were inoculated in 100 ml standard nutrient broth, and the encapsulation of bacteria followed the procedure above.
Three radish seeds and three amidated pectin beads containing immobilized GFP-labelled K. radicincitans (∼ 1.0 × 107 CFU per bead) were located in a Petri dish with 20 ml of agar media (1% w/v). After four days of incubation at 30 ± 1 °C, GFP tagged bacteria activity inside beads and their chemotactic interaction with radish seedlings was detected by multispectral and kinetic fluorescence imaging (PSI Open FluorCam FC 800-O, PSI, Brno, Czech Republic). The following parameters for capturing images were used: Reflectance mode: Blue light source (447 nm) at 5% intensity, bandpass filter (440/40 nm), shutter at 2 milliseconds and sensitivity at 0%; Fluorescence mode: GFP bandpass filter (517/20 nm): Blue excitation light (447 nm) at 100% intensity, shutter at 300 milliseconds and sensitivity at 38%. Further, root colonization by immobilized bacterial was recorded with a Zeiss LSM 510 META laser scanning confocal microscope (Carl Zeiss Jena GmbH). Bacterial eGFP fluorescence signals were captured using argon laser excitation at 488 nm (BP505-550 180 filter, Plan Apo 63/1.4 oil lens), and root images were taken using bright-field settings [55,58].
2.8. Statistical analysis
Data were analyzed using the SPSS Statistics v.22 software (SPSS, Chicago, IL), and are presented as mean values ± standard deviations (SD). Data were checked for normality and homogeneity of variance using the Shapiro-Wilk and Bartlett test, respectively. Means were tested for significant differences by one-way analysis of variance (ANOVA) followed by a Tukey post hoc test. Data from glasshouse experiments were subjected to Duncan post-hoc test. In this study, the level of significance was set at p < 0.05.
3. Results
3.1. Hydroxyectoine uptake by K. radicincitans
The accumulation of hydroxyectoine during the cultivation at high salinities previously to encapsulation was assessed with HPLC. In response to high salinity during the exponential growth phase in DM at 4% NaCl, bacterial cells amassed this osmolyte after 21 h at 235.09 ± 17.56 (n = 3) μmol g-1 dry weight cells. Osmolyte content in K. radicincitans cells increased over time, >500 μmol per gram of dry biomass at 24 h. No hydroxyectoine was detectable in cells grown in DM in the absence of salt.
3.2. Screening of pectin materials references as a nutrient source
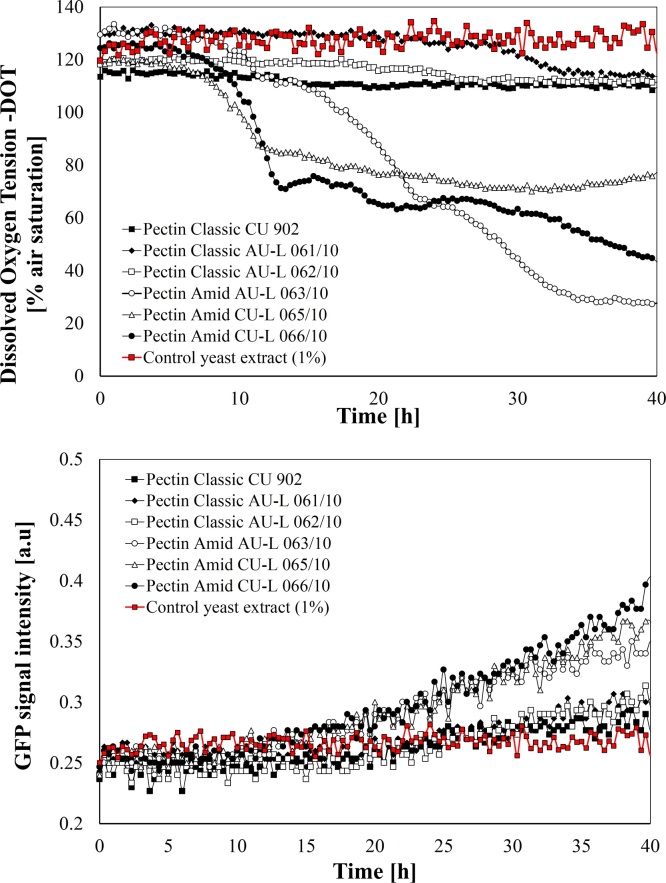
Since the early days during plant growth are considered crucial to provide an open window to the pathogen-endophytes entrance, a rapid bead activation through water uptake and internal bacteria proliferation are essential [1]. Then, to assess the affinity of selected pectin materials to serve as a nutrient source for encapsulation procedures, a high-throughput microfermentation study was applied. Dissolved oxygen tension and GFP signal intensity curves demonstrated that pectin references with a degree of amidation (DA) favored DOT activity and further bacterial growth (F3, 17 = 59.21; p < 0.05). Noticeable, DA in combination with a high galacturonic acid content in pectin, posse a significant effect on kinetic growth and oxygen consumption during K. radicincitans cultivation (F5, 17 = 30.45; p < 0.05, Fig. 1 A–B). Thus, GFP intensity curves confirmed that the pectin amid references AU-L 063/10, CU-L065/10 and CU-L-066/10, are assimilated by K. radicincitans, which included the combination of DA and galacturonic acid content (DA/GA) at 15%/83%, 11%/89% and 24%/91% respectively (Fig. 1B). Interestingly, CU-L-066/10 showed the shortest lag phase among the biopolymers tested with ∼ 6 h in comparison to 9.3 h required for AU-L 063/10. Yeast extract alone at 1% cannot support growth under the evaluated conditions.
Fig. 1.
Screening of selected pectin references as carries through the cultivation of K. radicincitans. A) Dissolved oxygen tension and B) Fluorescence intensity of GFP signal. BioLector approach (means ± SD, n = 3).
3.3. Encapsulation efficiency
The utility of a biopolymer hydrogel for providing a delivery system relies on the entrapment effectiveness of the active ingredient and additives [33,59]. Thus, to look into the efficiency of amidated pectin beads with CU-L-066/10 for encapsulating K. radicincitans cells and formulation additives, the concentration of these parameters before and after the entrapment was determined. The initial cell count of K. radicincitans before beads preparation was 9.46 ± 0.28 log CFU ml-1. The encapsulation efficiency for bacteria cells using amidated pectin beads was 98.37 ± 1.39% (n = 4). Regarding sorbitol encapsulation efficiency, HPLC showed that 48 ± 1.39% (n = 4) of this polyol remains into the amidated pectin beads after the cross-linking reaction and further drying procedure. Beads diameter before and after drying were 3.38 ± 0.31 mm and 2.26 ± 0.26 mm, respectively (n>8). Drying survival results are shown in Fig. S1. The endophyte was quite sensitive to the drying process, since no viable cells from the free-living treatment after the desiccation procedure were recovered.
3.4. Plant growth promotion in radish
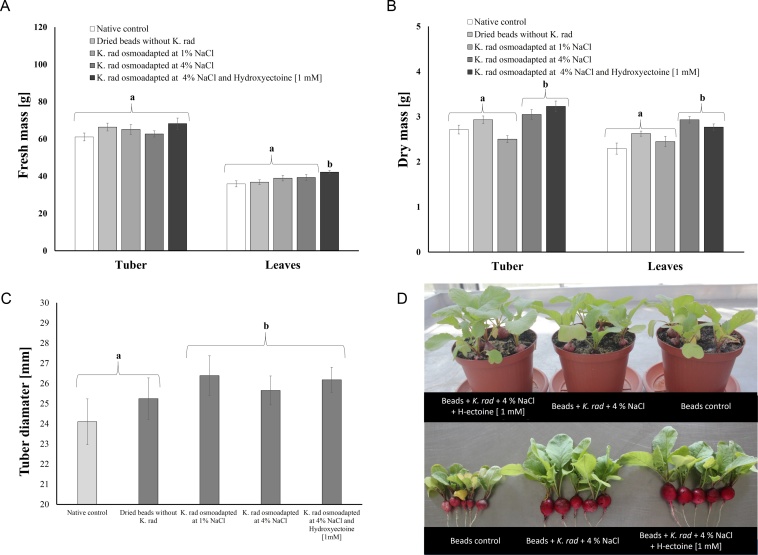
Generally, in both glasshouse experiments, plant growth was promoted by amidated pectin dried beads containing K. radicincitans cells. Higher radish yields in plants inoculated compared to non-inoculated native plants were found. The dried beads for each treatment contained the same log unit of concentration ∼ 2.0 × 106 viable cells per bead. Noticeably, when hydroxyectoine at 1 mM was added during the pre-conditioning step, the fresh matter of leaves increased significantly by 17.45% in comparison to the absolute native control treatment (F4, 39 = 3.15; p = 0.0259, Fig. 2A, D). Noteworthy, either the dry matter of tuber or leave increased significantly by 18.93% (F4, 39 = 9.66; p < 0.0001) and 20.68% (F4, 39 = 7.74; p = 0.0001) respectively, contrasting the absolute native control treatment (Fig. 2B). Considering the dried beads control (without bacterium) as reference, the increments were 2.97% and 5.48% for weight gain in the tuber and leaves respectively. The tuber diameter was also significantly increased in all cases when K. radicincitans cells were presented in comparison to the native control and dried beads control (F4, 39 = 7.64, p = 0.0002). In line with the plant weight gain, the root length after eight days of planting was significantly longer in the case of hydroxyectoine treatment at 3.99 ± 1.07 cm, in comparison to the native control at 2.83 ± 0.65 cm (F4, 39 = 9.67, p < 0.001, Fig. S2). Tuber diameter varied significantly from 24.10 ± 1.14 mm for the native control up to 26.18 ± 0.62 mm for the hydroxyectoine treatment (F4, 39 = 7.86, p = 0.0001) (Fig. 2C, D). Free-living cells treatment showed higher radish yields in comparison to encapsulated approaches; thereby, fresh and dry matter of leaves increased by 27.18% and 43.42% respectively.
Fig. 2.
Growth promotion in glasshouse radish plants inoculated with amidated pectin dried beads containing pre-conditioning K. radicincitans cells by osmoadaptation at 1% NaCl, 4% NaCl and 4% NaCl + hydroxyectoine [1 mM]. A) Fresh mass of tuber and leaves. Fresh tuber mass (F4, 39 = 1.47, P = 0.2321); leaves fresh mass (F4,39 = 3.15, P = 0.0259) B) Dried mass of tuber and leaves. Dry tuber mass (F4, 39 = 9.66, P < 0.0001) and dry leaves mass (F4, 39 = 7.74, P = 0.001). C). Tuber diameter after 5 weeks of planting (F4, 39 = 7.64, P = 0.0002). D). Glasshouse-grown radish plants inoculated with pre-conditioned K. radicincitans cells encapsulated in amidated pectin dried beads. Beads control (without bacterium), beads with pre-conditioned bacterial cells in DM at 4% NaCl and DM at 4% NaCl with the addition of hydroxyectoine [1 mM]. Different letters represent significant differences according to post hoc Dunnett’s test. at p < 0.05 (means ± SD, n = 8).
3.5. K. radicincitans plant colonization
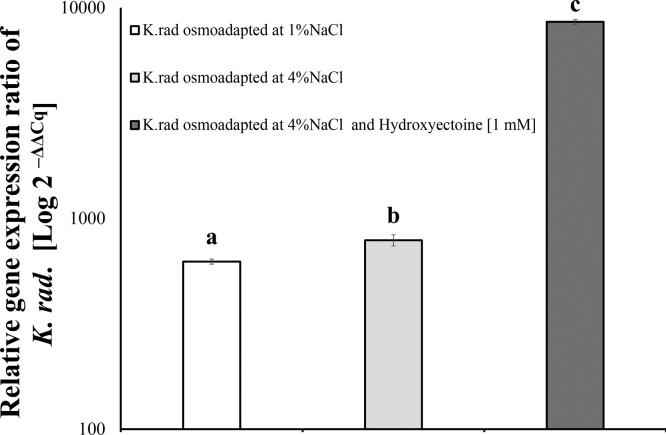
In general, K. radicincitans cells encapsulated in dried amidated pectin beads can colonize plant tissue. Thereby, bacterial cells could leave the beads and settle into the eight days seedlings (Fig. 4). Thus, regarding relative K. radicincitans gene copy number response, dried amidated pectin beads containing pre-conditioned bacteria cells with 4% NaCl, significantly enhanced the plant tissue colonization, in comparison to non-pre-conditioned immobilized cells at 1% NaCl (F2, 11 = 2460.71; p < 0.05, Fig. 3). Consistent with biomass production of radish plants, the plant colonization was stronger with intracellular hydroxyectoine in K. radicincitans cells osmoadapted at 4% NaCl, which the roughly 10-fold DNA copy number increment was significantly under the evaluated conditions (F3, 15 = 10477.33; p < 0.0001, Fig. 3). Free-living cells were capable of colonizing early plant tissue copiously in comparison to entrapped cells, with values ∼100 fold higher than encapsulated treatments (Fig. S3), indicating the role of rapid bacteria activation for settling. The effects of salt pre-conditioning and hydroxyectoine inclusion in free-living cells on endophytic ability were previously discussed [28].
Fig. 4.
Interaction of encapsulated bacteria cells in amidated pectin dried beads with radish seedlings (gnotobiotic system), phase-contrast microscopy approach (EVOS® XL core, Life Technologies). K. radicincitans colonizing root hairs and secondary root surface 4 dpi. Scale bar: 5000 μm.
Fig. 3.
Accumulation of K. radicincitans DNA in inoculated radish plants with amidated pectin dried beads. Effect of pre-conditioning of K. radicincitans in culture media by osmoadaptation at 1% NaCl, at 4% NaCl, and 4% NaCl + hydroxyectoine [1 mM], on relative gene expression in plant tissue. Different letters above bars indicate significant differences of treatments according to Tukey post hoc test at p < 0.05, (means ± SD, n = 4).
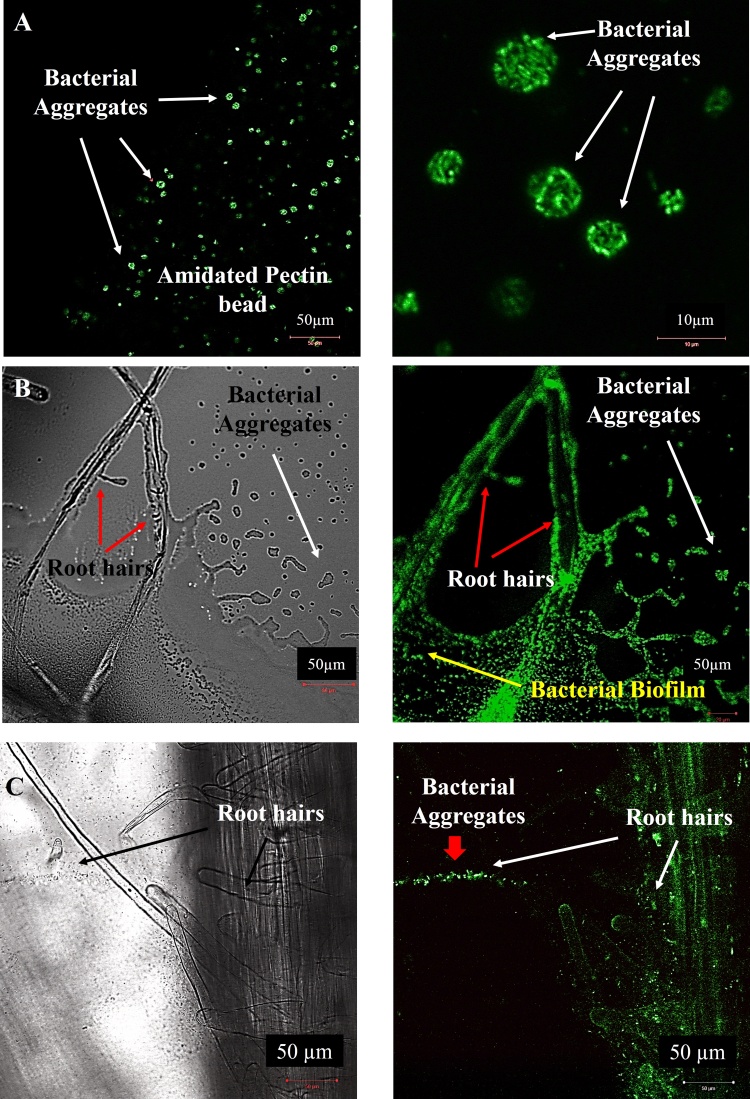
In vitro assessments suggested that the immobilization in amidated pectin beads allowed the successful endophytic colonization, through first the root adhesion to the round shape beads and further, the establishments of root hairs and secondary roots within the bead matrix (Fig. 4). The bacterial aggregates formation within the beads (Fig. 5A–B) along plant interaction is quite interesting, since these aggregates ∼10 μm were also observed in both colonization stages, forming biofilms after seed sprouting in either roots hairs and secondary roots (Figs. 5C and S4). These advantageous sites for colonizing are facilitating by the junction, where lateral root emerges through the endodermis, the cortex and the epidermis (Fig. S4).
Fig. 5.
Confocal laser scanning micrographs showing the inner colonization of radish seedlings by encapsulated K. radicincitans cells expressing eGFP. A. Bacterial aggregates formation inside and at the edge of amidated pectin bead [Sliced 20 μm, CM 1800 microtome (Leice Instruments, Nussloch, Germany)]. B. Amidated pectin-encapsulated GFP-tagged K. radicincitans cells colonizing root hairs by forming aggregates 4 dpi. C.K. radicincitans colonizing root hairs and secondary root epidermis at 4 dpi. In all cases were visualized the formation of bacterial aggregates and biofilms in plant tissue. Scale bar: 50 μm.
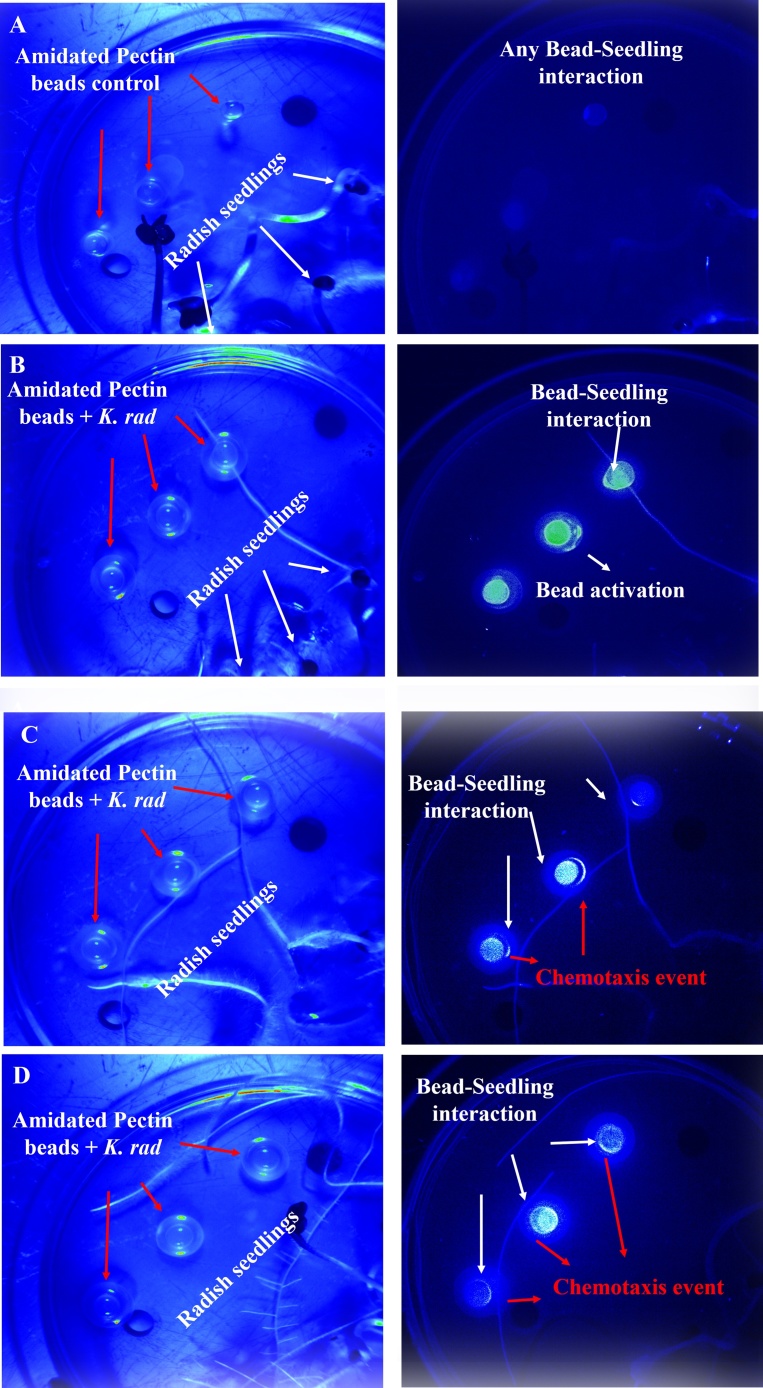
In Fig. 6, the mapping of chemotaxis of encapsulated K. radicincitans cells was evident, since GFP fluorescence images demonstrated the activation of beads, the localization of high bacterial density and the affinity of cells movement towards radish seedlings roots.
Fig. 6.
Mapping of the chemoattraction effect of K. radicincitans GFP-tagged cells encapsulated in pectin beads. Left column represents blue reflectance images. Right column represents GFP fluorescence images A) Control beads without K. radicincitans. B) Beads with K. radicincitans pre-conditioning at NaCl 1% C) Beads with K. radicincitans pre-conditioning at NaCl 4%. D) Beads with K. radicincitans pre-conditioning at NaCl 4% + hydroxyectoine [1 mM]. Color bar indicates emitted light intensity for both image types.
4. Discussion
Exploitation and manipulation of beneficial bacterial endophytes can be a sustainable alternative to cope with the demanding eco-friendly and productive agriculture. The formulation of bacterial endophytes can enhance the bio-prospection of this emerging low-input strategy. Formulation approaches for endophytic bacteria for applying as plant growth stimulators or biocontrol agents were established. These strategies include wettable powders [60], pellets [61], gel-based inoculants [62] and foliar sprays [63]. However, few studies have dealt with the entrapment or encapsulation of endophytic bacteria as proper application technology, using mainly the coating of seed by a bacteria-calcium alginate mix [14,64]. Herein, this research extends the knowledge of manipulated cultivations and further cells encapsulation by ionic gelation as an alternative to formulate bacterial endophytes. Thus, the effectiveness of amidated pectin as a nutrient source and entrapment biopolymer for K. radicincitans cells was established.
Though the evident capability of K. radicincitans to promote radish growth, the results suggest that integrate cells osmoadaptation during the cultivation and the uptake of advantageous compatible solutes such hydroxyectoine are beneficial to the endophytic activity. Noteworthy, both environment variations during growth may lead to greater phenotypic plasticity [65]. Thereby, previous studies discussed the beneficial effects on bacteria endophytes caused by salt stress and the uptake of hydroxyectoine, including metabolic reordering and enhancements of phosphatases activity [28,29]. Hydroxyectoine as osmolyte provides significant osmotic-stress reliving features, superior drying tolerance due to high glass-forming temperature, protein stabilization and water-binding, among others [[66], [67], [68]]. Despite the relatively high cost of hydroxyectoine, biotechnological applications are growing, which are facilitating by the advent of high-yielded optimized production processes and the ectoine genome-modified strains usage [69,70].
Entrapping bacterial cells in amidated pectin beads is a promising alternative to support plant colonization. Moreover, immobilized bacterial cells in pectin-based beads endure the drying process in comparison to fresh free-living cells, indicating an advantage for bioproduct development. Thus, encapsulated bacterial cells in dried amidated pectin beads maintain the capability to promote growth in radish plants. The yields are comparable to Berger et al. (2015), who reported increments in radish weight in tuber and leaves from 20 to roughly 50%, using fresh cultivated cells applying either seed-inoculated or two-leaf sprayed plants. Outstanding, the results of the present study were achieved by applying dried beads, since it is clear that encapsulation of cells and drying intrinsically depressed motility, which is highly implied with the plant interaction and colonization. In addition, dried beads may require a longer establishment to swell and uptake water for the surroundings, providing porous within the gel-matrix, facilitating the increment of internal aw for K. radicincitans cells multiplication [71]. The latter supported the higher radish yields compared to free-living cell treatment. The free-living cells may have a longer time than entrapped cells on seeds surrounding to take advantage of the essential early colonization sites, during seed sprout and seedlings developing. These results indicate that rapid colonization by bacterial cells plays a key role in the final plant growth performance. Embedded cells require an additional step for sensing root exudates through the polymeric matrix. They must first take up water from the soil to release the cells from the beads.
The osmoadaptation as a sub-lethal pre-conditioning procedure along with osmolytes addition could modify the identity and functioning of metabolites that bacteria cells produce. These alterations favor the competitiveness and the establishment in the rhizosphere before colonizing the plant endosphere [28,29]. Hence, osmotic unbalanced can modify mechanisms of quorum sensing (QS), quenching (QQ), or extracellular polymeric substances (EPS). The effects occur during the biofilm formation and further signaling steps for endophytic establishment [[72], [73], [74]]. Therefore, small groups of cells aggregates and biofilm formation, mediated through QS, provide advantages to capitalize on favorable environments or withstand stressful conditions [73].
The successful bacterial establishment could also involve indirect mechanisms provided by the formulation such the additives into the bead. Pectin is one of the major components in plant cell walls, and it might serve as an environmental factor in the stimulation of bacterial biofilm formation, during plant colonization, mimicking natural conditions and triggering the bacterial enzymatic arsenal [75,76].
This study demonstrated that amidated pectins with a high content of galacturonic acid could provide an advantageous nutrient source for endophytic bacteria, supporting the early establishment in the soil. Indeed, K. radicincitans can encode for pectinases secretion and utilizes D-galactose precursor of D-galacturonic acid as a sole carbon source [58,77]. Moreover, other studies in spruce provide evidence of D-galacturonic acid and D-sorbitol utilization as a trait for contributing to the endophytic lifestyle and proliferation in highly reducing microsites in plants [78,79]. Besides, amidated pectin beads have also successfully used as a carrier for delivering fungi endophyte in potato plants [32].
Regarding the other components in the bead, monosodium glutamate has been demonstrated as a nutrient source for plant bio-stimulation, enhancing soil microbial activity and soil respiration [80]. Maltodextrine- amyloglucosidase combination included within the beads may boost an additional C-source for bacteria proliferation. Considering that bead components may modify the nutrient niche of root surfaces and subsequent soil microbiome, they could influence the switch of K. radicincitans from bead- rhizosphere to endophytic lifestyles. This effect could alter radish carbon metabolism, including secondary metabolites such as glucosinolates and inducing priming of defense responses [[81], [82], [83]].
In line with biomass production of radish plants, entrapped cells colonization ability increased with hydroxyectoine inclusion in K. radicincitans cultivation, in which the roughly 10-fold specific DNA copy number increment was significantly under the glasshouse conditions. Similar results with free-living cells were found [28]. This finding indicates that plant colonization improved by synergistic effects of pre-conditioned cells by osmoadaptation and formulation performance. The hydroxyectoine amassing during cultivation may support the endophyte persistence in plant tissue, since this osmolyte may confer protection from osmotic stress during biofilms formation [84]. The best of our knowledge, the current study is the first to deal with the pre-conditioning of cells and pectin-based beads as a formulation alternative for supporting endophytic performance.
This research provides further details on the colonization patterns of GFP-labeled K. radicincitans in radish seedlings. Thus, bacteria cells entrapped in pectin beads can colonize radish seedlings and show an endophytic lifestyle by two main pathways. First, through root hairs during the first stage of root development. In contact with beads, root hairs establish mainly nearby the frontiers of growth out the bead and eventually into the bead by multiple adhesions entrance events. In these zones, K. radicincitans cells are predominant planktonic cells and forming aggregates thereafter. Secondly, bacteria cells are capable of colonizing the secondary roots, either the region of cell maturation (the basis) or the root cap (the tip) reaching the cortical tissue. This mechanism occurs during the second stage of root development. Surprisingly, secondary roots penetrate the bead matrix, and they can establish into it. K. radicincitans aggregates embedded into the bead, proliferate and migrated through the capsule material, colonizing the secondary root. Unusually, since groups of cells appeared at depths > 100 μm, the location of bacterial aggregates was not restricted to the bead borders, where the oxygen concentration could be higher [85]. The bacterial aggregates distribution suggested the facultative capability that allows bacterium the anoxic growth and the entire exploitation of bead structure. This feature could favor the proliferation through the redox gradient between anoxic sites and the microaerobic parenchymatic tissue environment [86]. Altogether, pectin hydrogels could consider as an in-vivo-like biofilm system, diffusion-limited, wherein bacterial endophytes growth exhibits central features of in-vivo biofilms showed during plant colonization.
Pectin as a biopolymer for entrapping bacteria could trigger the enzymatic arsenal of the bacterium. The early barrier encountered of this polysaccharide may mimic natural conditions and activate the plant cell wall-degrading apparatus. These mechanisms include the secretion of endopolygalacturonase and pectin esterases to degrade the backbone of α-D-galacturonic acid, which are considering as physiological traits in endophytes [87]. Besides, other important enzymes that K. radicincitans DSM16656T could encode and facilitate plant colonization are glycoside hydrolases (>127 proteins), β- glucosidases, mannosidases, galactosidases and glucanases [58,76,79].
The root exudates that diffuse to the neighborhood of the bead could be the driven force for entrapped K. radicincitans cells to swim towards seedlings, since the fluorescence images showed higher GFP intensities in the bead’s boundary pointed to the radish roots. Indeed, the induction of chemotactic response of bacterial endophytes to root exudates was reported [88] and is presented as the first step for colonizing the rhizoplane region [89]. Although all beads containing bacteria endophyte showed chemotactic activity, the images of pre-osmoadapted cells with hydroxyectoine suggested that these cells might have advantages for sensing the roots exudates. Questions remain regarding this hypothesis, to delve into the capability of osmotic stress to modify the flagella apparatus, the bacteriocin such kosakonicin, the metabolite profile and the quorum sensing in bacteria endophytes [86,90,91]. Noticeably, with this study, the use of multispectral-kinetic fluorescence imaging emerges as an applicable methodology for targeting analysis of GFP-labelled bacterial endophytes and plant host interaction.
5. Conclusions
This study showed that physiological modifications by osmotic stress, the accumulation of compatible solutes during cultivation, and the entrapment of these pre-conditioned cells in amidated pectin beads enclosed a feasible strategy to improve bacterial endophyte-host interactions. For the first time, a successful endophytic activity of K. radicincitans cells encapsulated in amidated pectin dried beads (DA = 24%; GA = 91%) was demonstrated. Besides, their capability to proliferate as aggregates, to migrate through the biopolymer matrix and to promote radish growth under glasshouse conditions was elucidated. The phenotypic plasticity of K. radicincitans DSM16656T triggered by osmoadaptation and providing exogenously hydroxyectoine during cultivation persists in entrapped cells for increasing plant bio-stimulation and endophytic performance. These findings advance inoculant technology for plant growth-promoting bacterial endophytes.
Funding
This research was funded by the University of Applied Sciences in Bielefeld, Leibnitz Institute of Vegetable and Ornamental Crops, Grossbeeren, ABiTEP GmbH in Germany; Universidad Nacional, AGROSAVIA and Minciencias (formerly Colciencias) (Grant 642) in Colombia.
Declaration of Competing Interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
CRediT authorship contribution statement
Mauricio Cruz Barrera: Conceptualization, Investigation, Writing - original draft. Desiree Jakobs-Schoenwandt: Conceptualization, Writing - review & editing, Funding acquisition, Writing - original draft. Martha Isabel Gómez: Conceptualization, Funding acquisition, Writing - original draft. Juan Serrato: Investigation, Writing - review & editing, Writing - original draft. Silke Ruppel: Writing - original draft. Anant V. Patel: Writing - original draft.
Acknowledgements
The authors want to thank Birgit Wernitz for technical assistance during greenhouse and qPCR experiments at IGZ. In addition, Dr. Jan Gräfe for his support during the FluorCam imaging capturing and Dr. Philip Albers during the CLSM analysis.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00463.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Kandel S.L., Joubert P.M., Doty S.L. Bacterial Endophyte Colonization and Distribution within Plants. Microorganisms. 2017;5(4) doi: 10.3390/microorganisms5040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenblueth M., Martinez-Romero E. Bacterial endophytes and their interactions with hosts. Mol Plant-Microbe Interact. 2006;19(8):827–837. doi: 10.1094/mpmi-19-0827. [DOI] [PubMed] [Google Scholar]
- 3.Reinhold-Hurek B., Hurek T. Living inside plants: bacterial endophytes. Current Opinion in Plant Biol. 2011;14(4):435–443. doi: 10.1016/j.pbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Hallmann J., QuadtHallmann A., Mahaffee W.F., Kloepper J.W. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43(10):895–914. doi: 10.1139/m97-131. [DOI] [Google Scholar]
- 5.Wilson D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos. 1995;73(2):274–276. doi: 10.2307/3545919. [DOI] [Google Scholar]
- 6.Ryan R.P., Germaine K., Franks A., Ryan D.J., Dowling D.N. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278(1):1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindow S.E., Leveau J.H. Phyllosphere microbiology. Curr Opin Biotechnol. 2002;13(3):238–243. doi: 10.1016/S0958-1669(02)00313-0. [DOI] [PubMed] [Google Scholar]
- 8.Paul E.A. Fourth Edition. Academic Press; Boston: 2015. Chapter 1 - Soil Microbiology, Ecology, and Biochemistry: An Exciting Present and Great Future Built on Basic Knowledge and Unifying Concepts, Soil Microbiol, Ecol Biochem; pp. 1–14. [Google Scholar]
- 9.Wieland G., Neumann R., Backhaus H. Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl Enviro Microbiol. 2001;67(12):5849–5854. doi: 10.1128/aem.67.12.5849-5854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao L., Sun M.H., Liu X.Z., Che Y.S. Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi. Mycol Res. 2007;111:87–92. doi: 10.1016/j.mycres.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Ren C., Wang T., Xu Y., Deng J., Zhao F., Yang G. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. Forest Ecol Manag. 2018;409:170–178. doi: 10.1016/j.foreco.2017.11.011. [DOI] [Google Scholar]
- 12.Bashan Y., de-Bashan L.E., Prabhu S.R., Hernandez J.P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998-2013) Plant Soil. 2014;378(1–2):1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- 13.Santoyo G., Moreno-Hagelsieb G., Orozco-Mosqueda MdC, Glick B.R. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Lally R.D., Galbally P., Moreira A.S., Spink J., Ryan D., Germaine K.J. Application of Endophytic Pseudomonas fluorescens and a Bacterial Consortium to Brassica napus Can Increase Plant Height and Biomass under Greenhouse and Field Conditions. Front Plant Sci. 2017;8 doi: 10.3389/fpls.2017.02193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma S.K., Kingsley K., Bergen M., English C., Elmore M., Kharwar R.N. Bacterial endophytes from rice cut grass (Leersia oryzoides L.) increase growth, promote root gravitropic response, stimulate root hair formation, and protect rice seedlings from disease. Plant Soil. 2018;422(1–2):223–238. doi: 10.1007/s11104-017-3339-1. [DOI] [Google Scholar]
- 16.Rho H., Hsieh M., Kandel S.L., Cantillo J., Doty S.L., Kim S.-H. Do Endophytes Promote Growth of Host Plants Under Stress? A Meta-Analysis on Plant Stress Mitigation by Endophytes. Microb Ecol. 2018;75(2):407–418. doi: 10.1007/s00248-017-1054-3. [DOI] [PubMed] [Google Scholar]
- 17.Brady C., Cleenwerck I., Venter S., Coutinho T., De Vos P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): Proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov. Syst Appl Microbiol. 2013;36:309–319. doi: 10.1016/j.syapm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Ruppel S., Ruehlmann J., Merbach W. Quantification and localization of bacteria in plant tissues using quantitative real-time PCR and online emission fingerprinting. Plant Soil. 2006;286(1–2):21–35. doi: 10.1007/s11104-006-9023-5. [DOI] [Google Scholar]
- 19.Berger B., Wiesner M., Brock A.K., Schreiner M., Ruppel S. K-radicincitans, a beneficial bacteria that promotes radish growth under field conditions. Agron Sustain Dev. 2015;35(4):1521–1528. doi: 10.1007/s13593-015-0324-z. [DOI] [Google Scholar]
- 20.Berger B., Patz S., Ruppel S., Dietel K., Faetke S., Junge H. Successful Formulation and Application of Plant Growth-Promoting Kosakonia radicincitans in Maize Cultivation. Biomed Res Inter. 2018 doi: 10.1155/2018/6439481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schilling G., Gransee A., Deuhel A., Ležoviž G., Ruppel S. Phosphorus availability, root exudates, and microbial activity in the rhizosphere. Zeitschrift für Pflanzenernährung und Bodenkunde. 1998;161(4):465–478. doi: 10.1002/jpln.1998.3581610413. [DOI] [Google Scholar]
- 22.Ruppel S., Merbach W. Effects of different nitrogen sources on nitrogen fixation and bacterial growth of Pantoea agglomerans and Azospirillum sp. in bacterial pure culture: An investigation using 15N2 incorporation and acetylene reduction measures. Microbiol Res. 1995;150(4):409–418. doi: 10.1016/S0944-5013(11)80023-6. [DOI] [Google Scholar]
- 23.Scholz-Seidel C., Ruppel S. Nitrogenase- and Phytohormone Activities of Pantoea agglomerans in Culture and their Reflection in Combination with Wheat Plants. Zentralblatt für Mikrobiologie. 1992;147(5):319–328. doi: 10.1016/S0232-4393(11)80395-1. [DOI] [Google Scholar]
- 24.Garima G., Nath J.P. Screening of potential PGPR candidates as future biofertilizers-A strategic approach from lab to field. Res J Biotechnol. 2015;10(11):48–62. [Google Scholar]
- 25.Berger B., Baldermann S., Ruppel S. The plant growth-promoting bacterium Kosakonia radicincitans improves fruit yield and quality of Solanum lycopersicum. J Sci Food Agric. 2017 doi: 10.1002/jsfa.8357. [DOI] [PubMed] [Google Scholar]
- 26.Qurashi A.W., Sabri A.N. Osmoadaptation and plant growth promotion by salt tolerant bacteria under salt stress. Afr J Microbiol Res. 2011;5(21):3546–3554. doi: 10.5897/AJMR11.736. [DOI] [Google Scholar]
- 27.Cabrefiga J., Frances J., Montesinos E., Bonaterra A. Improvement of Fitness and Efficacy of a Fire Blight Biocontrol Agent via Nutritional Enhancement Combined with Osmoadaptation. Appl Environ Microbiol. 2011;77(10):3174–3181. doi: 10.1128/aem.02760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrera M.C., Jakobs-Schoenwandt D., Gamez M.I., Becker M., Patel A.V., Ruppel S. Salt stress and hydroxyectoine enhance phosphate solubilisation and plant colonisation capacity of Kosakonia radicincitans. J Adv Res. 2019;19:91–97. doi: 10.1016/j.jare.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz Barrera M., Jakobs-Schoenwandt D., Persicke M., Gómez M.I., Ruppel S., Patel A.V. Anhydrobiotic engineering for the endophyte bacterium Kosakonia radicincitans by osmoadaptation and providing exogenously hydroxyectoine. World J Microbiol Biotechnol. 2019;36(1):6. doi: 10.1007/s11274-019-2780-0. [DOI] [PubMed] [Google Scholar]
- 30.Vemmer M., Patel A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol Control. 2013;67(3):380–389. doi: 10.1016/j.biocontrol.2013.09.003. [DOI] [Google Scholar]
- 31.Krell V., Jakobs-Schoenwandt D., Persicke M., Patel A.V. Endogenous arabitol and mannitol improve shelf life of encapsulated Metarhizium brunneum. World J Microbiol Biotechnol. 2018;34(8) doi: 10.1007/s11274-018-2492-x. [DOI] [PubMed] [Google Scholar]
- 32.Krell V., Jakobs-Schoenwandt D., Vidal S., Patel A.V. Cellulase enhances endophytism of encapsulated Metarhizium brunneum in potato plants. Fungal Biol. 2018;5(122):373–378. doi: 10.1016/j.funbio.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Bashan Y., Hernandez J.P., Leyva L.A., Bacilio M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biol Fert Soils. 2002;35(5):359–368. doi: 10.1007/s00374-002-0481-5. [DOI] [Google Scholar]
- 34.Trivedi P., Pandey A., Palni L.M.S. Carrier-based preparations of plant growth-promoting bacterial inoculants suitable for use in cooler regions. World J Microbiol Biotechnol. 2005;21(6–7):941–945. doi: 10.1007/s11274-004-6820-y. [DOI] [Google Scholar]
- 35.Przyklenk M., Vemmer M., Hanitzsch M., Patel A. A bioencapsulation and drying method increases shelf life and efficacy of Metarhizium brunneum conidia. J Microencapsul. 2017;34(5):498–512. doi: 10.1080/02652048.2017.1354941. [DOI] [PubMed] [Google Scholar]
- 36.Huynh U.T.D., Assifaoui A., Chambin O. Pellets based on polyuronates: Relationship between gelation and release properties. J Food Eng. 2017;199:27–35. doi: 10.1016/j.jfoodeng.2016.12.004. [DOI] [Google Scholar]
- 37.Munarin F., Tanzi M.C., Petrini P. Advances in biomedical applications of pectin gels. Int J Biol Macromol. 2012;51(4):681–689. doi: 10.1016/j.ijbiomac.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Sato A.C.K., Oliveira P.R., Cunha R.L. Rheology of mixed pectin solutions. Food Biophys. 2008;3(1):100–109. doi: 10.1007/s11483-008-9058-7. [DOI] [Google Scholar]
- 39.Chan S.Y., Choo W.S., Young D.J., Loh X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr Polym. 2017;161:118–139. doi: 10.1016/j.carbpol.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 40.Sriamornsak P. Application of pectin in oral drug delivery. Expert Opinion on Drug Delivery. 2011;8(8):1009–1023. doi: 10.1517/17425247.2011.584867. [DOI] [PubMed] [Google Scholar]
- 41.Kosaraju S.L. Colon targeted delivery systems: Review of polysaccharides for encapsulation and delivery. Crit Rev Food Sci Nutr. 2005;45(4):251–258. doi: 10.1080/10408690490478091. [DOI] [PubMed] [Google Scholar]
- 42.Liu L.S., Fishman M.L., Kost J., Hicks K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials. 2003;24(19):3333–3343. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Bepeyeva A., de Barros J.M.S., Albadran H., Kakimov A.K., Kakimov Z.K., Charalampopoulos D. Encapsulation of Lactobacillus casei into Calcium Pectinate-Chitosan Beads for Enteric Delivery. J Food Sci. 2017;82(12):2954–2959. doi: 10.1111/1750-3841.13974. [DOI] [PubMed] [Google Scholar]
- 44.Sandoval-Castilla O., Lobato-Calleros C., Garcia-Galindo H.S., Alvarez-Ramirez J., Vernon-Carter E.J. Textural properties of alginate-pectin beads and survivability of entrapped Lb. casei in simulated gastrointestinal conditions and in yoghurt. Food Res Int. 2010;43(1):111–117. doi: 10.1016/j.foodres.2009.09.010. [DOI] [Google Scholar]
- 45.Mishra R.K., Singhal P., Datt M., Banthia A.K. Amidated pectin based hydrogels: synthesis, characterization and cytocompatibility study. J Appl Biomater Biomech. 2007;5(2):88–94. doi: 10.1177/228080000700500204. [DOI] [PubMed] [Google Scholar]
- 46.Sunnotel O., Nigam P. Pectinolytic activity of bacteria isolated from soil and two fungal strains during submerged fermentation. World J Microbiol Biotechnol. 2002;18(9):835–839. doi: 10.1023/A:1021209123641. [DOI] [Google Scholar]
- 47.Yadav S., Yadav P.K., Yadav D., Yadav K.D.S. Purification and characterization of an alkaline pectin lyase from Aspergillus flavus. Process Biochem. 2008;43(5):547–552. doi: 10.1016/j.procbio.2008.01.015. [DOI] [Google Scholar]
- 48.Kunte H.J., Galinski E.A., Truper H.G. A modified fmoc-method for the detection of amino acid-type osmolytes and tetrahydropyrimidines (ectoines) J Microbiol Methods. 1993;17(2):129–136. doi: 10.1016/0167-7012(93)90006-4. [DOI] [Google Scholar]
- 49.Teixido N., Canamas T.P., Usall J., Torres R., Magan N., Vinas I. Accumulation of the compatible solutes, glycine-betaine and ectoine, in osmotic stress adaptation and heat shock cross-protection in the biocontrol agent Pantoea agglomerans CPA-2. Lett Appl Microbiol. 2005;41(3):248–252. doi: 10.1111/j.1472-765X.2005.01757.x. [DOI] [PubMed] [Google Scholar]
- 50.Humbert P., Vemmer M., Giampa M., Bednarz H., Niehaus K., Patel A.V. Co-encapsulation of amyloglucosidase with starch and Saccharomyces cerevisiae as basis for a long-lasting CO2 release. World J Microbiol Biotechnol. 2017;33(4) doi: 10.1007/s11274-017-2237-2. [DOI] [PubMed] [Google Scholar]
- 51.Humbert P., Przyklenk M., Vemmer M., Patel A.V. Calcium gluconate as cross-linker improves survival and shelf life of encapsulated and dried Metarhizium brunneum and Saccharomyces cerevisiae for the application as biological control agents. J Microencapsul. 2017;34(1):47–56. doi: 10.1080/02652048.2017.1282550. [DOI] [PubMed] [Google Scholar]
- 52.Mater D.D.G., Jean-Noël B., José Edmundo N.S., Nicole T., Daniel T. Effect of gelation temperature and gel-dissolving solution on cell viability and recovery of two Pseudomonas putida strains co-immobilized within calcium alginate or κ-carrageenan gel beads. Biotechnol Tech. 1995;9(10):747–752. doi: 10.1007/BF00159242. [DOI] [Google Scholar]
- 53.Haghshenas B., Abdullah N., Nami Y., Radiah D., Rosli R., Khosroushahi A.Y. Microencapsulation of probiotic bacteria Lactobacillus plantarum 15HN using alginate-psyllium-fenugreek polymeric blends. J Appl Microbiol. 2015;118(4):1048–1057. doi: 10.1111/jam.12762. [DOI] [PubMed] [Google Scholar]
- 54.Hoagland D.R., Arnon D.I. College of Agriculture, University of California; Berkeley, Calif: 1950. The water-culture method for growing plants without soil. [Google Scholar]
- 55.Witzel K., Strehmel N., Baldermann S., Neugart S., Becker Y., Becker M. Arabidopsis thaliana root and root exudate metabolism is altered by the growth-promoting bacterium Kosakonia radicincitans DSM 16656(T) Plant Soil. 2017;419(1–2):557–573. doi: 10.1007/s11104-017-3371-1. [DOI] [Google Scholar]
- 56.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25(4):402–408. doi: 10.1007/s11104-017-3371-1. [DOI] [PubMed] [Google Scholar]
- 57.Lagendijk E.L., Validov S., Lamers G.E.M., de Weert S., Bloemberg G.V. Genetic tools for tagging Gram-negative bacteria with mCherry for visualization in vitro and in natural habitats, biofilm and pathogenicity studies. FEMS Microbiol Lett. 2010;305(1):81–90. doi: 10.1111/j.1574-6968.2010.01916.x. [DOI] [PubMed] [Google Scholar]
- 58.Becker M., Patz S., Becker Y., Berger B., Drungowski M., Bunk B. Comparative Genomics Reveal a Flagellar System, a Type VI Secretion System and Plant Growth-Promoting Gene Clusters Unique to the Endophytic Bacterium Kosakonia radicincitans. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poncelet D. Production of alginate beads by emulsification/internal gelation. In: Hunkeler D., Cherrington A., Prokop A., Rajotte R., editors. Bioartificial Organs Iii: Tissue Sourcing, Immunoisolation, and Clinical Trials. New York Acad Sciences; New York: 2001. pp. 74–82. [Google Scholar]
- 60.Cheng H., Li L.L., Hua J., Yuan H.H., Cheng S.Y. A Preliminary Preparation of Endophytic Bacteria CE3 Wettable Powder for Biological Control of Postharvest Diseases. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2015;43(1):159–164. doi: 10.15835/nbha4319699. [DOI] [Google Scholar]
- 61.Hu X.J., Roberts D.P., Maul J.E., Emche S.E., Liao X., Guo X.L. Formulations of the endophytic bacterium Bacillus subtilis Tu-100 suppress Sclerotinia sclerotiorum on oilseed rape and improve plant vigor in field trials conducted at separate locations. Can J Microbiol. 2011;57(7):539–546. doi: 10.1139/w11-041. [DOI] [PubMed] [Google Scholar]
- 62.da Silva M.F., Antonio C.D., de Oliveira P.J., Xavier G.R., Rumjanek N.G., Soares L.H.D. Survival of endophytic bacteria in polymer-based inoculants and efficiency of their application to sugarcane. Plant Soil. 2012;356(1–2):231–243. doi: 10.1007/s11104-012-1242-3. [DOI] [Google Scholar]
- 63.Bejarano A., Sauer U., Preininger C. Design and development of a workflow for microbial spray formulations including decision criteria. Appl Microbiol Biotechnol. 2017;101(19):7335–7346. doi: 10.1007/s00253-017-8447-6. [DOI] [PubMed] [Google Scholar]
- 64.Oteino N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. 2015:6. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulz B., Boyle C. The endophytic continuum. Mycol Res. 2005;109:661–686. doi: 10.1017/s095375620500273x. [DOI] [PubMed] [Google Scholar]
- 66.Tanne C., Golovina E.A., Hoekstra F.A., Meffert A., Galinski E.A. Glass-forming property of hydroxyectoine is the cause of its superior function as a desiccation protectant. Front Microbiol. 2014:5. doi: 10.3389/fmicb.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lippert K., Galinski E.A. Enzyme stabilization be ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol. 1992;37(1):61–65. doi: 10.1007/bf00174204. [DOI] [Google Scholar]
- 68.Czech L., Hermann L., Stoveken N., Richter A.A., Hoppner A., Smits S.H.J. Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis. Genes. 2018;9(4) doi: 10.3390/genes9040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parwata I.P., Wahyuningrum D., Suhandono S., Hertadi R. Heterologous Ectoine Production in Escherichia coli: Optimization Using Response Surface Methodology. Int J Microbiol. 2019;2019 doi: 10.1155/2019/5475361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen W.-C., Hsu C.-C., Wang L.-F., Lan J.C.-W., Chang Y.-K., Wei Y.-H. Exploring useful fermentation strategies for the production of hydroxyectoine with a halophilic strain, Halomonas salina BCRC 17875. J biosci bioeng. 2019;128(3):332–336. doi: 10.1016/j.jbiosc.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 71.Young C.C., Rekha P., Lai W.A., Arun A.B. Encapsulation of plant growth-promoting bacteria in alginate beads enriched with humic acid. Biotechnol Bioeng. 2006;95(1):76–83. doi: 10.1002/bit.20957. [DOI] [PubMed] [Google Scholar]
- 72.Bacon C.W., White J.F. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis. 2016;68(1–3):87–98. doi: 10.1007/s13199-015-0350-2. [DOI] [Google Scholar]
- 73.Dulla G., Lindow S.E. Quorum size of Pseudomonas syringae is small and dictated by water availability on the leaf surface. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):3082–3087. doi: 10.1073/pnas.0711723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vu B., Chen M., Crawford R.J., Ivanova E.P. Bacterial Extracellular Polysaccharides Involved in Biofilm Formation. Molecules. 2009;14(7):2535–2554. doi: 10.3390/molecules14072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu K., Fang Z.Y., Guo R., Pan B., Shi W., Yuan S.F. Pectin Enhances Bio-Control Efficacy by Inducing Colonization and Secretion of Secondary Metabolites by Bacillus amyloliquefaciens SQY 162 in the Rhizosphere of Tobacco. Plos One. 2015;10(5) doi: 10.1016/j.procbio.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deboy R.T., Mongodin E.F., Fouts D.E., Tailford L.E., Khouri H., Emerson J.B. Insights into plant cell wall degradation from the genome sequence of the soil bacterium Cellvibrio japonicus. J Bacteriol. 2008;190(15):5455–5463. doi: 10.1128/jb.01701-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kampfer P., Ruppel S., Remus R. Enterobacter radicincitans sp nov., a plant growth promoting species of the family Enterobacteriaceae. Syst Appl Microbiol. 2005;28(3):213–221. doi: 10.1016/j.syapm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Shishido M., Breuil C., Chanway C.P. Endophytic colonization of spruce by plant growth-promoting rhizobacteria. FEMS Microbiol Ecol. 1999;29(2):191–196. doi: 10.1016/S0168-6496(99)00011-2. [DOI] [Google Scholar]
- 79.Yang H., Puri A., Padda K.P., Chanway C.P. Substrate utilization by endophytic bacteria Paenibacillus polymyxa P2b-2R that may facilitate bacterial entrance and survival inside diverse plant hosts. Facets. 2017;2 doi: 10.1139/facets-2016-0031. [DOI] [Google Scholar]
- 80.Singh S., Rekha P.D., Arun A.B., Young C.C. Impacts of monosodium glutamate industrial wastewater on plant growth and soil characteristics. Ecol Eng. 2009;35(10):1559–1563. doi: 10.1016/j.ecoleng.2009.06.002. [DOI] [Google Scholar]
- 81.Brader G., Compant S., Mitter B., Trognitz F., Sessitsch A. Metabolic potential of endophytic bacteria. Curr Opin Biotechnol. 2014;27:30–37. doi: 10.1016/j.copbio.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brock A.K., Berger B., Mewis I., Ruppel S. Impact of the PGPB Enterobacter radicincitans DSM 16656 on Growth, Glucosinolate Profile, and Immune Responses of Arabidopsis thaliana. Microb Ecol. 2013;65(3):661–670. doi: 10.1007/s00248-012-0146-3. [DOI] [PubMed] [Google Scholar]
- 83.Saminathan T., Garcia M., Ghimire B., Lopez C., Bodunrin A., Nimmakayala P. Metagenomic and Metatranscriptomic Analyses of Diverse Watermelon Cultivars Reveal the Role of Fruit Associated Microbiome in Carbohydrate Metabolism and Ripening of Mature Fruits. Front Plant Sci. 2018;9 doi: 10.3389/fpls.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mosier A.C., Justice N.B., Bowen B.P., Baran R., Thomas B.C., Northen T.R. Metabolites Associated with Adaptation of Microorganisms to an Acidophilic, Metal-Rich Environment Identified by Stable-Isotope-Enabled Metabolomics. mBio. 2013;4(2):8. doi: 10.1128/mBio.00484-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sonderholm M., Kragh K.N., Koren K., Jakobsen T.H., Darch S.E., Alhede M. Pseudomonas aeruginosa Aggregate Formation in an Alginate Bead Model System Exhibits In Vivo-Like Characteristics. Appl Environ Microbiol. 2017;83(9) doi: 10.1128/aem.00113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sessitsch A., Hardoim P., Doring J., Weilharter A., Krause A., Woyke T. Functional Characteristics of an Endophyte Community Colonizing Rice Roots as Revealed by Metagenomic Analysis. Mol Plant-Microbe Interact. 2012;25(1):28–36. doi: 10.1094/mpmi-08-11-0204. [DOI] [PubMed] [Google Scholar]
- 87.Walitang D.I., Kim K., Madhaiyan M., Kim Y.K., Kang Y., Sa T. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of Rice. Bmc Microbiol. 2017;17 doi: 10.1186/s12866-017-1117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bacilio-Jimenez M., Aguilar-Flores S., Ventura-Zapata E., Perez-Campos E., Bouquelet S., Zenteno E. Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil. 2003;249(2):271–277. doi: 10.1023/a:1022888900465. [DOI] [Google Scholar]
- 89.Ray S., Mishra S., Bisen K., Singh S., Sarma B.K., Singh H.B. Modulation in phenolic root exudate profile of Abelmoschus esculentus expressing activation of defense pathwa. Microbiol Res. 2018;207:100–107. doi: 10.1016/j.micres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 90.van Kessel J.C., Rutherford S.T., Cong J.P., Quinodoz S., Healy J., Bassler B.L. Quorum Sensing Regulates the Osmotic Stress Response in Vibrio harveyi. J Bacteriol. 2015;197(1):73–80. doi: 10.1128/jb.02246-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patz S., Becker Y., Richert-Pöggeler K.R., Berger B., Ruppel S., Huson D.H. Phage tail-like particles are versatile bacterial nanomachines – A mini-review. J Adv Res. 2019 doi: 10.1016/j.jare.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.