Abstract
Oxidative stress is a common feature of tuberculosis (TB), and persons with reduced antioxidants are at more risk of TB. TB patients with relatively severe oxidative stress had also more advanced disease as measured by the Karnofsky performance index. Since adverse effects from anti-TB drugs are also mediated by free radicals, TB patients are prone to side effects, such as hearing loss. In previous articles, researchers appealed for clinical trials aiming at evaluating N-acetyl cysteine (NAC) in attenuating the dreaded hearing loss during multidrug-resistant TB (MDR-TB) treatment. However, before embarking on such trials, considerations of NAC's overall impact on TB treatment are crucial. Unfortunately, such a comprehensive report on NAC is missing in the literature and this manuscript reviews the broader effect of NAC on TB treatment. This paper discusses NAC's effect on mycobacterial clearance, hearing loss, drug-induced liver injury, and its interaction with anti-TB drugs. Based on the evidence accrued to date, NAC appears to have various beneficial effects on TB treatment. However, despite the favorable interaction between NAC and first-line anti-TB drugs, the interaction between the antioxidant and some of the second-line anti-TB drugs needs further investigations.
1. Introductions
Oxidative stress is a common feature of tuberculosis (TB) [1] and is evidenced by elevated lipid peroxidation products such as malondialdehyde (MDA) as well as reduced antioxidant capacity. Compared to healthy individuals, TB patients have low vitamins A, C, and E, selenium, and glutathione (GSH) amounts [2, 3]. TB patients in developing countries have even worse oxidative stress compared with their counterparts in developed nations [2]. After successful treatment with anti-TB drugs, the elevated oxidative stress in TB patients returns to normal [1]. Individuals with diminished antioxidant capacity and increased oxidative stress are also predisposed to TB [4], and TB patients with relatively advanced oxidative stress are more likely to have a severe form of the disease as measured by the Karnofsky performance index [2].
Anti-TB drugs induce several adverse effects in TB patients, and oxidative stress is implicated in mediating these adverse effects. Drug-induced liver injury (DILI) [5] and hearing loss [6] are some of these untoward effects that follow treatment with anti-TB drugs, and both adverse effects are believed to be mediated through oxidative stress. TB patients have already high oxidative stress and hence are prone to these side effects. Hence, antioxidants could potentially mitigate adverse effects induced by anti-TB drugs and facilitate recovery from TB.
Researchers previously appealed for clinical trials aiming at evaluating N-acetyl cysteine (NAC) in attenuating hearing loss in multidrug-resistant TB (MDR-TB) patients owing to the gravity of the problem [7, 8]. However, before testing NAC in TB patients for its hearing loss protective effect, considerations of how NAC would impact other aspects of TB treatment are critical. Unfortunately, there are no such comprehensive scientific reports on NAC and hence this manuscript reviews the broader effects of NAC on TB treatment. This paper discusses NAC's effect on mycobacterial clearance, hearing loss, and DILI as well as the antioxidant's interaction with anti-TB drugs. The safety of NAC itself is not covered here as it has been reviewed elsewhere [7].
2. Antimycobacterial Properties of N-Acetyl Cysteine
NAC demonstrated antimycobacterial properties in previous studies of different models [9–11]. NAC cleared mycobacteria through several mechanisms including immunomodulation [12], enhancement of GSH level [13], and direct antimycobacterial effects [14].
2.1. Antimycobacterial Mechanisms of NAC
NAC is a precursor of GSH [15], and GSH, in turn, has demonstrated direct antimycobacterial effects. NAC is deacetylated, and the resulting cysteine is used to synthesize GSH [15]. GSH has both direct and indirect antimycobacterial effects. The direct effect of GSH includes enhancing the effect of nitric oxide (NO), one of the immune effector molecules produced by the immune effector cells. Normally, NO had a short-lived antimycobacterial effect but when it is combined with GSH and forms S-nitrosoglutathione (GSSNO) [16], it was persistently released from GSSNO and this prolonged its antimycobacterial effect [17]. Additionally, GSH, being a thiol-containing molecule, might also create a redox imbalance in the mycobacteria since the bacteria use mycothiol [18, 19] as an antioxidant, eventually causing growth inhibition. Another antimycobacterial effect of GSH is through enhancing immune cell activity and cytokine production [20].
NAC also has a direct antimycobacterial effect [14] independent of GSH as well as its free radical-scavenging effects. NAC maintained similar antimycobacterial effects in the presence and absence of the nicotinamide adenine dinucleotide phosphate (NADPH) system in a study that used a knockout mouse model [14]. Had NAC exclusively depended on GSH for its antimycobacterial effect, the absence of NADPH could have diminished its antimycobacterial effect since the recycling of GSH from its oxidized form to its reduced form needs NADPH [21]. In Escherichia coli, intracellular cysteine-induced reactive oxygen species (ROS) leading to DNA damage [22] and a similar mechanism of cysteine were confirmed later in mycobacterium [23]. Moreover, cysteine and any other thiol-containing molecules prevented mycobacterial persistence, a state where the bacteria decrease its metabolic rate and become refractory to killing by anti-TB drugs [23]. By avoiding persistence, NAC can also facilitate sterilization by anti-TB drugs and prevent drug resistance.
Moreover, NAC modulated immunity against tuberculosis where it increased interleukin-2 (IL-2), IL-12, and interferon gamma (INF-γ) production [9, 20, 24] and these cytokines are important for suppressing mycobacterial proliferation. Additionally, NAC reduced the production of IL-10, a cytokine that favors mycobacterial proliferation [10, 11]. The antioxidant also reduced the proinflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor alpha (TNF-α), cytokines known to exacerbate oxidative stress. NAC also increased the immunological activities of various cells including natural killer (NK) cells and macrophages against mycobacterium [13, 25].
2.2. Evidence from Studies Other than the In Vitro Model
NAC demonstrated antimycobacterial effects in animal studies [14, 26]. In guinea pigs infected with Mycobacterium tuberculosis (MTB), 60 days of oral NAC treatment partially restored red blood cell GSH concentrations and serum total antioxidant capacity. NAC also reduced spleen mycobacterial load as well as lesion burden and the severity in the lungs [26]. However, in this study, NAC did not reduce the mycobacterial load in the lungs. A different study in C57BL/6 mice NAC significantly reduced the mycobacterial load in the lungs after 7 days of treatment with the antioxidant [14].
The difference in the two studies, concerning the reduction in lung mycobacterial load, could be due to species variation, the difference in infection methodology, and the duration of infection. Guinea pigs are very susceptible to infection by MTB and clearing the mycobacteria may be difficult for them while mice are resistant to mycobacteria [27]. The study in guinea pigs also used aerosolization for infecting the animals while the mouse study used intratracheal inoculation. Moreover, the guinea pig study assessed the effect of NAC at 30 and 60 days after infection while the mice study assessed the effect only seven days postinoculation.
NAC also demonstrated several beneficial effects including facilitation of mycobacterial clearance in a clinical trial. In a double-blind, randomized clinical trial of 67 newly diagnosed TB patients, NAC as an adjunct to anti-TB drugs improved smear conversions 3 weeks after initiation of DOTs (directly observed therapies) [28]. NAC at an oral dose of 1200 mg/day resulted in a smear conversion rate of 95.8%, while the conversion rate was 58.3% in the control group. At the end of two months, smear conversion rates were 100% for the NAC group and 91.7% for the control group. Additionally, NAC improved weight gain and response to treatment as assessed by radiology. However, the attrition rate in this study was very high (28%). Two additional trials trying to assess the efficacy and safety of NAC in newly diagnosed TB patients are ongoing (NCT03281226, NCT03702738).
Despite numerous in vitro animal studies and a clinical trial which reported antimycobacterial effects of NAC, studies by Khameneh et al. and Vilchèze et al. did not confirm the antimycobacterial effects of the antioxidant in their experiments [23, 29] (Table 1). According to Khameneh et al., NAC did not show antimycobacterial effects against H37Rv even at a concentration of up to 40 mg/ml. However, both studies reported that NAC increased the antimycobacterial effects of anti-TB drugs. What is common in these two studies is that they used TB culture media, Middlebrook 7H9 Broth (7H9) and Lowenstein Jensen (LJ) media, in reporting the sole effect of NAC against TB. From these observations, we can estimate that for its direct anti-TB effect, NAC will probably need macrophages or other similar immune cells, and in the absence of these cells, it can only enhance the effects of other anti-TB drugs.
Table 1.
Summary of studies on anti-TB effects of NAC against MTB.
| Author | Year | Type of study | Anti-TB effect | Effect measurement | Type of immune cells | Type of MTB | Conc. of NAC | Study subjects | Remark | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Venketaraman et al. | 2006 | Cell culture | Yes | CFU reduction, effect size not indicated | Human Øs | H37Rv | 10 mM | HIV +ve and -ve | [9] | |
| Cao et al. | 2018 | Cell culture | Yes | 50% CFU reduction | THP-1 | Erdman strain | 10 mM | NA | Also showed that anti-TB drugs and NAC have synergistic effects | [10] |
| Venketaraman et al. | 2008 | Cell culture | Yes | CFU reduction, effect size not indicated | Human Øs | H37Rv | 10 mM | HIV -ve | Used whole blood culture | [11] |
| Guerra et al. | 2011 | Cell culture | Yes | CFU reduction, effect size not indicated | Human NK cells | H37Rv | 20 mM | HIV +ve and -ve | NK cells pretreated with NAC | [20] |
| Morris et al. | 2013 | Cell culture | Yes | CFU against control, effect size not indicated | Human Øs | H37Rv | 10 mM | HIV +ve and -ve | [13] | |
| Amaral et al. | 2016 | Cell culture | Yes | CFU vs. control, effect size not indicated | THP-1 | H37Rv, avium, Beijing 1471, M. bovis | 10 mM | HIV -ve | [14] | |
| Guerra et al. | 2011 | Cell culture | Yes | CFU reduction, effect size not indicated | Human Øs | H37Rv | 5, 10, 20 mM | HIV +ve and -ve | [20] | |
| Vilchèze et al. | 2017 | Cell culture & 7H9 | No | 4-5 log CFU reduction | J774 Øs | H37Rv | 5-10 mM | NA | Anti-TB effect when only combined with RIF/INH | [23] |
| Lamprecht et al. | 2016 | 7H9 medium | Yes | CFU reduction, sterilized | NA | H37Rv | 5 mM | NA | Combined with BDQ, Q203, and CFZ | [30] |
| Teskey et al. | 2018 | Cell culture & animal study | Yes | CFU reduction | Human PBMCs | Erdman strain | 10 mM | Healthy and T2DM patients, rats | [24] | |
| Palanisamy et al. | 2011 | Animal study | Yes | CFU reduction | NA | H37Rv | 400 mg/kg/day for 60 days | Guinea pigs | [26] | |
| Mahakalkar et al. | 2017 | Clinical trial | Yes | Sputum conversion | NA | NA | 600 mg/day | Smear +ve TB patients | [28] | |
| Khameneh et al. | 2016 | LJ media | No | NA | NA | H37Rv | 0.04-40 mg/ml | NA | Anti-TB effect only when combined with RIF or INH | [29] |
CFU: colony-forming unit; Øs: macrophages; NK cells: natural killer cells; LJ: Lowenstein Jensen; PBMC: peripheral blood mononuclear cells; T2DM: type 2 diabetes mellitus; NAC: N-acetyl cysteine; INH: isoniazid; RIF: rifampicin; BDQ: bedaquiline; CFZ: clofazimine; Q203: investigational product.
2.3. Interaction with Anti-TB Drugs
NAC showed additive/synergistic antimycobacterial interaction with all first-line and some second-line anti-TB drugs [10, 24, 30]. Administration of NAC and suboptimal concentrations of isoniazid (INH) or rifampicin (RIF) to peripheral blood mononuclear cells (PBMCs) infected with mycobacterium completely cleared mycobacterium from the culture [24]. According to this study, NAC also enhanced the antimycobacterial effect of ethambutol (EMB) in a statistically significant manner. When NAC and anti-TB drugs were also added to the cell culture of macrophages infected with MTB, NAC further reduced the viability of the bacteria in the macrophages [10]. In this study, the interaction of NAC with INH, RIF, EMB, and pyrazinamide (PZA) resulted in more than a fourfold further reduction of the CFU. Moreover, coadministration of NAC with clofazimine, bedaquiline, and Q203, an investigational agent, to a culture medium inoculated with MTB resulted in the sterilization of the culture medium [30]. According to the study, in the absence of NAC, the three drugs were not able to sterilize the culture media.
The synergistic effect of NAC with INH in clearing mycobacterium could probably be a paradox in light of previous findings on how INH works [31, 32]. INH is a prodrug requiring oxidative activation by the catalase-peroxidase hemoprotein, KatG, and the activation of INH by KatG is enhanced in the presence of a superoxide. This enhancement is evidenced by an observation that plumbagin and clofazimine, which are superoxide generators, increased the antimycobacterial effect of INH [31]. As an antioxidant, NAC reduces the free radical level, and hence, the antimycobacterial effect of INH could be expected to diminish when coadministered with NAC.
As mentioned earlier, NAC prevents a state of TB persistence, and through this mechanism, the antioxidant has the potential to reduce the duration of anti-TB treatment, reduce the rate of relapse, and prevent resistance against anti-TB drugs [23, 33, 34]. Persisters are subpopulations of the MTB colonies which are metabolically inactive and respond poorly to anti-TB drugs. These subpopulations are also the reason for prolonged treatment with anti-TB drugs and an important source of drug resistance. Persisters also do contribute to TB relapses [33]. According to Valchèze et al., the administration of exogenous reducing substances, such as NAC, switched persisters to metabolically active bacteria [23].
Despite synergism of NAC with the first-line anti-TB drugs and clofazimine as well as bedaquiline, assessing NAC's interactions with the remaining second-line drugs is imperative since NAC seemingly antagonized some of the antibiotics [35, 36]. Based on various reports, NAC increased the minimal inhibitory concentrations (MICs) of fluoroquinolones and aminoglycosides against various non-TB bacteria [35]. According to these reports, these effects of NAC are peculiar to the type of organisms since the antioxidant either increased or decreased the MICs depending on the type of organisms [35]. However, Rodriguez et al. explained the counterproductive effects of NAC on the antibiotics' MICs to be due to the acidic pH of the culture medium and adjustment of the medium pH to neutral avoided NAC's negative effect of the antibiotics' MICs [37]. Landini et al. also confirmed that 10 mM of NAC did not adversely affect the MIC of the antibiotics [38]. To further clear the confusion, it would be advisable to test the interaction between NAC and the remaining second-line anti-TB drugs before conducting a clinical trial of NAC in MDR-TB patients.
3. Protective Effects of NAC against Ototoxicity
Aminoglycosides are injectable anti-TB drugs used for the treatment of MDR-TB and are associated with ototoxicity [6]. The antibiotics cause loss of hair cells in the cochlea, involved in hearing, and in the vestibular apparatus, involved in maintaining balance. Early damage in the cochlea is limited to the hair cells in the basal region resulting in high-frequency losses in the inaudible range and then involves the apex affecting the low frequencies in the audible range [39]. Initially, the patient's hearing may not be affected but failure to promptly discontinue the antibiotics would diminish the patient's hearing. Ototoxicity caused by aminoglycosides is also irreversible and cumulative [40, 41].
A significant proportion of TB patients undergoing treatment with aminoglycosides develop ototoxicity [6, 42, 43]. Studies suggested that ototoxicity is more frequent in resource-limited settings than in developed nations [39]. The method of diagnosis also has an impact on the proportion of patients identified as having ototoxicity. Diagnosis with high-frequency audiometers is sensitive [44] and gives the true picture of the rates of aminoglycoside-induced ototoxicity than does clinical diagnosis, which underestimates the problem.
Several risk factors are associated with the development of aminoglycoside-induced ototoxicity. Patients with advanced age and low body mass index (BMI) are at a higher risk of ototoxicity [6]. Prolonged duration of treatment with aminoglycosides increases the probability of developing aminoglycoside-induced ototoxicity [44, 45]. Based on a systematic review, MDR-TB patients coinfected with HIV have 22% more risk of their treatment being complicated with ototoxicity [46].
The rate of ototoxicity also varies with the particular aminoglycosides used in the MDR-TB treatment. In a study comparing the rate of ototoxicity induced by capreomycin versus amikacin, the risk of ototoxicity in patients treated with amikacin was increased five times compared with those treated with capreomycin [47]. In another study, in Namibian MDR-TB patients, amikacin was associated with more risk of ototoxicity than kanamycin [48]. Moreover, according to a review on ototoxicity induced by injectable anti-MDR TB drugs, ototoxicity rates caused by streptomycin, kanamycin, and amikacin were 11.8%, 13.3%, and 19.7%, respectively [44]. However, the review seemed to overestimate the ototoxicity rate for capreomycin (25%) probably due to the enrollment in the review of only four patients treated with capreomycin.
NAC attenuated aminoglycoside-induced ototoxicity in various studies [49, 50], and its mechanism of ototoxicity attenuation could be through scavenging free radicals and inhibition of downstream molecular mechanisms of apoptosis induced by oxidative stress. Apart from its free radical-scavenging properties, NAC was shown to inhibit the activation of p38 mitogen-activated protein kinase (MAPK) [51]. The p38 MAPK and its downstream molecular mechanisms, such as activation of caspases and cytochrome c, were also shown to be responsible for aminoglycoside-induced ototoxicity [52].
Aminoglycosides cause ototoxicity through the generation of free radicals and these radicals, in turn, attack the hair cells of the vestibulocochlear nerve [53]. In this process, an iron-aminoglycoside complex is formed and unsaturated fatty acids donate electron in the process of ROS generation [54]. The free radicals cause cochlear and vestibular hair cell loss [55] through apoptosis due to the activation of the p38 MAPK system [56].
In animal studies, enhancing the antioxidant systems and/or free radical-scavenging capacity protected against aminoglycoside-induced ototoxicity. Animals overexpressing superoxide dismutase, the enzyme responsible for scavenging ROS, were resistant to kanamycin-induced ototoxicity [57]. NAC also protected gentamycin- and neomycin-induced ototoxicity in rats and zebrafish [49, 58, 59]. In a rat model which used both NAC and vitamin A for the prevention of gentamycin-induced ototoxicity, both interventions prevented ototoxicity but N-acetyl cysteine showed more protective effect [49]. However, according to an earlier study in guinea pigs by Bock et al., NAC rather worsened kanamycin-induced ototoxicity [60].
NAC protected against aminoglycoside-induced hearing loss in clinical trials of non-TB patients [50, 61, 62] (Table 2). In renal failure patients who developed peritonitis after undergoing peritoneal dialysis and were treated with aminoglycosides, NAC attenuated aminoglycoside induced-ototoxicity. In these studies, gentamycin and amikacin were administered along with NAC for four to six weeks. In a meta-analysis of these clinical trials, NAC protected 86% of the ototoxicity that might have been induced by aminoglycosides [7]. Vural et al. also confirmed that NAC protected against aminoglycoside-induced ototoxicity during a one-month treatment period with the antioxidant [63]. However, the protective effect diminished after one year. In this trial, treatments with aminoglycosides and NAC were completed at the same time. This finding probably suggests that for maximal protection with the antioxidant, we need to continue the administration of NAC for a little longer after completion of aminoglycoside administration. According to pharmacokinetic studies, aminoglycosides accumulate in the hair cells slowly and their half-life in these cells is prolonged [64, 65]. This means aminoglycosides could continue to cause hair cell damages even after they are discontinued.
Table 2.
Clinical trials conducted to investigate the otoprotective effect of 600 mg twice a day NAC against AGs in non-TB patients.
| Author | Year | Effect measurement | Duration of protection | Study subjects | Sample size | Type of aminoglycoside | Country of study | Remark | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Feldman et al. | 2007 | 25% vs. 60% in favor of NAC | 6 weeks | Hemodialysis patients | 40 | Gentamycin | Israel | [61] | |
| Tokgoz et al. | 2011 | 3.3% vs. 70% in favor of NAC | 4 weeks | Peritoneal dialysis patients | 60 | Amikacin | Turkey | Amikacin combined with vancomycin | [62] |
| Kocyigit et al. | 2015 | In favor of NAC | 4 weeks | Peritoneal dialysis patients | 46 | Amikacin | Turkey | [50] | |
| Vural et al. | 2018 | In favor of NAC | 4 weeks | Peritoneal dialysis patients | 40 | Amikacin | Turkey | Reassessment after 12 months showed reduced effects | [63] |
NAC: N-acetyl cysteine; AGs: aminoglycosides.
4. Protective Effect of NAC against DILI
DILI is one of the major adverse effects of treatment with anti-TB drugs. Patients treated with anti-TB drugs may experience DILI ranging from simple hepatic enzyme elevations to severe clinical hepatitis. In the presence of hepatitis symptoms, such as abdominal pain, nausea, vomiting, unexplained fatigue, or jaundice, hepatotoxicity is defined as an elevation of liver enzymes, alanine (ALT) and/or aspartate (AST) transaminase, more than three times the upper limit of the normal range (ULN). But in the absence of symptoms, either of the enzymes must be elevated more than five times ULN [66, 67]. The culprits causing DILI among the first-line anti-TB drugs are INH, PZA, and RIF while from the second line drugs, the list includes fluoroquinolones, ethionamide, prothionamide, amoxicillin-clavulanate, and para-aminosalicylic acid [68]. The rate of DILI in TB patients could sometimes go up as high as 27% [69].
DILI could have serious implications on the outcome of TB patient treatment [5, 70]. Some patients with DILI may develop acute liver failure, ascites, and hepatic encephalopathy, and yet others may manifest only with simple enzyme elevations. A study in China demonstrated more than a 9-fold risk of unsuccessful treatment and more than a 2-fold increased risk of prolonged intensive phase treatment in patients with DILI compared to TB patients without DILI [70]. Anti-TB associated DILI could also result in death, and this was more common in patients who developed encephalopathy, ascites, and jaundice [5]. The mortality rate in patients who developed anti-TB-associated DILI could also be as high as 23%.
Different risk factors are associated with the development of anti-TB DILI. Various studies suggested advanced age as one of the risk factors [71, 72] even if other studies did not confirm age as a risk factor [73, 74]. Some studies also indicated HIV to be a risk factor for anti-TB-associated DILI [75, 76]. Additionally, other risk factors associated with anti-TB DILI include low BMI [77, 78], viral hepatitis [79], INH acetylation status [80], and genetic makeup of the patients [81].
NAC attenuates anti-TB DILI through scavenging free radicals formed during the metabolism of the drugs as well as through enhancing the synthesis of GSH [82]. Anti-TB drugs induce liver injury via the formation of free radicals and then the free radicals damage different cellular parts [83]. The free radicals are probably generated during the metabolism of the various anti-TB drugs [67]. Normally, these molecules are detoxified by the host's antioxidant system including GSH. However, if the antioxidant system is compromised for various reasons, the free radicals could damage different structures within the cells and perpetuate the oxidative stress through lipid peroxidation.
According to studies on genetic polymorphisms of oxidant-antioxidant systems, mutations leading to reduced antioxidant enzymes or increased prooxidant enzymes resulted in increased susceptibility to anti-TB DILI [81, 84, 85]. A loss of function mutation on MAFK encoding MafK (small Maf basic leucine zipper proteins) increased susceptibility to anti-TB DILI [81] (Figure 1). Normally, the binding of MafK with Nrf2 (nuclear factor erythroid 2-related factor 2) leads to the upregulation of antioxidant enzymes. Similarly, a gain of function mutations on NOS2A, encoding inducible nitric oxide synthase (iNOS) and BACH1, encoding BTB and CNC homology 1 (bach1), increased susceptibility to anti-TB DILI [81]. NOS2A encodes the enzyme nitric oxide synthase while BACH1 encodes Bach1, whose binding with Nrf2 leads to the downregulation of antioxidant enzymes. Additionally, loss of function mutations on HMOX1, the gene encoding for heme oxygenase 1 recognized as phase II antioxidant enzyme, and NQO1, which encodes NAD (P)H: quinone oxidoreductase 1, were also associated with susceptibility to anti-TB DILI [84]. A meta-analysis also confirmed the association of polymorphisms of GSTM1 and GSTT1, glutathione S-transferase encoding genes, with anti-TB DILI [85].
Figure 1.
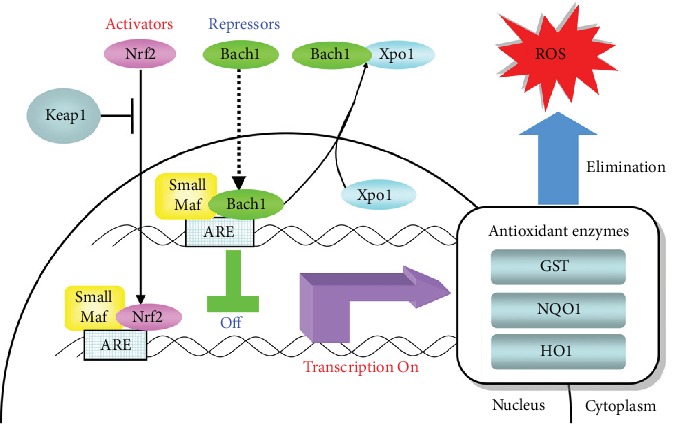
Activator and repressor arms in the antioxidant pathway. Schematic representation indicates the location and translocation of relevant genes involved in the activator arm (Nrf2/small Mafs/Xpo1) and repressor arm (Bach1/small Mafs/Keap1) in the antioxidant pathway as well as the transcriptional regulation of antioxidant enzymes (NQO1/HO1) against oxidative stress in hepatocytes. Nrf2: nuclear factor erythroid 2-related factor 2; Keap1: Kelch-like ECH-associated protein 1; Bach1: BTB and CNC homology 1; Xpo1: exportin 1; ARE: antioxidant-responsive element; GST: glutathione S-transferase; NQO1: NAD (P) H dehydrogenase quinone 1; HO1: heme oxygenase 1; ROS: reactive oxygen species [81].
NAC prevented anti-TB drug-induced hepatotoxicity in animal studies [82, 83] (Table 3). A study in rats demonstrated that INH and RIF each at a dose of 50 mg/kg depleted GSH as well as other antioxidants and increased lipid peroxidation in the liver tissue [83]. The changes in antioxidants levels were also accompanied by histopathologic changes such as portal triaditis, lobular inflammation, and patchy necrosis. NAC at a dose of 100 mg/kg prevented all these pathologic changes and attenuated the ALT elevation from 102 IU/l to 28 IU/l and the AST elevation from 578 IU/l to 165 IU/l at 3 weeks. Another study in rats confirmed the protective effect of NAC on RIF-induced hepatotoxicity. However, the latter study did not evidence a reduction in the level of GSH after treatment with RIF alone [82]. An in vitro study in human liver cancer cell line (hepg2) cells also demonstrated a protective property of NAC against anti-TB-induced hepato-/cytotoxicity [86] (Table 3).
Table 3.
List of studies on the hepatoprotective effect NAC on anti-TB drugs.
| Author | Year | Type of study | Types of animals or cells | Dose or concentration of NAC | Type of anti-TB | Protection by NAC | Reference |
|---|---|---|---|---|---|---|---|
| Attri et al. | 2000 | Animal study | Wistar rats | 100 mg/kg | INH (50 mg/kg), RIF (50 mg/kg) | Yes | [83] |
| Rana et al. | 2006 | Animal study | Wistar rats | 100 mg/kg | RIF (50 mg/kg) | Yes | [82] |
| Singh et al. | 2012 | In vitro | HepG2 | 10 μM | INH (100, 200 mM), RIF (50 mM), PZA (100, 200 mM) | Yes | [86] |
| Baniasadi et al. | 2011 | Clinical trial | NA | 600 mg PO BID | INH, RIF, PZA, ETH | Yes | [87] |
NAC: N-acetyl cysteine; INH: isoniazid; RIF: rifampicin; PZA: pyrazinamide; hepG2: human liver cancer cell line.
A clinical trial of NAC in newly diagnosed TB patients also showed protective effects against hepatotoxicity [87] (Table 3). Sixty patients, aged greater than 60, were recruited in this trial and followed for two weeks. The study was not blinded and controls did not receive placebo. NAC was administered orally at dose of 600 mg twice daily, and the incidence of hepatotoxicity in the control group was 37.5% and none in the NAC group. As the authors themselves acknowledged, these results need to be interpreted with caution and there is a need for well-controlled larger trials with longer follow-up.
5. Conclusions
Based on the evidence accrued to date, NAC appears to have various beneficial effects on TB treatment and its evaluation in clinical trials is justifiable. For maximal protection by NAC against anti-TB-induced hearing loss, we need to continue the administration of NAC for a little longer after completion of aminoglycoside administration. Moreover, the interaction between NAC and some of the second-line anti-TB drugs needs further investigations despite favorable interaction between NAC and first-line anti-TB drugs, bedaquiline and clofazimine.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Vidhya R., Rathnakumar K., Balu V., Pugalendi K. V. Oxidative stress, antioxidant status and lipid profile in pulmonary tuberculosis patients before and after anti-tubercular therapy. The Indian Journal of Tuberculosis. 2019;66(3):375–381. doi: 10.1016/j.ijtb.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Madebo T., Lindtjorn B., Aukrust P., Berge R. K. Circulating antioxidants and lipid peroxidation products in untreated tuberculosis patients in Ethiopia. The American Journal of Clinical Nutrition. 2003;78(1):117–122. doi: 10.1093/ajcn/78.1.117. [DOI] [PubMed] [Google Scholar]
- 3.Muzembo B. A., Mbendi N. C., Ngatu N. R., Suzuki T., Wada K., Ikeda S. Serum selenium levels in tuberculosis patients: a systematic review and meta-analysis. Journal of Trace Elements in Medicine and Biology. 2018;50:257–262. doi: 10.1016/j.jtemb.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Soh A. Z., Chee C. B. E., Wang Y. T., Yuan J. M., Koh W. P. Dietary intake of antioxidant vitamins and carotenoids and risk of developing active tuberculosis in a prospective population-based cohort study. American Journal of Epidemiology. 2017;186(4):491–500. doi: 10.1093/aje/kwx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devarbhavi H., Singh R., Patil M., Sheth K., Adarsh C. K., Balaraju G. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. Journal of Gastroenterology and Hepatology. 2013;28(1):161–167. doi: 10.1111/j.1440-1746.2012.07279.x. [DOI] [PubMed] [Google Scholar]
- 6.Sogebi O. A., Adefuye B. O., Adebola S. O., Oladeji S. M., Adedeji T. O. Clinical predictors of aminoglycoside-induced ototoxicity in drug-resistant tuberculosis patients on intensive therapy. Auris Nasus Larynx. 2017;44(4):404–410. doi: 10.1016/j.anl.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Kranzer K., Elamin W. F., Cox H., Seddon J. A., Ford N., Drobniewski F. A systematic review and meta-analysis of the efficacy and safety of N-acetylcysteine in preventing aminoglycoside-induced ototoxicity: implications for the treatment of multidrug-resistant TB. Thorax. 2015;70(11):1070–1077. doi: 10.1136/thoraxjnl-2015-207245. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Prats A. J., Schaaf H. S., Hesseling A. C. The safety and tolerability of the second-line injectable antituberculosis drugs in children. Expert Opinion on Drug Safety. 2016;15(11):1491–1500. doi: 10.1080/14740338.2016.1223623. [DOI] [PubMed] [Google Scholar]
- 9.Venketaraman V., Rodgers T., Linares R., et al. Glutathione and growth inhibition of Mycobacterium tuberculosis in healthy and HIV infected subjects. AIDS Research and Therapy. 2006;3(1):p. 5. doi: 10.1186/1742-6405-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao R., Teskey G., Islamoglu H., et al. Characterizing the effects of glutathione as an immunoadjuvant in the treatment of tuberculosis. Antimicrobial Agents and Chemotherapy. 2018;62(11) doi: 10.1128/AAC.01132-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venketaraman V., Millman A., Salman M., et al. Glutathione levels and immune responses in tuberculosis patients. Microbial Pathogenesis. 2008;44(3):255–261. doi: 10.1016/j.micpath.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Guerra C., Johal K., Morris D., et al. Control of Mycobacterium tuberculosis growth by activated natural killer cells. Clinical and Experimental Immunology. 2012;168(1):142–152. doi: 10.1111/j.1365-2249.2011.04552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris D., Guerra C., Khurasany M., et al. Glutathione supplementation improves macrophage functions in HIV. Journal of Interferon & Cytokine Research. 2013;33(5):270–279. doi: 10.1089/jir.2012.0103. [DOI] [PubMed] [Google Scholar]
- 14.Amaral E. P., Conceição E. L., Costa D. L., et al. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiology. 2016;16(1):p. 251. doi: 10.1186/s12866-016-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whillier S., Raftos J. E., Chapman B., Kuchel P. W. Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Report. 2009;14(3):115–124. doi: 10.1179/135100009X392539. [DOI] [PubMed] [Google Scholar]
- 16.Balazy M., Kaminski P. M., Mao K., Tan J., Wolin M. S. S-Nitroglutathione, a product of the reaction between peroxynitrite and glutathione that generates nitric oxide. The Journal of Biological Chemistry. 1998;273(48):32009–32015. doi: 10.1074/jbc.273.48.32009. [DOI] [PubMed] [Google Scholar]
- 17.Venketaraman V., Dayaram Y. K., Talaue M. T., Connell N. D. Glutathione and nitrosoglutathione in macrophage defense against Mycobacterium tuberculosis. Infection and Immunity. 2005;73(3):1886–1889. doi: 10.1128/IAI.73.3.1886-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderberg S. J., Newton G. L., Fahey R. C. Mycothiol biosynthesis and metabolism. Cellular levels of potential intermediates in the biosynthesis and degradation of mycothiol in Mycobacterium smegmatis. The Journal of Biological Chemistry. 1998;273(46):30391–30397. doi: 10.1074/jbc.273.46.30391. [DOI] [PubMed] [Google Scholar]
- 19.Morris D., Khurasany M., Nguyen T., et al. Glutathione and infection. Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(5):3329–3349. doi: 10.1016/j.bbagen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Guerra C., Morris D., Sipin A., et al. Glutathione and adaptive immune responses against Mycobacterium tuberculosis infection in healthy and HIV infected individuals. PLoS One. 2011;6(12, article e28378) doi: 10.1371/journal.pone.0028378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S. C. Regulation of glutathione synthesis. Molecular Aspects of Medicine. 2009;30(1-2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S., Imlay J. A. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. Journal of Bacteriology. 2003;185(6):1942–1950. doi: 10.1128/jb.185.6.1942-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilcheze C., Hartman T., Weinrick B., et al. Enhanced respiration prevents drug tolerance and drug resistance inMycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(17):4495–4500. doi: 10.1073/pnas.1704376114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teskey G., Cao R., Islamoglu H., et al. The synergistic effects of the glutathione precursor, NAC and first-line antibiotics in the granulomatous response against Mycobacterium tuberculosis. Frontiers in Immunology. 2018;9:p. 2069. doi: 10.3389/fimmu.2018.02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen M., Bailey C., Cahatol I., et al. Mechanisms of control of Mycobacterium tuberculosis by NK cells: role of glutathione. Frontiers in Immunology. 2015;6 doi: 10.3389/fimmu.2015.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palanisamy G. S., Kirk N. M., Ackart D. F., Shanley C. A., Orme I. M., Basaraba R. J. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PLoS One. 2011;6(10, article e26254) doi: 10.1371/journal.pone.0026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dharmadhikari A. S., Nardell E. A. What animal models teach humans about tuberculosis. American Journal of Respiratory Cell and Molecular Biology. 2008;39(5):503–508. doi: 10.1165/rcmb.2008-0154TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahakalkar S. M., Nagrale D., Gaur S., Urade C., Murhar B., Turankar A. N-acetylcysteine as an add-on to directly observed therapy short-I therapy in fresh pulmonary tuberculosis patients: a randomized, placebo-controlled, double-blinded study. Perspectives in Clinical Research. 2017;8(3):132–136. doi: 10.4103/2229-3485.210450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khameneh B., Fazly Bazzaz B. S., Amani A., Rostami J., Vahdati-Mashhadian N. Combination of anti-tuberculosis drugs with vitamin C or NAC against different Staphylococcus aureus and Mycobacterium tuberculosis strains. Microbial Pathogenesis. 2016;93:83–87. doi: 10.1016/j.micpath.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Lamprecht D. A., Finin P. M., Rahman M. A., et al. Turning the respiratory flexibility of _Mycobacterium tuberculosis_ against itself. Nature Communications. 2016;7(1) doi: 10.1038/ncomms12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulatovic V. M., Wengenack N. L., Uhl J. R., et al. Oxidative stress increases susceptibility of Mycobacterium tuberculosis to isoniazid. Antimicrobial Agents and Chemotherapy. 2002;46(9):2765–2771. doi: 10.1128/aac.46.9.2765-2771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J. Y., Burger R. M., Drlica K. Role of superoxide in catalase-peroxidase-mediated isoniazid action against mycobacteria. Antimicrobial Agents and Chemotherapy. 1998;42(3):709–711. doi: 10.1128/aac.42.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Yew W. W., Barer M. R. Targeting persisters for tuberculosis control. Antimicrobial Agents and Chemotherapy. 2012;56(5):2223–2230. doi: 10.1128/AAC.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yew W. W., Chan D. P., Chang K. C., Zhang Y. Does oxidative stress contribute to antituberculosis drug resistance? Journal of Thoracic Disease. 2019;11(7):E100–E102. doi: 10.21037/jtd.2019.06.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang F., Liu L. H., Li X. P., et al. Short communication: N-acetylcysteine-mediated modulation of antibiotic susceptibility of bovine mastitis pathogens. Journal of Dairy Science. 2016;99(6):4300–4302. doi: 10.3168/jds.2015-10756. [DOI] [PubMed] [Google Scholar]
- 36.Goswami M., Jawali N. N-acetylcysteine-mediated modulation of bacterial antibiotic susceptibility. Antimicrobial Agents and Chemotherapy. 2010;54(8):3529–3530. doi: 10.1128/AAC.00710-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Beltran J., Cabot G., Valencia E. Y., et al. N-acetylcysteine selectively antagonizes the activity of imipenem in Pseudomonas aeruginosa by an OprD-mediated mechanism. Antimicrobial Agents and Chemotherapy. 2015;59(6):3246–3251. doi: 10.1128/AAC.00017-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landini G., Di Maggio T., Sergio F., Docquier J. D., Rossolini G. M., Pallecchi L. Effect of high N-acetylcysteine concentrations on antibiotic activity against a large collection of respiratory pathogens. Antimicrobial Agents and Chemotherapy. 2016;60(12):7513–7517. doi: 10.1128/AAC.01334-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Huang W. G., Zha D. J., et al. Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hearing Research. 2007;226(1-2):178–182. doi: 10.1016/j.heares.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Kemp D. T. Otoacoustic emissions, their origin in cochlear function, and use. British Medical Bulletin. 2002;63(1):223–241. doi: 10.1093/bmb/63.1.223. [DOI] [PubMed] [Google Scholar]
- 41.Guthrie O. W. Aminoglycoside induced ototoxicity. Toxicology. 2008;249(2-3):91–96. doi: 10.1016/j.tox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Aznar M. L., Rando Segura A., Moreno M. M., et al. Treatment outcomes and adverse events from a standardized multidrug-resistant tuberculosis regimen in a rural setting in Angola. The American Journal of Tropical Medicine and Hygiene. 2019;101(3):502–509. doi: 10.4269/ajtmh.19-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad R., Singh A., Srivastava R., Hosmane G. B., Kushwaha R. A. S., Jain A. Frequency of adverse events observed with second-line drugs among patients treated for multidrug-resistant tuberculosis. The Indian Journal of Tuberculosis. 2016;63(2):106–114. doi: 10.1016/j.ijtb.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Sarin R., Behera D., Khanna A., Singh V., Narang P., Deepak T. S. Second-line injectable induced ototoxicity in drug resistant tuberculosis: a systematic review of Indian studies. The Indian Journal of Tuberculosis. 2019;66(2):279–287. doi: 10.1016/j.ijtb.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Modongo C., Pasipanodya J. G., Zetola N. M., Williams S. M., Sirugo G., Gumbo T. Amikacin concentrations predictive of ototoxicity in multidrug-resistant tuberculosis patients. Antimicrobial Agents and Chemotherapy. 2015;59(10):6337–6343. doi: 10.1128/AAC.01050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong H., Budhathoki C., Farley J. E. Increased risk of aminoglycoside-induced hearing loss in MDR-TB patients with HIV coinfection. The International Journal of Tuberculosis and Lung Disease. 2018;22(6):667–674. doi: 10.5588/ijtld.17.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold A., Cooke G. S., Kon O. M., et al. Adverse effects and choice between the injectable agents amikacin and capreomycin in multidrug-resistant tuberculosis. Antimicrobial Agents and Chemotherapy. 2017;61(9) doi: 10.1128/AAC.02586-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagwa E. L., Ruswa N., Mavhunga F., Rennie T., Leufkens H. G. M., Mantel-Teeuwisse A. K. Comparing amikacin and kanamycin-induced hearing loss in multidrug-resistant tuberculosis treatment under programmatic conditions in a Namibian retrospective cohort. BMC Pharmacology and Toxicology. 2015;16(1):p. 36. doi: 10.1186/s40360-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aladag I., Guven M., Songu M. Prevention of gentamicin ototoxicity with N-acetylcysteine and vitamin A. The Journal of Laryngology and Otology. 2016;130(5):440–446. doi: 10.1017/S0022215116000992. [DOI] [PubMed] [Google Scholar]
- 50.Kocyigit I., Vural A., Unal A., et al. Preventing amikacin related ototoxicity with N-acetylcysteine in patients undergoing peritoneal dialysis. European Archives of Oto-Rhino-Laryngology. 2015;272(10):2611–2620. doi: 10.1007/s00405-014-3207-z. [DOI] [PubMed] [Google Scholar]
- 51.Gong X., Celsi G., Carlsson K., Norgren S. Protective effects of N-acetylcysteine amide (NACA) on gentamicin-induced apoptosis in LLC-PK1 cells. Renal Failure. 2012;34(4):487–494. doi: 10.3109/0886022X.2012.655684. [DOI] [PubMed] [Google Scholar]
- 52.Wei X., Zhao L., Liu J., Dodel R. C., Farlow M. R., Du Y. Minocycline prevents gentamicin-induced ototoxicity by inhibiting p 38 MAP kinase phosphorylation and caspase 3 activation. Neuroscience. 2005;131(2):513–521. doi: 10.1016/j.neuroscience.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Forge A., Schacht J. Aminoglycoside antibiotics. Audiology & Neuro-Otology. 2000;5(1):3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- 54.Priuska E. M., Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochemical Pharmacology. 1995;50(11):1749–1752. doi: 10.1016/0006-2952(95)02160-4. [DOI] [PubMed] [Google Scholar]
- 55.Jiang H., Sha S. H., Forge A., Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death and Differentiation. 2006;13(1):20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kralova J., Dvorak M., Koc M., Kral V. p38 MAPK plays an essential role in apoptosis induced by photoactivation of a novel ethylene glycol porphyrin derivative. Oncogene. 2008;27(21):3010–3020. doi: 10.1038/sj.onc.1210960. [DOI] [PubMed] [Google Scholar]
- 57.Sha S. H., Zajic G., Epstein C. J., Schacht J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiology & Neuro-Otology. 2001;6(3):117–123. doi: 10.1159/000046818. [DOI] [PubMed] [Google Scholar]
- 58.Somdas M. A., Korkmaz F., Gurgen S. G., Sagit M., Akcadag A. N-acetylcysteine prevents gentamicin ototoxicity in a rat model. The Journal of International Advanced Otology. 2015;11(1):12–18. doi: 10.5152/iao.2015.650. [DOI] [PubMed] [Google Scholar]
- 59.Wu C. Y., Lee H. J., Liu C. F., Korivi M., Chen H. H., Chan M. H. Protective role of L-ascorbic acid, N-acetylcysteine and apocynin on neomycin-induced hair cell loss in zebrafish. Journal of Applied Toxicology. 2015;35(3):273–279. doi: 10.1002/jat.3043. [DOI] [PubMed] [Google Scholar]
- 60.Bock G. R., Yates G. K., Miller J. J., Moorjani P. Effects of N-acetylcysteine on kanamycin ototoxicity in the guinea pig. Hearing Research. 1983;9(3):255–262. doi: 10.1016/0378-5955(83)90030-8. [DOI] [PubMed] [Google Scholar]
- 61.Feldman L., Efrati S., Eviatar E., et al. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney International. 2007;72(3):359–363. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- 62.Tokgoz B., Ucar C., Kocyigit I., et al. Protective effect of N-acetylcysteine from drug-induced ototoxicity in uraemic patients with CAPD peritonitis. Nephrology, Dialysis, Transplantation. 2011;26(12):4073–4078. doi: 10.1093/ndt/gfr211. [DOI] [Google Scholar]
- 63.Vural A., Kocyigit I., San F., et al. Long-term protective effect of N-acetylcysteine against amikacin-induced ototoxicity in end-stage renal disease: a randomized trial. Peritoneal Dialysis International. 2018;38(1):57–62. doi: 10.3747/pdi.2017.00133. [DOI] [PubMed] [Google Scholar]
- 64.Aran J. Current perspectives on inner ear toxicity. Otolaryngology and Head and Neck Surgery. 1995;112(1):133–144. doi: 10.1016/S0194-5998(95)70313-6. [DOI] [PubMed] [Google Scholar]
- 65.Tan K. H., Mulheran M., Knox A. J., Smyth A. R. Aminoglycoside prescribing and surveillance in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2003;167(6):819–823. doi: 10.1164/rccm.200109-012CC. [DOI] [PubMed] [Google Scholar]
- 66.Tostmann A., Boeree M. J., Aarnoutse R. E., de Lange W. C., van der Ven A. J., Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. Journal of Gastroenterology and Hepatology. 2008;23(2):192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 67.Ramappa V., Aithal G. P. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. Journal of Clinical and Experimental Hepatology. 2013;3(1):37–49. doi: 10.1016/j.jceh.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keshavjee S., Gelmanova I. Y., Shin S. S., et al. Hepatotoxicity during treatment for multidrug-resistant tuberculosis: occurrence, management and outcome. The International Journal of Tuberculosis and Lung Disease. 2012;16(5):596–603. doi: 10.5588/ijtld.11.0591. [DOI] [PubMed] [Google Scholar]
- 69.Gafar F., Arifin H., Jurnalis Y. D., et al. Antituberculosis drug-induced liver injury in children: incidence and risk factors during the two-month intensive phase of therapy. The Pediatric Infectious Disease Journal. 2019;38(1):50–53. doi: 10.1097/INF.0000000000002192. [DOI] [PubMed] [Google Scholar]
- 70.Shang P., Xia Y., Liu F., et al. Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (ATLI) in China. PLoS One. 2011;6(7, article e21836) doi: 10.1371/journal.pone.0021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warmelink I., ten Hacken N. H., van der Werf T. S., van Altena R. Weight loss during tuberculosis treatment is an important risk factor for drug-induced hepatotoxicity. The British Journal of Nutrition. 2011;105(3):400–408. doi: 10.1017/S0007114510003636. [DOI] [PubMed] [Google Scholar]
- 72.Singla R., Sharma S. K., Mohan A., et al. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity. The Indian Journal of Medical Research. 2010;132:81–86. [PubMed] [Google Scholar]
- 73.Isa S. E., Ebonyi A. O., Shehu N. Y., et al. Antituberculosis drugs and hepatotoxicity among hospitalized patients in Jos, Nigeria. International Journal of Mycobacteriology. 2016;5(1):21–26. doi: 10.1016/j.ijmyco.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Abera W., Cheneke W., Abebe G. Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro zone, South Ethiopia: a cohort study. International Journal of Mycobacteriology. 2016;5(1):14–20. doi: 10.1016/j.ijmyco.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Possuelo L. G., Castelan J. A., de Brito T. C., et al. Association of slow N-acetyltransferase 2 profile and anti-TB drug-induced hepatotoxicity in patients from southern Brazil. European Journal of Clinical Pharmacology. 2008;64(7):673–681. doi: 10.1007/s00228-008-0484-8. [DOI] [PubMed] [Google Scholar]
- 76.Yimer G., Aderaye G., Amogne W., et al. Anti-tuberculosis therapy-induced hepatotoxicity among Ethiopian HIV-positive and negative patients. PLoS One. 2008;3(3, article e1809) doi: 10.1371/journal.pone.0001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ali A. H., Belachew T., Yami A., Ayen W. Y. Anti-tuberculosis drug induced hepatotoxicity among TB/HIV co-infected patients at Jimma University Hospital, Ethiopia: nested case-control study. PLoS One. 2013;8(5, article e64622) doi: 10.1371/journal.pone.0064622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makhlouf H. A., Helmy A., Fawzy E., El-Attar M., Rashed H. A. A prospective study of antituberculous drug-induced hepatotoxicity in an area endemic for liver diseases. Hepatology International. 2008;2(3):353–360. doi: 10.1007/s12072-008-9085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lomtadze N., Kupreishvili L., Salakaia A., et al. Hepatitis C virus co-infection increases the risk of anti-tuberculosis drug-induced hepatotoxicity among patients with pulmonary tuberculosis. PLoS One. 2013;8(12, article e83892) doi: 10.1371/journal.pone.0083892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ben Mahmoud L., Ghozzi H., Kamoun A., et al. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatotoxicity in Tunisian patients with tuberculosis. Pathologie Biologie. 2012;60(5):324–330. doi: 10.1016/j.patbio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Nanashima K., Mawatari T., Tahara N., et al. Genetic variants in antioxidant pathway: risk factors for hepatotoxicity in tuberculosis patients. Tuberculosis (Edinburgh, Scotland) 2012;92(3):253–259. doi: 10.1016/j.tube.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 82.Rana S. V., Attri S., Vaiphei K., Pal R., Attri A., Singh K. Role of N-acetylcysteine in rifampicin-induced hepatic injury of young rats. World Journal of Gastroenterology. 2006;12(2):287–291. doi: 10.3748/wjg.v12.i2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Attri S., Rana S. V., Vaiphei K., et al. Isoniazid- and rifampicin-induced oxidative hepatic injury--protection by N-acetylcysteine. Human & Experimental Toxicology. 2000;19(9):517–522. doi: 10.1191/096032700674230830. [DOI] [PubMed] [Google Scholar]
- 84.Yang M., Zhang H., Tao B., et al. Possible association of HMOX1 and NQO1 polymorphisms with anti-tuberculosis drug-induced liver injury: a matched case-control study. Journal of Clinical Pharmacy and Therapeutics. 2019;44(4):534–542. doi: 10.1111/jcpt.12818. [DOI] [PubMed] [Google Scholar]
- 85.Zhang M., Wu S. Q., He J. Q. Are genetic variations in glutathione S-transferases involved in anti-tuberculosis drug-induced liver injury? A meta-analysis. Journal of Clinical Pharmacy and Therapeutics. 2019;44(6):844–857. doi: 10.1111/jcpt.13006. [DOI] [PubMed] [Google Scholar]
- 86.Singh M., Sasi P., Gupta V. H., Rai G., Amarapurkar D. N., Wangikar P. P. Protective effect of curcumin, silymarin and N-acetylcysteine on antitubercular drug-induced hepatotoxicity assessed in an in vitro model. Human & Experimental Toxicology. 2012;31(8):788–797. doi: 10.1177/0960327111433901. [DOI] [PubMed] [Google Scholar]
- 87.Baniasadi S., Eftekhari P., Tabarsi P., et al. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. European Journal of Gastroenterology & Hepatology. 2010;22(10):1235–1238. doi: 10.1097/MEG.0b013e32833aa11b. [DOI] [PubMed] [Google Scholar]


