Abstract
Background
Shear stress is an effective modulator of endothelial progenitor cells (EPCs) and has been suggested to play an important role in angiogenesis. The phosphatase and tensin homolog (PTEN)/Akt and guanosine triphosphate cyclohydrolase (GTPCH)/tetrahydrobiopterin (BH4) pathways regulate the function of early EPCs. However, the role of these pathways in the shear stress-induced angiogenesis of late EPCs remains poorly understood. Therefore, we aim to investigate whether shear stress could upregulate the angiogenesis capacity of late EPCs and to further explore the possible underlying mechanisms.
Methods
Late EPCs were subjected to laminar shear stress (LSS), and their in vitro migration, proliferation, and tube formation capacity were determined. In addition, the in vivo angiogenesis capacity was explored, along with the expression of molecules involved in the PTEN/Akt and GTPCH/BH4 pathways.
Results
LSS elevated the in vitro activities of late EPCs, which were accompanied by downregulated PTEN expression, accelerated Akt phosphorylation, and GTPCH/BH4 pathway activation (all P < 0.05). Following Akt inhibition, LSS-induced upregulated GTPCH expression, BH4, and NO level of EPCs were suppressed. LSS significantly improved the migration, proliferation, and tube formation ability (15 dyn/cm2 LSS vs. stationary: 72.2 ± 5.5 vs. 47.3 ± 7.3, 0.517 ± 0.05 vs. 0.367 ± 0.038, and 1.664 ± 0.315 vs. 1 ± 0, respectively; all P < 0.05) along with the in vivo angiogenesis capacity of late EPCs, contributing to the recovery of limb ischemia. These effects were also blocked by Akt inhibition or GTPCH knockdown (P < 0.05, respectively).
Conclusions
This study provides the first evidence that shear stress triggers angiogenesis in late EPCs via the PTEN/Akt/GTPCH/BH4 pathway, providing a potential nonpharmacologic therapeutic strategy for promoting angiogenesis in ischemia-related diseases.
1. Background
Tissue ischemia and hypoxia induced by vascular disease are an important pathophysiological mechanism of ischemic disease. Thus, achieving an improved angiogenesis response to tissue ischemia is an effective therapeutic strategy to reduce organ damage in ischemia diseases [1, 2]. Accumulating evidence suggests that adult angiogenesis is not solely the result of endothelial cell (EC) proliferation but also related to the neovascularization function of circulating endothelial progenitor cells (EPCs) [3–6]. In addition, at least two different types of EPCs, early EPCs and late EPCs (or outgrowth EPCs), were recently identified in an in vitro cell culture system [7] with distinct cellular properties and biological functions. Early EPCs emerge during the early culture period within 4–7 days, with faint positive staining of VE-cadherin and KDR, low proliferation potential, and strong cytokine release, and are mainly involved in the repair of the injured vascular endothelium. By contrast, late EPCs (or outgrowth EPCs) emerge in the late culture period at up to 2–4 weeks and show stronger expression of VE-cadherin, KDR, and vWF, with high competency to produce endogenous nitric oxide (NO) and enhanced neovasculogenesis. Moreover, the proliferation, migration, and adhesion of late EPCs are promoted by the hypoxia in ischemic tissues, resulting in improved tubular formation ability and enhanced EPC-related angiogenesis to exacerbate the condition [7–9]. Therefore, gaining a better understanding of the mechanisms underlying angiogenesis derived from late EPCs could provide a basis for a novel therapeutic strategy for ischemia diseases.
It is now well established that shear stress has a beneficial effect on homeostasis of the vascular endothelium and also acts as the key trigger for new vessel formation [10, 11], and the beneficial effects of shear stress on ECs and EPCs are mediated exclusively by laminar shear stress (LSS), not turbulent/oscillatory flow [12–16]. In line with previous investigations, we found that LSS increased the migratory, adhesive, and proliferative activities of human early EPCs, which were accompanied by the upregulated expression of tissue type plasminogen activator and enhanced levels of endothelial nitric oxide synthase (eNOS) and superoxide dismutase [17–23]. Thus, LSS is an important nonpharmacological means of modulating the function of EPCs. However, studies on the individual effect of LSS on late EPCs and their angiogenesis capacity are limited.
The tumor suppressor phosphatase and tensin homolog (PTEN), an endogenous inhibitor of the PI3K/Akt/eNOS pathway, constitutes a major determinant of neovascularization at ischemic sites and has been shown to be associated with the angiogenesis functions of ECs and EPCs [24–26]. Hamada et al. revealed that PTEN deficiency in ECs accelerates tumor growth by promoting angiogenesis [24], and Koide et al. found that apoptosis regulator through modulating IAP expression (ARIA) increases membrane-associated PTEN expression while its knockdown leads to an opposite effect, therefore amplifying PI3K/Akt signaling, identifying that ARIA enhances PTEN activation and consequently reduces the PI3K/Akt signaling in ECs and EPCs, leading to the negative regulation in angiogenesis and vasculogenesis [26]. Those studies suggested from different perspectives that PTEN plays as a negative regulator of neovascularization in regard to its expression in ECs or EPCs. In addition, the PTEN/Akt signaling pathway plays important roles in multiple biological processes such as cell proliferation and growth and participates in prototypical endothelial functions such as the regulation of vascular tone, angiogenesis, control of adhesion, and recruitment of leukocytes to the vessel wall [27, 28]. And another study established that the expression and activity of GTP cyclohydrolase I (GTPCH I) and biosynthesis of tetrahydrobiopterin (BH4) were upregulated in human EPCs by treatment with a selective PPARδ agonist GW501516, which downregulated PTEN and thereby increased Akt phosphorylation and consequently enhanced the in vivo regenerative capacity of the EPCs [29].
Our previous study demonstrated that reduced GTPCH expression and decreased BH4 level contribute to impaired early EPCs in a condition of hypertension, and LSS upregulates GTPCH/BH4 pathway by activating sGC/cGMP and suppressing TSP-1 expression, thus enhancing reendothelialization capacity of early EPCs [23]. Considering the similarity between early and outgrowth EPCs, we hypothesized that shear stress may also regulate the expression of the PTEN/Akt and GTPCH/BH4 signaling pathways in late EPCs, which could further enhance their in vitro activity and in vivo angiogenesis capacity. To test these hypotheses, in the present study, we exposed late EPCs to LSS and examined their in vitro function and in vivo angiogenesis capacity. We further investigated the role of the PTEN/Akt and GTPCH/BH4 pathways in LSS-mediated alterations of the angiogenesis capacity of late EPCs in a hindlimb ischemia murine model. These findings may provide a novel therapeutic approach to restore the deteriorated EPC function in ischemic disease.
2. Material and Methods
2.1. Cell Culture and Identification of Late EPCs
This study was approved by the Ethics Committee of Xiangya Medical School of Central South University. Blood samples were obtained from volunteers after receiving informed consent, and late EPCs were isolated and cultured according to our previously reported method [30].
2.2. EPC Migration, Proliferation, and Tube Formation In Vitro
EPC migration was determined using a modified Boyden chamber placed in a 24-well culture dish containing 500 ml EBM-2 and supplemented with vascular endothelial growth factor (VEGF). Proliferation was determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and capillary tube formation was measured as previously reported [17]. Matrigel was added to a 24-well plate to form collagen gels, which were then overlaid with 100 μl cultured EPCs resuspended to 106 cells/ml and incubated at 37°C in an atmosphere of 5% CO2. Images of the gel were captured under a phase-contrast microscope. The total length of the tube-like structures in each image was measured and further normalized to the control group and presented into relative tube length according to previous research reports [31, 32].
2.3. Laminar Shear Stress Assay
The EPCs were exposed to laminar shear stress with a flow chamber loading device as previously described [18, 23, 30]. The seeded cells were exposed to 5, 15, and 25 dyn/cm2 laminar shear stress for up to 15 h or to 15 dyn/cm2 laminar shear stress for 5, 10, and 15 h, respectively. The EPCs in the control group were maintained in a static condition. All experiments were performed at 37°C in a CO2 incubator.
2.4. GTPCH I Knockdown and Pharmacologic Inhibition of Akt
Shear stress experiments were also performed with cells after GTPCH I knockdown by viral transduction using mission shRNA lentiviral transduction particles according to the manufacturer instructions (30MOI, Sunbio Medical Biotechnology Co., Ltd., Shanghai, China) [23]. The target sequence of GTPCH-I was 5′-CcggGCCGCTACCTACTAATGAATTCAAGAGATTCA-TTAGTAGGTAGCGGCTTTTTTg-3′, and the target sequence of the negative control was 5′-CcggTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTg-3′. After transduction, the cells were washed with phosphate-buffered saline (PBS) and incubated with EPC medium for 48 h; the effect of shRNA transduction on reducing the GTPCH I expression level was confirmed by polymerase chain reaction (PCR).
In addition, the cultured EPCs were preincubated with 10 μmol of the Akt phosphorylation inhibitor LY 294002 (Calbiochem) for 1 h before shear stress stimulation as described previously [18].
2.5. Western Blot Analysis
The cells were washed with PBS twice, and total EPC proteins were harvested by cell lysis buffer (#9803, Cell Signaling Technology Inc., Danvers, MA, USA). Protein extracts were subjected to 12% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Immobilon-P, Merck-Millipore, Darmstadt, Germany). The following antibodies were used for western blot analysis: rabbit anti-PTEN (1 : 1000; #9559, Cell Signaling Technology Inc., Danvers, MA, USA), rabbit anti-phosphorylated Akt (Ser473) (1 : 1000; #9271, Cell Signaling Technology Inc., Danvers, MA, USA), rabbit anti-total Akt antibody (1 : 1000; #9272, Cell Signaling Technology Inc., Danvers, MA, USA), mouse anti-GTPCH I (1 : 1000; sc-271482, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and rabbit anti-β-actin (1 : 1000; #4970, Cell Signaling Technology Inc., Danvers, MA, USA). Proteins were visualized with horseradish peroxidase- (HRP-) conjugated anti-rabbit IgG (1 : 2000; #7074, Cell Signaling Technology Inc., Danvers, MA, USA) or HRP-conjugated anti-mouse IgG (1 : 2000; #7076,Cell Signaling Technology Inc., Danvers, MA, USA), followed by use of the ECL chemiluminescence system (#6883, Cell Signaling Technology Inc., Danvers, MA, USA). The intensity of immunoreactive bands was analyzed and expressed as the ratio of PTEN, anti-phosphorylated Akt, Akt, and GTPCH I to β-actin protein in human EPCs.
2.6. Intracellular BH4 and NO Measurement
Intracellular BH4 concentrations were measured according to our previous report and another study [23, 33], by subtracting the level of BH2 plus oxidized biopterin from total biopterins, expressed as pmol/mg protein. The intracellular NO level in EPCs was evaluated by fluorescence microscopy using DAF-FM (Invitrogen) fluorescence as described previously [23] and expressed as a percentage change in fluorescence with respect to cells used as a time/vehicle control. The data were statistically compared relative to those of the static condition group.
2.7. Murine Hindlimb Ischemia Model
All animal care protocols and experiments were reviewed and approved by the Animal Care and Use Committee of the Laboratory Animal Research Center at Xiangya Medical School of Central South University. All of the mice were maintained in the specific pathogen-free facility of the Laboratory Animal Research Center at Central South University. Hindlimb ischemia was induced in 8-10-week-old male NRMInu/nu athymic nude mice. In brief, the mice were anesthetized with pentobarbital sodium (0.5%, 50 mg/kg) by intraperitoneal injection, and the surgical procedures were performed under sterile conditions. A vertical longitudinal incision was made in the left hindlimb, and the femoral artery and its branches were then dissected and ligated [18, 30, 34].
2.8. Laser Speckle Imaging
The blood flow was measured by scanning both rear paws with laser-speckle contrast imaging (PeriCam PSI Z, Perimed, Sweden) before and after the surgical procedure (days 0, 3, 7, 14, and 21). During the procedure, the animals were kept under pentobarbital sodium anesthesia and their body temperatures were strictly maintained between 36.5°C and 37.5°C [35]. Plantar perfusion was quantified within anatomically defined regions of interest (ROIs). The resulting data are reported as the ratio of blood flow in the left to right (L/R) hindlimb.
2.9. Immunohistochemistry
The gastrocnemius muscles were harvested at day 14 after femoral artery ligation. The midzone of the muscles (the 5 mm wide centermost section) was trimmed. The samples were embedded in paraffin, and 4 μm thick cross-sections were made to prepare for hematoxylin and eosin (H&E) staining. The number of collateral arteries per field was measured under a microscope at 40x magnification. For immunohistochemistry, we used antibodies against CD31 (1 : 200, Abcam, UK). The paraffin section was rehydrated, and endogenous peroxidase activity was blocked for 30 min in methanol containing 0.3% hydrogen peroxide. The section was incubated with the primary antibody at 4°C overnight, followed by 60 min of incubation with the biotinylated secondary antibody (1 : 500, Abcam, UK) [36]. All specimens were counterstained with hematoxylin staining solution (Beyotime Institute of Biotechnology, China) and then sealed with neutral gum for storage. Analyses of morphology and degree of angiogenesis were performed after scanning the section through OLYMPUS CX41 and Leica Application Suite 4.0 software.
2.10. Statistical Analysis
All the data were statistically analyzed using GraphPad Prism 6.0 software with one-way analysis of variance for comparisons of three or more groups, followed by the least significant difference post hoc test. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. Laminar Shear Stress Enhanced the Functions of Late EPCs In Vitro
The migration (Figures 1(a) and 2(a)), proliferation (Figures 1(b) and 2(b)), and tube formation (Figures 1(c) and 2(c)) of EPCs increased after laminar shear stress in a dose-dependent and time-dependent manner.
Figure 1.
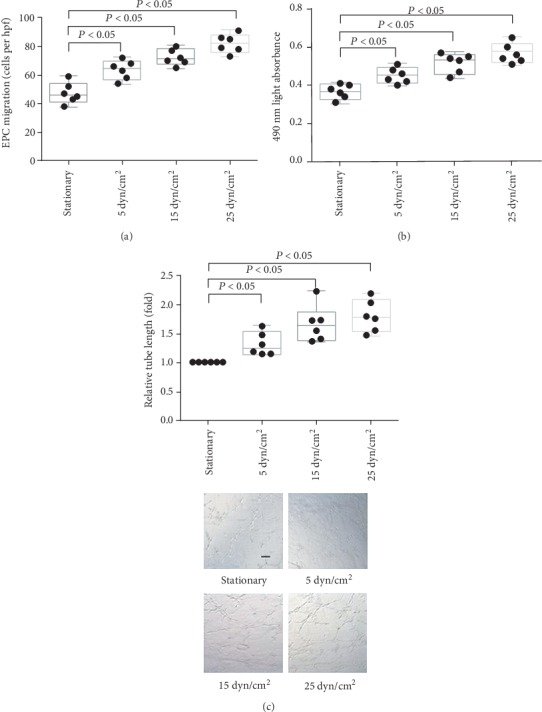
The effect of different levels of LSS on in vitro function of late EPCs. (a) Quantification analysis of VEGF-induced migration of late EPCs (∗P < 0.05 vs. EPCs under static condition group, n = 6 per group). (b) Quantification analysis of proliferative activity of late EPCs (∗P < 0.05 vs. EPCs under static condition group, n = 6 per group). (c) Quantification analysis and representative photograph tube information of late EPCs (represented as relative value to EPCs under static condition group, ∗P < 0.05 vs. EPCs under static condition group, n = 6 per group). Scale bar 200 μm. Least significant difference was applied for the post hoc test in statistical analysis. hpf = high power field.
Figure 2.
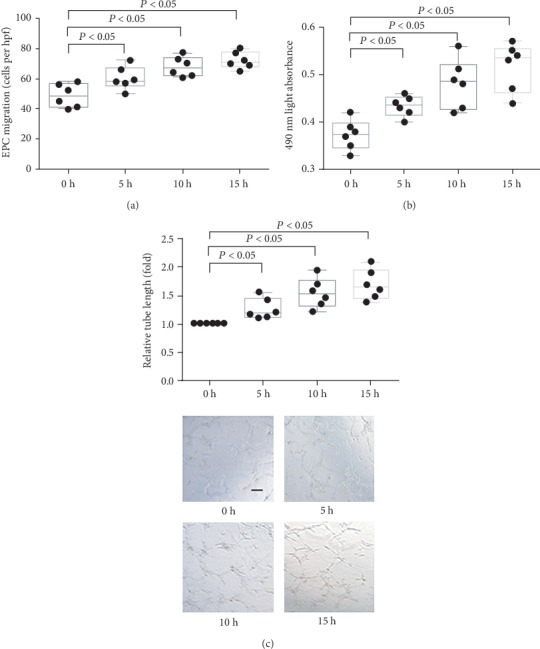
The effect of LSS on in vitro function of late EPCs at different time points. (a) Quantification analysis of VEGF-induced migration of late EPCs (∗P < 0.05 vs. EPCs at 0 h, n = 6 per group). (b) Quantification analysis of proliferative activity of late EPCs (∗P < 0.05 vs. EPCs at 0 h, n = 6 per group). (c) Quantification analysis and representative photograph tube information of late EPCs (represented as relative value to EPCs at 0 h, ∗P < 0.05 vs. EPCs at 0 h, n = 6 per group). Scale bar 200 μm. Least significant difference was applied for the post hoc test in statistical analysis. hpf = high power field.
3.2. Laminar Shear Stress Regulated the PTEN/Akt and GTPCH/BH4 Pathways in EPCs
As shown in Figure 3, after laminar stress treatment, the PTEN expression level decreased and the Akt phosphorylation level increased in dose- and time-dependent manners. In addition, GTPCH I expression and the intracellular BH4 and NO levels in late EPCs were also dose- and time-dependently increased in response to laminar shear stress. Collectively, these data demonstrated that LSS decreased PTEN expression, induced Akt phosphorylation, and subsequently induced GTPCH/BH4 pathway activation in late EPCs.
Figure 3.
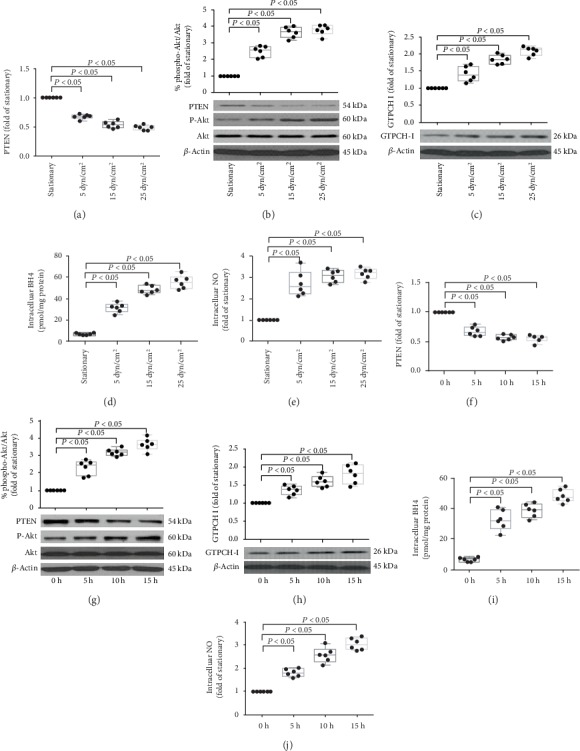
The effect of LSS on PTEN/Akt and GTPCHI/BH4 pathway in EPCs. (a, b) Representative photographs and quantification analysis of PTEN protein expression (a) and phosphorylation of Akt (b) of late EPCs treated with 5, 15, and 25 dyn/cm2 shear stress for 15 hours or under stationary condition, respectively (∗P < 0.05 vs. EPCs under static condition group, n = 6). (c) Representative photographs and quantification analysis of GTPCH I protein expression of late EPCs treated with 15 dyn/cm2 shear stress for 5, 10, and 15 hours (∗P < 0.05 vs. EPCs under static condition group, n = 6). (d, e) Quantitative analyses of intracellular BH4 (d) and NO (e) levels in late EPCs treated with 15 dyn/cm2 shear stress for 5, 10, and 15 hours, respectively (∗P < 0.05 vs. EPCs under static condition group, n = 6). (f, g) Representative photographs and quantification analysis of PTEN protein expression (f) and phosphorylation of Akt (g) of late EPCs treated with 5, 15, and 25 dyn/cm2 shear stress for 15 hours or under stationary condition, respectively (∗P < 0.05 vs. EPCs at 0 h, n = 6 per group). (h) Representative photographs and quantification analysis of GTPCH I protein expression of late EPCs treated with 15 dyn/cm2 shear stress for 5, 10, and 15 hours (∗P < 0.05 vs. EPCs at 0 h, n = 6 per group). (i, j) Quantitative analyses of intracellular BH4 (i) and NO (j) levels in late EPCs treated with 15 dyn/cm2 shear stress for 5, 10, and 15 hours, respectively (∗P < 0.05 vs. EPCs at 0 h, n = 6 per group). Least significant difference was applied for the post hoc test.
3.3. Laminar Shear Stress Triggered PTEN/Akt Activation Upstream of the GTPCH/BH4 Pathway in Late EPCs
As expected, pretreatment with GTPCH I shRNA did not prevent the laminar shear stress-induced reduction of PTEN expression and Akt phosphorylation elevation, and inhibition of Akt phosphorylation also did not change the expression level of PTEN under LSS (Figures 4(a) and 4(b)). In contrast, shRNA-mediated knockdown of GTPCH I significantly abolished the LSS-induced GTPCH I expression, leading to reductions in both the intracellular BH4 and NO levels in late EPCs. The same effects were observed after treatment of the Akt phosphorylation-specific inhibitor LY (Figures 4(c)–4(e)). These results demonstrated that laminar shear stress activates the GTPCH/BH4 pathway through suppressing PTEN expression and inducing Akt phosphorylation; in other words, PTEN/Akt acts as upstream of GTPCH/BH4 in the regulation of LSS on EPCs.
Figure 4.
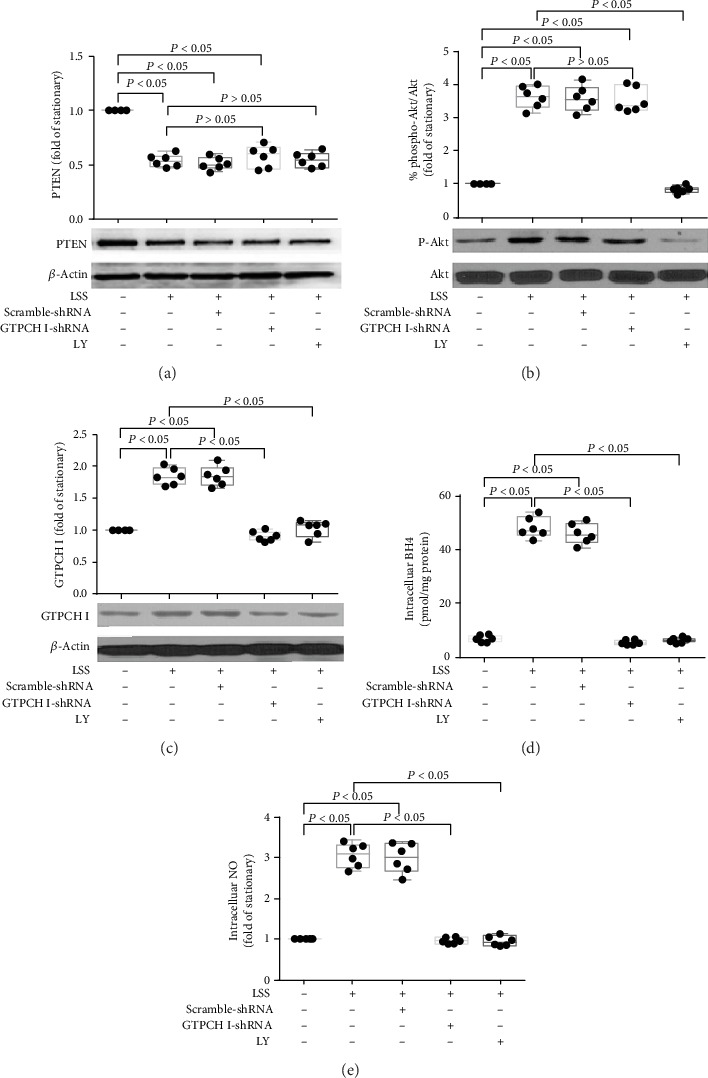
The relationship between PTEN/Akt and GTPCHI/BH4 pathway in EPCs in response to LSS. (a) Representative photograph and quantitative analysis of the effects of Akt inhibition or GTPCH I knockdown on LSS-regulated PTEN protein expression in late EPCs (∗P < 0.05 vs. EPCs under static condition group, n = 6; NS: no significant difference vs. LSS+EPC group, n = 6 per group). (b) Representative photograph and quantitative analysis of the effects of Akt inhibition or GTPCH I knockdown on LSS-regulated Akt phosphorylation of EPCs in late EPCs (∗P < 0.05 vs. EPCs under static condition group, n = 6; NS: no significant difference vs. LSS+EPC group, n = 6 per group). (c) Representative photograph and quantitative analysis of the effects of Akt inhibition or GTPCH I knockdown on LSS-regulated GTPCH I protein expression in late EPCs (∗P < 0.05 vs. EPCs under static condition group, n = 6; #P < 0.05 vs. LSS+EPC group, n = 6 per group). (d, e) Quantitative analyses of the effects of Akt inhibition or GTPCH I knockdown on LSS-regulated intracellular BH4 (d) and NO (e) levels in late EPCs (∗P < 0.05 vs. EPCs under static condition group, n = 6; #P < 0.05 vs. LSS+EPC group, n = 6 per group). Least significant difference was applied for the post hoc test in statistical analysis. Concentration of LY 294002: 10 μmol/l. LSS = laminar shear stress.
3.4. The PTEN/Akt/GTPCH/BH4 Pathway Mediated Shear Stress-Enhanced In Vitro Function and In Vivo Angiogenesis Capacity of Late EPCs
Then, we evaluated the role of the PTEN/Akt/GTPCH/BH4 pathway in EPC function. Pretreatment with GTPCH I shRNA or LY attenuated the laminar shear stress-induced migration, proliferation, and tube formation activity of late EPCs (Figure 5). Consistent with the in vitro results, laminar shear stress remarkably improved the functional recovery of limb ischemia after femoral artery ligation in the mouse model (Figure 6). Moreover, this functional recovery was abolished by GTPCH I shRNA or LY.
Figure 5.
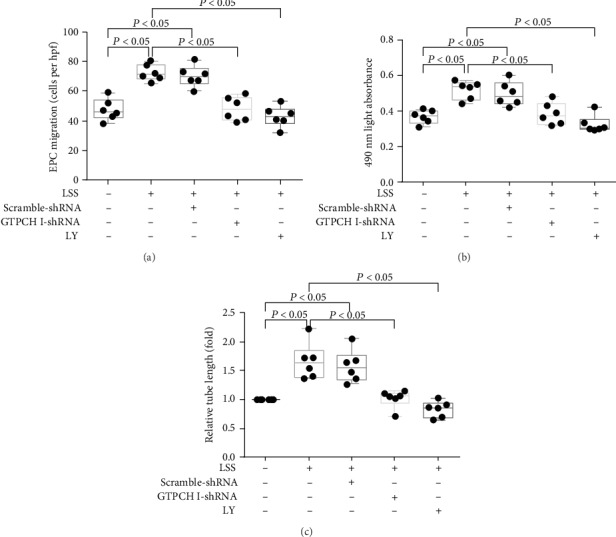
PTEN/Akt and GTPCH/BH4 pathway blockade inhibits in vitro function of EPCs treated with LSS. Quantification analysis of migration (a), proliferative activity (b), and tube formation (represented as relative value to EPCs under static condition group) of late EPCs (c) of EPCs treated with GTPCH I knockdown and Akt inhibition (∗P < 0.05 vs. EPCs under static condition group, n = 6; #P < 0.05 vs. LSS+EPC group, n = 6 per group). Least significant difference was applied for the post hoc test in statistical analysis. Concentration of LY 294002: 10 μmol/l. LSS = laminar shear stress.
Figure 6.
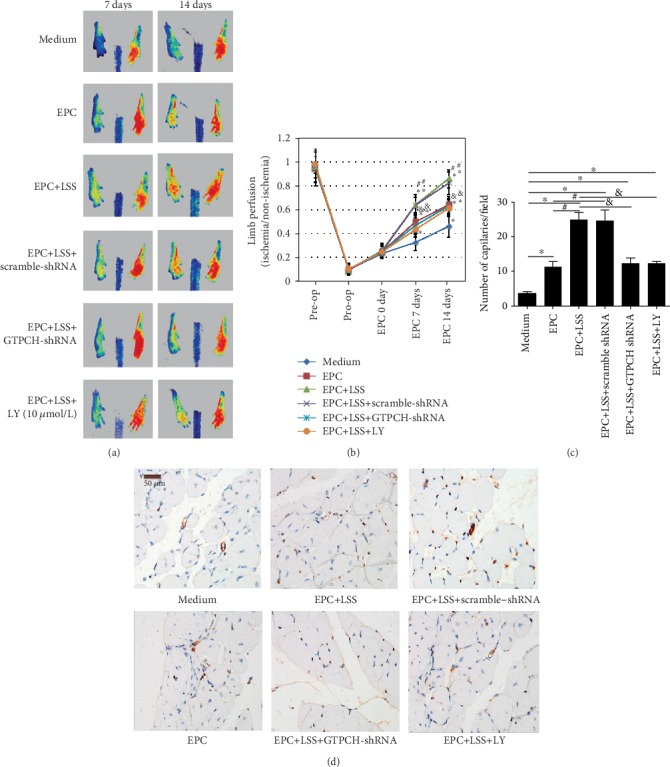
PTEN/Akt and GTPCH/BH4 pathway blockade inhibits in vivo angiogenesis of EPCs treated with LSS. Representative photographs (a) and quantification analysis showing in vivo angiogenesis capacity of EPCs treated with GTPCH I knockdown and Akt inhibition for improving blood perfusion in ischemic hind limb (b, c) (∗P < 0.05 vs. medium, n = 6; #P < 0.05 vs. EPCs under static condition group, n = 6; &P < 0.05 vs. LSS+EPC group, n = 6 per group). Least significant difference was applied for the post hoc test in statistical analysis. Concentration of LY 294002: 10 μmol/l. (d) Representative photographs and quantification analysis showing in vivo angiogenesis capacity of EPCs by CD31 staining (∗P < 0.05 vs. medium, n = 6; #P < 0.05 vs. EPCs under static condition group, n = 6; &P < 0.05 vs. LSS+EPC group, n = 6 per group). Scale bar 50 μm. Concentration of LY 294002: 10 μmol/l. LSS = laminar shear stress; hpf = high power field.
Finally, CD31 staining of the gastrocnemius muscles on the 14th day after femoral artery ligation showed that LSS significantly enhanced angiogenesis in the ischemic limbs of mice, which was inhibited by GTPCH I shRNA or LY (Figure 6(d)). Collectively, these results confirmed that LSS triggered late EPC function both in vivo and in vitro via the PTEN/Akt/GTPCH/BH4 pathway.
4. Discussion
In this study, we observed that LSS downregulated PTEN expression, induced Akt phosphorylation, activated the GTPCH/BH4 signaling pathway, and elevated the in vitro activities and angiogenesis ability of late EPCs. Thus, LSS triggered late EPC-associated angiogenesis via the PTEN/Akt/GTPCH/BH4 pathway, revealing a novel mechanism underlying the beneficial effect of LSS on late EPCs. This indicates that shear stress can act as a potential therapeutic option for ischemic diseases.
EPCs derived from the bone marrow play pivotal roles in promoting neovascularization, repairing endothelial damage, and improving endothelial function [37–39]. Both EPC number and function are impaired in cardiovascular disease and associated risk factors [40–42], constituting an important mechanism of vascular injury. Previous studies further revealed that late EPCs exhibit high angiogenic potential and enhanced neovascularization in critical limb ischemia or myocardial infarction via directly engrafting into newly formed host vessels and promoting paracrine function [3, 43]. Accordingly, the angiogenesis capacity of late EPCs plays a fundamental role in the pathophysiology of vascular injury in ischemic diseases, thus showing promise as an effective therapeutic approach for ischemic diseases.
Accumulating data suggest that shear stress modulates the morphology, functions, and expression of functional genes in ECs [44, 45], thus emerging as an important nonpharmacological approach for vascular repair. We previously reported that LSS increased the proliferative and migratory activities and enhanced the repair ability of early EPCs in the injured endothelium by upregulating the expression of eNOS, Tie2, and GTPCH I [18, 20–23, 46]. Shear stress was also found to regulate the differentiation of late EPCs via upregulation of integrins or cytoskeletal rearrangement [47, 48]. However, the effect of LSS on the late EPC-mediated angiogenesis capacity has remained unclear. The present study demonstrated that LSS enhanced the proliferation, migration, and tube formation ability of late EPCs in vitro. Furthermore, 15 dyn/cm2 LSS treatment for 15 h significantly increased the angiogenesis of late EPCs in a murine hindlimb ischemic model. Thus, LSS upregulates the angiogenesis process mediated by late EPCs, further supporting its beneficial effect on EPC function and shedding light on the potential application of LSS as a nonpharmacological treatment option in ischemic disease. Combined with the research results showing that LSS could induce restoration of in vitro migration, proliferation, and adhesion of early EPCs by upregulating GTPCH/BH4 pathway in our previous research, we come to conclusion that LSS could stimulate both the in vitro function of early EPCs and late EPCs through GTPCH/BH4 pathway and improve their in vivo reendothelialization or angiogenesis function, respectively (since their different fundamental physiological functions in vivo).
PTEN participates in the angiogenic function of ECs as well as postnatal neovascularization [24, 49] and acts on EPC-mediated angiogenesis via PI3K/Akt/eNOS [26], which performed as increased PTEN suppresses PI3K and Akt activation, and inhibited PTEN expression gets the opposite effect. A previous study revealed that the GTPCH/BH4 pathway is associated with the early EPC-mediated endothelial repair capacity in hypertension [50], and we also found that LSS ameliorates the reendothelialization capacity of early EPCs by upregulating the GTPCH/BH4 pathway [23], confirming the essential role of this pathway in maintaining early EPC function. Moreover, the expression and activity of GTPCH I and biosynthesis of BH4 are stimulated under the condition of PTEN downregulation and subsequent Akt activation in late EPCs, thus enhancing their regenerative function [29]. Accordingly, we speculated that LSS affects the functional activity of late EPCs through the PTEN/Akt/GTPCH/BH4 pathway, which was supported by the fact that LSS downregulated PTEN expression, accelerated Akt phosphorylation, and increased GTPCH and BH4 levels in late EPCs. This effect was diminished by inhibiting Akt phosphorylation, indicating that GTPCH/BH4 acts downstream of the PTEN/Akt pathway of late EPCs in response to shear stress. This was further confirmed by the suppression of LSS-induced enhancement of BH4 and intracellular NO levels by GTPCH I knockdown; however, GTPCH I did not affect PTEN expression or Akt phosphorylation in late EPCs. Furthermore, inhibition of Akt and blockage of the GTPCH/BH4 pathway attenuated the LSS-induced increased in vitro activities and the in vivo neovascularization capacity or vascular density of late EPCs. Thus, these results highlight the importance of the PTEN/Akt and GTPCH/BH4 pathways in the LSS-mediated regulation of EPC in vitro function and in vivo neovascularization capacity.
Our previous study revealed that LSS can augment eNOS mRNA expression [20], activate the Tie2/Akt/eNOS signaling pathway [18], and upregulate the GTPCH/BH4 pathway [23] in early EPCs with subsequent enhancement of their endothelial repair capacity, indicating that LSS-stimulated NO production in early EPCs involves both posttranscriptional (including mRNA expression and subsequent phosphorylation of functional proteins involved in the NO production) and transcriptional modulation. It presented that LSS enhanced the phosphorylation of Tie2 and Akt, and Tie2 knockdown or Akt inhibition suppressed LSS-induced phosphorylation of Akt and eNOS in EPCs, which subsequently inhibited reendothelialization capacity of EPCs [18]. Moreover, it was found that LSS restored the Janus kinase 2 (JAK2) phosphorylation via upregulation of SDF-1/CXCR4 and then stimulated EPC-mediated reendothelialization. By contrary, CXCR4 knockdown by sh-RNA or JAK2 inhibition diminished this beneficial effect [30]. Other investigations demonstrated the roles of CXCR4 and CXCR7 in EPC homing and angiogenesis, through inhibition of SDF-1 inducing EPC activities by blocking CXCR4 or CXCR7 with their antibodies or antagonists [51], which showed an effect of depression on EPC migration, adhesion, and tube formation by both CXCR4 or CXCR7 inhibition. Among those researches, it was also suggested that Akt acts as a downstream signaling of angiopoietin-2/Tie2 [18] and CXCR7/SDF-1 pathway [51, 52] and regulates both in vitro functions and in vivo reendothelialization capacity of EPCs [30]. The present study further expounds that the LSS-induced increase in late EPC function and angiogenesis capacity is related to Akt and the GTPCH/BH4 pathway, suggesting that posttranscriptional regulation may play a crucial role in LSS-induced NO biosynthesis in late EPCs. Consistent with our previous research [23], it verified that eNOS/NO is the downstream signaling of GTPCH/BH4 pathway of EPCs. Therefore, modulation of the NO production of EPCs by LSS may be mediated by multiple mechanisms, and NO-dependent pathway is probably the common downstream target in modulating early or late EPC functions.
There are certain limitations of our study that should be acknowledged. First, we did not conduct a gene transfection experiment of the negative regulatory factor PTEN to confirm its role in the upregulation of EPC function mediated by shear stress. Second, fluorescence tracing of EPCs was not performed in this study. Previous studies used fluorescence tracing to demonstrate that after EPC injection, late EPCs reside in the capillaries of the ischemic muscle and differentiate into endothelial cells to significantly improve blood flow recovery [23, 53, 54]. This evidence reveals the fate of late EPCs in EPC-mediated neovascularization in response to ischemia. Third, because we did not have access to an oscillatory flow apparatus, we were unable to investigate the effect of oscillatory shear stress on late EPCs. Previous studies demonstrated that the effect of oscillatory shear on the endothelium is associated with an atheroprone endothelial phenotype, which is distinct from the atheroprotective potential exerted by laminar shear stress [55–57]. Therefore, it is possible that oscillatory shear stress inhibits the functional activity of late EPCs, thereby opposing the effects of laminar shear stress-mediated regulation; however, further investigation is needed to investigate this possibility.
5. Conclusion
In conclusion, the current study is the first to demonstrate that shear stress enhanced the in vitro function and in vivo neovascularization capacity of late EPCs via the PTEN/Akt/GTPCH/BH4 pathway. This investigation provides novel insights into the protective effect of shear stress on EPC-mediated angiogenesis, suggesting shear stress as an important nonpharmacologic therapeutic strategy for ischemic diseases.
Acknowledgments
This work was supported by the grants from the National Natural Scientific Foundation of China (81670220), the project of Guangdong Provincial Science and Technology Plan (2015A020212013), the Science and Technology Project of Guangzhou City (201803010008), the Fundamental Research Funds for the Central Universities in Sun Yat-sen University (17ykzd18), and the International Scientific and Technological Cooperation Project of Guangzhou Economic and Technological Development Zone (2017GH13).
Abbreviations
- EPCs:
Endothelial progenitor cells
- NO:
Nitric oxide
- eNOS:
Endothelial nitric oxide synthase
- PTEN:
Tumor suppressor phosphatase and tensin homolog
- PI3K:
Phosphatidylinositol 3′-kinase
- GTPCH I:
GTP cyclohydrolase I
- BH4:
Tetrahydrobiopterin
- LSS:
Laminar shear stress.
Contributor Information
Ling-Ping Zhu, Email: 158102107@csu.edu.cn.
Zhen Yang, Email: yangzhen10710710@163.com.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Ethical Approval
This study was approved by the Ethics Committee of Xiangya Medical School of Central South University.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Zhen Yang designed and supervised the experiment. Shao-Hong Wu, Feng Zhang, and Ling-Ping Zhu performed the experiments, analyzed the data, plotted the figures, and wrote the manuscript. Shun Yao, Lu Tang, and Hai-Tao Zeng performed some of the experiments and analyzed the data. All authors contributed intellectually to the manuscript. All authors read and approved the final manuscript. Shao-Hong Wu and Feng Zhang contributed equally to this work.
References
- 1.Zhao L., Johnson T., Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Research & Therapy. 2017;8(1):p. 125. doi: 10.1186/s13287-017-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tournois C., Pignon B., Sevestre M. A., et al. Cell therapy in critical limb ischemia: a comprehensive analysis of two cell therapy products. Cytotherapy. 2017;19(2):299–310. doi: 10.1016/j.jcyt.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Minami Y., Nakajima T., Ikutomi M., et al. Angiogenic potential of early and late outgrowth endothelial progenitor cells is dependent on the time of emergence. International Journal of Cardiology. 2015;186:305–314. doi: 10.1016/j.ijcard.2015.03.166. [DOI] [PubMed] [Google Scholar]
- 4.You J., Sun J., Ma T., et al. Curcumin induces therapeutic angiogenesis in a diabetic mouse hindlimb ischemia model via modulating the function of endothelial progenitor cells. Stem Cell Research & Therapy. 2017;8(1):p. 182. doi: 10.1186/s13287-017-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng J., Wang L., Qu M., et al. Endothelial progenitor cells transplantation attenuated blood-brain barrier damage after ischemia in diabetic mice via HIF-1α. Stem Cell Research & Therapy. 2017;8(1):p. 163. doi: 10.1186/s13287-017-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin T. C., Lin C. S., Tsai T. N., et al. Stimulatory influences of far infrared therapy on the transcriptome and genetic networks of endothelial progenitor cells receiving high glucose treatment. Acta Cardiologica Sinica. 2015;31(5):414–428. doi: 10.6515/acs20141201c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon C. H., Hur J., Park K. W., et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 8.Hur J., Yoon C. H., Kim H. S., et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(2):288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y., Weisdorf D. J., Solovey A., Hebbel R. P. Origins of circulating endothelial cells and endothelial outgrowth from blood. The Journal of Clinical Investigation. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu S., Li X., LaPenna K. B., Yokota S. D., Huke S., He P. New insights into shear stress-induced endothelial signalling and barrier function: cell-free fluid versus blood flow. Cardiovascular Research. 2017;113(5):508–518. doi: 10.1093/cvr/cvx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama K. H., Surya V. N., Gole M., et al. Nanoscale patterning of extracellular matrix alters endothelial function under shear stress. Nano Letters. 2016;16(1):410–419. doi: 10.1021/acs.nanolett.5b04028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souilhol C., Serbanovic-Canic J., Fragiadaki M., et al. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nature Reviews Cardiology. 2019;31 doi: 10.1038/s41569-019-0239-5. [DOI] [PubMed] [Google Scholar]
- 13.Kutikhin A. G., Sinitsky M. Y., Yuzhalin A. E., Velikanova E. A. Shear stress: an essential driver of endothelial progenitor cells. Journal of Molecular and Cellular Cardiology. 2018;118:46–69. doi: 10.1016/j.yjmcc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Baratchi S., Khoshmanesh K., Woodman O. L., Potocnik S., Peter K., McIntyre P. Molecular sensors of blood flow in endothelial cells. Trends in Molecular Medicine. 2017;23(9):850–868. doi: 10.1016/j.molmed.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Chistiakov D. A., Orekhov A. N., Bobryshev Y. V. Effects of shear stress on endothelial cells: go with the flow. Acta Physiologica (Oxford, England) 2017;219(2):382–408. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 16.Baeyens N., Bandyopadhyay C., Coon B. G., Yun S., Schwartz M. A. Endothelial fluid shear stress sensing in vascular health and disease. The Journal of Clinical Investigation. 2016;126(3):821–828. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K., Takahashi T., Asahara T., et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. Journal of Applied Physiology. 2003;95(5):2081–2088. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z., Xia W. H., Zhang Y. Y., et al. Shear stress-induced activation of Tie2-dependent signaling pathway enhances reendothelialization capacity of early endothelial progenitor cells. Journal of Molecular and Cellular Cardiology. 2012;52(5):1155–1163. doi: 10.1016/j.yjmcc.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Obi S., Yamamoto K., Shimizu N., et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. Journal of Applied Physiology. 2009;106(1):203–211. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z., Tao J., Wang J. M., et al. Shear stress contributes to t-PA mRNA expression in human endothelial progenitor cells and nonthrombogenic potential of small diameter artificial vessels. Biochemical and Biophysical Research Communications. 2006;342(2):577–584. doi: 10.1016/j.bbrc.2006.01.172. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z., Wang J. M., Wang L. C., et al. In vitro shear stress modulates antithrombogenic potentials of human endothelial progenitor cells. Journal of Thrombosis and Thrombolysis. 2007;23(2):121–127. doi: 10.1007/s11239-006-9045-0. [DOI] [PubMed] [Google Scholar]
- 22.Tao J., Yang Z., Wang J. M., et al. Shear stress increases Cu/Zn SOD activity and mRNA expression in human endothelial progenitor cells. Journal of Human Hypertension. 2007;21(5):353–358. doi: 10.1038/sj.jhh.1002147. [DOI] [PubMed] [Google Scholar]
- 23.Bai Y. P., Xiao S. H., Tang Y. B., et al. Shear stress-mediated upregulation of GTP cyclohydrolase/tetrahydrobiopterin pathway ameliorates hypertension-related decline in reendothelialization capacity of endothelial progenitor cells. Journal of Hypertension. 2017;35(4):784–797. doi: 10.1097/HJH.0000000000001216. [DOI] [PubMed] [Google Scholar]
- 24.Hamada K., Sasaki T., Koni P. A., et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes & Development. 2005;19(17):2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morello F., Perino A., Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovascular Research. 2009;82(2):261–271. doi: 10.1093/cvr/cvn325. [DOI] [PubMed] [Google Scholar]
- 26.Koide M., Ikeda K., Akakabe Y., et al. Apoptosis regulator through modulating IAP expression (ARIA) controls the PI3K/Akt pathway in endothelial and endothelial progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(23):9472–9477. doi: 10.1073/pnas.1101296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang H., Lee O. H., Lee Y., et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. Journal of Pineal Research. 2016;60(3):336–347. doi: 10.1111/jpi.12316. [DOI] [PubMed] [Google Scholar]
- 28.Luo M., Tan X., Mu L., et al. MiRNA-21 mediates the antiangiogenic activity of metformin through targeting PTEN and SMAD7 expression and PI3K/AKT pathway. Scientific Reports. 2017;7(1, article 43427) doi: 10.1038/srep43427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He T., Smith L. A., Lu T., Joyner M. J., Katusic Z. S. Activation of peroxisome proliferator-activated receptor–δ enhances regenerative capacity of human endothelial progenitor cells by stimulating biosynthesis of tetrahydrobiopterin. Hypertension. 2011;58(2):287–294. doi: 10.1161/HYPERTENSIONAHA.111.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia W. H., Yang Z., Xu S. Y., et al. Age-related decline in reendothelialization capacity of human endothelial progenitor cells is restored by shear stress. Hypertension. 2012;59(6):1225–1231. doi: 10.1161/HYPERTENSIONAHA.111.179820. [DOI] [PubMed] [Google Scholar]
- 31.Han J. K., Lee H. S., Yang H. M., et al. Peroxisome proliferator–activated receptor-δ agonist enhances vasculogenesis by regulating endothelial progenitor cells through genomic and nongenomic activations of the phosphatidylinositol 3-kinase/Akt pathway. Circulation. 2008;118(10):1021–1033. doi: 10.1161/CIRCULATIONAHA.108.777169. [DOI] [PubMed] [Google Scholar]
- 32.Wang H. W., Huang T. S., Lo H. H., et al. Deficiency of the microRNA-31–microRNA-720 pathway in the plasma and endothelial progenitor cells from patients with coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(4):857–869. doi: 10.1161/ATVBAHA.113.303001. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa T., Imamura K., Kondo T., et al. Genetic and pharmacological correction of aberrant dopamine synthesis using patient iPSCs with BH4 metabolism disorders. Human Molecular Genetics. 2016;25(23):5188–5197. doi: 10.1093/hmg/ddw339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L. P., Zhou J. P., Zhang J. X., et al. mir-15b-5p regulates collateral artery formation by targeting ATK3 (protein kinase B-3) Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(5):957–968. doi: 10.1161/ATVBAHA.116.308905. [DOI] [PubMed] [Google Scholar]
- 35.Postnov D. D., Sosnovtseva O., Tuchin V. V. Improved detectability of microcirculatory dynamics by laser speckle flowmetry. Journal of Biophotonics. 2015;8(10):790–794. doi: 10.1002/jbio.201500152. [DOI] [PubMed] [Google Scholar]
- 36.Herold J., Nowak S., Kostin S., et al. Factor VII activating protease (FSAP) influences vascular remodeling in the mouse hind limb ischemia model. American Journal of Translational Research. 2017;9(6):3084–3095. [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta J. L., Szwedo J. Circulating endothelial progenitor cells, microparticles and vascular disease. Journal of Hypertension. 2010;28(8):1611–1613. doi: 10.1097/HJH.0b013e32833bcfe9. [DOI] [PubMed] [Google Scholar]
- 38.Du F., Zhou J., Gong R., et al. Endothelial progenitor cells in atherosclerosis. Frontiers in Bioscience. 2012;17(7):2327–2349. doi: 10.2741/4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fadini G. P., Losordo D., Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circulation Research. 2012;110(4):624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z., Chen L., Su C., et al. Impaired endothelial progenitor cell activity is associated with reduced arterial elasticity in patients with essential hypertension. Clinical and Experimental Hypertension. 2010;32(7):444–452. doi: 10.3109/10641961003686435. [DOI] [PubMed] [Google Scholar]
- 41.Lee P. S., Poh K. K. Endothelial progenitor cells in cardiovascular diseases. World Journal of Stem Cells. 2014;6(3):355–366. doi: 10.4252/wjsc.v6.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao J., Wang Y., Yang Z., Tu C., Xu M. G., Wang J. M. Circulating endothelial progenitor cell deficiency contributes to impaired arterial elasticity in persons of advancing age. Journal of Human Hypertension. 2006;20(7):490–495. doi: 10.1038/sj.jhh.1001996. [DOI] [PubMed] [Google Scholar]
- 43.Dubois C., Liu X., Claus P., et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. Journal of the American College of Cardiology. 2010;55(20):2232–2243. doi: 10.1016/j.jacc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 44.Obi S., Yamamoto K., Ando J. Effects of shear stress on endothelial progenitor cells. Journal of Biomedical Nanotechnology. 2014;10(10):2586–2597. doi: 10.1166/jbn.2014.2014. [DOI] [PubMed] [Google Scholar]
- 45.Ando J., Yamamoto K. Vascular mechanobiology endothelial cell responses to fluid shear stress. Circulation Journal. 2009;73(11):1983–1992. doi: 10.1253/circj.CJ-09-0583. [DOI] [PubMed] [Google Scholar]
- 46.Tao J., Yang Z., Wang J. M., Tu C., Pan S. R. Effects of fluid shear stress on eNOS mRNA expression and NO production in human endothelial progenitor cells. Cardiology. 2006;106(2):82–88. doi: 10.1159/000092636. [DOI] [PubMed] [Google Scholar]
- 47.Cui X. D., Zhang X. Y., Guan X., et al. Shear stress augments the endothelial cell differentiation marker expression in late EPCs by upregulating integrins. Biochemical and Biophysical Research Communications. 2012;425(2):419–425. doi: 10.1016/j.bbrc.2012.07.115. [DOI] [PubMed] [Google Scholar]
- 48.Cheng M., Guan X., Li H., et al. Shear stress regulates late EPC differentiation via mechanosensitive molecule-mediated cytoskeletal rearrangement. PLoS One. 2013;8(7, article e67675) doi: 10.1371/journal.pone.0067675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y., Dowbenko D., Spencer S., et al. Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. The Journal of Biological Chemistry. 2000;275(28):21477–21485. doi: 10.1074/jbc.M909741199. [DOI] [PubMed] [Google Scholar]
- 50.Xie H. H., Zhou S., Chen D. D., Channon K. M., Su D. F., Chen A. F. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension. Hypertension. 2010;56(6):1137–1144. doi: 10.1161/HYPERTENSIONAHA.110.160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dai X., Tan Y., Cai S., et al. The role of CXCR7 on the adhesion, proliferation and angiogenesis of endothelial progenitor cells. Journal of Cellular and Molecular Medicine. 2011;15(6):1299–1309. doi: 10.1111/j.1582-4934.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odemis V., Boosmann K., Heinen A., Kury P., Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. Journal of Cell Science. 2010;123(7):1081–1088. doi: 10.1242/jcs.062810. [DOI] [PubMed] [Google Scholar]
- 53.Kebir A., Harhouri K., Guillet B., et al. CD146 short isoform increases the proangiogenic potential of endothelial progenitor cells in vitro and in vivo. Circulation Research. 2010;107(1):66–75. doi: 10.1161/CIRCRESAHA.109.213827. [DOI] [PubMed] [Google Scholar]
- 54.Harhouri K., Kebir A., Guillet B., et al. Soluble CD146 displays angiogenic properties and promotes neovascularization in experimental hind-limb ischemia. Blood. 2010;115(18):3843–3851. doi: 10.1182/blood-2009-06-229591. [DOI] [PubMed] [Google Scholar]
- 55.Lee J. Y., Chung J., Kim K. H., et al. Fluid shear stress regulates the expression of Lectin-like oxidized low density lipoprotein receptor-1 via KLF2-AP-1 pathway depending on its intensity and pattern in endothelial cells. Atherosclerosis. 2018;270:76–88. doi: 10.1016/j.atherosclerosis.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 56.Green J. P., Souilhol C., Xanthis I., et al. Atheroprone flow activates inflammation via endothelial ATP-dependent P2X7-p38 signalling. Cardiovascular Research. 2018;114(2):324–335. doi: 10.1093/cvr/cvx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ajami N. E., Gupta S., Maurya M. R., et al. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(41):10990–10995. doi: 10.1073/pnas.1707517114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.


