Abstract
Growing worldwide, the genus Ephedra (family Ephedraceae) had a medicinal, ecological, and economic value. The extraordinary morphological diversity suggests that Ephedra was survivor of an ancient group, and its antiquity is also supported by fossil data. It has recently been suggested that Ephedra appeared 8–32 million years ago, and a few megafossils document its presence in the Early Cretaceous. Recently, the high analytical power provided by the new mass spectrometry (MS) instruments is making the characterization of Ephedra metabolites more feasible, such as ephedrine series. In this regard, the chemical compounds isolated from crude extracts, fractions, and few isolated compounds of Ephedra species were characterized by MS-based techniques (LC-MS, LC-ESI-MS, HPLC-PDA-ESI/MS, LC-DAD-ESI/MSn, LC/Orbitrap MS, etc.). Moreover, we carry out an exhaustive review of the scientific literature on biomedicine and pharmacotherapy (anticancer, antiproliferative, anti-inflammatory, antidiabetic, antihyperlipidemic, antiarthritic, and anti-influenza activities; proapoptotic and cytotoxic potential; and so on). Equally, antimicrobial and antioxidant activities were discussed. This review is focused on all these topics, along with current studies published in the last 5 years (2015–2019) providing in-depth information for readers.
1. Introduction
It is becoming increasingly clear that plants, ranging from across the plant kingdom, produce a unique diversity of secondary metabolites that can be exploited for the discovery of new drugs, bio-sourced materials, nutraceuticals, or cosmetics [1–5]. For finding new molecules, plant natural products are undoubtedly good sources of chemical diversity [6–10]. It is estimated that over 200,000 primary and secondary metabolites may be present in the plant kingdom [11–15]. Medicinal plant is the product of long-term medical practice worldwide, with the advantages of outstanding curative properties and less side effects [16–21]. Containing many natural products and their derivatives of therapeutic value, medicinal plants are considerate as main source of remedies able to protect human body against diseases.
As medicinal plant, enclosing over 50 species, Ephedra genus belongs to the family Ephedraceae which in turn represents one of three families in the order Gnetales [22–26]. Ephedra is common in cold and dry places in both the Old and the New Worlds; the Gnetaceae members live in warm and humid tropical/subtropical forests of Asia, Africa, and South America [27]. The shrubs, which reach approximately one meter in height, grow in semiarid and desert conditions in both hemispheres across six continents [25, 26]. Known as Ma Huang, E. sinica is one of the oldest medicinal herbs in traditional Chinese medicine [28–31]. E. sinica preparations have been used for over 5000 years as stimulants and as antiasthmatics and are traditionally used to treat cold, bronchial asthma, cough, fever, flu, headache, edema, and allergies [32, 33]. It can also be used to lose weight by increasing sweating and basal metabolism and by stimulating the central nervous system [34, 35]. Moreover, it has also been combined with cardiovascular drugs to treat cardiovascular diseases [36, 37]. For years, ephedrine series [(-)-ephedrine, (+)-pseudoephedrine, (-)-N-methylephedrine, (+)-N-methylpseudoephedrine, (-)-norephedrine, (+)-norpseudoephedrine] were considered to be the main Ephedra constituents [38, 39]. Nowadays, at the side of pharmacological effects, there has been considerable research on the phytochemistry and bioactivities of genus Ephedra, including their antibacterial and primarily antioxidant activity [40–44]. From the entire plant, a wide range of Ephedra natural products including alkaloids, tannins, saponins, proanthocyanidins, phenolic acids, flavonoids, and essential oils have been mentioned and the plants-derived polyphenols are of great importance for their biological and pharmacological potential [45–50].
However, these numbers may be underestimated since many metabolites have not been characterized yet and new publications appear continuously with numerous new structures. In the last two decades, there was a quick development of mass spectrometric techniques allowing analysis of Ephedra natural products. Mass spectrometry is currently one of the most versatile and sensitive instrumental methods applied to structural characterization of Ephedra metabolite [51–55].
Although the analysis of Ephedra natural products has been investigated for many years, there is not a review in the literature focusing on the great pharmacological and biological potential and applications of high-resolution mass spectrometry. In this review, we will provide information about these topics and their advances and applications in the last five years (2015–2019) that could be interesting for botanical, analytical chemistry, and natural products communities.
2. Ephedra History Evolution
Containing approximately fifty species, the genus Ephedra (Family Ephedraceae) was distributed in arid and semiarid regions of Asia, Europe, northern Africa, southwestern North America, and South America [56–60]. In fact, Ephedra was distributed from the northern temperate zone (from the Canary Islands through the Mediterranean region and Central Asia to Shandong in China) to the arid regions of USA and Mexico, and to alpine area of the Andes in South America [26, 27,61–64]. This wide range is at least partially attributable to its effective dispersal syndromes.
Fossil evidence has been playing important roles in understanding early evolution of the Ephedra gnetophytes [65, 66]. The early evolution and diversification of the Ephedra have increasingly become clear because of recently reported macrofossils from the Early Cretaceous strata of Asia, Australia, Europe, and Americas [67, 68]. Ephedra macrofossils, especially female cones, provide a historical perspective for the early evolution, taxonomy, and biogeography of this genus. In this respect, by using molecular sequence data (rbcL) and assuming a constant rate of evolution calculated by landmark event calibration, the corresponding age of extant Ephedra was recently estimated to be 8–32 million years [69–72]. Macrofossils of female cones were found in the Early Cretaceous of South America [73, 74], Mongolia [75, 76], and adjacent Northeast China [60, 77–80]. Early Ephedra might have transformed bracts of female cones into vivid color to assist seed dispersal by birds, wind, or seed-caching rodents resulting in a wide intercontinental distribution [59, 81, 82].
On the other hand, phylogenetic analysis resulted in well-supported subgroups of Ephedra that correspond to geographical regions [69, 83–85] with African-Mediterranean species in a basal grade or clade and Asian species forming two well-supported clades [72, 86]. New World species are monophyletic and comprise a South American clade [72, 86] and a nonmonophyletic grade of North American species [86]. As reported by Crane [87], a possible origin of Ephedra in Africa is interesting as the diversity of ephedroid pollen grains is particularly high in Early Cretaceous palaeoequatorial regions of Africa and South America. In this context, African species constitute a basal grade or clade within Ephedra [72]: some of the basal species are limited to Africa (E. altissima); others have a broader distribution in the Old World, extending from Africa into Asia or southern Europe. In recent decades, various Ephedra and Ephedra-like meso- and macrofossils have been reported from the Early Cretaceous of South Europe, Northeast China [79], Mongolia [88], North America, and South America [25, 56, 89]. Seed mesofossils with in situ pollen were reported from the Early Cretaceous of North America [72] and Portugal (South Europe) [60].
3. Ephedra Extracts Phytochemical Content
From a chemical point of view, previous studies conducted on Ephedra showed that it contained different types of polyphenols, flavonoids, and anthocyanins [41, 42, 90–97]. For quantitative measurement, gallic acid (quercetin or catechin) and cyanidin-3-glucoside were used as standard compounds (references) to quantify total polyphenol, total flavonoid, and total anthocyanins content, respectively.
In a study carried out by Danciu et al. [90] on ethanolic extracts of the aerial part of E. alata Decne., an amount equal to 156.22 mg of gallic acid equivalents/g dry sample (mg GAE/g) was reported for total polyphenol (TPC). Jaradat et al. [91] have analyzed the phytochemical composition of E. alata Decne., by using water, MeOH, and EtOH for the extraction. The study reports that, when water was used, total polyphenols could not be detected in the extract, and the EtOH extract was 19.175 mg GAE/g. On the other hand, Alali et al. [92] and Nasar et al. [93] reported that water extracts of E. alata Decne. and E. procera C. A. Mey showed a TPC of 16.2 and 117.01 mg GAE/g, respectively.
Al-Rimawi et al. [41] analyzed extracts of E. alata Decne. collected from the southern part of the West Bank, Palestine. These authors used three different solvents, namely, water, 100% EtOH, and 80% EtOH, in order to observe which solvent leads to the highest amounts of total flavonoid contents (TFC). The results showed that TFC was higher in case of 100% EtOH (19.5 ± 0.3 mg catechin/g dry weight). Aghdasi et al. [42] studied the variation of TFC of Iranian E. major during May to October from Bojnoord. In fact, TFC exhibited a variation during sampling period and ranged from 4.63 to 8.4 mg QE/g. Mellado et al. [94] analyzed flavonoids in E. chilensis K Presl, a Chilean endemic plant. These authors reported significant differences in the CH2Cl2 extracts (P < 0.05) compared to the hexane and EtOH extracts. The total phenolic content in both CH2Cl2 extract and EtOH extract shows significant differences (P < 0.05) with the hexanoic extract. In the study of Al-Trad et al. [95], the butanolic extract from the stem of Jordanian E. alte had a phenolic content of 404.001 ± 5.53 mg/g gallic acid and flavonoids of 40.73 ± 6.59 mg/g quercetin [95].
Hegazy et al. [96] observed that the total anthocyanins content (TAC) of Saudi E. foeminea, collected from Shada Mountain, southwest Saudi Arabia, was 0.14 mg cy-3-glu/100 g. TAC of Lebanese E. campylopoda was detected in the MeOH extracts but not in EtOH and aqueous fractions [97].
4. Recent Applications of High-Resolution Mass Spectrometry for Ephedra Extract Characterization
Although the analysis of natural products from Ephedra species has been investigated for many years, there is not a review in the literature focusing on the great possibilities and applications of high-resolution mass spectrometry. This review is devoted to chemical identification using mass spectrometry as the most powerful technique of qualitative analysis. It is evident in the fact that the terms of “identification” and “mass spectrometry” occur together in more than a million scientific reports returned in the search results performed by Google Scholar engine [98]. The reason for the potency of MS is that it is superior to other analytical techniques in the combination of features, such as multianalytic property, sensitivity, selectivity, possibility of compounds identification by molecular mass or formula, and possibility of combining with chromatography.
The most prominent methods include MS/MS (tandem mass spectrometry); LC-ESI/MS/MSn (liquid chromatography–electrospray ionization/multistage mass spectrometry); LC-PDA (liquid chromatography coupled to photodiode array); HPLC-PDA-ESI/MS (high-performance liquid chromatography coupled to photodiode array and electrospray ionization mass spectrometric); LC-DAD-ESI/MSn (high-performance liquid chromatography with diode array detection coupled to tandem mass spectrometry analysis with electrospray ionization); and LC/Orbitrap MS (liquid chromatography Orbitrap Fusion Tribrid tandem mass spectrometry). In this section, we will provide information about this topic and its advances and applications in the last five years (2015–2019) that could be interesting for both the analytical chemistry and the natural products communities, from Ephedra species collected from the five continents of the world. The ephedrine alkaloids (Figure 1) are considered the active constituents of plants belonging to the genus Ephedra. (−)-Ephedrine is the major isomer; the minor alkaloids include (−)-norephedrine, (+)-norpseudoephedrine, (+)-pseudoephedrine, and (−)-methylephedrine [99].
Figure 1.
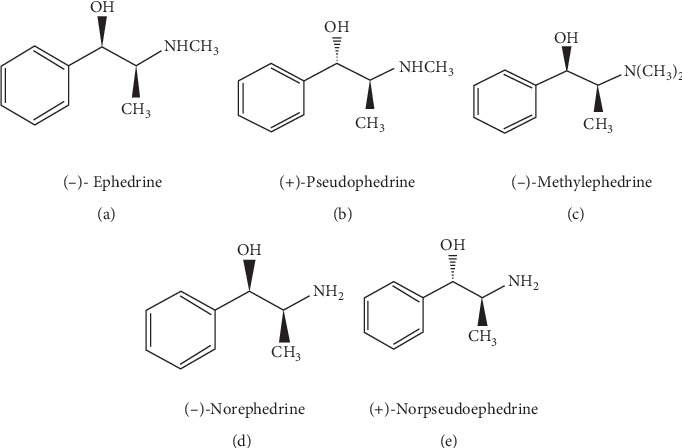
Chemical structures of ephedrine alkaloids.
As a summary, Table 1 shows some of the most commonly used methods to identify and quantify phenolic compounds (chromatographic conditions; mobile phase and gradient, quantification and detection, and analytical method) from Ephedra species extracts.
Table 1.
Selected high-resolution MS applications in the characterization of Ephedra species compounds (published in the period 2015–2019).
| Source | Part | Solvent | Analyte | Mobile phase and gradient program | Analytical method | Detection (nm) | Reference |
|---|---|---|---|---|---|---|---|
| Tunisia | Aerial parts of E. alata Decne | 70% EtOH | Gallic acid, protocatechuic acid, caffeic acid, epicatechin, p-coumaric acid, ferulic acid, rutin, rosmarinic acid, resveratrol, quercetin and kaempferol | A: water acidified with formic acid at pH 3; B: acetonitrile acidified with formic acid at pH 3 : 0.01–20 min, 5% B; 20.01–50 min, 5–40% B; 50–55 min, 40–95% B; and 55–60 min 95% B | LC-MS | 280–320 | [90] |
| Aerial parts of E. alata | EtOH/water (50 : 50 v/v) | Quinic acid, gallic acid, 4-O-caffeoylquinic acid, syringic acid, p-coumaric acid, trans-ferulic acid, catechin, epicatechin, rutin, quercitrin (quercetin-3-O-rhamnoside), apigenin-7-O-glucoside, kaempferol, naringenin, luteolin, cirsilineol | The mobile phase was composed of A (0.1% formic acid in H2O, v/v) and B (0.1% formic acid in methanol, v/v): linear gradient elution: 0–45 min, 10–100% B; 45–55 min, 100% B | LC-ESI/MS/MSn | 280 | [23] | |
| Aerial parts of E. alata | 70% MeOH then fractionation with hexane, DCM, EAc BuOH, and water | Quinic acid (1), gallic acid (2), protocatechuic acid (3), chlorogenic acid (3-O-caffeoylquinic acid) (4), caffeic acid (5), syringic acid (6), p-coumaric acid (7), trans-ferulic acid (8), o-coumaric acid (9), transcinnamic acid (10), 4-O-caffeoylquinic acid (11), 1,3-di-O-caffeoylquinic acid (12), 3,4-di-O-caffeoylquinic acid (13), 4,5-di-O-caffeoylquinic acid (14), rosmarinic acid (15), salvianolic acid (16), (+)-catechin (17), (−)-epicatechin (18), acacetin (19), apigetrin (apigenin-7-O-glucoside) (20), apigenin (21), quercitrin (quercetin-3-o rhamnoside) (22), kaempferol (23), cirsilineol (24), cirsiliol (25), hyperoside (quercetin-3-O-galactoside) (26), cynaroside (luteolin-7-O-glucoside) (27), luteolin (28), Naringenin (29), naringin (naringenin-7-O-rutinoside) (30), quercitrin (quercetin-3-O-rhamnoside) (31), rutin (quercetin-7-O-rutinoside) (32), and silymarin (33) |
The mobile phase: A (0.2% acetic acid in 95% water and 5% MeOH) and B (0.2% acetic acid in 50% water and 50% acetonitrile) with a linear gradient elution: 0–45 min, 10–20% B; 45–85 min, 20–55% B; 85–97 min, 55–100% B; 97–110 min, 100% B; the initial conditions were held for 10 min as a re-equilibration step | HPLC-PDA-ESI/MS | (1) 240; (2) 272–218; (3) 259–294-220; (4)230–280; (5) 327–245-295; (6) 327–295-245; (7) 324–295-220; (8) 274–220; (9) 322–302–245–218; (10) 230–279; (11) 309–229-298; (12) 322–240-295; (13) 255–356; (14) 355–256; (15) 275–325-230; (16) 347–253-267; (17) 329–295–245–221; (18) 329–290-245; (19) 329–290-245; (20) 212–226–282–328; (21) 336–67; (22) 325–295–245–221; (23) 287–254–309–228; (24) 275–222-215; (25) 275–365; (26) 254–290- 366; (27) 228–288-332; (28) 337–267-225; (29) 287–231; (30) 347–253-266; (31) 344–273-225; (32) 343–247-225; and (33) 331–268 | [45] | |
| Algeria | Whole plant E. alata Decne ssp. alenda | - Infusion - Decoction - 80% EtOH |
10 phenolic compounds: 2 myricetin-C-hexoside isomers (1 and 2); biochanin A 7-O-glucoside (Sissotrin) (3); 2 hydroxydaidzein-8-C-glucoside isomers (4 and 5); 5,5′-dihydroxy-methoxy-isoflavone-O-glucoside (6); hydroxydaidzein-8-C-glucoside isomer (7); quercetin-3-O-rutinoside (8); isorhamnetin-3-O-glucoside (9); and kaempferol-O-di-deoxyhexoside (10) | (A) 0.1% formic acid in water, and (B) acetonitrile: 15% B (0–5 min), 15%–20% B (5–10 min), 20–25% B (10–20 min), 25–35% B (20–30 min), and 35–50% B (30–40 min) | LC-DAD-ESI/MSn | (1) 291, 340; (2) 290, 340 (3) 255, 320; (4) 262,340 (5) 262,340 ; (6) 263,336 (7) 262,340 ; (8) 351 (9) 368; and (10) 263,348 |
[100] |
| Austria | Aerial parts of E. sinica | PET, DCM, EtOAc n-BuOH, EtOH, MeOH, or water | Epigallocatechin-4β-benzylthioether, epigallocatechin-4-benzylthioether stereoisomers, and epicatechin-4β-benzylthioether |
A = water, B = acetonitrile. 0 min: 35% B, 20 min: 35% B, 30 min: 45% B, 40 min: 45% B, 45 min: 98% B60 min: S top; post- time 15 min |
HPLC and HPLC-MS | 254 | [101] |
| Austria/Germany | Aerial parts of 8 Ephedra spp. | HCl (6.2%, v/v) | Ephedrine and pseudoephedrine | Acetonitrile, tetrahydrofuran, and water (38 : 5:57, v/v/v) | UPLC-UV | 208 | [102] |
| Palestine | Aerial parts of E. alata | EtOH, EtOH (80%), and water | Luteolin-7-O-glucuronide, myricetin-3-rhamnoside | The start was a 100% (A) that descended to 70% (A) in 40 minutes, then to 40% (A) in 20 minutes, and finally to 10% (A) in 2 minutes and stayed there for 6 minutes and then back to the initial conditions in 2 minutes | HPLC/PDA and HPLC/MS | 350 | [41] |
| Iran | Green stems from E. major | MeOH (80%) | Ephedrine | A mixture of 0/1% phosphoric acid (pH 4), 25 mM SDS, and 40% acetonitrile (10 : 1 v/v) | HPLC | 210 | [42] |
| Pakistan | Aerial plant of E. intermedia | 70% EtOH and MeOH 70% | Ephedrine and pseudoephedrine | Buffer solution of H3PO4 at 0.25 M (pH 5.3), methanol, and acetonitrile in ratio 1 : 1: 8 | HPLC | 210 | [103] |
| Korea | Aerial parts of E. intermedia | 30% EtOH | Ephedrine and pseudoephedrine | 60% solvent A (0–25 min), 60–40% solvent A (25–35 min), 40% solvent A (35–40 min), 40–20% solvent A (40–50 min), and 20% solvent A (50–60 min) | HPLC-UVD | 210 and 254 | [104] |
| E. sinica | Distilled water for 22 h at 95 °C | Ephedrine (1), pseudoephedrine (2), rhein (3), aloe-emodin (4), emodin (5), chrysophanol (6), and physcion (7) | -For (1) and (2) the mixtures of HPLC-grade H2O buffered with 25 mM sodium dodecyl sulfate (solvent A) and acetonitrile (AcCN, solvent B) -For (3), (4), (5), (6), and (7) the mixtures of H2O, AcCN, and phosphoric acid (850 : 150 : 1) for 20 min - For (1) and (2): 60% solvent A for 40 min |
HPLC | (1) 215; (2) 215 (3) 254; (4) 254 (5) 254; (6) 254, and (7) 254 |
[105] | |
| Stems of E. intermedia | 70% EtOH | Ephedrine, pseudoephedrine, N-methylephedrine, N-methylpseudoephedrine, norephedrine, and norpseudoephedrine | Isocratic gradient 25 mM SDS in water (A) and acetonitrile (B) | HPLC-UV | 215 | [106] | |
| Japan | E. sinica | Water at 95°C | Syringin; kaempferol 3-O-rhamnoside 7-O-glucoside; isovitexin 2″-O-rhamnoside; cinnamic acid; 6-hydroxykynurenic acid; 6-methoxykynurenic acid | 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B): 5% B (0–10 min), 5–75% B (10–70 min), 75–100% B (70–80 min), 100% B (80–90 min) | LC-PDA | 210 | [107] |
| E. sinica | Hot water at 95 °C for 1 h | Vicenin-2 and isovitexin 2″-O-rhamnoside | 0.1% formic acid (HCOOH) in water (A) − 0.1% HCOOH in MeOH (B) 5% B (0 min) ⟶ 50% B (40 min) ⟶ 100% B (50 min) ⟶ 100% B (55 min) ⟶ 5% B (55.1 min) ⟶ 5% B (60 min) | LC/Orbitrap MS | 200–400 | [108] | |
| Taiwan | Aerial parts of Ephedra | Boiling | Ephedrine, amygdalin, glycyrrhizic acid, and carvedilol | 5 mM NH4CH3CO2 (0.1% formic acid) as the aqueous phase (A) and 100% methanol (0.1% formic acid) as the organic phase (B); 20–70% B at 0–1 min, 70–90% B at 1–4 min, 90% B at 4–9 min, 90–20% B 9–10 min, 20% B at 10–13 min | UHPLC–MS/MS | [109] | |
| China | Aerial parts of Ephedra | Water | Norephedrine, norpseudoephedrine, ephedrine, pseudoephedrine, and methylephedrine | A mixture of KH2PO4 (20 mmol/L)-acetonitrile (96 : 4, v/v) | HPLC | 210 | [110] |
| Ephedra herb | ACN-ammonium acetate | Methylephedrine, ephedrine, and pseudoephedrine | Acetonitrile-ammonium acetate (pH 5.0; 0.195 M) (95 : 5, v/v) | HPLC | 208 | [111] | |
| Stems of E. sinica | EtOH, EtOAc, and BuOH | (S)–N-((1R,2S)-1-hydroxy-1-phenylpropan-2-yl)-5-oxopyrrolidine-2-carboxamide (1) and (3R)-3-O-β-D-glucopyranosyl-3-phenylpropanoic acid (2) | ∗CH3OH/H2O (23%, v/v) (1) ∗ 25% MeOH in H2O, containing 0.1% formic acid (2) |
LC/MSD | 280 | [112] |
A phytochemical characterization of the hydroalcoholic (70% EtOH) extract of the aerial part of Tunisian E. alata Decne was reported by Danciu et al. [90]. Using LC-MS, detected individual polyphenols were gallic acid, protocatechuic acid, caffeic acid, coumaric acid, ferulic acid, rosmarinic acid, epicatechin, rutin, resveratrol, quercetin, and kaempferol. Under the same operating conditions, individual polyphenols were determined using two different C18 chromatographic columns: Adsorbosphere UHS C18 and EC 150/2 NUCLEODUR C18 Gravity SB. On both columns, identified compounds were rosmarinic acid (0.013 µg/mg), resveratrol (0.223 µg/mg), quercetin (2.63 µg/mg), and kaempferol (15.55 µg/mg). Caffeic acid and p-coumaric acid were identified in small quantities, respectively, at 0.014 and 0.05 µg/mg. These compounds were identified only on the Adsorbosphere UHS C18 column, while epicatechin was identified on the NUCLEODUR C18 Gravity SB column. Using liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS) analysis, Benabderrahim et al. [23] determined that Tunisian E. alata ethanol (50%) extracts, collected from Saharan regions of South Tunisia, showed medium levels of quinic acid, p-coumaric acid, epicatechin, rutin, luteolin, and cirsilineol. Extracted by LC/PDA/ESI (−)/MS method, 24 phenolic compounds were found in the hydromethanol E. alata crude extract [45]. These phenolics were 10 phenolic acids (quinic acid, gallic acid, protocatechuic acid, chlorogenic acid, 4-O-caffeoylquinic acid, syringic acid, caffeic acid, p-coumaric acid, trans-ferulic acid, and trans-cinnamic acid); 5 flavones (apigenin, luteolin, cirsiliol, cirsilineol, and acacetin); 2 flavonols (quercetin and kaempferol); 2 flavan-3-ols ((+)-catechin and epicatechin); 2 flavonol glycosides (rutin and quercitrin); 2 flavone glycosides (apigenin-7-O-glucoside and naringin); and 1 flavanone (naringenin). For derivative fractions of MeOH crude extract, 19 phenolic compounds were detected in the EAc and BuOH, whereas 18 compounds were identified in the water and only 14 compounds were detected in the DCM. The EAc and the BuOH contained almost the same detected compounds (18 compounds among 19 identified). The main phenolic compounds in the EAc were (−)-epicatechin (5864.24 μg/g dry extract (DE), quercetin-3-O-rhamnoside (3647.49 μg/g DE), and (+)-catechin (3289.03 μg/g DE). The principle phenolic compounds identified in the BuOH were quinic acid (847.79 μg/g DE), naringin (682.98 μg/g DE), (−)-epicatechin (363.15 μg/g DE), quercetin-3-O-rhamnoside (309.59 μg/g DE), (+)-catechin (201.0915 μg/g DE), and apigenin-7-O-glucoside (99.51 μg/g DE). In the aqueous fraction, the major phenolic compounds were quinic acid (2533.89 μg/g DE), naringin (230.34 μg/g DE), trans-cinnamic acid (71.87 μg/g DE), and syringic acid (46.73 μg/g DE). The most important phenolic compounds detected in DCM were trans-cinnamic acid, naringin, and trans-ferulic acid detected, respectively, at 2064.35, 1920.11, and 1406.31 μg/g DE. Ziani et al. [100] reported that ten phenolic compounds, five isoflavones, and five flavones were characterized and performed by applying LC-DAD-ESI/MSn to three different extracts obtained from infusion, decoction, and maceration in hydroethanolic mixtures of Algerian E. alata.
Collected from Austria, dry herbs of E. major, E. distachya subsp. Helvetica, E. monosperma, E. fragilis, E. foeminea, E. alata, E. altissima, and E. foliate were used to separate and quantify ephedrine (E) and pseudoephedrine (PE) by UPLC-UV [102]. Using 5 ng as a limit of detection, among the analyzed species, E is the dominant alkaloid in E. major, E. fragilis, and E. distachya subsp. Helvetica. E. monosperma was the only species with a higher PE content. E and PE were not detected in E. foeminea.
Palestinian E. alata extracted with water, 80% ethanol, and 100% ethanol was rich in flavonoid glycosidic compounds. In fact, the full scanned LC-MS using the positive and negative electrospray ionization modes revealed the presence of luteolin-7-O-glucuronide flavonoid, myricetin 3-rhamnoside, and some other major polyphenolic compounds that share myricetin skeleton [41] Collected from Bojnoord (Iran), stems and seeds of E. major were soaked in 80% MeOH, and the amounts of ephedrine were determined by HPLC [42]. Data from HPLC analysis revealed that while root is depleted of ephedrine, the ephedrine amount in stem organ ranged from 1.50 to 2.12 mg/g dry weight. To assess the alkaloids present in Pakistani E. intermedia, the HPLC method was used for the quantitative analysis of ephedrine and pseudoephedrine [103]. This study showed that average alkaloid substance in E. intermedia was as follows: pseudoephedrine (0.209%, 0.238%, and 0.22%) and ephedrine (0.0538%, 0.0666%, and 0.0514%).
Hyuga et al. [107] described the preparation of an ephedrine alkaloids-free Japanese Ephedra herb extract (EFE) by ion-exchange column chromatograph. In this study, LC-PDA analysis of aqueous Ephedra herb extract and EFE was used. In fact, the Ephedra herb extract standard revealed the presence of ephedrine alkaloids (ephedrine, pseudoephedrine, norephedrine, and methylephedrine), 6-hydroxykynurenic acid, syringin, kaempferol 3-O-rhamnoside-7-O-glucoside, 6-methoxykynurenic acid, isovitexin 2″-O-rhamnoside, and cinnamic acid. However, ephedrine alkaloids, 6-hydroxykynurenic acid, and 6-methoxykynurenic acid were not present in the EFE chromatogram. Later, in 2018, Oshima et al. [108] analyzed Ephedra herb extracts grown in different habitats and collection years in Japan by liquid chromatography/high-resolution mass spectrometry (LC/HRMS). These authors detected two notable peaks common to each extract. These peaks were identified as vicenin-2 (1) and isovitexin 2″-O-rhamnoside (2). Quantitative analyses using the isocratic condition of LC/MS showed that the content percentages of 1 and 2 in EFE were 0.140–0.146% and 0.350–0.411%, respectively. Furthermore, Oshima et al. [108] analyzed apigenin (3), an aglycon common to 1 and 2. In 2016, Mei et al. [110] reported that the HPLC analysis determined five activity components of Ephedra-Gypsum extract. They were norephedrine (NE), norpseudoephedrine (NPE), ephedrine (E), pseudoephedrine (PE), and methylephedrine (ME) with contents of 0.143, 0.065, 1.723, 0.794, and 0.165 mg/g, respectively. A rapid hydrophilic interaction liquid chromatographic (HILIC) method has been developed and validated for simultaneous quantitative analysis of methylephedrine, ephedrine, and pseudoephedrine in Chinese Ephedra herb and its preparations [111]. The chromatographic method was validated for specificity, linearity and range, limit of detection and quantification, precision, stability, repeatability, and accuracy. The main parameters were specificity (peak purity match factors were >980), linearity (r > 0.9996), intra- and interday precisions (RSD%: 0.48–1.70 and 0.81∼1.86, respectively), and limit of detection and quantifications (29.49 and 98.31 ng/mL for methylephedrine; 47.74 and 159.1 ng/mL for ephedrine; 121.8 and 406.0 ng/mL for pseudoephedrine). On the other hand, two new compounds of phenylpropanoids, (S)–N-((1R,2S)-1-hydroxy-1-phenylpropan-2-yl)-5-oxopyrrolidine-2-carboxamide (1) and (3R)-3-O-β-d-glucopyranosyl-3-phenylpropanoic acid (2), were isolated from the Chinese E. sinica stems. Their structures were elucidated by in-depth examination of spectroscopic data, mainly including those from the 1D and 2D NMR technique, high-resolution electron spray ionization mass spectrum (HRESIMS) technique, and chemical method [112].
5. Biological Activities
5.1. Antioxidant Activity
The antioxidant activity of Ephedra was evaluated by cupric ion reducing capability in the presence of neocuproine: CUPRAC method, DPPH (2.2-diphenyl-1-picrylhydrazyl), ABTS (2.2′-azino-Bis(3-ethylbenzothiazoline-6-sulphonic acid), TAC (total antioxidant capacity), FRAP (ferric-reducing antioxidant), reducing power assay, β-carotene bleaching inhibition, ferrous ion chelating, hydroxyl radical, hydrogen peroxide scavenging activity, and metal chelating activity. The antioxidant activities of Ephedra reported in the literature was illustrated in Table 2. Among these studies, Danciu et al. [90] showed that the Tunisian aerial parts of E. alata Decne, extracted with EtOH 70%, have an important antioxidant activity (CUPRAC) which is around 7453.18 µmol Trolox/g. Also, Benabderrahim et al. [23] found that the antioxidant contents, expressed by DPPH and ABTS, of EtOH/water (v/v) extracts of E. alata Decne were, respectively, 33.51 ± 0.05 mg TEAC/100 g and 37.86 ± 0.03 mg TEAC/100 g. Mighri et al. [45] showed that the chloroform fraction of Tunisian aerial parts of E. alata exhibited the highest antioxidant activity (TAC and DPPH) compared to methanol extract and butanol, ethyl acetate, and water fractions. The authors of this study reported that, compared with the methanol extract, butanol, aqueous, and chloroform fractions, ethyl acetate showed higher FRAP activity (21.36 mM TEq/g). From Algerian E. alata Decne ssp. alenda, Ziani et al. [100] found that the EtOH/water extract displayed the highest DPPH, reducing power, and β-carotene bleaching inhibition.
Table 2.
Antioxidant activity of Ephedra species (published in the period 2015–2019).
| Source | Part | Extraction | Method | Activity | References | |
|---|---|---|---|---|---|---|
| Tunisia | Aerial parts of E. alata Decne | EtOH 70% | CUPRAC | 7453.18 ± 2.5 µmol trolox/g | [90] | |
| Aerial parts of E. alata | EtOH/water (v/v) | DPPH | 33.51 ± 0.05 mg TEAC/100 g | [23] | ||
| ABTS | 37.86 ± 0.03 mg TEAC/100 g | |||||
| Aerial parts of E. alata | MeOH 70% (I); CHCL3 (II)EtOAc (III); BuOH (IV); and water (V) | TAC | (I) | 125.50 ± 3.50 mg aa eq/g | [45] | |
| (II) | 221.71 ± 8.90 mg aa eq/g | |||||
| (III) | 145.71 ± 13.1 mg aa eq/g | |||||
| (IV) | 130.29 ± 2.60 mg aa eq/g | |||||
| (V) | 56.29 ± 4.50 mg aa eq/g | |||||
| DPPH | (I) | 0.330 ± 0.004 mg/mL | ||||
| (II) | 0.454 ± 0.008 mg/mL | |||||
| (III) | 0.180 ± 0.002 mg/mL | |||||
| (IV) | 0.176 ± 0.002 mg/mL | |||||
| (V) | - | |||||
| FRAP | (I) | 10.38 ± 0.04 mM TEq/g | ||||
| (II) | 18.32 ± 0.07 mM TEq/g | |||||
| (III) | 21.36 ± 0.04 mM TEq/g | |||||
| (IV) | 4.14 ± 0.03 mM TEq/g | |||||
| (V) | 0.82 ± 0.02 mM TEq/g | |||||
| Algeria | Whole plant of E. alataDecne ssp. alenda | Water (boiling) | DPPH (EC50) | 450 ± 7 μg/mL | [100] | |
| Reducing power (EC50) | 108 ± 1 μg/mL | |||||
| β-carotene bleaching inhibition | 131 ± 1 μg/mL | |||||
| Water (decoction) | DPPH (EC50) | 455 ± 6 μg/mL | ||||
| Reducing power (EC50) | 109 ± 3 μg/mL | |||||
| β-Carotene bleaching inhibition (EC50) | 173 ± 3 μg/mL | |||||
| EtOH/water | DPPH (EC50) | 540 ± 3 μg/mL | ||||
| Reducing power (EC50) | 377 ± 4 μg/mL | |||||
| β-carotene bleaching inhibition (EC50) | 502 ± 8 μg/mL | |||||
| Jordan | Stems of E. alata | Petroleum ether and MeOH | DPPH (IC50) | 66.4 ± 0.55 μg/mL | [95] | |
| ABTS (IC50) | 50.2 ± 1.2 μg/mL | |||||
| Ferrous ion (Fe2+) chelating | 77.1 ± 1.1 μg/mL | |||||
| (IC50) | ||||||
| Hydroxyl radical (IC50) | 43.5 ± 1.14 μg/mL | |||||
| Saudi Arabia | Ripe fruits of E. foeminea | MeOH | DPPH (1 mg/mL) | 68% | [96] | |
| Total antioxidant activity | 60% | |||||
| Hydrogen peroxide scavenging activity (1 mg/mL) | 68% | |||||
| Palestine | Leaves of E. alata | MeOH | DPPH (IC50) | 15.85 μg/mL | [113] | |
| Aerial parts of E. alata | MeOH | DPPH (100 μg/mL) | 75.02 ± 1.67% | [114] | ||
| Lebanon | Fresh stems of E. campylopoda | Water | DPPH(IC50) | 300 ± 4.4 μg/mL | [97] | |
| Metal chelating activity (IC50) | >1.5 mg/mL | |||||
| EtOH | DPPH (IC50) | 125 ± 4.4 μg/mL | ||||
| Metal chelating activity (IC50) | >1.5 mg/mL | |||||
| MeOH | DPPH (IC50) | 150 ± 5.1 μg/mL | ||||
| Metal chelating activity (IC50) | 1 ± 1.2 mg/mL | |||||
| Pakistan | Root and stem of E. gerardiana | MeOH | DPPH (root) (IC50) | 14.94 ± 3.54 μg/mL | [115] | |
| Water | DPPH (stem) (IC50) | 3.44 ± 0.69 μg/mL | ||||
| n-Hx | DPPH (roots) (IC50) | 21.49 ± 6.26 μg/mL | ||||
| DPPH (stem) (IC50) | 13.92 ± 6.04 μg/mL | |||||
| CHCl3 | DPPH (roots) (IC50) | 6.38 ± 1.59 μg/mL | ||||
| DPPH (stem) (IC50) | 22.73 ± 6.92 μg/mL | |||||
| EtOAc | DPPH (roots) (IC50) | 2.96 ± 0.39 μg/mL | ||||
| DPPH (stem) (IC50) | 2.73 ± 0.84 μg/mL | |||||
| n-BuOH | DPPH (roots) (IC50) | 13.74 ± 2.71 μg/mL | ||||
| DPPH (stem) (IC50) | 2.69 ± 0.26 μg/mL | |||||
| Aerial parts of Ephedra | EtOH/MeOH/water | DPPH (100 μg/mL) | 90.08 ± 1.37% | [103] | ||
| Korea | Stem of E. sinica | EtOH | DPPH (1 mg/mL) | 75% | [116] | |
| ABTS (1 mg/mL) | 80% | |||||
| Chile | Aerial parts of Ephedra chilensis | n-Hx | DPPH (IC50) | 13.77 ± 0.37 mg/mL | [94] | |
| FRAP | 3.90 ± 0.20TEAC mM | |||||
| TRAP | 0.28 ± 0.05TEAC mM | |||||
| CH2Cl2 | DPPH (IC50) | 3.02 ± 0.02 mg/mL | ||||
| FRAP | 21.05 ± 0.18TEAC mM | |||||
| TRAP | 1.40 ± 0.07TEAC mM | |||||
| EtOH | DPPH (IC50) | 0.68 ± 0.01 mg/mL | ||||
| FRAP | 24.00 ± 0.43TEAC mM | |||||
| TRAP | 1.53 ± 0.06TEAC mM) | |||||
| Leaves and stems of E. chilensis | EtOH | DPPH (1 mg/mL) | 82% | [117] | ||
From northern Jordan, the in vitro antioxidant activities of the butanolic extract from the stem of E alte were assessed against DPPH, ABTS, and hydroxyl radicals [95]. In fact, butanolic extract showed different levels of radicals scavenging activity in a dose-dependent manner over the range of 5–500 μg/mL concentration, indicating the high antioxidative capacity of the extract. The IC50 values of the extract were 66.4, 50.2, 43.5, and 77.1 μg/mL for DPPH, ABTS, hydroxyl radicals, and the ferrous ion chelating activity, respectively. Hegazy et al. [96] evaluated the antioxidant activities of the five wild underutilized fruits in the mountains of southwest Saudi Arabia (Coccinia grandis (L.) Voigt, Diospyros mespiliformis Hochst. ex A. DC., Cissus rotundifolia (L.), E. foeminea Forssk., and Grewia villosa Willd.). Corresponding to this study, methanol extract of E. foeminea displayed antioxidant activity higher than 50% even at low concentration of 0.6 mg/mL. Shawarb et al. [113] and Jaradat et al. [114] found that the leaves of Palestinian E. alata showed a good antioxidant activity. This activity was evaluated by the free radical scavenging as 15.85 μg/mL (IC50) and 75.02% at 100 μg/mL, respectively. Kallassy et al. [97] evaluated the antioxidant capacity of various solvent (distilled water, ethanol, and methanol) extracts of Lebanese E. campylopoda stems. The different extracts showed varying antioxidant potential, and their DPPH scavenging capacities were in the following order: ethanolic extract (IC50 = 125 μg/mL) >methanolic extract (IC50 = 150 μg/mL) >aqueous extract (IC50 = 300 μg/mL). On the other hand, this study showed that methanolic extract had the most efficient Fe2+ chelating capacity (IC50 = 1 mg/mL) in comparison to both the ethanolic and the aqueous extracts, which presented IC50 values of more than 1.5 mg/mL.
Khan et al. [115] reported that the ethyl acetate fraction of Pakistani E. gerardiana (root and stem) presented more significant free radical scavenging potential than the methanol extract, chloroform, n-hexane, and n-butanol fractions, and the mean values ranged, respectively, from 21.49 to 2.96 μg/mL. It should be noted that the stem of E. gerardiana gave the maximum antioxidant yield and chloroform gave the lowest one (IC50 = 22.73 μg/mL). Extracted by solvent mixture of ethanol/methanol/water of aerial parts of Pakistani E. intermedia, the antioxidant activity, tested by DPPH radical procedure, was 90.08 at 100 μg/mL [103].
Mellado et al. [94] studied the antioxidant (DPPH, FRAP, and TRAP assays) activity of Chilean E. chilensis K. The DPPH assay showed that the hexanoic extract had a poor activity (P < 0.05) compared with the positive controls (trolox and gallic acid). Dichloromethane (CH2Cl2) and ethanolic extracts showed similar activities, and these activities are different from the activities of trolox and gallic acid (P < 0.05). For the FRAP assay, the CH2Cl2 and EtOH extracts show better antioxidant activity than the positive controls (P < 0.05). Concerning the TRAP assay, Hex extract was the least active of all of the tested extracts compared with the positive controls (gallic acid and BHT) with significant differences (P < 0.05).
5.2. Antimicrobial Activity
Antimicrobial efficacy of Ephedra species extracts has been described in several studies using in vitro methods such as agar disc diffusion assays and/or minimum inhibitory concentration (MIC). The in vitro antimicrobial activity against a number of pathogenic and drug-resistant bacteria and fungi is presented in Table 3. Using both in vitro agar diffusion and MIC (minimum inhibitory concentration) Danciu et al. [90] showed that the hydroalcoholic extract of E. alata Decne, collected from Djerba (Tunisia), had a bactericidal effect against Staphylococcus aureus ATCC 25923 and Enterococcus faecalis ATCC 51299 and fungicide impacts on Candida albicans ATCC 10231 and Candida parapsilosis ATCC 22019. Palici et al. [118] studied the antibacterial activity of ethanol/water extract of E. alata var. alenda, collected from Tunisian region of Sahara. This studied extract demonstrated a notable inhibition against methicillin-resistant Staphylococcus aureus (MRSA) ATCC 29213. Other authors demonstrated that the decoction and infusion of hydroethanolic extract of E. alata exhibited a MIC value of 5 mg/mL against methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) [100]. In this study, infusions and decoctions had a weak effect, except against E. coli strains, which was the most susceptible microorganism with a MIC value of 0.625 mg/mL. On the contrary, high antibacterial and antifungal effects of this plant were previously reported in extracts prepared with water, methanol, and acetonitrile, with the latter exhibiting the most potent effect against all the microorganisms supplied by the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt [121].
Table 3.
Antimicrobial activity extract from different species of the worldwide genus of Ephedra.
| Source | Part | Extraction | Target microorganism | Activity | References | |
|---|---|---|---|---|---|---|
| MIC | IZ (mm) | |||||
| Tunisia | Aerial parts of E. alata Decne | EtOH 70% | Klebsiella pneumonia ATCC 700603 | 200 μg/mL | 7 | [90] |
| Shigella flexneri ATCC 12022 | 200 μg/mL | 7 | ||||
| Salmonella enterica ATCC 14028 | 200 μg/mL | 7 | ||||
| Escherichia coli ATCC 25922 | 200 μg/mL | 7 | ||||
| Pseudomonas aeruginosa ATCC 27853 | 200 μg/mL | 7 | ||||
| Staphylococcus aureus ATCC 25923 | 50 μg/mL | 9 | ||||
| Enterococcus faecalis ATCC 51299 | 100 μg/mL | 7 | ||||
| Candida albicans ATCC 10231 | 50 μg/mL | 10 | ||||
| Candida parapsilosis ATCC 22019 | 50 μg/mL | 10 | ||||
| Aerial parts of E. alata var. alenda | EtOH–water (1 : 1) - |
50 mg/mL | 9.5 mm | [118] | ||
| Bacillus subtilis ATCC 6633 | ||||||
| Moraxella catarrhalis ATCC 25238 | - | 7.5 mm | ||||
| Methicillin-resistant Staphylococcus aureus ATCC 43300 | >5 | 14.5 mm | ||||
| Staphylococcus aureus ATCC 29213 | - | 9.5 mm | ||||
| Algeria | Whole plant of E. alata Decne ssp. alenda | Water (infusion | Escherichia coli ESBL | 20 mg/mL | [100] | |
| Escherichia coli | 20 mg/mL | |||||
| Klebsiella pneumoniae ESBL | 20 mg/mL | |||||
| Klebsiella pneumoniae | 20 mg/mL | |||||
| Morganella morganii | 20 mg/mL | |||||
| Pseudomonas aeruginosa | >20 mg/mL | |||||
| Enterococcus faecalis | 20 mg/mL | |||||
| Listeria monocytogenes | 20 mg/mL | |||||
| Methicillin-resistant S. aureus | 10 mg/mL | |||||
| Methicillin-susceptible Staphylococcus aureus | 10 mg/mL | |||||
| Water (decoction) | Escherichia coli ESBL | 20 mg/mL | ||||
| Escherichia coli | 20 mg/mL | |||||
| Klebsiella pneumoniae ESBL | 20 mg/mL | |||||
| Klebsiella pneumoniae | 20 mg/mL | |||||
| Morganella morganii | >20 mg/mL | |||||
| Pseudomonas aeruginosa | >20 mg/mL | |||||
| Enterococcus faecalis | 20 mg/mL | |||||
| Listeria monocytogenes | 20 mg/mL | |||||
| Methicillin-resistant S. aureus | 20 mg/mL | |||||
| Methicillin-susceptible Staphylococcus aureus | 20 mg/mL | |||||
| EtOH/H2O | Escherichia coli ESBL | 5 mg/mL | ||||
| Escherichia coli | 5 mg/mL | |||||
| Klebsiella pneumoniae ESBL | 10 mg/mL | |||||
| Klebsiella pneumoniae | 10 mg/mL | |||||
| Morganella morganii | 20 mg/mL | |||||
| Pseudomonas aeruginosa | 20 mg/mL | |||||
| Enterococcus faecalis | 10 mg/mL | |||||
| Listeria monocytogenes | 10 mg/mL | |||||
| Methicillin-resistant S. aureus | 5 mg/mL | |||||
| Methicillin-susceptible Staphylococcus aureus | 5 mg/mL | |||||
| Iran | E. sinica | EtOH | Pseudomonas aeruginosa ATCC 27853 | 12.5 μg/mL | [119] | |
| Pakistan | Aerial parts of E. procera | H2O | B. subtilis ATCC 6633 | 11.33 μg/mL | 15.2 | [93] |
| (5 μL (100 μg/disc)) | P. aeruginosa ATCC 9721 | 100 μg/mL | 11 | |||
| E. coli ATCC 25922 | 11.12 μg/mL | 19.2 | ||||
| S. epidermidis ATCC 12228 | - | - | ||||
| K. pneumoniae ATCC 1705) | 33.3 μg/mL | 14.2 | ||||
| S. aureus ATCC 6538 | - | - | ||||
| A. fumigatus FCBP 66 | 13 | |||||
| A. flavus FCBP 0064 | 14.2 | |||||
| A. Niger FCBP 0198 | 15.8 | |||||
| Mucor spp. FCBP 0300 | 11 | |||||
| Pakistan | Dry stems of E. vulgaris | MeOH | S. pneumoniae | 15.36 | [120] | |
| Pseudomonas aeruginosa | 10.36 | |||||
| Klebsiella pneumoniae | 12.70 | |||||
| EtOH | S. pneumoniae | 15.30 | ||||
| Pseudomonas aeruginosa | 8.70 | |||||
| Klebsiella pneumoniae | 11.60 | |||||
| CHCl3 | S. pneumoniae | 17.16 | ||||
| Pseudomonas aeruginosa | 0 | |||||
| Klebsiella pneumoniae | 12.63 | |||||
| Water | S. pneumoniae | 13.26 | ||||
| P. aeruginosa | 0 | |||||
| K. pneumoniae | 13.70 | |||||
Ethanolic and hydroalcoholic herb extract of Iranian E. sinica was assayed against standard and clinical Pseudomonas aeruginosa, and then the MIC and MBC (minimum bactericidal concentration) were assayed [119]. The results showed the lowest MIC values of ethanolic herb extract were, respectively, 25 and 12.5 μg/mL, but the lowest MIC values of the hydroalcoholic herb extract were 25 and 25 μg/mL, respectively. Equally, the lowest MBC values of ethanolic herb extract on clinical and standard strains of P. aeruginosa were 50 and 25 μg/mL, respectively; however, the lowest MBC values were 25 and 25 μg/mL, respectively. The biosynthesized E. procera nanoparticles (EpNPs) exhibited considerable activity against E. coli ATCC 25922 and B. subtilis ATCC 6633 with MICs of 11.12 μg/mL and 11.33 μg/mL, respectively. Nevertheless, EpNPs showed moderate activity against P. aeruginosa while the S. epidermidis and S. aureus strains were found resistant. Equally, EpNPs showed considerable antifungal activity against A. flavus and A. Niger, but moderate activity against Mucor spp [120]. These authors have proven that EpNPs showed antifungal activity against A. flavus, A. Niger, and Mucor spp. with a diameter of inhibitory zone equal to 14.2, 15.8, and 11 mm, respectively. Four extracts of Pakistani E. vulgaris (CHCl3, MeOH, EtOH, and water) were used against three pathogen bacteria, namely, Streptococcus pneumonia, Pseudomonas aeruginosa, and Klebsiella pneumonia [122]. It was noted from the results that chloroform and aqueous extracts have no inhibition effects against P. aeruginosa. The maximum inhibitory effects (17.16 mm inhibition zone) in chloroform extract against S. pneumoniae and minimum inhibition activities (8.70 mm zone of inhibition) in the extract of ethanol against P. aeruginosa were observed.
6. Biomedicine and Pharmacotherapy Activity
Pharmacological activities of extracts from different species of the worldwide genus Ephedra, published in the period 2015–2019, are well documented in Table 4. The evaluation of the antiproliferative, proapoptotic, and cytotoxic potential against the MCF-7 breast cancer cell line of the ethyl acetate (EA) extract of the aerial part of E. alata Decne was reported by Danciu et al. [90]. In this study, the antiproliferative activity of EA started from a concentration of 10 μg/mL, with a cell growth inhibition of 19.68%. For the highest tested concentration, 30 μg/mL, the growth inhibition percentage was 56.45. The cytotoxicity assessment revealed that the EA manifested a significant difference in cytotoxic potential, displaying a cytotoxicity percentage above 13%. The potential antimigratory activity of the EA extract on MCF-7 human breast adenocarcinoma cells was verified by means of a wound-healing technique. In this regard, on the MCF-7 cells' migration EA had a strong inhibitory effect and showed a wound-healing rate below 5% after an interval of 24 h. The cytotoxicity assessment revealed that the EA extract manifested a significant difference in the cytotoxic potential when compared to the positive control (DMSO), displaying a cytotoxicity percentage of ∼13%. To investigate the apoptotic potential of EA at the selected concentration, MCF-7 cells were treated with 30 μg/mL for 72 h, and the cells' nuclei were analyzed by DAPI (4′,6′-diamidino–2-phenylindole) staining. In this line, Danciu et al. [90] showed that the control cells exhibit a normal organization, with a large, round nucleus, a clear nucleolus, and uniform chromatin density. However, after treatment, the MCF-7 cells manifested morphological changes distinctive for apoptosis induction, such as chromatin condensation.
Table 4.
Pharmacological activity of extracts from different species of the worldwide genus Ephedra (published in the period 2015–2019).
| Source | Part | Extract | Therapy | Model | References |
|---|---|---|---|---|---|
| Tunisia | Aerial part of E. alata | Ethyl acetate | Antiproliferative, proapoptotic, and cytotoxic potential | MCF-7 human breast cancer cells | [90] |
| Palestine | Aerial parts of E. alata | Decoction | Anticancer | 115 breast cancer patients | [114] |
| Jordan | Aerial parts of E. aphylla | MeOH, methanol, CHCl3, EtOAc, n-Hx, and water | Anti-inflammatory | The inhibition of albumin denaturation assay | [122] |
| Antiproliferative | Breast cancer cell lines (T47D, MCF-7) and Vero cell line (African green monkey kidney) | ||||
| Lebanon | Stems of E. campylopoda | EtOH, MeOH and water | Anti-inflammatory | RAW 264.7, a murine monocyte/macrophage cell line | [97] |
| Antiproliferative | Human leukemic T cell line | ||||
| Iran | Stems and leaves of E. sarcocarpa | Water | Anticancer | Human breast adenocarcinoma (MCF-7) and human normal breast epithelial (MCF10A) cell lines | [123] |
| Aerial parts of Ephedra | Water | Antidiabetic and antihyperlipidemic | 40 male BALB/cArc Wistar rats aged eight to ten weeks (200 to 250 g) | [124] | |
| Pakistan | Aerial parts of E. gerardiana | EtOH 70%, EtOAc, n-BuOH, and water | Antiarthritic | -Young and healthy male and female Sprague-Dawley rats -Human red blood cell (HRBC) -Egg albumin -Protein (BSA) |
[125] |
| Korea | Stem of E. sinica | Water | Antineuroinflammatory | Mouse primary microglia and immortal BV-2 mouse microglial cells | [116] |
| Dried stems and leaves of E. sinica Stapf., E. intermedia Schrenk, E. equisetina | MeOH | Antihyperlipidemic | 6-week-old male ICR mice weighing 20 to 25 g | [126] | |
| Japan | Ephedra | Water | Analgesic | Specific pathogen-free ddY mice (5 weeks old, male) | [127] |
| E. sinica | Water | Anti-influenza | Madin–Darby canine kidney (MDCK) cells | [107] | |
| Anticancer | MDA-MB-231 human breast cancer cells | ||||
| Analgesic | ICR male mice (5 weeks of age, 8 mice per group) | ||||
| E. sinica | Water | Antiproliferative | H1975 non-small-cell lung cancer (NSCLC) cell line | [128] | |
| Taiwan | Ephedra | Water | Antipyretic | Male Sprague-Dawley rats (200–250 g) | [129] |
| Antitussive | The eligible Guinea pigs | ||||
| China | Ephedra | Water | Antipyretic and antiasthmatic | Male Wistar rats (6 weeks old, 160–200 g, license number: SCXK 2011–0015), male SD rats (5 weeks old, 100–150 g, license number: SCXK 2011– 0015) |
[110] |
| Chile | Aerial parts of E. chilensis | Hexane, dichloromethane and EtOH | Antiproliferative | MCF-7 (human breast cancer), HT-29 (human colon cancer), PC-3 and DU-145 (human prostate cancer), and CoN (human colon epithelial cells CCD 841) | [94] |
Jaradat et al. [114] investigated the use of herbal remedies by women living with breast cancer in the West Bank of Palestine by a questionnaire-based cross-sectional descriptive study; the questionnaire was distributed to 115 patients. This study revealed that E. alata was the most commonly used plant species in the treatment of breast cancer. Leaves and seeds of E. alata were the most commonly used parts, and decoction was the most commonly used method of preparation. Jordanian E. aphylla extracts (methanol, chloroform, ethyl acetate, n-hexane, and water) were tested to evaluate antiproliferative potential [122]. The authors observed that all extracts displayed strong antiproliferative potential against the tested cell lines (breast cancer cell lines (T47D, MCF-7) and Vero cell line (African green monkey kidney)). Moreover, E. aphylla extracts showed a little cytotoxicity activity against the Vero normal cell line. The antiproliferative activity of various solvent extracts against MFC7 cell line was in the order of aqueous > methanol > chloroform > ethyl acetate > n-hexane [122].
Kallassy et al. [97] studied the anti-inflammatory and antiproliferative potential of ethanol, methanol, and water extracts of Lebanese E. campylopoda stems. The anti-inflammatory capacity was estimated both by evaluating RAW 264.7 murine macrophage cells-mediated secretion of PGE2 using ELISA technique, and by quantifying the mRNA level of the proinflammatory cytokines (IL-α, IL-β, and IL-6), chemokines (CCL3 and CCL4), and inflammation-inducible COX-2 and iNOS enzymes using quantitative real-time PCR (qRT-PCR). By using the XTT viability assay, the antiproliferative potential of E. campylopoda was determined. This study confirmed that the alcoholic extracts showed more potent anti-inflammatory and antiproliferative capacities than aqueous extract [97]. Hoshyar et al. [123] examined the anticancer effects of E. sarcocarpa on proliferation of breast cancer, MCF-7, and epithelial normal MCF-10A cells. These authors evaluated the effect of E. sarcocarpa aqueous extracts on cell proliferation and investigated the cytotoxic effects at concentrations ranging between 0 and 3 mg/mL on the growth of human breast cancer (MCF-7) and normal mammalian (MCF10-A) cells after different time incubation (0–72 h) using MTT (the methylthiazolyldiphenyl-tetrazolium bromide) assay. This study uncovers that the treatment of MCF-7 cells with the E. sarcocarpa aqueous extract (0.25, 0.5, and 0.75 mg/mL) significantly decreased cell viability and increased cell death percentage by increasing extract concentration after 72 h. Parallel treatments of the normal cells with this extract indicted a much less inhibitory effect on the viability of MCF10-A cells.
Park et al. developed E. sinica Stapf. (ES) extract-capped gold nanoparticles (ES-GNs) and investigated their antineuroinflammatory properties in microglia [116]. For this purpose, antineuroinflammatory properties of ES-GNs on production of proinflammatory mediators (nitric oxide, prostaglandin E2, and reactive oxygen species) and cytokines (tumor necrosis factor-α, IL-1β, and IL-6) in lipopolysaccharide- (LPS-) stimulated microglia were well investigated by ELISA and flow cytometry. In this regard, ES-GNs significantly attenuated LPS-induced production of proinflammatory mediators and cytokines, which was related to suppressed transcription and translation of inducible nitric oxide synthase and cyclooxygenase-2, determined by RT-PCR and western blotting. These authors hypothesized that antineuroinflammatory properties of ES-GNs were mediated by AMP-activated protein kinase and nuclear erythroid 2-related factor 2/antioxidant response element signaling. In 2017, Lee et al. [130] evaluated the effects and molecular targets of methanolic extract of dried stems and leaves of E. sinica Stapf. and E. intermedia Schrenk (EHM) on high-fat diet- (HFD-) induced hyperlipidemic ICR mice. According to these authors, results showed that EHM administration for 3 weeks significantly (P < 0.05) decreased total cholesterol (TC) and triglyceride levels without altering body weight (BW) in mice, and gene expression levels in the livers of EHM-treated mice were restored at 34.0% and 48.4% of those up- or downregulated by hyperlipidaemia, respectively. Hyuga et al. [107] confirmed that ephedrine alkaloids-free Ephedra herb extract (EFE) suppressed hepatocyte growth factor- (HGF-) induced cancer cell motility by preventing both HGF-induced phosphorylation of c-Met and its tyrosine kinase activity. Equally, this study displayed the analgesic effect of EFE and the anti-influenza virus activity by showing inhibition of MDCK cell infection in a concentration-dependent manner. All assessments of toxicity, even after repeated oral administration, suggest that EFE would be a safer alternative to Ephedra herb.
Oshima et al. [108] established a preparation method for EFE : ephedrine alkaloids-free Ephedra herb extract (EH) and revealed its chemical composition, including the content of herbacetin, a flavonoid aglycon. In addition, these authors showed the antiproliferative effects of EFE against the H1975 non-small-cell lung cancer (NSCLC) cell line. It should be noted that the antiproliferative effect of EFE against H1975 cells was comparable to that of EH extract. The Ephedra-Gypsum extracts at test dose (6, 12, 24 g/kg) significantly and dose-dependently attenuated yeast-induced fever in rats. The Ephedra-Gypsum extracts also prolonged the latent period, reduced ovalbumin- (OVA-) induced increases in eosinophils and white blood cell (WBC), and decreased the wet and dry weight ratio of the lungs in the antiasthmatic test.
7. Ephedra Toxicity
Although Ephedra metabolites are naturally occurring alkaloids that can be derived from evergreens worldwide and have been used as medicinals, recent studies reported that ephedrine has various adverse effects on organisms such as hepatitis, angle closure glaucoma, nephrolithiasis, neurodegenerative diseases, and cardiovascular toxicity. Few of these side effects are reversible whereas others are irreversible and may even lead to death [28]. Several recent reviews have documented the dangerous nature of using these “drugs” unsupervised, including multiple deaths, and the FDA is currently reviewing ephedrine's use in the alternative medicine industry. Powell et al. [131] reported the toxicity ephedrine nephrolithiasis in a patient using an energy supplement, Ma Huang extract, which contains ephedrine. Although previously not reported, The Louis C. Herring and Company kidney stone database shows that this is an endemic complication of ephedrine with hundreds of previous episodes.
Nauffal and Gabardi [132] found that the use of 40–3,000 mg/day of E. sinica for several months can cause nephrolithiasis with flank pain, hematuria, and renal dysfunction. On the other hand, recent studies have reported that Ephedra herb containing many pharmacologically active alkaloids, principally ephedrine, has been reported to cause acute hepatitis. In this context, Lee et al. [133] investigated hepatotoxicity and key regulation of mitophagy in ephedrine-treated LX-2 cells. However, mitochondrial swelling and autolysosome were observed in ephedrine-treated cells. Also, ephedrine inhibited mitochondrial biogenesis, and the mitochondrial copy number was decreased. Moreover, antioxidants can serve as therapeutic targets for ephedrine-induced hepatotoxicity [133]. Equally, it is important to note that Ephedra species have been implicated in causing liver injury in case reports [134]. Similarly, speculation has also indicated that the alkaloid ephedrine may be hepatotoxic, based upon case reports, and associated with liver injury [135]. An in vitro assay using human hepatoblastoma cells (HepG2) demonstrated that when Ephedra (Ma Huang) extracts were normalized for their ephedrine content, they displayed greater cytotoxicity relative to ephedrine itself, indicating that there may be other constituents responsible for toxicity [133]. While ephedrine and pseudoephedrine showed cytotoxicity in the HepG2 cell line, the concentrations required (i.e., >300 μg/mL) again indicate that such data are likely irrelevant to in vivo administration.
The reported adverse reactions principally involve the cardiovascular system and are, in general, similar to other sympathomimetics. The most common side effect is hypertension with a risk of hemorrhagic stroke. Also ischemic stroke due to vasoconstriction and likely platelet aggregation can occur after its consumption [136]. Although the risk of hemorrhagic stroke with pseudoephedrine seems to be lower, it can occur and might result in death. The adverse reactions after Ephedra administration can more easily occur when it is used in combination with caffeine. This combination increases the effect of sympathomimetics, and the mechanisms will be discussed later [136].
Ephedrine is also found in dietary supplements that promote short-term weight loss, but those are now illegal in the USA. However, in traditional Chinese medicines that contain ephedrine, it is legal. The ephedrine quantity in dietary supplements taken orally is about 20 mg per serving, and doses are taken up to two to three times per day. It has been shown that labels of dietary supplements do not list ephedrine content or incorrectly report the amount of ephedrine in these products. Ephedrine has been associated with cardiovascular dysfunction such as myocardial infarction, severe hypertension, myocarditis, and lethal cardiac arrhythmias. The typical dose of ephedrine used for bronchodilation is 25–50 mg, but ephedrine doses associated with adverse events were less than this amount [137].
The use of drug-herb interaction causes irreversible neurodegenerative diseases. For example, a 29-year-old male has been injected with an intravenous mixture of pseudoephedrine (extracted from Ephedra), potassium permanganate, and acetylsalicylic acid two to three times a day for 9 years and a half. Throughout these years, the patient developed many symptoms including speech disturbance, inability to walk independently, postural instability, tremor, and dystonia. The patient was diagnosed with manganese toxicity which leads to irreversible neurodegenerative disorder due to the long exposure to E. sinica [28].
8. Conclusion
Ephedra natural products have attracted more and more attention since they can exhibit complementary biological and therapeutic effects against diseases. Historically, Ephedra may even have been diverse and widespread at that time and the corresponding fossils document that Ephedra was already present in the Early Cretaceous. Further testing and development of methods for molecular dating will be needed to clarify conflicts between molecular signals and the fossil record.
In this review, we summarized the chemical components isolated and identified by MS. Further instrument sophistication in coupling several systems such as multidimensional chromatography with NMR and MS in series is already occurring. The prediction of the future for promising approaches involves the application of HPLC with ESI time of flight mass spectrometers and ESI FT ion cyclotron resonance mass spectrometers. An increased emphasis on microcapillary columns with nanotechnology ESI systems driven partly by environmental issues seems inevitable.
Additionally, biopharmacological effects, such as anticancer, anti-inflammatory, antitumor, hepatoprotective, antioxidant, and antimicrobial activities, have been well discussed. The relationship between the Ephedra natural products structure and its pharmacological activity needs to be further studied. In this context, the mechanisms of action of phytochemical Ephedra content can provide guidance for its clinical application.
Acknowledgments
This research was funded by the Tunisian Ministry of Higher Education and Scientific Research (Program contract 2015–2018) of the Laboratory of Microorganisms and Biomolecules (LR15CBS05) of the Center of Biotechnology of Sfax, Tunisia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Anisuzzman M., Hasan M. M., Acharzo A. K., Das A. K., Rahman S. In vivo and in vitro evaluation of pharmacological potentials of secondary bioactive metabolites of Dalbergia candenatensis leaves. Evidence-Based Complementary and Alternative Medicine. 2017;2017:10. doi: 10.1155/2017/5034827.5034827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarfraz I., Rasul A., Jabeen F., et al. Fraxinus: a plant with versatile pharmacological and biological activities. Evidence-Based Complementary and Alternative Medicine. 2017;2017:12. doi: 10.1155/2017/4269868.4269868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aktar K., Foyzun T. Phytochemistry and pharmacological studies ofCitrus macroptera: a medicinal plant review. Evidence-Based Complementary and Alternative Medicine. 2017;2017:7. doi: 10.1155/2017/9789802.9789802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sbhatu D. B., Abraha H. B. Preliminary antimicrobial profile of Solanum incanum L.: a common medicinal plant. Evidence-Based Complementary and Alternative Medicine. 2020;2020:6. doi: 10.1155/2020/3647065.3647065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas N. N., Acharzo A. K., Anamika S., Khushi S., Bokshi B. Screening of natural bioactive metabolites and investigation of antioxidant, antimicrobial, antihyperglycemic, neuropharmacological, and cytotoxicity potentials ofLitsea polyanthaJuss. Ethanolic root extract. Evidence-Based Complementary and Alternative Medicine. 2017;2017:11. doi: 10.1155/2017/3701349.3701349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blowman K., Magalhães M., Lemos M. F. L., Cabral C., Pires I. M. Anticancer properties of essential oils and other natural products. Evidence-Based Complementary and Alternative Medicine. 2018;2018:12. doi: 10.1155/2018/3149362.3149362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agyare C., Akindele A. J., Steenkamp V. Natural products and/or isolated compounds on wound healing. Evidence-Based Complementary and Alternative Medicine. 2019;2019:3. doi: 10.1155/2019/4594965.4594965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arena A. C., Kassuya C. A. L., Fernandes G. S. A., Scarano W. R. Toxic versus therapeutic effects of natural products on reproductive disorders. Evidence-Based Complementary and Alternative Medicine. 2019;2019:2. doi: 10.1155/2019/9791506.9791506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim N., Abdelhalim H., Gavande N., Khan I., Khan H. Natural products as an emerging therapeutic alternative in the treatment of neurological disorders. Evidence-Based Complementary and Alternative Medicine. 2018;2018:1–2. doi: 10.1155/2018/3056847.3056847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabral C., Efferth T., Pires I. M., Severino P., Lemos M. F. L. Natural products as a source for new leads in cancer research and treatment. Evidence-Based Complementary and Alternative Medicine. 2018;2018:2. doi: 10.1155/2018/8243680.8243680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Mao M., Liu S., Xu S., Yang J. A comparative study of bolus norepinephrine, phenylephrine, and ephedrine for the treatment of maternal hypotension in parturients with preeclampsia during cesarean delivery under spinal anesthesia. Medical Science Monitor. 2019;25:1093–1101. doi: 10.12659/msm.914143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di S., Wang Y., Han L., et al. The intervention effect of traditional Chinese medicine on the intestinal flora and its metabolites in glycolipid metabolic disorders. Evidence-Based Complementary and Alternative Medicine. 2019;2019:13. doi: 10.1155/2019/2958920.2958920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsayari A., Almghaslah D., Khaled A., et al. Community pharmacists’ knowledge, attitudes, and practice of herbal medicines in asir region, kingdom of Saudi Arabia. Evidence-Based Complementary and Alternative Medicine. 2018;2018:7. doi: 10.1155/2018/1568139.1568139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilahun M., Etifu M., Shewage T. Plant diversity and ethnoveterinary practices of Ethiopia: a systematic review. Evidence-Based Complementary and Alternative Medicine. 2019;2019:9. doi: 10.1155/2019/5276824.5276824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ninh S. A review on the medicinal plant Dalbergia odorifera species: phytochemistry and biological activity. Evidence-Based Complementary and Alternative Medicine. 2017;2017:27. doi: 10.1155/2017/7142370.7142370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali S. A., Sharief N. H., Mohamed Y. S. Hepatoprotective activity of some medicinal plants in Sudan. Evidence-Based Complementary and Alternative Medicine. 2019;2019:16. doi: 10.1155/2019/2196315.2196315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu C.-l., Li X.-f. A review ofOenanthe javanica(blume) DC. As traditional medicinal plant and its therapeutic potential. Evidence-Based Complementary and Alternative Medicine. 2019;2019:17. doi: 10.1155/2019/6495819.6495819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K., Park K.-I. A review of antiplatelet activity of traditional medicinal herbs on integrative medicine studies. Evidence-Based Complementary and Alternative Medicine. 2019;2019:18. doi: 10.1155/2019/7125162.125162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade-Cetto A., Cruz E. C., Cabello-Hernández C. A., Cárdenas-Vázquez R. Hypoglycemic activity of medicinal plants used among the Cakchiquels in Guatemala for the treatment of type 2 diabetes. Evidence-Based Complementary and Alternative Medicine. 2019;2019:7. doi: 10.1155/2019/2168603.2168603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y.-X., Zhang R.-Q., Rahman K., Cao Z.-X., Zhang H., Peng C. Diverse pharmacological activities and potential medicinal benefits of geniposide. Evidence-Based Complementary and Alternative Medicine. 2019;2019:15. doi: 10.1155/2019/4925682.4925682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsioutsiou E. E., Giordani P., Hanlidou E., Biagi M., De Feo V., Cornara L. Ethnobotanical study of medicinal plants used in central Macedonia, Greece. Evidence-Based Complementary and Alternative Medicine. 2019;2019:22. doi: 10.1155/2019/4513792.4513792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iqbal A., Khera R. A., Hanif M. A., Ayub M. A., Zafar M. N. Medicinal Plants of South Asia. Amsterdam, Netherlands: Elsevier; 2020. Ma-Huang; pp. 479–494. [Google Scholar]
- 23.Benabderrahim M. A., Yahia Y., Bettaieb I., Elfalleh W., Nagaz K. Antioxidant activity and phenolic profile of a collection of medicinal plants from Tunisian arid and Saharan regions. Industrial Crops and Products. 2019;138(111427):1–7. doi: 10.1016/j.indcrop.2019.05.076. [DOI] [Google Scholar]
- 24.Wang Q., Yang Y., Zhao X., et al. Chemical variation in the essential oil ofEphedra sinica from Northeastern China. Food Chemistry. 2006;98(1):52–58. doi: 10.1016/j.foodchem.2005.04.033. [DOI] [Google Scholar]
- 25.Schaneberg B. T., Crockett S., Bedir E., Khan I. A. The role of chemical fingerprinting: application to Ephedra. Phytochemistry. 2003;62(6):911–918. doi: 10.1016/s0031-9422(02)00716-1. [DOI] [PubMed] [Google Scholar]
- 26.Caveney S., Charlet D. A., Freitag H., Maier-Stolte M., Starratt A. N. New observations on the secondary chemistry of world Ephedra (Ephedraceae) American Journal of Botany. 2001;88(7):1199–1208. doi: 10.2307/3558330. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y., Lin L., Ferguson D. K., Wang Y. Macrofossil evidence unveiling evolution of male cones in Ephedraceae (Gnetidae) BMC Evolutionary Biology. 2018;18(1):1–9. doi: 10.1186/s12862-018-1243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Dhamen M., Ahmad R., Ahmad N., Naqvi A. A. Clinical uses and toxicity of Ephedra sinica: an evidence-based comprehensive retrospective review (2004-2017) Pharmacognosy Journal. 2019;11(2):439–444. [Google Scholar]
- 29.Eng Y. S., Lee C. H., Lee W. C., Huang C. C., Chang J. S. Unraveling the molecular mechanism of traditional Chinese medicine: formulas against acute airway viral infections as examples. Molecules. 2019;24(19):3505–3534. doi: 10.3390/molecules24193505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sõukand R., Pieroni A., Biró M., et al. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. Journal of Ethnopharmacology. 2015;170:284–296. doi: 10.1016/j.jep.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Hsu D.-Z., Liu C.-T., Chu P.-Y., Li Y.-H., Periasamy S., Liu M.-Y. Sesame oil attenuates ovalbumin-induced pulmonary edema and bronchial neutrophilic inflammation in mice. BioMed Research International. 2013;2013:7. doi: 10.1155/2013/481827.481827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakur A., Pathak S. R. In Synthesis of Medicinal Agents from Plants. Amsterdam, Netherlands: Elsevier; 2018. Introduction to medicinally important constituent from Chinese medicinal plants; pp. 333–349. [Google Scholar]
- 33.Lee M. The history of Ephedra (ma-huang) Journal of the Royal College of Physicians of Edinburgh. 2011;41(1):78–84. doi: 10.4997/jrcpe.2011.116. [DOI] [PubMed] [Google Scholar]
- 34.Al-Salihi B. Ma huang (ephedrae herba): setting the record straight. Journal of Chinese Medicine. 2016;110:18–30. [Google Scholar]
- 35.White L. B., Foster S. The Herbal Drugstore: The Best Natural Alternatives to Over-the-counter and Prescription Medicines! Newyork, PA, USA: Rodale; 2003. [Google Scholar]
- 36.Lipka A. F., Vrinten C., van Zwet E. W., et al. Ephedrine treatment for autoimmune Myasthenia gravis. Neuromuscular Disorders. 2017;27(3):259–265. doi: 10.1016/j.nmd.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Andraws R., Chawla P., Brown D. L. Cardiovascular effects of Ephedra alkaloids: a comprehensive review. Progress in Cardiovascular Diseases. 2005;47(4):217–225. doi: 10.1016/j.pcad.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Cruz A., Padilla-Martínez I. I., Bautista-Ramirez M. E. Ephedrines as chiral auxiliaries in enantioselective alkylation reactions of acyl ephedrine amides and esters: a Review. Current Organic Synthesis. 2018;15(1):38–83. doi: 10.2174/1570179414666170830125915. [DOI] [Google Scholar]
- 39.Cruz A., Esther Bautista Ramirez M. Ephedrines and their acyclic derivatives. Current Organic Synthesis. 2011;8(6):901–928. doi: 10.2174/157017911804586601. [DOI] [Google Scholar]
- 40.Rayan M., Abu-Farich B., Basha W., Rayan A., Abu-Lafi S. Correlation between antibacterial activity and free-radical scavenging: in-Vitro evaluation of polar/non-polar extracts from 25 plants. Processes. 2020;8(1):117–128. doi: 10.3390/pr8010117. [DOI] [Google Scholar]
- 41.Al-Rimawi F., Abu-Lafi S., Abbadi J., Alamarneh A. A. A., Sawahreh R. A., Odeh I. Analysis of phenolic and flavonoids of wild Ephedra alata plant extracts by LC/PDA and LC/MS and their antioxidant activity. African Journal of Traditional, Complementary and Alternative Medicines. 2017;14(2):130–141. doi: 10.21010/ajtcam.v14i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aghdasi M., Mofid Bojnoordi M., Mianabadi M., Nadaf M. Chemical components of theEphedra majorfrom Iran. Natural Product Research. 2015;30(3):369–371. doi: 10.1080/14786419.2015.1058794. [DOI] [PubMed] [Google Scholar]
- 43.Dehkordi N. V., Kachouie M. A., Pirbalouti A. G., Malekpoor F., Rabei M. Total phenolic content, antioxidant and antibacterial activities of the extract of Ephedra procera fisch. et mey. Acta Poloniae Pharmaceutica Drug Research. 2015;72:341–345. [PubMed] [Google Scholar]
- 44.Parsaeimehr A., Sargsyan E., Javidnia K. A comparative study of the antibacterial, antifungal and antioxidant activity and total content of phenolic compounds of cell cultures and wild plants of three endemic species of Ephedra. Molecules. 2010;15(3):1668–1678. doi: 10.3390/molecules15031668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mighri H., Akrout A., Bennour N., Eljeni H., Zammouri T., Neffati M. LC/MS method development for the determination of the phenolic compounds of Tunisian Ephedra alata hydro-methanolic extract and its fractions and evaluation of their antioxidant activities. South African Journal of Botany. 2019;124:102–110. doi: 10.1016/j.sajb.2019.04.029. [DOI] [Google Scholar]
- 46.Roy M., Datta A. Cancer Genetics and Therapeutics. 2019. Fundamentals of phytochemicals; pp. 49–81. [Google Scholar]
- 47.Alamgir A. N. M. Secondary metabolites: secondary metabolic products consisting of C and H. In Therapeutic Use of Medicinal Plants and their Extracts. 2018;2:165–309. [Google Scholar]
- 48.Bansal V., Kumar P., Tuteja S. K., Siddiqui M. W. Diverse utilization of plant-originated secondary metabolites. In Plant Secondary Metabolites. 2017;3:235–258. [Google Scholar]
- 49.Chebouat E., Gherraf N., Dadamoussa B., Allaoui M., Chirite A., Zellagui A. Chemical composition of the dichloromethane extract of Ephedra alata leaves and flowers. Der Pharmacia Letter. 2016;8(6):10–13. [Google Scholar]
- 50.Kumar A., Irchhaiya R., Yadav A., et al. Metabolites in plants and its classification. World Journal of Pharmaceutical Sciences. 2015;4(1):287–305. [Google Scholar]
- 51.Aydoğan C. Recent advances and applications in LC-HRMS for food and plant natural products: a critical review. Analytical and Bioanalytical Chemistry. 2020;412:1973–1991. doi: 10.1007/s00216-019-02328-6. [DOI] [PubMed] [Google Scholar]
- 52.Ersoy E., Eroglu Ozkan E., Boga M., Mat A. Evaluation of in vitro biological activities of three Hypericum species (H. calycinum, H. confertum, and H. perforatum) from Turkey. South African Journal of Botany. 2020;130:141–147. doi: 10.1016/j.sajb.2019.12.017. [DOI] [Google Scholar]
- 53.Kepceoğlu A., Gündoğdu Y., Ledingham K. W. D., Kilic H. S. Real-Time distinguishing of the xylene isomers using photoionization and dissociation mass spectra obtained by Femtosecond Laser Mass Spectrometry (FLMS) Analytical Letters. 2020;53(2):290–307. [Google Scholar]
- 54.Alvarez-Rivera G., Ballesteros-Vivas D., Parada-Alfonso F., Ibañez E., Cifuentes A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC Trends in Analytical Chemistry. 2019;112:87–101. doi: 10.1016/j.trac.2019.01.002. [DOI] [Google Scholar]
- 55.Ballesteros-Vivas D., Álvarez-Rivera G., Ibáñez E., Parada-Alfonso F., Cifuentes A. A multi-analytical platform based on pressurized-liquid extraction, in vitro assays and liquid chromatography/gas chromatography coupled to high resolution mass spectrometry for food by-products valorisation. part 2: characterization of bioactive compounds from goldenberry (Physalis peruviana L.) calyx extracts using hyphenated techniques. Journal of Chromatography A. 2019;1584:144–154. doi: 10.1016/j.chroma.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 56.Puebla G. G., Iglesias A., Gómez M. A., Prámparo M. B. Fossil record of Ephedra in the lower cretaceous (Aptian), Argentina. Journal of Plant Research. 2017;130(6):975–988. doi: 10.1007/s10265-017-0953-1. [DOI] [PubMed] [Google Scholar]
- 57.Wu H., Ma Z., Wang M.-M., Qin A.-L., Ran J.-H., Wang X.-Q. A high frequency of allopolyploid speciation in the gymnospermous genusEphedraand its possible association with some biological and ecological features. Molecular Ecology. 2016;25(5):1192–1210. doi: 10.1111/mec.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollander J. L., Vander Wall S. B., Baguley J. G. Evolution of seed dispersal in North American Ephedra. Evolutionary Ecology. 2010;24(2):333–345. [Google Scholar]
- 59.Hollander J. L., Vander Wall S. B. Dispersal syndromes in North American Ephedra. International Journal of Plant Sciences. 2009;170(3):323–330. doi: 10.1086/596334. [DOI] [Google Scholar]
- 60.Rydin C., Pedersen K. R., Crane P. R., Friis E. M. Former diversity of Ephedra (Gnetales): evidence from early cretaceous seeds from Portugal and North America. Annals of Botany. 2006;98(1):123–140. doi: 10.1093/aob/mcl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y., Ferguson D. K. Macrofossil evidence unveiling evolution and ecology of early Ephedraceae. Perspectives in Plant Ecology, Evolution and Systematics. 2015;17(5):331–346. doi: 10.1016/j.ppees.2015.06.006. [DOI] [Google Scholar]
- 62.Pearson H..H. W. Gnetales. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- 63.Foster A. S., Gifford E. M. Morphology and Evolution of Vascular Plants. San Francisco, USA: WH Freeman and Company; 1989. [Google Scholar]
- 64.Stapf O. “Die Arten der gattung Ephedra,” KK hof-und staatsdruckerei. Commission bei F. 1889;56:1–112. [Google Scholar]
- 65.Rothwell G. W., Stockey R. A. Evolution and phylogeny of gnetophytes: evidence from the anatomically preserved seed cone Protoephedrites eamesii gen. et sp. nov. and the seeds of several bennettitalean species. International Journal of Plant Sciences. 2013;174(3):511–529. doi: 10.1086/668688. [DOI] [Google Scholar]
- 66.Yang Y., Wang Q. The earliest fleshy cone of Ephedra from the early cretaceous Yixian Formation of northeast China. PLoS One. 2013;8(1):1–8. doi: 10.1371/annotation/d1b28569-d9c0-4812-8332-9a6bfa9eb27f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y., Lin L., Ferguson D. K. Parallel evolution of leaf morphology in gnetophytes. Organisms Diversity & Evolution. 2015;15(4):651–662. doi: 10.1007/s13127-015-0226-6. [DOI] [Google Scholar]
- 68.Ferguson K., Yang Y., Lin & David L. Org Divers Evol. 2015;15:651–662. [Google Scholar]
- 69.Loera I., Sosa V., Ickert-Bond S. M. Diversification in North American arid lands: niche conservatism, divergence and expansion of habitat explain speciation in the genus Ephedra. Molecular Phylogenetics and Evolution. 2012;65(2):437–450. doi: 10.1016/j.ympev.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 70.Rydin C., Khodabandeh A., Endress P. K. The female reproductive unit of Ephedra (Gnetales): comparative morphology and evolutionary perspectives. Botanical Journal of the Linnean Society. 2010;163(4):387–430. doi: 10.1111/j.1095-8339.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- 71.Ickert-Bond S. M., Rydin C., Renner S. S. A fossil-calibrated relaxed clock forEphedraindicates an Oligocene age for the divergence of Asian and New World clades and Miocene dispersal into South America. Journal of Systematics and Evolution. 2009;47(5):444–456. doi: 10.1111/j.1759-6831.2009.00053.x. [DOI] [Google Scholar]
- 72.Rydin C., Pedersen K. R., Friis E. M. On the evolutionary history of Ephedra: cretaceous fossils and extant molecules. Proceedings of the National Academy of Sciences. 2004;101(47):16571–16576. doi: 10.1073/pnas.0407588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kunzmann L., Mohr B. A., Bernardes-de-Oliveira M. E. Cearania heterophylla gen. nov. et sp. nov., a fossil gymnosperm with affinities to the Gnetales from the Early Cretaceous of northern Gondwana. Review of Palaeobotany and Palynology. 2009;158(1-2):193–212. doi: 10.1016/j.revpalbo.2009.09.001. [DOI] [Google Scholar]
- 74.Cladera G., del Fueyo G., Villar de Seoane L., Archangelsky S. Early cretaceous riparian vegetation in patagonia, Argentina. Revista del Museo Argentino de CIencias Naturales. 2007;9(1):49–58. doi: 10.22179/revmacn.9.364. [DOI] [Google Scholar]
- 75.Krassilov V. A. Diversity of Mesozoic gnetophytes and the first angiosperms. Paleontological Journal. 2009;43(10):1272–1280. doi: 10.1134/s0031030109100098. [DOI] [Google Scholar]
- 76.Krassilov V. Early cretaceous flora of Mongolia. Palaeontographica Abteilung B Palaeophytologie. 1982;181(1-3):1–43. [Google Scholar]
- 77.Yang Y. A review on gnetalean megafossils: problems and perspectives. Taiwania. 2010;55(4):346–354. [Google Scholar]
- 78.Rydin C., Friis E. A new Early Cretaceous relative of Gnetales: siphonospermum simplex gen. et sp. nov. from the Yixian Formation of Northeast China. BMC Evolutionary Biology. 2010;10(1):p. 183. doi: 10.1186/1471-2148-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y., Geng B.-Y., Dilcher D. L., Chen Z.-D., Lott T. A. Morphology and affinities of an early cretaceous Ephedra (Ephedraceae) from China. American Journal of Botany. 2005;92(2):231–241. doi: 10.3732/ajb.92.2.231. [DOI] [PubMed] [Google Scholar]
- 80.Liu H. M., Ferguson D. K., Hueber F. M., Li C. S., Wang Y. F. Taxonomy and systematics of Ephedrites cheniae and Alloephedra xingxuei (Ephedraceae) Taxon. 2008;57(2):577–582. [Google Scholar]
- 81.Meena B., Singh N., Mahar K. S., Sharma Y. K., Rana T. S. Molecular analysis of genetic diversity and population genetic structure in Ephedra foliata: an endemic and threatened plant species of arid and semi-arid regions of India. Physiology and Molecular Biology of Plants. 2009;25(3):753–764. doi: 10.1007/s12298-019-00648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuster F., Traveset A. Evidence for a double mutualistic interaction between a lizard and a Mediterranean gymnosperm, Ephedra fragilis. AoB Plants. 2019;11(1):p. 001. doi: 10.1093/aobpla/plz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ickert‐Bond S. M., Renner S. S. The Gnetales: recent insights on their morphology, reproductive biology, chromosome numbers, biogeography, and divergence times. Journal of Systematics and Evolution. 2016;54(1):1–16. [Google Scholar]
- 84.Wang X.-Q., Ran J.-H. Evolution and biogeography of gymnosperms. Molecular Phylogenetics and Evolution. 2014;75:24–40. doi: 10.1016/j.ympev.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Qin A. L., Wang M. M., Cun Y. Z., et al. Phylogeographic evidence for a link of species divergence of Ephedra in the Qinghai-Tibetan Plateau and adjacent regions to the Miocene Asian aridification. PloS One. 2013;8:1–13. doi: 10.1371/journal.pone.0056243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ickert-Bond S. M., Wojciechowski M. F. Phylogenetic relationships in Ephedra (Gnetales): evidence from nuclear and chloroplast DNA sequence data. Systematic Botany. 2004;29(4):834–849. doi: 10.1600/0363644042451143. [DOI] [Google Scholar]
- 87.Crane P. R. The fossil history of the Gnetales. International Journal of Plant Sciences. 1996;157:50–57. doi: 10.1086/297403. [DOI] [Google Scholar]
- 88.Wang X., Zheng S. Whole fossil plants of Ephedra and their implications on the morphology, ecology and evolution of Ephedraceae (Gnetales) Chinese Science Bulletin. 2010;55(15):1511–1519. doi: 10.1007/s11434-010-3069-8. [DOI] [Google Scholar]
- 89.Yang Y. A systematic classification of Ephedraceae: living and fossil. Phytotaxa. 2014;158(3):283–290. doi: 10.11646/phytotaxa.158.3.8. [DOI] [Google Scholar]
- 90.Pellati F., Benvenuti S. Determination of ephedrine alkaloids in Ephedra natural products using HPLC on a pentafluorophenylpropyl stationary phase. Journal of Pharmaceutical and Biomedical Analysis. 2008;48(2):254–263. doi: 10.1016/j.jpba.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 91.Jaradat N., Hussen F., Al Ali A. Preliminary phytochemical screening, quantitative estimation of total fl2avonoids, total phenols and antioxidant activity of Ephedra alata Decne. Journal of Materials and Environmental Science. 2015;6:1771–1778. [Google Scholar]
- 92.Alali F. Q., Tawaha K., El-Elimat T., Syouf M., et al. Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: an ICBG project. Natural Product Research. 2007;21(12):1121–1131. doi: 10.1080/14786410701590285. [DOI] [PubMed] [Google Scholar]
- 93.Nasar M. Q., Khalil A. T., Ali M., Shah M., Ayaz M., Shinwari Z. K. Phytochemical analysis, Ephedra Procera CA Mey. mediated green synthesis of silver nanoparticles, their cytotoxic and antimicrobial potentials. Medicina. 2019;55:1–17. doi: 10.3390/medicina55070369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mellado M., Soto M., Madrid A., et al. In vitro antioxidant and antiproliferative effect of the extracts of Ephedra chilensis K Presl aerial parts. BMC Complementary and Alternative Medicine. 2019;19:1–10. doi: 10.1186/s12906-019-2462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Trad B., A Al –Qudah M., Al Zoubi M., et al. In-vitro and in-vivo antioxidant activity of the butanolic extract from the stem of Ephedra alte. Biomedical and Pharmacology Journal. 2018;11(3):1239–1245. doi: 10.13005/bpj/1485. [DOI] [Google Scholar]
- 96.Hegazy A. K., Mohamed A. A., Ali S. I., Alghamdi N. M., Abdel-Rahman A. M., Al-Sobeai S. Chemical ingredients and antioxidant activities of underutilized wild fruits. Heliyon. 2019;5:1–8. doi: 10.1016/j.heliyon.2019.e01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kallassy H., Fayyad-Kazan M., Makki R., et al. Chemical composition and antioxidant, anti-inflammatory, and antiproliferative activities of Lebanese Ephedra Campylopoda plant. Medical Science Monitor Basic Research. 2017;23:313–325. doi: 10.12659/msmbr.905056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Milman B. L. General principles of identification by mass spectrometry. TrAC Trends in Analytical Chemistry. 2015;69:24–33. doi: 10.1016/j.trac.2014.12.009. [DOI] [Google Scholar]
- 99.Pellati F., Benvenuti S. Determination of ephedrine alkaloids in Ephedra natural products using HPLC on a pentafluorophenylpropyl stationary phase. Journal of Pharmaceutical and Biomedical Analysis. 2008;48(2):254–263. doi: 10.1016/j.jpba.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 100.Ziani B. E. C., Heleno S. A., Bachari K., et al. Phenolic compounds characterization by LC-DAD- ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Research International. 2019;116:312–319. doi: 10.1016/j.foodres.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 101.Schäfer S., Salcher S., Seiter M., et al. Characterization of the XIAP-inhibiting proanthocyanidin fraction of the aerial parts of Ephedra sinica. Planta Medica. 2016;82(11):973–985. doi: 10.1055/s-0042-107253. [DOI] [PubMed] [Google Scholar]
- 102.Ibragic S., Sofić E. Chemical composition of various Ephedra species. Bosnian Journal of Basic Medical Sciences. 2015;15:21–27. doi: 10.17305/bjbms.2015.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gul R., Jan S. U., Faridullah S., Sherani S., Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. The Scientific World Journal. 2017;2017:7. doi: 10.1155/2017/5873648.5873648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi S. Y., Jeong B., Jang H. S., et al. Simultaneous analysis of four standards of the herbal formula, DF-02, of Ephedra intermedia and Rheum palmatum, using by High Performance Liquid Chromatography-Ultraviolet Detector (HPLC-UVD) Natural Product Sciences. 2019;25(2):111–114. doi: 10.20307/nps.2019.25.2.111. [DOI] [Google Scholar]
- 105.Lim J., Lee H., Ahn J., et al. The polyherbal drug GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica inhibits hepatic steatosis and fibroinflammtion in high-fat diet-induced obese mice. Journal of Ethnopharmacology. 2018;225:31–41. doi: 10.1016/j.jep.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 106.Jeong B., Yoon Y., Shin S. S., Kwon Y. S., Yang H. Simultaneous determination of (+)-Pseudoephedrine and (-)-Ephedrine in Ephedra intermedia by HPLC-UV. Korean Journal of Pharmacognosy. 2017;48:93–96. [Google Scholar]
- 107.Hyuga S., Hyuga M., Oshima N., et al. Ephedrine alkaloids-free Ephedra herb extract: a safer alternative to Ephedra with comparable analgesic, anticancer, and anti-influenza activities. Journal of Natural Medicines. 2016;70(3):571–583. doi: 10.1007/s11418-016-0979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oshima N., Maruyama T., Yamashita T., et al. Two flavone C-glycosides as quality control markers for the manufacturing process of ephedrine alkaloids-free Ephedra herb Extract (EFE) as a crude drug preparation. Journal of Natural Medicines. 2018;72(1):73–79. doi: 10.1007/s11418-017-1111-8. [DOI] [PubMed] [Google Scholar]
- 109.Wang J.-W., Chiang M.-H., Lu C.-M., Tsai T.-H. Determination the active compounds of herbal preparation by UHPLC-MS/MS and its application on the preclinical pharmacokinetics of pure ephedrine, single herbal extract of Ephedra, and a multiple herbal preparation in rats. Journal of Chromatography B. 2016;1026:152–161. doi: 10.1016/j.jchromb.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 110.Mei F., Xing X.-f., Tang Q.-f., et al. Antipyretic and anti-asthmatic activities of traditional Chinese herb-pairs, Ephedra and Gypsum. Chinese Journal of Integrative Medicine. 2016;22(6):445–450. doi: 10.1007/s11655-014-1952-x. [DOI] [PubMed] [Google Scholar]
- 111.Lu N.-w., Li N., Dong Y.-m. A Rapid hydrophilic interaction liquid chromatographic method for simultaneous determination of three ephedrine alkaloids in Ephedra herb and its preparations. Journal of Liquid Chromatography & Related Technologies. 2015;38(15):1507–1514. doi: 10.1080/10826076.2015.1063509. [DOI] [Google Scholar]
- 112.Zhang D., Deng A.-J., Ma L., et al. Phenylpropanoids from the stems of Ephedra sinica. Journal of Asian Natural Products Research. 2016;18(3):260–267. doi: 10.1080/10286020.2015.1070831. [DOI] [PubMed] [Google Scholar]
- 113.Shawarb N., Jaradat N., Abu-Qauod H., Alkowni R., Hussein F. Investigation of antibacterial and antioxidant activity for methanolic extract from different edible plant species in Palestine. Moroccan Journal of Chemistry. 2017;5:573–579. [Google Scholar]
- 114.Jaradat N. A., Shawahna R., Eid A. M., Al-Ramahi R., Asma M. K., Zaid A. N. Herbal remedies use by breast cancer patients in the West Bank of Palestine. Journal of Ethnopharmacology. 2016;178:1–8. doi: 10.1016/j.jep.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 115.Khan A., Jan G., Khan A., Gul Jan F., Bahadur A., Danish M. In vitro antioxidant and antimicrobial activities of Ephedra gerardiana (root and stem) crude extract and fractions. Evidence-Based Complementary and Alternative Medicine. 2017;2017:6. doi: 10.1155/2017/4040254.4040254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park S. Y., Yi E. H., Kim Y., Park G. Anti-neuroinflammatory effects of Ephedra sinica Stapf extract-capped gold nanoparticles in microglia. International Journal of Nanomedicine. 2019;14:2861–2877. doi: 10.2147/ijn.s195218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gajardo S., Aguilar M., Stowhas T., et al. Determination of sun protection factor and antioxidant properties of six Chilean Altiplano plants. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2016;15:352–363. [Google Scholar]
- 118.Palici I. F., Liktor-Busa E., Zupkó I., et al. Study of in vitro antimicrobial and antiproliferative activities of selected Saharan plants. Acta Biologica Hungarica. 2015;66:385–394. doi: 10.1556/018.66.2015.4.3. [DOI] [PubMed] [Google Scholar]
- 119.Fazeli-Nasab B., Mousavi S. R. Antibacterial activities of Ephedra sinica herb extract on standard and clinical strains of Pseudomonas aeruginosa. Journal of Medical Bacteriology. 2019;8:40–48. [Google Scholar]
- 120.Mahmood N., Nazir R., Khan M., et al. Phytochemical screening, antibacterial activity and heavy metal analysis of ethnomedicinal recipes and their sources used against infectious diseases. Plants. 2019;8:14. doi: 10.3390/plants8110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ghanem S., El-Magly U. I. Antimicrobial activity and tentative identification of active compounds from the medicinal Ephedra alata male plant. Journal of Taibah University Medical Sciences. 2008;3:7–15. doi: 10.1016/s1658-3612(08)70039-8. [DOI] [Google Scholar]
- 122.Al-Awaida W., Al-Hourani B. J., Akash M., et al. In vitro anticancer, anti-inflammatory, and antioxidant potentials of Ephedra aphylla. Journal of Cancer Research and Therapeutics. 2018;14:1350–1354. doi: 10.4103/0973-1482.196760. [DOI] [PubMed] [Google Scholar]
- 123.Hoshyar R., Mostafavinia S. E., Zarban A., et al. Correlation of anticancer effects of 12 Iranian herbs on human breast adenocarcinoma cells with antioxidant properties. Free Radicals & Antioxidants. 2015;5:65–73. doi: 10.5530/fra.2015.2.4. [DOI] [Google Scholar]
- 124.Mohammad S., Masoumeh H., Gholamali J., Hoda T. Ephedraceae as a treatment for hyperlipidemia and hyperglycemia: an experimental study. Journal of Autoimmune Disorders. 2017;3:1–4. [Google Scholar]
- 125.Uttra A. M. Assessment of anti-arthritic potential of Ephedra gerardiana by in vitro and in vivo methods. Bangladesh Journal of Pharmacology. 2017;12:403–409. doi: 10.3329/bjp.v12i4.32798. [DOI] [Google Scholar]
- 126.Lee S. E., Lim C., Lim S., Lee B., Cho S. Effect of Ephedra herb methanol extract on high-fat diet-induced hyperlipidaemic mice. Pharmaceutical Biology. 2019;57:676–683. doi: 10.1080/13880209.2019.1666883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakamori S., Takahashi J., Hyuga S., et al. “Analgesic effects of Ephedra herb extract, ephedrine alkaloids–free Ephedra herb extract, ephedrine, and pseudoephedrine on formalin-induced Pain. Biological and Pharmaceutical Bulletin. 2019;42:1538–1544. doi: 10.1248/bpb.b19-00260. [DOI] [PubMed] [Google Scholar]
- 128.Oshima N., Yamashita T., Hyuga S., et al. Efficiently prepared ephedrine alkaloids-free Ephedra Herb extract: a putative marker and antiproliferative effects. Journal of Natural Medicines. 2016;70:554–562. doi: 10.1007/s11418-016-0977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin Y. C., Chang C. W., Wu C. R. Antitussive, anti-pyretic and toxicological evaluation of Ma-Xing-Gan-Shi-Tang in rodents. BMC Complementary and Alternative Medicine. 2016;16:1–9. doi: 10.1186/s12906-016-1440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee A. Y., Jang Y., Hong S. H., et al. Ephedrine-induced mitophagy via oxidative stress in human hepatic stellate cells. The Journal of Toxicological Sciences. 2017;42:461–473. doi: 10.2131/jts.42.461. [DOI] [PubMed] [Google Scholar]
- 131.Powell T., Hsu F. F., Turk J., Hruska K. Ma-huang strikes again: ephedrine nephrolithiasis. American Journal of Kidney Diseases. 1998;32:153–159. doi: 10.1053/ajkd.1998.v32.pm9669437. [DOI] [PubMed] [Google Scholar]
- 132.Nauffal M., Gabardi S. Nephrotoxicity of natural products. Blood Purification. 2016;41:123–129. doi: 10.1159/000441268. [DOI] [PubMed] [Google Scholar]
- 133.Lee M. K., Cheng B. W. H., Che C. T., Hsieh D. P. H. Cytotoxicity assessment of Ma-huang (Ephedra) under different conditions of preparation. Toxicological Sciences. 2000;56:424–430. doi: 10.1093/toxsci/56.2.424. [DOI] [PubMed] [Google Scholar]
- 134.Wu Z., Kong X., Zhang T., Ye J., Fang Z., Yang X. Pseudoephedrine/ephedrine shows potent anti-inflammatory activity against TNF-α-mediated acute liver failure induced by lipopolysaccharide/d-galactosamine. European Journal of Pharmacology. 2014;724:112–121. doi: 10.1016/j.ejphar.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 135.Zheng E., Navarro V. Liver injury due to herbal and dietary supplements: a review of individual ingredients. Clinical Liver Disease. 2016;7:80–83. doi: 10.1002/cld.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mladěnka P., Patočka L. A. J., et al. Comprehensive review of cardiovascular toxicity of drugs and related agents. Medicinal Research Reviews. 2018;38:1332–1403. doi: 10.1002/med.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hudson A., Lopez E., Almalki A. J., Roe A. L., Calderón A. I. A review of the toxicity of compounds found in herbal dietary supplements. Planta Medica. 2018;84(09/10):613–626. doi: 10.1055/a-0605-3786. [DOI] [PubMed] [Google Scholar]


