Abstract
Hepatocellular carcinoma (HCC) represents 80% of the primary hepatic neoplasms. It is the sixth most frequent neoplasm, the fourth cause of cancer-related death, and 7% of registered malignancies. Sorafenib is the first line molecular targeted therapy for patients in advanced stage of HCC. The present study shows that Sorafenib exerts free radical scavenging properties associated with the downregulation of nuclear factor E2-related factor 2 (Nrf2)-regulated thioredoxin 1 (Trx1) expression in liver cancer cells. The experimental downregulation and/or overexpression strategies showed that Trx1 induced activation of nitric oxide synthase (NOS) type 3 (NOS3) and S-nitrosation (SNO) of CD95 receptor leading to an increase of caspase-8 activity and cell proliferation, as well as reduction of caspase-3 activity in liver cancer cells. In addition, Sorafenib transiently increased mRNA expression and activity of S-nitrosoglutathione reductase (GSNOR) in HepG2 cells. Different experimental models of hepatocarcinogenesis based on the subcutaneous implantation of HepG2 cells in nude mice, as well as the induction of HCC by diethylnitrosamine (DEN) confirmed the relevance of Trx1 downregulation during the proapoptotic and antiproliferative properties induced by Sorafenib. In conclusion, the induction of apoptosis and antiproliferative properties by Sorafenib were related to Trx1 downregulation that appeared to play a relevant role on SNO of NOS3 and CD95 in HepG2 cells. The transient increase of GSNOR might also participate in the deactivation of CD95-dependent proliferative signaling in liver cancer cells.
Keywords: Apoptosis, Cell proliferation, CD95, GSNOR, Hepatocarcinoma, Nrf2, NOS3
Graphical abstract
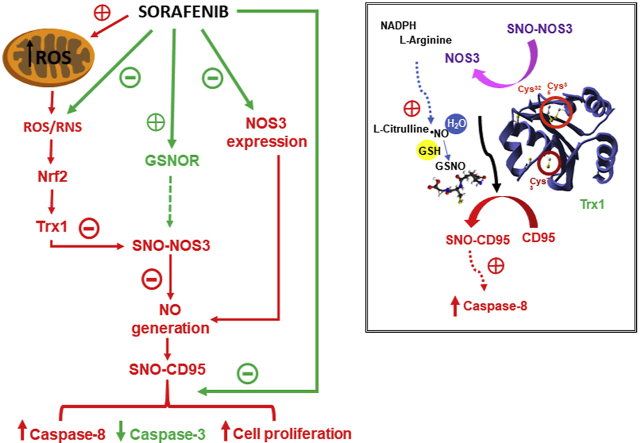
Graphical Abstract. Sorafenib reduced ROS/RNS cytoplasmic concentrations which were associated with decreased Nrf2 nuclear translocation, Trx1 expression/activity, and SNO–NOS3 expression and NO intracellular generation in HepG2. The reduction of SNO–NOS3 expression and NO generation might also be influenced by the induction of transient GSNOR expression/activity and reduced NOS3 expression by Sorafenib. The diminution of SNO-CD95 expression by Sorafenib involved reduction of caspase-8 activity and cell proliferation, and increased downstream caspase-3 activity in liver cancer cells.
Highlights
-
•
Sorafenib induces mitochondrial ROS generation, but also acts as nucleophilic scavenger.
-
•
Sorafenib reduces Nrf2-depenent Trx1 expression, and SNO–NOS3 and SNO-CD95 ratios.
-
•
Sorafenib-related antitumoral in vivo activity involves diminution of Trx1 and SNO-CD95.
List of abbreviations:
- HCC
hepatocellular carcinoma
- Nrf2
nuclear factor E2-related factor 2
- Trx1
thioredoxin 1
- SNO
S-nitrosation
- ROS
reactive oxygen species
- NOS3
nitric oxide synthase type 3
- GSNOR
S-nitrosoglutathione reductase
- DEN
diethylnitrosamine
- BCLC
Barcelona Clinic Liver Cancer
- c-MET
mesenchymal-epithelial transition factor receptor
- IGFR
insulin growth factor receptor
- FGFR
fibroblast growth factor receptor
- VEGFR
vascular endothelial growth factor receptor
- PDGFR-β
platelet-derived growth factor receptor-β
- JNK
c-Jun N-terminal
- MEK
mitogen-activated protein kinase kinase
- ERK
extracellular signalregulatedkinase
- AMPK
AMP-activated protein kinase
- NF-κB
nuclear factor-κB
- HIF-1
hypoxia inducible factor-1
- TrxR
thioredoxin reductase
- NO
nitric oxide
- Trx
thioredoxin
- VEGF
vascular endothelial growth factor
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- CMH
cyclic hydroxylamine 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine
- MitoSOX™
mitochondrial superoxide indicator
- DHE
dihydroethidium
- H2DCFDA
2,7-dichloro dihydro-fluorescein diacetate
- DAF-2
4,5-diaminofluorescein diacetate
- SFN
sulforaphane
- EPR
electron paramagnetic resonance
- O2•-
superoxide anion
- BrdU
bromodeoxyuridine
- ARE
antioxidant response elements
- H2O2
hydrogen peroxide
- OH•
hydroxyl radicals
- OH
hydroxyl anion
- GST
glutathione-S-transferase
- TrxR1
thioredoxin reductase type 1
- Gpx1
glutathione peroxidase type 1
- NQO1
NADPH-quinone oxidoreductase 1
- HO-1
heme oxygenase 1
- GCLC
glutamate-cysteine ligase catalytic subunit
- PKA
protein kinase type A
- PKC
protein kinase C
- AMPK
5′ adenosine monophosphate-activated protein kinase
- ERK
extracellular signal-regulated kinase
- SHP-1
SH2 domain-containing tyrosine phosphatase
- STAT3
signal transducers and activators of transcription type 3
- Mcl-1
induced myeloid leukemia cell differentiation protein-1
- RNS
reactive nitrogen species
1. Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer developed in the context of a cirrhotic liver (80–90%). Although major prevalent genetic mutations are related to telomerase (60%), Wnt-β-catenin (54%), PI3K-Akt-mTOR (51%), p53 (49%) and MAPK (43%) signaling, the alteration of redox regulatory mechanisms is also observed in a relevant proportion of patients (12%) [1]. Only one third of HCC patients are diagnosed at early stage (0 or A), according to the Barcelona Clinic Liver Cancer (BCLC) staging system, being eligible for potential curative therapies such as local ablation, resection or orthotopic liver transplantation with 5-years survival in 50–80% of patients [2]. Then, high proportion of patients are diagnosed at more advanced stages of the disease (BCLC B–C) [3]. Sorafenib is the recommended therapy for patients with locally advanced/metastatic disease (BCLC C) stage with mean overall survival around 11 months [4,5]. The resistance of HCC cells to Sorafenib has been related to the activation of survival cell signaling related to insulin growth factor receptor (IGFR) [6,7], mesenchymal-epithelial transition factor receptor (c-MET) [8], and fibroblast growth factor receptor (FGFR) [7].
Sorafenib simultaneously inhibits tyrosine kinase receptors such as vascular endothelial growth factor receptor (VEGFR) 2, VEGFR 3, platelet-derived growth factor receptor-β (PDGFR-β), Flt3 and c-Kit, as well as molecular components of the Raf-mitogen-activated protein kinase kinase (MEK)-extracellular signalregulatedkinase (ERK) signaling pathway [9]. The early induction by Sorafenib of endoplasmic reticulum stress, upregulation of c-Jun N-terminal (JNK) and AMP-activated protein kinase (AMPK)-dependent signaling was related to the induction of survival autophagic processes [10]. The induction of apoptosis by Sorafenib was associated with a decrease in S-nitrosation (SNO) of cell death receptors in HepG2 cells [11].
Cancer cells activate an adaptive program leading to the upregulation of reactive oxygen species (ROS)-scavenging systems, inhibition of apoptosis, and promoting advanced transformation, metastasis and resistance to anticancer drugs [12]. The adaptive response involves up-regulation of redox-sensitive transcription factors, such as nuclear factor-κB (NF-κB), nuclear factor E2-related factor 2 (Nrf2), c-jun and hypoxia inducible factor (HIF-1), which lead to the increased expression of antioxidant molecules such as SOD, catalase, thioredoxin 1 (Trx1) and the GSH antioxidant system, as well as survival factors such as Bcl-2 and induced myeloid leukemia cell differentiation protein-1 (Mcl-1) [13]. The thioredoxin (Trx) system, constituted by NADPH, FAD-containing thioredoxin reductase (TrxR) (EC 1.8.1.9) and Trx, plays a key role against oxidative stress regulating protein thiol/disulfide balance, supplying electrons to thiol-dependent peroxidases (peroxiredoxins), ribonucleotide reductase and methionine sulfoxide reductases, as well as regulating the activity of many redox-sensitive transcription factors, and selenium and nitric oxide (NO) metabolism [14]. The expression of Trx1 has been shown to be increased in HCC and cultured liver cancer cells [15,16]. The present study showed that the induction of apoptosis and antiproliferative properties by Sorafenib was related to Trx1 downregulation which played a relevant role in SNO of nitric oxide synthase (NOS) type 3 (NOS3) and CD95 in HepG2 cells. The regulation of SNO might be also influenced by the transient upregulation of GSNOR by Sorafenib in liver cancer cells.
2. Material and Methods
2.1. Chemical and cell lines
Sorafenib Tosylate was obtained from Carbosynth Limited (Berkshire, UK) (Supplementary Fig. 1). Xanthine oxidase (X2252), xanthine (X0626), 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) and cyclic hydroxylamine 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH) were obtained from Sigma-Aldrich (Missouri, USA). Red Mitochondrial Superoxide Indicator (MitoSOX™) (M36008), dihydroethidium (DHE) (D-11347), 2,7-dichloro dihydro-fluorescein diacetat (H2DCFDA) (D-399) and 4,5-diaminofluorescein diacetate (DAF-2) (D-23842) were obtained from Life Technologies (Thermo Fisher Scientific, Massachusetts, USA). Sulforaphane (SFN, Sigma-Aldrich) and vascular endothelial growth factor (VEGF, PrepoTech, New Jersey, USA) were used as inducers of Nrf2 activation.
HepG2 (HB-8065™) was obtained from ATCC/LGC Standards (Barcelona, Spain). JHH2, JHH-4, JHH-5 and Huh-1 were obtained from the Japanese Collection of Research Bioresources Cell Bank (Tokyo, Japan). SNU886 was obtained from the Korean Cell Lines Bank (Seoul, Korea). MHCC97H was obtained from Woodland Pharmaceuticals (Massachusetts, USA).
2.2. Measurement of reactive oxygen and nitrogen species
Cells were incubated with DHE (10 μM), MitoSOX™ (5 μM), H2DCFDA (10 μM) and DAF-2 (2.5 μM) for 30 min in culture medium for measuring superoxide anion (O2•-) in cytoplasm and mitochondria, hydrogen peroxide (H2O2) and NO, respectively. The production of NO was also measured by quantification of nitrite/nitrate in culture medium using the Griess reaction [17].
2.3. Evaluation of the scavenging properties of Sorafenib
The scavenging properties of Sorafenib were evaluated by electron paramagnetic resonance (EPR) using the spin probe CMH (100 μM) and spin trap DMPO (100 mM) to monitor O2•- formation. The xanthine (50 μM) and xanthine oxidase (5 mU/mL) system was used to generate ROS for 30 min. The formation of the radicals was monitored using bench-top EMXnano spectrometer (Bruker, Massachusetts, USA). Deferoxamine (25 μM) was added when required in order to prevent Fenton reaction.
2.4. mRNA and protein expression
RT-PCR was performed in StepOnePlus RT-PCR System (Thermo Fisher Scientific, Waltham, USA). Reactions were performed in 96-well plates with optical sealing tape (Applied Biosystem, Foster City, California, USA) in 10 μL total volume containing SYBR Green MasterMix and corresponding cDNA (obtained with High capacity cDNA Reverse Transcription Kit, Applied Biosystems). The conditions for amplification were as follows: denaturation step of 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 60 s. The melting temperature was fixed at 60 °C. All primers were designed using online Primer3 software.
Proteins were separated by Any kD™ Criterion™ TGX Stain-Free™ Protein Gel (BioRad) and transferred to PVDF membranes. The membranes were incubated with the corresponding commercial primary antibodies against Trx1 (Cat ATRX-04, IMCO Corporation Ltd, Stockholm, Sweden), NOS3 (sc-654), CD95 (sc-715) and caspase-8 (sc-7890) from Santa Cruz Biotechnology (Texas, USA); Nrf2 (#12721), Keap1 (#8047) obtained from Cell Signaling Technology (Massachusetts, USA).
The procedure for measuring SNO–NOS3 and SNO-CD95 was performed using biotin switch assay as previously described [18]. The obtained SNO-biotinylated fractions were incubated with 45 μL Neutravidin Plus UltralinK resin (Pierce, Appleton, Wisconsin, USA) for 1 h in agitation, washed and assessed by Western Blot analysis.
2.5. GSNOR and Trx1 activities
S-nitrosoglutathione reductase (GSNOR) and Trx1 activities were measured as previously described [19,20].
2.6. Cell death and proliferation
Caspase-8 and -3-associated activities were determined using Caspase-Glo® 8 (G8201) and Caspase-Glo® 3 (G8091) Assay Systems (Promega, Madison, USA). Bromodeoxyuridine (BrdU) incorporation was used as marker of cell proliferation (Roche Diagnostics, Indianapolis, USA) in cultured HepG2 cells. Cell proliferation was also assessed in tumor sections by the measurement of Ki67 expression by immunohistochemistry procedure using antibodies against Ki67 (sc-23900, Santa Cruz Biotechnology).
2.7. Nrf2 signaling using a reporter gene vector
HepG2 cells were transiently transfected with Antioxidant Response Elements (ARE)-luciferase plasmid (Dr J. Alam, Department of Molecular Genetics, Ochsner Clinic Foundation, USA). Cells were lysed and assayed for luciferase activity by Dual-Luciferase® Reporter (DLR™) Assay System (Promega). pTK-Renilla was used as an internal control vector (Promega).
2.8. Trx1 knock-down and overexpression
Human Trx1 was down-regulated in HepG2 cells using siRNA (ref L-006340, Dharmacon, GE Healthcare Life Sciences). Trx1 overexpressing plasmid was obtained after amplification of human Trx1 by PCR from a cDNA library from human testis (Clonteck, Human Testis QUICK-CloneTM cDNA), and cloned into the pcDNA MYC plasmid at the EcoR1 and XhoI sites.
2.9. Experimental models of liver cancer
Diethylnitrosamine (DEN, Sigma-Chemicals) (10 mg/kg day) was administered in drinking water (0.01% vol/vol) to Wistar rats (4 groups of 8 animals, n = 32; weighing 170-200 gr) for 15 weeks. Sorafenib (10 mg/kg day) was administered in edible hydrogel formulation 12 weeks after the initiation of DEN treatment and lasted until sacrifice of the animals at week 15th. The effect of Sorafenib was also evaluated in tumors-derived from subcutaneously implanted 10x106 HepG2 cells, or HepG2 cells stably transfected with Trx1 overexpressing or empty pcDNA MYC (mock) vectors in Hsd:Athymic Nude-Foxn1nu mice (Harlan Laboratories, Barcelona, Spain). When the tumors reached 5 mm, one group of animals received orally by gavage either Sorafenib (200 mg/kg) or solvent. The animals of each experimental group were sacrificed when tumor of animals from any experimental group reached 15 mm. All animal care and experimentation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences.
2.10. Statistical analysis
All results are expressed as mean ± SEM of independent experiments (n = 3–8). Data were compared using the analysis of variance (ANOVA) with the Least Significant Difference's test (homogeneity of variances) or Games-Howell (non-homogeneity of variances). If Shapiro-Wilks's test showed non-normal distribution of data non-parametric Kruskal-Wallis coupled to U-Man-Whitney post-hoc analysis with Finner's correction was done. All statistical analyses were performed using the IBM SPSS Statistics 19.0.0 (SPSS Inc., IBM, Armonk, NY, USA) software.
3. Results
3.1. Sorafenib induced mitochondrial O2•- generation, but diminished cytoplasmic H2O2, O2.- and NO in HepG2
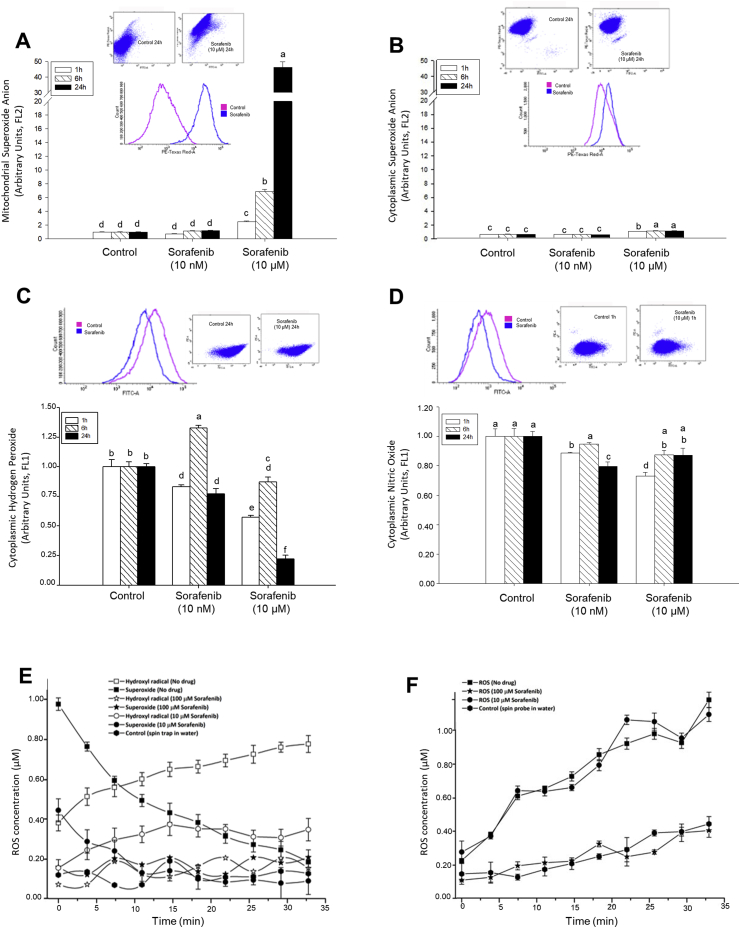
Coriat et al. [21] showed that Sorafenib increased intracellular generation of O2•-, H2O2 and NO in HepG2. O2• was greatly and dose-dependently generated by Sorafenib at mitochondrial level (Fig. 1A) but was scarcely detected at cytoplasmic level (Fig. 1B). Sorafenib tended to decrease the intracellular concentration of H2O2 (Fig. 1C) and NO (1 h, Fig. 1D) in HepG2 cells. The scavenging properties of Sorafenib were assessed by EPR. The nitrone spin trap DMPO in presence of xanthine/xanthine oxidase generating system promotes specific spin-adduct (DMPO-OOH) that rapidly decomposes to DMPO-OH. O2• induces decomposition of H2O2 to hydroxyl radical (OH•) and hydroxyl anion (OH−) through the coupled Fenton and Haber-Weiss reaction in the presence of transition metal traces. This process explained the inverse relationship among O2•- and OH• generation in absence of drug over time (Fig. 1E). Sorafenib significantly lessened or abolished O2•- generation depending on the dose used (10 μM and 100 μM), respectively (Fig. 1E). The spin probe CMH is oxidized by O2•- to the corresponding EPR active and stable nitroxide with coupled H2O2 generation. In this case, Sorafenib (100 μM but not 10 μM) reduced O2•- production by xanthine/xanthine oxidase system for 30 min (Fig. 1F). The differences found in the DMPO and CMH experiments were probably due to their different kinetics, being the last one more reactive than DMPO against ROS and consequently higher competitor than Sorafenib. These results suggest that O2•- generation by Sorafenib (10 μM) in the mitochondria is scavenged in a dose-dependent manner by the drug at the cytoplasmic level.
Fig. 1.
Effect of Sorafenib on free radical generation. The effect of Sorafenib on the mitochondrial (A) and cytoplasmic (B) O2•-, and cytoplasmic H2O2 (C) and NO (D) production in cultured HepG2, as well as in vitro scavenging properties of Sorafenib measured by spin trap (E) and spin probe (F) using a xanthine/xanthine oxidase system are shown. Mitochondrial and/or cytoplasmic ROS and reactive nitrogen species (RNS) were in situ determined using different fluorescent probes described in Material and Methods. The nitrone spin trap DMPO in presence of O2•- generating system promotes specific spin-adduct (DMPO-OOH) that rapidly decompose to DMPO-OH. O2• induces decomposition of H2O2 to hydroxil radical (O•) and hydroxyl anion (OH-) through the coupled Fenton and Haber-Weiss reaction in the presence of transition metal traces. This process explained the inversed relationship of detected O2•- and OH• in the absence of drug over time (E). The spin probe CMH is oxidized by O2•- to the corresponding EPR active and stable nitroxides with coupled H2O2 generation (F). Data are expressed as mean ± SEM of 6–8 independent experiments. The groups with different letters (a, b, c, d, e or f) were significantly different (p ≤ 0.05).
3.2. Sorafenib downregulated Nrf2 signalling and Trx1 expression
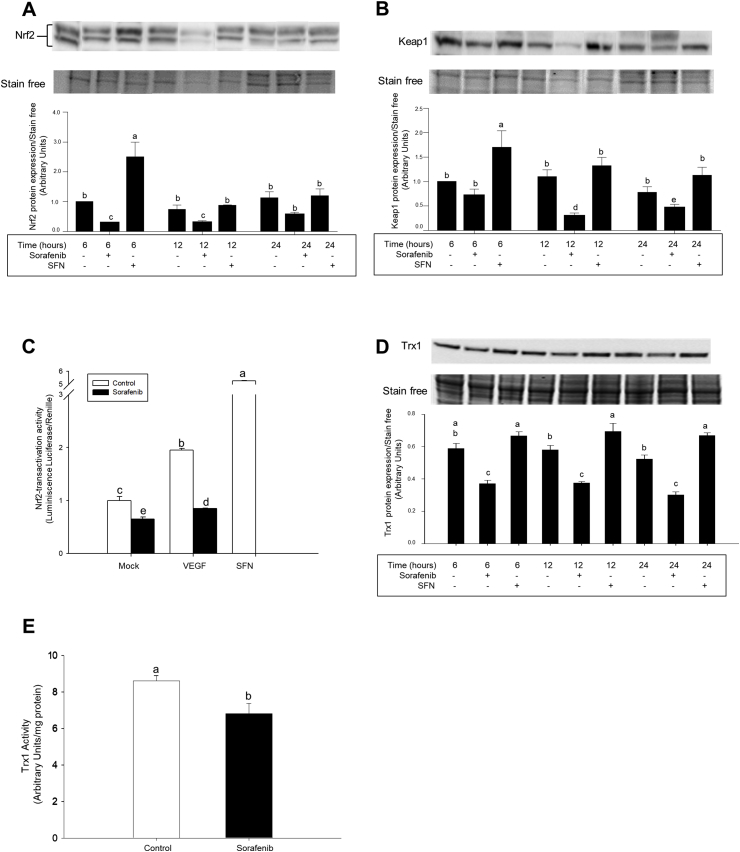
Sorafenib reduced the expression of Nrf2 (Fig. 2A) and Keap1 (Fig. 2B) in nuclear fractions from HepG2 cells. The transcriptional factor Nrf2 positively regulates the expression of enzymes involved in phase I to III detoxification systems, GSH- and Trx-related antioxidant systems, carbohydrate and lipid metabolism, and heme and iron metabolism [22]. Sorafenib decreased Nrf2-transactivation activity in HepG2 cells transfected with ARE/Luc overexpressing vector (Fig. 2C). In agreement, the expression (Fig. 2D) and activity (Fig. 2E) of Trx1 were reduced in Sorafenib-treated HepG2 cells. Sorafenib also decreased mRNA expression of glutathione-S-transferase (GST), thioredoxin reductase type 1 (TrxR1), glutathione peroxidase type 1 (Gpx1), NADPH-quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HO-1) and glutamate-cysteine ligase catalytic subunit (GCLC) in HepG2 cells (Supplementary Figs. 2A–2F).
Fig. 2.
Altered Nrf2-signaling pathway by Sorafenib in HepG2 cells. The reduction of Nrf2 (A) and Keap1 (B) expressions and Nrf2-transactivation activity (C) were associated with downregulation of Trx1 expression (D) and activity (E) in HepG2. Sorafenib (10 μM), VEGF (50 ng/ml) and SFN (10 μM) were administered 24 h after cell stabilization. The expression of Nrf2 and Keap1 in nuclear fraction, and Trx1 in cell lysate was measured by western-blot analysis as described in Material and Methods. Nrf2-transactivation activity was assessed using ARE-luc transfected HepG2 cells. pTK-Renilla was used as internal control vector. Trx1 activity was measured as detailed in Material and Methods. Data are expressed as mean ± SEM of 4 independent experiments. The groups with different letters (a, b, c, d or e) were significantly different (p ≤ 0.05).
3.3. Sorafenib transiently upregulated GSNOR and reduced NOS3 activity. Role of Trx1
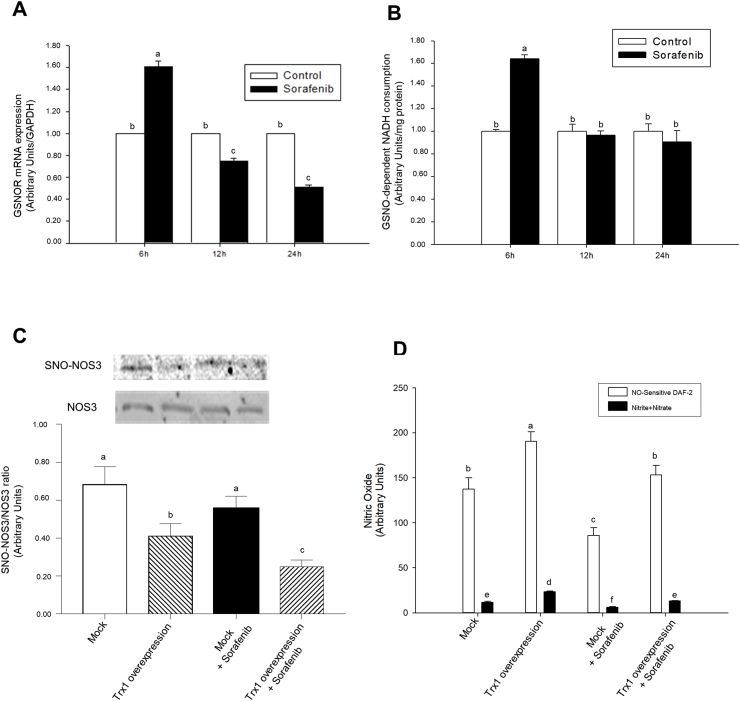
Thiol nitrosation (SNO) is a posttranslational modification that plays a critical role in health and disease [23]. GSNOR has been described as a highly conserved metabolizing enzyme involved in SNO homeostasis [24]. Intracellular GSNO exists in equilibrium with S-nitrosoproteins which are not substrates of GSNOR [24]. mRNA expression (Fig. 3A) and activity (Fig. 3B) of GSNOR were transiently increased after 6 h of Sorafenib treatment in HepG2 cells. NOS3 interacts with a variety of regulatory and structural proteins modulating its activity and traffic between plasma membrane, caveolae and intracellular membrane structures whose activity is tightly regulated by SNO [25]. Trx1 overexpression diminished SNO–NOS3 (Fig. 3C) and increased NO production (Fig. 3D) in both, control and Sorafenib-treated HepG2 cells. Sorafenib appeared to reduce NOS3 expression (Fig. 3C) and NO generation (Fig. 3D) in control and Sorafenib-treated HepG2 cells.
Fig. 3.
Sorafenib transitory increased GSNOR expression and activity in HepG2. Trx1 overexpression reduced SNO–NOS3/NOS3 ratio and NO generation in HepG2 cells. Effect of Sorafenib in the mRNA expression (A) and activity (B) of GSNOR, as well as Trx1 overexpression on SNO–NOS3 (C), and NO in situ generation and NO-end products concentration in culture medium (D). Sorafenib (10 μM) was administered 24 h after cell stabilization. The SNO–NOS3 was determined by biotin-switch assay coupled to western-blot analysis as described in Material and Methods. NO generation was determined by the in-situ reaction with DAF-2, as well as nitrites + nitrates concentration in culture medium was determined by the Griess reagent as described in Material and Methods. Results are expressed as mean ± SEM of 4 independent experiments. The groups with different letters (a, b, c, d, e or f) were significantly different (p ≤ 0.05).
3.4. Sorafenib reduced SNO-CD95, caspase-8 activity and cell proliferation, and increased caspase-3 activity. Trx1 overexpression prevented the beneficial effect of Sorafenib in HepG2 cells
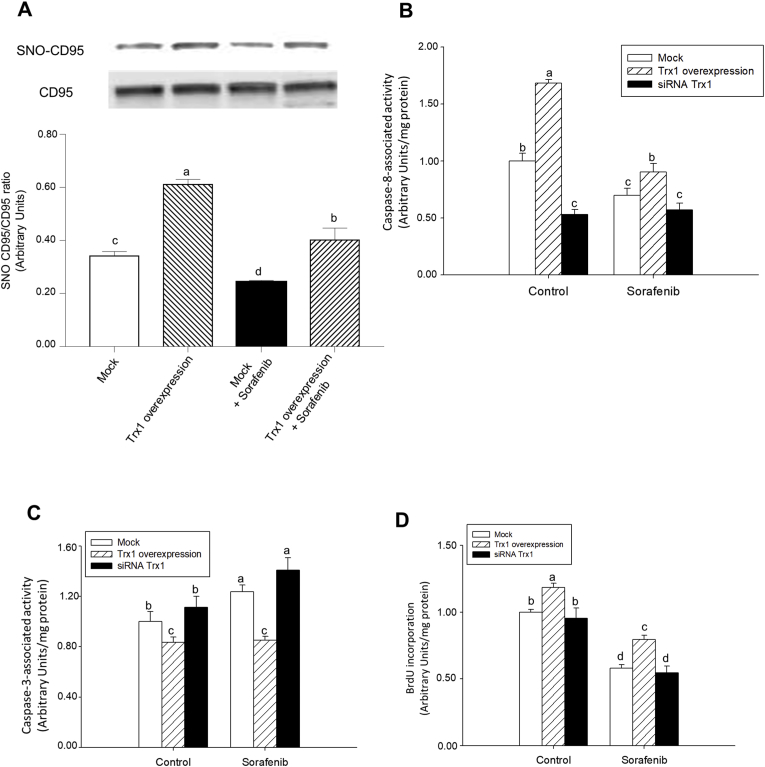
The induction of cell death by Sorafenib was related to a decrease in SNO-CD95 and a shift from caspase-8 to caspase-3 in HepG2 cells [11]. Sorafenib reduced SNO-CD95/CD95 ratio in control and Trx1-overexpressing HepG2 cells (Fig. 4A). Trx1 overexpression increased SNO-CD95 in control and Sorafenib-treated cells (Fig. 4A).
Fig. 4.
Sorafenib reduced, but Trx1 had the opposite effect, SNO-CD95/CD95 ratio, caspase-8 activity, and cell proliferation, while increased caspase-3 activity in HepG2. Effect of Trx1 overexpression or siRNA Trx1 on SNO-CD95/CD95 ratio (A), caspase-8 activity (B), caspase-3 activity (C) and cell proliferation (D) in control and Sorafenib (10 μM)-treated hepG2 cells. Cell transfection using Trx1 overexpressing plasmid or siRNA Trx1, and Sorafenib administration were done as described in Material and Methods. The SNO-CD95 was determined by biotin-switch assay coupled to western-blot analysis, as well as caspase-8 and -3 activities and BrdU incorporation were measured at 24 h after Sorafenib administration as described in Material and Methods. Results are expressed as mean ± SEM of 4 independent experiments. The groups with different letters (a, b, c or d) were significantly different (p ≤ 0.05).
The increase in SNO-CD95 was directly related to caspase-8 activity (Fig. 4B) and cell proliferation (Fig. 4D), but inversely with caspase-3 activity (Fig. 4C). The overexpression of Trx1 increased caspase-8 activity (Fig. 4B) and cell proliferation (Fig. 4D) but decreased caspase-3 activity (Fig. 4C) in control and Sorafenib-treated cells. We addressed the question whether Trx1 downregulation by Sorafenib played a role during the induction of apoptosis and/or reduction of cell proliferation in HepG2 cells. The silencing of Trx1 by the siRNA strategy downregulated caspase-8 activity (Fig. 4B), but had no effect on caspase-3 activity (Fig. 4C) and cell proliferation (Fig. 4D) in control cells. The downregulation of Trx1 did not change these parameters in Sorafenib-treated HepG2 cells (Fig. 4B–D).
3.5. Role of Nrf2 in the proapoptotic properties of Sorafenib
The expression of Nrf2 and TrxR-Trx1 sytem has been related to resistance of cancer cells to chemotherapy [[26], [27], [28]]. The oxidative stress pathway is altered in a high number of HCC (12%) mainly related to KEAP1 mutations [1]. We tested whether HCC cells harboring inhibitory Keap1 mutations (JHH5, MHCC97H and Huh-1) altered the regulation of Trx1 expression, apoptosis and cell proliferation by Sorafenib in comparison with wild type Keap1 cancer cells (JHH2, JHH4 and SNU886). Sorafenib downregulated Nrf2 expression in Keap1 wild type HCC cell lines (JHH-2, JHH-4 and SNU886) (Supplementary Fig. 3A). Sorafenib downregulated the expression of Trx1 in all analyzed HCC cell lines (Supplementary Fig. 3B) that was related to increased caspase-3 (Supplementary Fig. 3C) and reduced cell proliferation (Supplementary Fig. 3D). Basal protein expression levels of Nrf2 appeared to be lower in Keap1-mutated cells than in cells with wild type Keap1 (Supplementary Fig. 3A) that were related to increased Sorafenib-dependent caspase-3 activation (Supplementary Fig. 3C).
3.6. The reduction of tumor growth by Sorafenib was related to decrease in Trx1 and SNO-CD95 expression, and caspase-8 activity in different experimental models of hepatocarcinogenesis
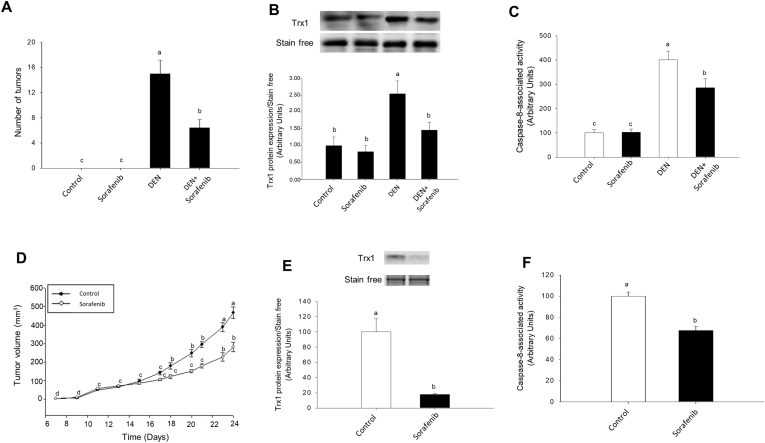
Sorafenib (200 mg/kg) reduced the number of tumor nodules (Fig. 5A), which was associated with a reduction of Trx1 expression (Fig. 5B), SNO-CD95 (Supplementary Fig. 4A) and caspase-8 activity (Fig. 5C) in animals treated with DEN for 17 weeks. The administration of Sorafenib (200 mg/kg) reduced tumor volume by 50% at 24 days after subcutaneous implantation of HepG2 cells (Fig. 5D). This effect was parallel to a decrease in the expression of Trx1 (Fig. 5E), SNO-CD95 (Supplementary Fig. 4B), and caspase-8 activity (Fig. 5F).
Fig. 5.
The antitumoral properties of Sorafenib were associated with downregulation of Trx1 expression and caspase-8 activity in two xenograft mouse models. Effect of Sorafenib on the number of tumors induced by DEN (A) and tumor volume derived from subcutaneous HepG2 implantation (D), and their association with Trx1 expression (B and E, respectively) and caspase-8 activity (C and F, respectively). The administration of DEN (10 mg/kg day) was carried out during 17 weeks in Wistar rats (170 gr) in drinking water (0.01% vol/vol) or solvent. Sorafenib (10 mg/kg day) was administered 12 weeks after the initiation of DEN treatment DEN administration when liver nodules were evident (A, B and C). HepG2 cells (10 × 106) were subcutaneously implanted in male Hsd:Athymic Nude-Foxn1nu mice. When the tumors reached 5 mm, one group of animals received orally by gavage either Sorafenib (200 mg/kg) or solvent (D, E and F). Trx1 expression and caspase-8 activity were measured as described in Material and Methods. Animals were sacrificed when tumors reached 15 mm. Results are expressed as mean ± SEM of six independent animals. The groups with different letters (a, b, c or d) were significantly different (p ≤ 0.05).
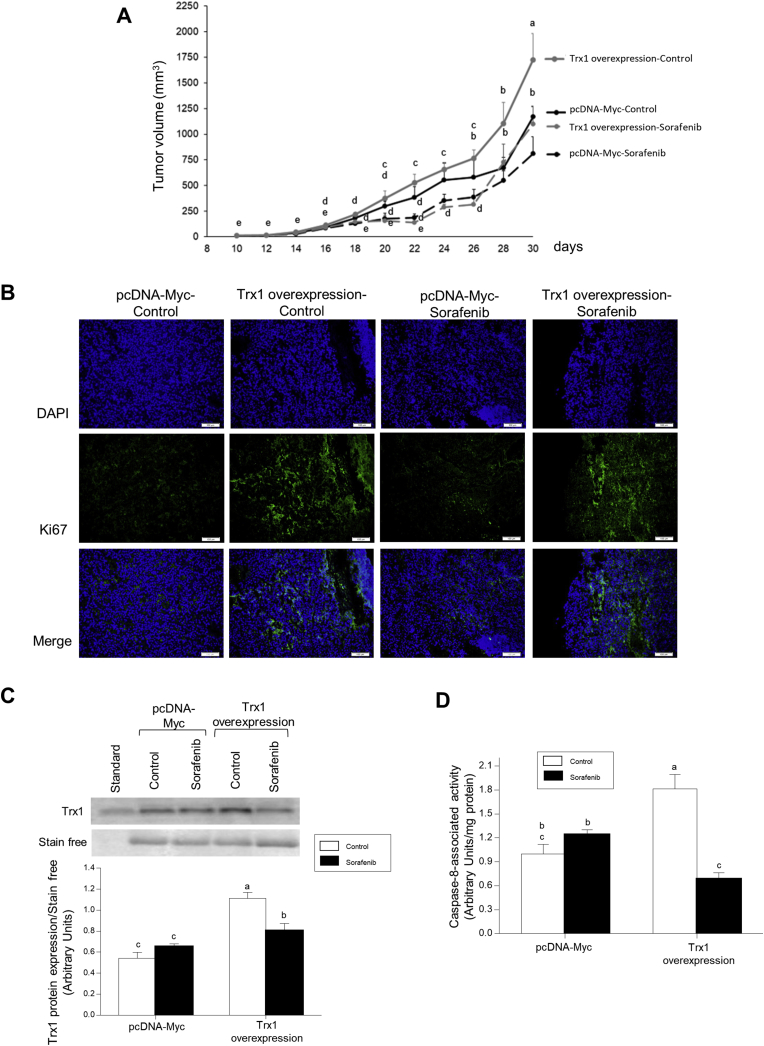
Trx1 overexpression increased tumor growth (Fig. 6A) and ki67 expression (Fig. 6B) in control and Sorafenib-treated animals. Sorafenib slowed tumor growth up to day 26th after implantation of Trx1 overexpressing HepG2 cells, while a strong relapse of tumor growth occurred from this time to the day of sacrifice (Fig. 6A). Sorafenib administration reduced Trx1 expression (Fig. 6C) and caspase-8 activity (Fig. 6D) in tumors derived from Trx1 overexpressing transfected HepG2 cells.
Fig. 6.
The reduction of tumor volume by Sorafenib was associated with downregulation of cell proliferation, Trx1 expression and caspase-8 activity in Trx1 overexpressing HepG2 cells subcutaneously implanted in nude mice. Effect of Sorafenib in tumor volume (A), Ki67 (B) and Trx1 (C) expressions, and caspase-8 activity in tumors derived from subcutaneous implantation of HepG2 cells stably transfected with either Trx1 overexpressing or empty pcDNA Myc (mock) vectors in nude mice. HepG2 cells (10 × 106) were subcutaneously implanted in male Hsd:Athymic Nude-Foxn1nu mice. When the tumors reached 5 mm, one group of animals received orally by gavage either Sorafenib (200 mg/kg) or solvent. Ki67 and Trx1 expressions, and caspase-8 activity were measured as described in Material and Methods. Animals were sacrificed when tumors reached 15 mm. Results are expressed as mean ± SEM of six independent animals. The groups with different letters (a, b, c, d or e) were significantly different (p ≤ 0.05).
4. Discussion
Sorafenib has demonstrated a moderate but significant increase in median overall survival in patients with HCC and good liver function [29,30]. However, a proportion of patients (2%) are resistant to treatment [31] which has been related to the activation of cell survival IGFR [6,7], c-MET [8], and FGFR [7] signalings. The alteration of redox regulatory mechanisms mainly leading to Nrf2 activation by Keap1 mutation is observed in 12% of patients with HCC [1]. The induction of cell death by Sorafenib has been associated with the generation of oxidative stress in HepG2 cells [21]. In concordance with Rahmani et al. [32], Sorafenib induced O2•- generation in mitochondria and cytoplasm fractions, but we observed that the magnitude of this increase was much higher in the mitochondria than in the cytoplasm, which was accompanied with a decrease in cytoplasmic H2O2 and NO (Fig. 1A–D). The results are compatible with the active antioxidant properties of Sorafenib in cytoplasm. In fact, Sorafenib exerted dose-dependent ROS scavenging action in vitro (Fig. 1E and F). It is interesting to note that the clinical effectiveness of the drug has also been related to the lessening of oxidative stress in peripheral blood monocytic cells obtained from Sorafenib-responder HCC patients [33]. Sorafenib includes a trifluoromethylated or CF3− anion in its molecule that involves an increased antitumoral potency [34] (Supplementary Fig. 1). This CF3− group might be involved in the radical scavenger properties of Sorafenib through a nucleophilic mechanism based on the donation of one electron to the free radical (O2•- or OH•) [35].
Transcription factor Nrf2 is the master regulator of antioxidant response through ARE-dependent transcriptional activation of several genes involved in phase I to III detoxification systems, antioxidants, and transporters that protect cells from toxic and carcinogenic compounds [36]. The transactivation activity of Nrf2 is regulated by Keap1, which sequesters Nrf2 in the cytoplasm [37]. The generation of oxidative stress or pharmacological induction promotes oxidation of critical cysteines C273 and C288 of Keap1 that leads to Nrf2 escaping from Keap1 retention and allowing its translocation into the nucleus where it heterodimerizes with small Maf proteins and transactivates ARE driven gene expression [38]. The administration of Sorafenib reduced the nuclear translocation of Nrf2 in wild type Keap 1 cells such as HepG2 (Fig. 2) and JHH-2, JHH-4 and SNU886 (Supplementary Fig. 3). Nrf2 downregulation in nuclear fraction induced by Sorafenib was associated with reduced ARE-dependent Nrf2 transactivation capacity (Fig. 2C), Trx1 expression (Fig. 2D) and Trx1 activity (Fig. 2E), as well as the mRNA expression of GST, TrxR1, Gpx1, NQO1, HO-1 and GCLC in control and VEGF-stimulated HepG2 cells (Supplementary Fig. 2).
Redox modifications of protein cysteine thiols, and SNO in particular, is a posttranslational regulatory mechanism that plays an important role in the modulation of cellular processes by ROS and RNS with effects in health and disease [23]. Interestingly, mRNA expression and activity (Fig. 3A and B) of GSNOR were transiently increased in Sorafenib-treated HepG2 cells. The expression of NOS and NO generation within different cell types present in the tumor microenvironment, such as tumor, endothelial and inflammatory cells, as well as fibroblasts, regulate tumor growth, migration, invasion, survival, angiogenesis, and metastasis in cancer [39]. The expression of NOS2 has been related to poor prognosis in HCC [40].
NOS3 activity is tightly modulated by its phosphorylation state under control of protein kinase type A (PKA) and C (PKC), Akt, 5′ adenosine monophosphate-activated protein kinase (AMPK), Ca2+/calmodulin-dependent kinase II, etc [41]. However, exposure of intact endothelial cells, plasma membranes, or purified NOS to NO also reduces NOS catalytic activity [25] by inducing a change from dimeric (active) to monomeric (inactive) form [42]. NO donors induce SNO–NOS3 in multiple cysteines, such as those located at the positions 93 and 98 residues that are involved in its dimerization through the zinc tetrathiolate center [43]. Trx1 has two cysteines at its active site, Cys32-Gly-Pro-Cys35, that can catalytically reduce specific protein disulfide bonds and other oxidative cysteine modifications, and can serve as a denitrosylase towards specific SNO-proteins [44] (Fig. 7, Graphical Abstract). In addition, three structural Cys residues at positions 62, 69, and 73 are also present in Trx1. Cys73 displays transnitrosylating activity when the active site Cys32/Cys35 is in the oxidized disulfide form and the reductase activity is blocked. The inhibition of caspase-3 activity by SNO has been shown to be mediated by Trx1 [44]. Sorafenib reduced NO generation probably though NOS3 downregulation moreover than altering SNO–NOS3 in control cells (Fig. 3D and C, respectively). Trx1 overexpression reduced SNO–NOS3 and increased NO generation (Fig. 3C and D, respectively).
Trx1 plays a key role against oxidative stress by regulating protein thiol/disulfide balance, supplying electrons to thiol-dependent peroxidases (peroxiredoxins), ribonucleotide reductase, methionine sulfoxide reductases, and regulating the activity of many redox-sensitive transcription factors, selenium and nitric oxide metabolism [14]. TrxR1, glutathione reductase and Nrf2-driven antioxidant systems attenuate potentially carcinogenic oxidative damage but also protect cancer cells from oxidative death. The expression of Nrf2 and TrxR-Trx1 system has been related to resistance of cancer cells to chemotherapy [[26], [27], [28]]. McLouglin et al. [45] showed that the frequency of DEN-induced cancer initiation decreased in TrxR1-null livers. Stable transfection of murine NIH 3T3 fibroblast-like cells and human MCF-7 breast cancer cells with cDNA encoding for human wild-type Trx increased cell proliferation, whereas redox-inactive mutant Trx acted in a dominant negative manner [46].
The induction of NO-related posttranslational modifications in components of cell death pathways promotes different outcomes in terms of cell death or survival [39]. In particular, the prevention of SNO-CD95 by mutation of Cys199 or Cys304 residues avoids its recruitment and activation, respectively [47]. Sorafenib reduced SNO-CD95 (Fig. 4A), caspase-8 (Fig. 4B), and cell proliferation (Fig. 4D) while increased caspase-3 (Fig. 4C). Although CD95 is a prototypical trigger of the extrinsic apoptotic pathway, it has also been shown to be required for optimal cell proliferation, motility and invasion in tumor cells [48].
The overexpression of Trx1 increased caspase-8 activity and cell proliferation, but decreased caspase-3 in control and Sorafenib-treated HepG2 cells (Fig. 4). The downregulation of Trx1 by siRNA strategy diminished caspase-8 activity (Fig. 4A), but had no effect on caspase-3 activity (Fig. 4C) and cell proliferation (Fig. 4D) in control cells. These results suggest that CD95-dependent caspase-8 activity might be the primary target of Trx1 downregulation. Silencing of Trx1 did not change these parameters in Sorafenib-treated HepG2 cells (Fig. 4B–D) that might suggest a critical role of Trx1 downregulation in the reduction of caspase-8 activity by Sorafenib in HepG2 cells (Fig. 4B). The inverse relationship between caspase-8 and caspase-3 during Sorafenib treatment has been previously shown in HepG2 cells [11].
Several tumorigenic signals such as NF-kB, ERK, and caspase-8 are downstream effectors of CD95 [49]. This protumorigenic phenotype has been related to tyrosine phosphorylation of caspase-8 by src-family kinases [50], as well as by oxidative stress-dependent YES activation of EGFR and CD95 tyrosine phosphorylation [51]. In addition, Chen et al. [52] showed that Sorafenib enhances TRAIL-induced cell death through SH2 domain-containing tyrosine phosphatase (SHP-1) dependent decrease in the phosphorylation of signal transducers and activators of transcription type 3 (STAT3) and downstream-regulated proteins such as Mcl-1, survivin, and cyclin D1 in hepatoma cells. STAT3 is a pro-survival transcription factor which is constitutively activated in human cancer cell lines. Tyrosine phosphorylation of STAT3 is dependent on the thiol redox state modulated by H2O2 and peroxiredoxin-2 levels and the Trx system activity [53]. We have recently shown that the treatment with Sorafenib induced thiol redox reductive changes in STAT3, and Trx1 downregulation counteracted this effect of the drug in HCC cell lines [16]. We are deeply investigating CD95-related downstream events leading to regulation by Sorafenib of cell proliferation in liver cancer cells.
The reduction of Trx1 expression and activity appears to be essential in the antitumoral action of Sorafenib. Two independent experimental models of HCC showed that the antitumoral properties of Sorafenib were related to a decrease in Trx1 expression, SNO-CD95 and caspase-8 activity (Fig. 5 and Supplementary Fig. 4). The in vivo experiment involving subcutaneous implantation of Trx1 overexpressing HepG2 cells further supports the relevant role of Trx1 in tumor growth. Sorafenib reduced tumor growth, Ki67 and Trx1 expressions and caspase-8 activity in tumors derived from Trx1-overexpressing HepG2 cells in nude mice (Fig. 6).
In conclusion, the antioxidant properties of Sorafenib might positively influence the downregulation of Nrf2-dependent Trx1 expression which plays a relevant role in the deactivation of NOS3 and CD95 through SNO. CD95 deactivation could also be achieved by transient upregulation of GSNOR and downregulation of CD95 by Sorafenib in HepG2 cells. Decreased expression of SNO-CD95 by Sorafenib involved reduction of caspase-8 activity and cell proliferation, and increased downstream caspase-3 activity in liver cancer cells (Fig. 7, Graphical Abstract). The mechanism supports the inverse relationship between caspase-8 and caspase-3 during Sorafenib treatment in HepG2 cells [11]. The relevance of Trx1, SNO-CD95 and caspase-8 downregulation in the antitumoral properties of Sorafenib was demonstrated in three independent experimental models of hepatocarcinogenesis.
Financial support
This study was funded by Institute of Health Carlos III (ISCIII) (PI13/00021, PI15/00107, PI16/00090 and PI19/01266), Spanish Ministry of Economy and Competitiveness (SAF2015-71208-R, BFU2016-8006-P, PGC2018-094276-B-I00 and RED2018-102576-T), Andalusian Ministry of Economy, Innovation, Science and Employment (BIO-216 and CTS-6264) and Andalusian Ministry of Equality, Health and Social Policies (PI-00025-2013 and PI-0198-2016). P de la C–O was supported by FPU predoctoral fellowship (FPU17/00026) from Ministry of Education, Culture and Sports. We thank the Biomedical Research Network Center for Cardiovascular Diseases (CIBERCV), and the Biomedical Research Network Center for Liver and Digestive Diseases (CIBERehd) founded by the ISCIII and co-financed by European Development Regional Fund “A way to achieve Europe” ERDF for their financial support.
Acknowledgments
We thank Prof. Jessica Zucman-Rossi and Dr. Sandra Rebouissou for supplying and providing information regarding the use of JHH2, JHH-4, JHH-5, Huh-1, SNU886 and MHCC97H cell lines.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101528.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Schulze K., Imbeaud S., Letouze E., Alexandrov L.B., Calderaro J., Rebouissou S., Couchy G. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The L., European Organisation For R., Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Stravitz R.T., Heuman D.M., Chand N., Sterling R.K., Shiffman M.L., Luketic V.A., Sanyal A.J. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am. J. Med. 2008;121:119–126. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J., Reig M., Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 6.Huynh H. AZD6244 (ARRY-142886) enhances the antitumor activity of rapamycin in mouse models of human hepatocellular carcinoma. Cancer. 2010;116:1315–1325. doi: 10.1002/cncr.24863. [DOI] [PubMed] [Google Scholar]
- 7.Tovar V., Cornella H., Moeini A., Vidal S., Hoshida Y., Sia D., Peix J. Tumour initiating cells and IGF/FGF signalling contribute to sorafenib resistance in hepatocellular carcinoma. Gut. 2017;66:530–540. doi: 10.1136/gutjnl-2015-309501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huynh H., Ngo V.C., Koong H.N., Poon D., Choo S.P., Thng C.H., Chow P. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J. Cell Mol. Med. 2009;13:2673–2683. doi: 10.1111/j.1582-4934.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervello M., Bachvarov D., Lampiasi N., Cusimano A., Azzolina A., McCubrey J.A., Montalto G. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle. 2012;11:2843–2855. doi: 10.4161/cc.21193. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Hernandez M.A., Gonzalez R., de la Rosa A.J., Gallego P., Ordonez R., Navarro-Villaran E., Contreras L. Molecular characterization of autophagic and apoptotic signaling induced by sorafenib in liver cancer cells. J. Cell. Physiol. 2018;234:692–708. doi: 10.1002/jcp.26855. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Hernandez A., Navarro-Villaran E., Gonzalez R., Pereira S., Soriano-De Castro L.B., Sarrias-Gimenez A., Barrera-Pulido L. Regulation of cell death receptor S-nitrosylation and apoptotic signaling by Sorafenib in hepatoblastoma cells. Redox Biol. 2015;6:174–182. doi: 10.1016/j.redox.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Sanchez G., Giuliani A. Cellular redox status regulates hypoxia inducible factor-1 activity. Role in tumour development. J. Exp. Clin. Canc. Res. 2007;26:39–50. [PubMed] [Google Scholar]
- 13.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 14.Lu J., Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H., Masutani H., Tagaya Y., Yamauchi A., Inamoto T., Nanbu Y., Fujii S. Expression and growth-promoting effect of adult T-cell leukemia-derived factor. A human thioredoxin homologue in hepatocellular carcinoma. Cancer. 1992;69:2091–2097. doi: 10.1002/1097-0142(19920415)69:8<2091::aid-cncr2820690814>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.López-Grueso M.J., González R., Muntané J., Bárcena J.A., Padilla C.A. Thioredoxin downregulation enhances sorafenib effects in hepatocarcinoma cells. Antioxidants. 2019;8 doi: 10.3390/antiox8100501. 501-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 18.Izquierdo-Álvarez A., Tello D., Cabrera-García J.D., Martínez-Ruiz A. Identification of S-nitrosylated and reversibly oxidized proteins by fluorescence switch and complementary techniques. Methods Mol. Biol. 2018;1747:73–87. doi: 10.1007/978-1-4939-7695-9_7. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Sanchez L.M., Corrales F.J., Gonzalez R., Ferrin G., Munoz-Castaneda J.R., Ranchal I., Hidalgo A.B. Alteration of S-nitrosothiol homeostasis and targets for protein S-nitrosation in human hepatocytes. Proteomics. 2008;8:4709–4720. doi: 10.1002/pmic.200700313. [DOI] [PubMed] [Google Scholar]
- 20.Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 1979;254:9627–9632. [PubMed] [Google Scholar]
- 21.Coriat R., Nicco C., Chereau C., Mir O., Alexandre J., Ropert S., Weill B. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol. Canc. Therapeut. 2012;11:2284–2293. doi: 10.1158/1535-7163.MCT-12-0093. [DOI] [PubMed] [Google Scholar]
- 22.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Stamler J.S., Lamas S., Fang F.C. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 24.Liu L., Hausladen A., Zeng M., Que L., Heitman J., Stamler J.S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 25.Patel J.M., Zhang J., Block E.R. Nitric oxide-induced inhibition of lung endothelial cell nitric oxide synthase via interaction with allosteric thiols: role of thioredoxin in regulation of catalytic activity. Am. J. Respir. Cell Mol. Biol. 1996;15:410–419. doi: 10.1165/ajrcmb.15.3.8810647. [DOI] [PubMed] [Google Scholar]
- 26.Wang X.J., Sun Z., Villeneuve N.F., Zhang S., Zhao F., Li Y., Chen W. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnér E.S.J. vol. 136. 2017. pp. 139–151. (Targeting the Selenoprotein Thioredoxin Reductase 1 for Anticancer Therapy). [DOI] [PubMed] [Google Scholar]
- 28.Roh J.L., Jang H., Kim E.H., Shin D. Targeting of the glutathione, thioredoxin, and Nrf2 antioxidant systems in head and neck cancer. Antioxidants Redox Signal. 2017;27:106–114. doi: 10.1089/ars.2016.6841. [DOI] [PubMed] [Google Scholar]
- 29.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 31.Villanueva A., Llovet J.M. Second-line therapies in hepatocellular carcinoma: emergence of resistance to sorafenib. Clin. Canc. Res. 2012;18:1824–1826. doi: 10.1158/1078-0432.CCR-12-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiou J.F., Tai C.J., Wang Y.H., Liu T.Z., Jen Y.M., Shiau C.Y. Sorafenib induces preferential apoptotic killing of a drug- and radio-resistant Hep G2 cells through a mitochondria-dependent oxidative stress mechanism. Canc. Biol. Ther. 2009;8:1904–1913. doi: 10.4161/cbt.8.20.9436. [DOI] [PubMed] [Google Scholar]
- 33.Caraglia M., Giuberti G., Marra M., Addeo R., Montella L., Murolo M., Sperlongano P. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis. 2011;2:e150. doi: 10.1038/cddis.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y., Wang J., Gu Z., Wang S., Zhu W., Aceña J.L., Soloshonok V.A. Next generation of fluorine-containing Pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 2016;116:422–518. doi: 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]
- 35.Chu L., Qing F.-L. Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (Trifluoromethyl)trimethylsilane as a nucleophilic CF3 source. Acc. Chem. Res. 2014;47:1513–1522. doi: 10.1021/ar4003202. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi M., Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxidants Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 37.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez R., Molina-Ruiz F.J., Barcena J.A., Padilla C.A., Muntane J. Regulation of cell survival, apoptosis, and epithelial-to-mesenchymal transition by nitric oxide-dependent post-translational modifications. Antioxidants Redox Signal. 2018;29(13):1312–1332. doi: 10.1089/ars.2017.7072. [DOI] [PubMed] [Google Scholar]
- 40.Rahman M.A., Dhar D.K., Yamaguchi E., Maruyama S., Sato T., Hayashi H., Ono T. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin. Canc. Res. 2001;7:1325–1332. [PubMed] [Google Scholar]
- 41.Hu Z., Chen J., Wei Q., Xia Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: co-commitment and interplay of Akt and AMPK. J. Biol. Chem. 2008;283:25256–25263. doi: 10.1074/jbc.M802455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravi K., Brennan L.A., Levic S., Ross P.A., Black S.M. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tummala M., Ryzhov V., Ravi K., Black S.M. Identification of the cysteine nitrosylation sites in human endothelial nitric oxide synthase. DNA Cell Biol. 2008;27:25–33. doi: 10.1089/dna.2007.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell D.A., Marletta M.A. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat. Chem. Biol. 2005;1:154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 45.McLoughlin M.R., Orlicky D.J., Prigge J.R., Krishna P., Talago E.A., Cavigli I.R., Eriksson S. TrxR1, Gsr, and oxidative stress determine hepatocellular carcinoma malignancy. Proc. Natl. Acad. Sci. U. S. A. 2019 doi: 10.1073/pnas.1903244116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallegos A., Gasdaska J.R., Taylor C.W., Paine-Murrieta G.D., Goodman D., Gasdaska P.Y., Berggren M. Transfection with human thioredoxin increases cell proliferation and a dominant-negative mutant thioredoxin reverses the transformed phenotype of human breast cancer cells. Canc. Res. 1996;56:5765–5770. [PubMed] [Google Scholar]
- 47.Leon-Bollotte L., Subramaniam S., Cauvard O., Plenchette-Colas S., Paul C., Godard C., Martinez-Ruiz A. S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology. 2011;140:2009–2018. doi: 10.1053/j.gastro.2011.02.053. 2018 e2001-2004. [DOI] [PubMed] [Google Scholar]
- 48.Barnhart B.C., Legembre P., Pietras E., Bubici C., Franzoso G., Peter M.E. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004;23:3175–3185. doi: 10.1038/sj.emboj.7600325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin-Villalba A., Llorens-Bobadilla E., Wollny D. CD95 in cancer: tool or target? Trends Mol. Med. 2013;19:329–335. doi: 10.1016/j.molmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Barbero S., Mielgo A., Torres V., Teitz T., Shields D.J., Mikolon D., Bogyo M. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Canc. Res. 2009;69:3755–3763. doi: 10.1158/0008-5472.CAN-08-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ta N.L., Chakrabandhu K., Huault S., Hueber A.O. The tyrosine phosphorylated pro-survival form of Fas intensifies the EGF-induced signal in colorectal cancer cells through the nuclear EGFR/STAT3-mediated pathway. Sci. Rep. 2018;8:12424. doi: 10.1038/s41598-018-30804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen K.F., Tai W.T., Liu T.H., Huang H.P., Lin Y.C., Shiau C.W., Li P.K. Sorafenib overcomes TRAIL resistance of hepatocellular carcinoma cells through the inhibition of STAT3. Clin. Canc. Res. 2010;16:5189–5199. doi: 10.1158/1078-0432.CCR-09-3389. [DOI] [PubMed] [Google Scholar]
- 53.Sobotta M.C., Liou W., Stöcker S., Talwar D., Oehler M., Ruppert T., Scharf A.N.D. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015;11:64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.