Highlights
-
•
The functional network of children with childhood absence epilepsy is less efficiently organized in terms of clustering and small-worldness.
-
•
Longer path lengths (i.e. less efficient organization) of the functional network relate to a longer duration of childhood absence epilepsy.
-
•
Longer path lengths of the functional network relate to a higher seizure frequency in childhood absence epilepsy.
Keywords: Graph theory, Network analysis, Cognitive performance, Seizures, Functional mri
Abstract
While cognitive impairments are not generally considered to be part of the childhood absence epilepsy (CAE) syndrome, some recent studies report cognitive, mainly attentional, deficits. Here we set out to investigate the whole brain functional network of children with CAE and controls. Furthermore, the possible relation of the functional network abnormalities with epilepsy and neurocognitive characteristics is studied.
Seventeen children with childhood CAE (aged 9.2 ± 2.1 years) and 15 controls (aged 9.8 ± 1.8 years) were included. Resting state functional MRI was acquired to study the functional network. Using graph theoretical analysis, three global metrics of the functional network were investigated: the characteristic path length, the clustering coefficient, and the small-worldness. A multivariable linear regression model including age, sex, and subject motion as covariates was used to investigate group differences in the graph metrics. Subsequently, relations of the graph metrics with epilepsy and neurocognitive characteristics were assessed.
Longer path lengths, weaker clustering and a lower small-world network topology were observed in children with CAE compared to controls. Moreover, longer path lengths were related to a longer duration of CAE and a higher number of absence seizure per hour. Clustering and small-worldness were not significantly related to epilepsy or neurocognitive characteristics.
The organization of the functional network of children with CAE is less efficient compared to controls, and is related to disease duration. These preliminary findings suggest that CAE is associated with alterations in the functional network.
1. Introduction
Childhood absence epilepsy (CAE) constitutes approximately 12% of all types of childhood epilepsies (Berg et al., 1999). Children with CAE suffer from frequent absence seizures that occur multiple times per day (Curwood et al., 2015; Li et al., 2015), often in clusters. Absence seizures typically last around 10 s, during which the child is unresponsive to the environment (Kessler et al., 2017). At these times, generalized 3 Hz spike-and-wave discharges (GSWD) can be observed in an electroencephalogram (EEG) (Li et al., 2015; Luo et al., 2011). The onset of CAE is usually between the ages of 5 and 10 years (Curwood et al., 2015; Guerrini, 2006). Most children with CAE will outgrow the seizures when reaching adulthood, therefore CAE has traditionally been considered a benign syndrome (Vining and Thio, 2013). However, recent reports indicate a broad spectrum of abnormal behavioural and cognitive performance in CAE, including deficits in attention, memory, processing speeds, and language (Caplan et al., 2008; Loughman et al., 2014). Furthermore, these deficits in cognitive performance may persist, even after becoming seizure-free (Fonseca Wald et al., 2019).
Previous research has shown that children with CAE have altered resting-state functionality in selected networks of the brain (Li et al., 2015; Luo et al., 2014; Wang et al., 2017). Differences in functional connectivity have been reported using independent component analysis (ICA), particularly in dorsal attention, salience, and default mode networks (Li et al., 2015). Furthermore, disruptions of hubs in the functional network were found for the default mode network and the thalamus (Wang et al., 2017). Moreover, a less efficiently organized whole brain structural network in children with CAE was previously reported using diffusion weighted imaging (Qiu et al., 2017). Since prior cohort studies in children with epilepsy suggested that early changes of functional brain organization are followed by changes of structural connectivity (Besseling et al., 2014; Overvliet et al., 2013), and based on the clinical profile and widespread cognitive comorbidities of this allegedly benign syndrome (Caplan et al., 2008; Loughman et al., 2014; Verrotti et al., 2015), we would also expect whole brain functional disruptions. While the whole brain functional network has already been shown to be reorganized during absence seizures (Liao et al., 2014), currently the involvement of the resting state functional network in CAE (i.e. inter-ictally) on a whole brain level remains undetermined. Whole brain network metrics can be calculated using graph theoretical analysis. Graph theory was developed as a way of representing pairwise relations between objects, using vertices (or nodes) and their relations defined as edges (or connections) (Onias et al., 2014). For the brain, the nodes represent brain regions and the edges are the connections between the brain regions (Telesford et al., 2013). In case of functional magnetic resonance imaging (fMRI), these connections do not describe the physical structural connections, but rather their temporal correlations (e.g. functional connectivity).
In this study, we will investigate characteristics of functional connectivity in children with CAE and controls by means of graph theoretical analysis. Two global graph metrics, the characteristic path length and the clustering coefficient, will be used to quantify the functional brain network in terms of integration and segregation, respectively (Onias et al., 2014; Rubinov and Sporns, 2010). Moreover, the overall topology of the networks will be assessed using the small-worldness, which is indicative of the overall organization of the networks (Watts and Strogatz, 1998). Subsequently, we will assess whether the global graph metrics differ between children with CAE and controls. Thereafter, we aim to explore the potential relation of the global graph metrics with epilepsy characteristics and neurocognitive performance.
2. Methods
2.1. Participants
Seventeen children with a clinical diagnosis of CAE (aged 6–12y) were prospectively included. Children with a clinical diagnosis of CAE were included based on the following criteria: 1) Primarily presenting with daily occurring episodes of brief loss of consciousness in an otherwise normal child and an EEG showing ictal 3 Hz (2.5–4.5 Hz) generalized rhythmic spike-and-wave complexes with a discharge duration of at least 3 s on a present or former EEG (in accordance to ILAE statements for CAE (Berg et al., 2010; Fisher et al., 2017)). 2) Early absence epilepsy, defined as a confirmed diagnosis or seizure onset within 2 years. 3) Six to twelve years of age (Drenthen et al., 2019). Additionally, fifteen controls (aged 7–12y) were included for comparison, the control subjects were carefully selected such that, on average, the age and sex of the two groups matched approximately. All included children were following regular education, except one child included in the CAE group who followed special needs education. All caregivers, and participants aged ≥12 years old gave written permission prior to inclusion in the study and this research was approved by the medical ethics committee azM/UM NL55455.068.15/METC152055 and is listed at clinicaltrials.gov under NCT02954107. The following epilepsy characteristics were recorded: age at onset, duration of epilepsy and number of GSWD per hour. The latter was determined using a 24h-EEG, where the number of GSWD that lasted at least 3 s were counted in three separate and randomly chosen hours during which the patient was in a wakeful state. The mean of the three measurements was used as a measure of seizure frequency. Furthermore, nine of the children with CAE were taking Ethosuximide (range 14.7 - 27.2 mg/kg), three were taking Valproïc Acid (range 13.3 - 30.8 mg/kg), two were taking Ethosuximide + Valproïc Acid (1. 31.8 mg/kg ETM and 22.7 mg/kg VPA; 2. 25.7 mg/kg ETM and 14.12 mg/kg VPA) and one was taking Lamotrigine + Clobazam (1.4 + 0.42 mg/kg). At the time of the MRI, two children were still drug-naïve, whereas 15 were on anti-epileptic drug treatment. Five children with CAE were already successfully treated with anti-epileptic drugs (AEDs), and were therefore omitted from any subsequent analysis with seizure frequency.
For all participants, general intelligence and processing speed was determined using six subtests (mean ± SD = 10 ± 3) of the Dutch version of the Wechsler Intelligence Scale for Children third edition (WISC-III) (similarities, vocabulary, picture completion, coding, block design, and symbol search). Furthermore, the Bourdon-Vos, a paper and pencil cancelation test, was used to test for sustained visual attention and vigilance (Vos, 1988). The time needed to complete the test and the errors made are recorded. The subject characteristics are shown in Table 1. Group differences in sex, handedness, schooling and family history were assessed using the Chi-square test of independence. Prior to assessing group-differences between the rest of the subject characteristics, the Anderson-Darling test of normality was performed (Anderson and Darling, 1952). For the normally distributed characteristics, general intelligence and processing speed, differences were assessed using Student t-tests, while differences between the non-normally distributed characteristics, age, and Bourdon-Vos duration and errors, were assessed using Mann–Whitney U tests. The two groups did not differ significantly regarding age, sex, and general intelligence. Processing speed index (mean ± SD = 100 ± 15) was significantly lower for children with CAE compared to controls (95 ± 15 vs. 108 ± 14, p = .03). Furthermore, the children with CAE took longer to finish the Bourdon-Vos test (12 ± 3.3 min vs. 9.5 ± 2.0 min, p = .04), while no difference in the number of errors was found.
Table 1.
Subject characteristics of children with CAE and controls.
| CAE | Controls | p-value | |
|---|---|---|---|
| Number (#) | 17 | 15 | – |
| Age (y, mean ± SD) | 9.2 ± 2.1 | 9.8 ± 1.8 | .36 |
| Sex (M/F) | 12/5 | 11/4 | .86 |
| Handedness (R/L) | 16/1 | 11/4 | .11 |
| Age of onset (y, mean ± SD) | 8.0 ± 2.0 | – | – |
| Duration of epilepsy (y, mean ± SD) | 1.2 ± 0.74 | – | – |
| Average GSWD per hour (mean ± SD) | 6.3 ± 7.0 | – | – |
| Schooling (regular/special) | 16/1 | 15/0 | .34 |
| Family history of epilepsy (Yes/No) | 2/15 | 1/14 | .62 |
| WISC-III Subtests (mean ± SD) | 9.6 ± 1.9 | 10.9 ± 2.3 | .09 |
| WISC-III Processing speed index (mean ± SD) | 95 ± 15 | 108 ± 14 | .03 |
| Bourdon-Vos duration (min, mean ± SD) | 12 ± 3.3 | 9.5 ± 2.0 | .04 |
| Bourdon-Vos errors (#, mean ± SD) | 17 ± 12 | 13 ± 12 | .06 |
Y, years; SD, standard deviation; L, left; R, right; CAE, childhood absence epilepsy; WISC-III, Wechsler Intelligence Scale for Children, third edition; GSWD, generalized spike wave discharges.
2.2. MRI acquisition
All subjects were scanned on a 3.0 T unit (Philips Achieva, Best, the Netherlands) using a 32-element phased array coil. To minimize moving during the MRI exam, children were carefully prepared beforehand using a combination of video and written information. Furthermore, parents were instructed to practice laying still with the child at home, and be present with them in the magnet room during scanning. First, for anatomical reference and segmentation, T1-weighted (T1w) 3D turbo field echo images were acquired (repetition time (TR) = 8.36 ms, echo time (TE) = 3.84 ms, flip angle (FA) = 8°, voxel size = 1 × 1 × 1 mm, field of view = 240 × 240 × 180 mm). Functional MR images were acquired in resting state with eyes closed using a single-shot echo planar imaging (EPI) sequence (TR = 2000 ms, 31 slices, TE = 35 ms, pixel size = 2 × 2 mm, 4 mm thick transverse slices, field of view = 212 × 256 × 124 mm, and 195 acquisitions).
2.3. Analysis
2.3.1. Preprocessing
Preprocessing of the fMRI data and T1w structural images was performed using the Statistical Parametric Mapping software package, SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) in Matlab R2016b. The structural images were automatically parcellated into 68 cortical and 14 sub-cortical regions using Freesurfer (version 5.1) based on the Desikan-Killiany atlas (Desikan et al., 2006; Fischl, 2004). The resulting parcellations were visually checked and corrected manually where appropriate.
First, a slice timing correction was applied to the functional images. Second, to correct for head displacement, all slices were computationally realigned to the first volume in the sequence. Third, the functional images were smoothed through convolution with a 6 mm full-width half maximum (FWHM) Gaussian kernel. Fourth, a band-pass filter of 0.01 to 0.1 Hz was applied to extract the frequency band of interest. Fifth, the first 5 vol of the functional images were discarded to ensure steady-state longitudinal magnetization of the blood-oxygen level dependent (BOLD) signal (Li et al., 2015; Liao et al., 2010). Last, the time signals of each region of interest were averaged and corrected for head motion (relative translational movement (Van Dijk et al., 2012)) as well as white matter and cerebrospinal fluid (CSF) signals via linear regression in order to reduce the contribution of physiological noise (Onias et al., 2014). To cope with EPI distortions in the fMRI data, the T1w image and corresponding Freesurfer atlas were non-linearly co-registered to the functional images using Elastix v4.9.0 (Klein et al., 2010). Subsequently, a mask based on the outline of the brain in the fMRI data was used to prevent that fMRI voxels outside brain regions were included.
Since we included young children in our study, subject motion is likely to affect our results. Therefore, mean relative motion parameters for translational and rotational movement were included in the statistical analysis as covariates (Van Dijk et al., 2012).
2.3.2. Functional network construction
For each pair of regions, the connection strength was calculated using the Pearson's correlation coefficients of the fMRI signals, resulting in a weighted adjacency matrix representing the functional network. Only those region pairs with a significant Pearson's correlation coefficient (p < .05) are added as an edge to the network. Furthermore, the Pearson's correlation coefficients are used as weights, where a higher correlation is considered a ‘stronger’ connection. Negative correlations were set to zero because currently the role of negative weights in the functional network is unclear (Rubinov and Sporns, 2010). On average, only 2% of the correlations were negative. Additionally, to cope with noise and false positive connections, non-significant correlation coefficients in the connectivity matrix were set to zero. Functional network construction was performed in Matlab R2016b.
2.3.3. Network analysis
The weighted functional networks are quantitatively described by two of the most robust and widely applied global graph metrics, the characteristic path length (L) and clustering coefficient (C) (Onnela et al., 2005; Watts and Strogatz, 1998). L provides insight into how well information can spread throughout a network, while C is a measure of local information processing. A network with strongly clustered modules and relative short path length between nodes is considered an efficiently organized small-world network. Graph analysis is highly dependent on the topology of the network (van Wijk et al., 2010). To obtain global graph metrics that are normalized with respect to variations in topology, the global graph metrics are determined relative to the average of 100 random networks with similar degree distribution (λ=L/Lrand and γ=C/Crand). Random networks were constructed by iteratively rewiring the connections, each edge is rewired approximately 10 times (Maslov and Sneppen, 2002). A measure of small-worldness can now be defined as σ=γ/λ, where σ>1 indicates that a network has a small-world topology. Furthermore, only networks with the same number of nodes and edges (i.e. the networks are equally sparse) will be compared. Moreover, to decrease the occurrence of false positives and false negatives in the network, only the nodes and edges present in the network of at least half of the subjects are considered in the graph analysis (i.e. group thresholding) (Reus and Heuvel, 2013). The number of edges in each network is varied such that the functional networks are 60–90% sparse, with intervals of 1%. Networks sparser than 90% would lead to disconnected nodes, hindering the graph analysis. Graph metrics were calculated using the Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net) in Matlab R2016b.
2.3.4. Statistical analysis
Between-group differences in the global graph metrics were assessed using multivariable linear regression models. To account for demographical differences and head motion, age, sex and the two mean motion parameters were added to the models as covariates. However, since the motion parameters are inherently correlated (r = 0.88, p < .01), only the mean translation motion parameter was added to the model to prevent multicollinearity in the regression model. Prior to the analysis, the non-normally distributed mean motion parameters and number of GSWD per hour were transformed using the Box-Cox transformation (Box and Cox, 1964).
To assess whether the graph metrics relate to epilepsy and cognitive characteristics, linear multivariable regression models were used, correcting for the effects of age, sex and head motion. For the children with CAE, the relation of the global graph metrics with the duration of CAE, number of GSWD per hour, general intelligence, and processing speed index, was assessed using linear regression models, correcting for the effects of age, sex and head motion. Moreover, the relation of the global graph metrics and errors of the Bourdon-Vos test was assessed using linear regression models, correcting for the effects of age, sex, head motion and the duration of the Bourdon-Vos test. Furthermore, in the control group, the relation of global graph metrics with the general intelligence, and processing speed index was assessed using linear regression models, correcting for the effects of age, sex, and head motion. Moreover, the relation of the global graph metrics and errors of the Bourdon-Vos test was assessed using linear regression models, correcting for the effects of age, sex, head motion and the duration of the Bourdon-Vos test.
Statistical significance was inferred when p < .05.
3. Results
3.1. Between-group differences
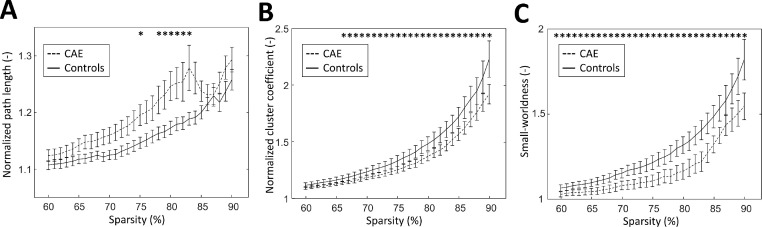
The graph metrics of children with CAE and controls are plotted with respect to the sparsity level in Fig. 1. The normalized characteristic path length was significantly higher in children with CAE compared to controls for networks that are 75% and 78–83% sparse. The normalized clustering coefficient was found to be lower in children with CAE compared to controls, reaching statistical significance over a wide sparsity range (67–90%). Similarly, the small-worldness was significantly lower in children with CAE compared to controls, over nearly the whole sparsity range (62–90%).
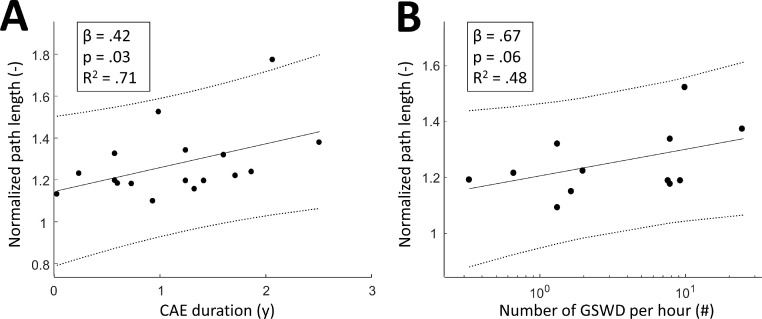
Fig. 2.
The relationship of normalized path length for networks that are 82% sparse with A) the duration of CAE and B) the number of GSWD per hour. A least squares line and 95% confidence intervals are added for visualization. Note that the latter is boxcox transformed and therefore displayed on a log-scale. CAE, childhood absence epilepsy; GSWD, generalized spike-wave discharges.
Fig. 1.
A) Normalized path length, B) normalized cluster coefficient and C) normalized small-worldness as a function of sparsity. Mean and standard errors for children with CAE (dashed) and controls (solid) are shown. Asterisks indicate a significant difference between the two groups (p < .05).
The mean motion parameters did not significantly differ between the children with CAE and control subjects (data not shown).
3.2. Epilepsy and neurocognitive characteristics
The normalized path length was found to be positively related to the duration of CAE, reaching significance for sparsity values 82% and 83%. Furthermore, the normalized path length related significantly to the number of GSWD per hour for sparsity values 68%, and 70–81%. The normalized clustering coefficient and small-worldness did not significantly relate to either the duration of CAE nor number of GSWD per hour (p > .10). Fig. 2 shows the normalized path length of networks for a representative sparsity of 82% as a function of the duration of CAE (A) and as a function of the GSWD per hour (B).
The graph metrics did not relate significantly to general intelligence, processing speed index or number of errors of the Bourdon-Vos test in either the control or the CAE group.
The mean motion parameters did not significantly correlate to the epilepsy variables (data not shown).
4. Discussion
4.1. Current findings
In the current study, we aimed to study alterations in the global functional network of children with CAE compared to controls on a whole brain level using graph theoretical analysis. We have shown that the functional network organization is impaired in children with CAE, revealing a less efficiently organized network in terms of a weaker clustering and less small-world organized networks. Furthermore, a longer duration of CAE and higher number of GSWD per hour is related to longer path lengths (i.e. more deviant compared to controls), illustrating a relationship with disease development. Clustering and small-worldness were not significantly related to epilepsy characteristics. None of the graph metrics were related to general intelligence in either the controls or children with CAE.
4.2. Between-group differences
Weaker clustering and lower small-worldness are reported in children with CAE compared to controls, showing that the network topology exhibits a less small-world organization. These results indicate that the functional brain network of children with CAE is organized in a less efficient manner compared to controls. Previously, similar findings of weaker clusters and lower small-worldness were reported in the structural network of children with CAE using diffusion weighted imaging (Qiu et al., 2017). Combined with the results from our study, this implies a disruption of the brain network on a functional as well as structural level. To date, reports on functional network changes in CAE have mainly focussed on the default mode network and the attention networks (dorsal and salience) (Bear, 2019; Li et al., 2015; Luo et al., 2014, 2011; Wang et al., 2017). Possibly, our results could be related to some extent to the underlying impairments of either the default mode or attention networks. However, here we showed that the functional alterations are not limited to certain resting state networks, but rather extend to the functional brain network as a whole. This could provide additional insights that not focus on particular (e.g. attentional) deficits in CAE, but rather on the broad spectrum of behavioural, cognitive and linguistic comorbidities of CAE (Caplan et al., 2008). Previously, it was shown that the whole brain functional network reorganized during and after a GSWD (Liao et al., 2014). The results of this study extend upon this by showing that the inter-ictal functional brain network also seems to be affected.
4.3. Relationship of network metrics with epilepsy characteristics
Longer path lengths were found to be associated with a longer duration of CAE and more GSWD per hour. Networks with high degrees of clustering and short path lengths are considered efficient networks with a small-world organization (Watts and Strogatz, 1998). Therefore, longer paths indicate a less optimal network organization and could be a contributing factor to the pathology. Previously using fMRI, the local functional network disruptions in CAE were also related to the duration of CAE. More specifically, a longer duration of CAE was related to a lower degree centrality in the precuneus (Wang et al., 2017), left-lateralization of functional connectivity in the anterior insula (Luo et al., 2014) and decreased functional connectivity in the superior frontal gyrus and lateral parietal cortex (Luo et al., 2011). While these studies vary in methodology, they share that more aberrant network properties from the ‘normal situation’ (i.e. controls) is related to longer duration of CAE. Similarly, in the current study the children with CAE showed longer path lengths compared to controls, indicating that suffering from CAE for a longer time is associated with a more abnormal state of the functional network. Moreover, our study extends upon the previous work by showing that abnormalities in the whole-brain functional network related to the duration of CAE, rather than specific ROIs. Furthermore, a less efficiently and more randomly integrated network was previously associated with poor seizure control in temporal lobe epilepsy (TLE) patients (Park et al., 2017). Although the clinical profile of TLE differs from our study, this further indicates that an altered integration might be related to a worse seizure control.
4.4. Neurocognitive performance
A lower neurocognitive performance for the processing speed index and a trend towards lower general intelligence was observed in children with CAE. However, on average the processing speed index of the children with CAE is still within the normal range (e.g. > 85). Moreover, the children with CAE took longer to finish the Bourdon-Vos test, while not performing better. This hints towards a worse sustained attention in children with CAE. This is in agreement with previous studies on neurocognition in CAE (Loughman et al., 2014; Masur et al., 2013). Furthermore, more efficient global functional networks in terms of clustering and higher degrees of small-world topology are believed to be related to better cognitive performance. For example, previous research has shown that a more efficient global functional network in terms of integration was related to better intellectual performance (Heuvel et al., 2009). Therefore, as the functional brain network of children with CAE were less efficiently organized, there might be a relation between the impaired neurocognitive performance in children with CAE and the whole-brain functional network efficiency. However, in the current study no relation was found between the neurocognitive performance and global graph metrics.
4.5. Study considerations
Our study has important strengths. The study used well defined inclusion criteria for children with CAE in agreement with current ILAE standards. Moreover, the carefully selected controls enabled us to make reliable group-level comparisons. Furthermore, to minimize the influence of subject motion, which is an inherent problem when scanning young children, the head motion was carefully considered in both preprocessing and statistical analysis. Last, the 24-hour EEG's allowed for a quantitative assessment of the number of GSWD per hour. The main limitation of this study is the relatively low sample size, although even with our small sample size we already report between-group differences as well as relations with the duration of epilepsy. However, a larger sample is required for a more in depth analysis of the relation of neurocognitive characteristics. Moreover, the cortical parcellation by Freesurfer is based on an adult template, which could result in errors in a pediatric cohort. This is especially the case if the images are also corrupted by subject motion (Phan et al., 2018), accordingly in this study we attempted to minimize subject motion by instructing parents to practice laying still with the child. However, even with these precautions, we cannot entirely exclude group-differences in vigilance and subject motion, and as such, our results could be partly confounded by subject motion. Furthermore, it cannot be excluded that at least some of the children with CAE suffered from GSWD during the fMRI acquisition. This could have a bearing on our results, as previously it was shown that GSWD could have an effect on the graph theoretical metrics of functional networks (Liao et al., 2014). However, since the fMRI exam lasts over 6 min, while absence seizures generally last a few seconds, the effects are likely to be minimal. Last, during the MRI examination 15 out of 17 children with CAE were on anti-epileptic drug (AED) treatment. Since Ethosuximide and Valproic acid have been associated with neurocognitive side effects (Ijff et al., 2016; Masur et al., 2013) and changes in functional connectivity (Salinas and Szabó, 2017; Tenney et al., 2018), it might have a bearing on our results. However, since the current guidelines prescribe the use of these AED's, our results reflect children with CAE at this point in time.
5. Concluding remark
Disruptions of the functional network in terms of longer path lengths, weaker clustering and a less small-world network topology were observed in children with CAE compared to controls. Moreover, longer path lengths are related to a longer duration of CAE and a higher number of GSWD per hour. Furthermore, cognitive performance was found to be impaired in the children with CAE, though a relation between the impaired cognitive performance and global graph metrics could not be formally established. These results hint that less efficiently organized functional networks are a characteristic of CAE which is related to disease. However, future prospective studies with larger cohorts are needed to confirm our findings.
Declarations of interest
None
Acknowledgements
The authors thank Remco Berting, Henri Saes, Paul Hofman, Marjolein van Dijk and other staff of Kempenhaeghe for their valuable contributions or support. We thank all the subjects who agreed to participate in the study. The authors are grateful for the guidance of Prof. J.H.S. Vles in the early stages of the project.
This work was supported by Stichting Vooruit.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102264.
Appendix. Supplementary materials
References
- Anderson T.W., Darling D.A. Asymptotic Theory of Certain “Goodness of Fit” Criteria Based on Stochastic Processes. Ann. Math. Stat. 1952;23:193–212. doi: 10.1214/aoms/1. 177,729,437. [DOI] [Google Scholar]
- Bear J.J. The epileptic network and cognition: What functional connectivity is teaching us about the childhood epilepsies. Epilepsia. 2019;60:1491–1507. doi: 10.1111/epi.16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A.T., Berkovic S.F., Brodie M.J., Buchhalter J., Cross J.H., Van Emde Boas W., Engel J., French J., Glauser T.A., Mathern G.W., Moshé S.L., Nordli D., Plouin P., Scheffer I.E. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528. 1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Berg A.T., Shinnar S., Levy S.R., Testa F.M. Newly Diagnosed Epilepsy in Children: Presentation at Diagnosis. Epilepsia. 1999;40:445–452. doi: 10.1111/j.1528-1157.1999.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Besseling R.M.H., Jansen J.F.A., Overvliet G.M., van der Kruijs S.J.M., Ebus S.C.M., de Louw A.J.A., Hofman P.A.M., Aldenkamp A.P., Backes W.H. Delayed convergence between brain network structure and function in rolandic epilepsy. Front. Hum. Neurosci. 2014;8:704. doi: 10.3389/fnhum.2014.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box G.E.P., Cox D.R. An analysis of transformations. J. R. Stat. Soc. Ser. B. 1964;26:211–246. [Google Scholar]
- Caplan R., Siddarth P., Stahl L., Lanphier E., Vona P., Gurbani S., Koh S., Sankar R., Shields W.D. Childhood absence epilepsy: Behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49:1838–1846. doi: 10.1111/j.1528. 1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- Curwood E.K., Pedersen M., Carney P.W., Berg A.T., Abbott D.F., Jackson G.D. Abnormal cortical thickness connectivity persists in childhood absence epilepsy. Ann. Clin. Transl. Neurol. 2015;2:456–464. doi: 10.1002/acn3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Drenthen G.S., Fonseca Wald E.L.A., Backes W.H., Debeij - Van Hall M.H.J.A., Hendriksen J.G.M., Aldenkamp A.P., Vermeulen R.J., Klinkenberg S., Jansen J.F.A. Lower myelin‐water content of the frontal lobe in childhood absence epilepsy. Epilepsia. 2019;60:1689–1696. doi: 10.1111/epi.16280. [DOI] [PubMed] [Google Scholar]
- Fischl B. Automatically Parcellating the Human Cerebral Cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fisher R.S., Cross J.H., French J.A., Higurashi N., Hirsch E., Jansen F.E., Lagae L., Moshé S.L., Peltola J., Roulet Perez E., Scheffer I.E., Zuberi S.M. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- Fonseca Wald E.L.A., Klinkenberg S., Voncken T.P.C., Ebus S.C.M., Aldenkamp A.P., Vles J.S.H., Vermeulen R.J., Hendriksen J.G.M, Hall M.H.J.A.D.-V. Cognitive development in absence epilepsy during long-term follow-up. Child Neuropsychol. 2019;25:1003–1021. doi: 10.1080/09,297,049.2019.1614156. [DOI] [PubMed] [Google Scholar]
- Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524. doi: 10.1016/S0140-6736(06)68,182-8. [DOI] [PubMed] [Google Scholar]
- Heuvel M.P.Van Den, Stam C.J., Kahn S., Pol H.E.H. Efficiency of Functional Brain Networks and Intellectual Performance. J. Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijff D.M., van Veenendaal T.M., Debeij-van Hall M.H., Jansen J.F.A., de Louw A.J.A., Majoie M.H.J.M., Aldenkamp A.P. The Cognitive Profile of Ethosuximide in Children. Pediatr. Drugs. 2016;18:379–385. doi: 10.1007/s40272-016. -0187-z. [DOI] [PubMed] [Google Scholar]
- Kessler S.K., Dlugos D., Conry J., Hirtz D.G., Moshé S.L., Clark P. Pretreatment seizure semiology in childhood absence epilepsy. Neurology. 2017;89:673–679. doi: 10.1212/WNL.0000000000004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Staring M., Murphy K., Viergever M.A., Pluim J. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging. 2010;29:196–205. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- Li Q., Cao W., Liao X., Chen Z., Yang T., Gong Q., Zhou D., Luo C., Yao D. Altered resting state functional network connectivity in children absence epilepsy. J. Neurol. Sci. 2015;354:79–85. doi: 10.1016/j.jns.2015.04.054. [DOI] [PubMed] [Google Scholar]
- Liao W., Zhang Z., Mantini D., Xu Q., Han G.J., Wang J., Wang Z., Chen G., Tian L., Jiao Q., Lu G. Dynamical intrinsic functional architecture of the brain during absence seizures. Brain Struct Funct. 2014;219:2001–2015. doi: 10.1007/s00429-013-0619-2. [DOI] [PubMed] [Google Scholar]
- Liao W., Zhang Z., Pan Z., Mantini D., Ding J., Duan X., Luo C., Lu G., Chen H. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5:27–29. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughman A., Bowden S.C., D'Souza W. Cognitive functioning in idiopathic generalised epilepsies: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2014;43:20–34. doi: 10.1016/j.neubiorev.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Luo C., Li Q., Lai Y., Xia Y., Qin Y., Liao W., Li S., Zhou D., Yao D., Gong Q. Altered functional connectivity in default mode network in absence epilepsy: A resting-state fMRI study. Hum. Brain Mapp. 2011;32:438–449. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Yang T., Tu S., Deng J., Liu D., Li Q., Dong L., Goldberg I., Gong Q., Zhang D., An D., Zhou D., Yao D. Altered intrinsic functional connectivity of the salience network in childhood absence epilepsy. J. Neurol. Sci. 2014;339:189–195. doi: 10.1016/j.jns.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Maslov S., Sneppen K. Specificity and stability in topology of protein networks. Science (80-.) 2002;296:910–913. doi: 10.1126/science.1065103. [DOI] [PubMed] [Google Scholar]
- Masur D., Shinnar S., Cnaan A., Shinnar R.C., Clark P., Wang J., Weiss E.F., Hirtz D.G., Glauser T.A. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology. 2013;81:1572–1580. doi: 10.1212/WNL.0b013e3182a9f3ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onias H., Viol A., Palhano-Fontes F., Andrade K.C., Sturzbecher M., Viswanathan G., de Araujo D.B. Brain complex network analysis by means of resting state fMRI and graph analysis: Will it be helpful in clinical epilepsy? Epilepsy Behav. 2014;38:71–80. doi: 10.1016/j.yebeh.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Onnela J., Saramäki J., J K., Kaski K. Intensity and coherence of motifs in weighted complex networks. Phys Rev E 71. 2005;065:103. doi: 10.1103/PhysRevE.71.065103. [DOI] [PubMed] [Google Scholar]
- Overvliet G.M., Besseling R.M.H., Jansen J.F.A., Van Der Kruijs S.J.M., Vles J.S.H., Hofman P.A.M., Ebus S.C.M., de Louw A., Aldenkamp A.P., Backes W.H. Early onset of cortical thinning in children with rolandic epilepsy. NeuroImage Clin. 2013;2:434–439. doi: 10.1016/j.nicl.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Choi Y.S., Jung A., Chung H., Kim H.J., Yoo J.H., Lee H.W. Seizure Control and Memory Impairment Are Related to Disrupted Brain Functional Integration in Temporal Lobe Epilepsy. Neuropsychiatry Clin Neurosci. 2017;29:343–350. doi: 10.1176/appi.neuropsych.16100216. [DOI] [PubMed] [Google Scholar]
- Phan T.V., Smeets D., Talcott J.B., Vandermosten M. Processing of structural neuroimaging data in young children: Bridging the gap between current practice and state-of-the-art methods. Dev. Cogn. Neurosci. 2018;33:206–223. doi: 10.1016/j.dcn.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W., Yu C., Gao Y., Miao A., Tang L., Huang S. Disrupted topological organization of structural brain networks in childhood absence epilepsy. Sci. Rep. 2017:1–10.. doi: 10.1038/s41598-017-10,778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus M.A.De, Heuvel M.P., Van Den Estimating false positives and negatives in brain networks. Neuroimage. 2013;70:402–409. doi: 10.1016/j.neuroimage.2012.12.066. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Salinas F.S., Szabó C.Á. Resting-state functional connectivity changes due to acute and short-term valproic acid administration in the baboon model of GGE. NeuroImage Clin. 2017;16:132–141. doi: 10.1016/j.nicl.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford Q.K., Burdette J.H., Laurienti P.J. An exploration of graph metric reproducibility in complex brain networks. Front. Neurosci. 2013;7:1–9. doi: 10.3389/fnins.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney J.R., Kadis D.S., Agler W., Rozhkov L., Altaye M., Xiang J., Vannest J., Glauser T.A. Ictal connectivity in childhood absence epilepsy: Associations with outcome. Epilepsia. 2018;59:971–981. doi: 10.1111/epi.14067. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk B.C.M., Stam C.J., Daffertshofer A. Comparing Brain Networks of Different Size and Connectivity Density Using Graph Theory. PLoS One. 2010;5:e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrotti A., Matricardi S., Rinaldi V.E., Prezioso G., Coppola G. Neuropsychological impairment in childhood absence epilepsy: Review of the literature. J. Neurol. Sci. 2015;359:59–66. doi: 10.1016/j.jns.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Vining E.P.G., Thio L.L. Absence in childhood absence epilepsy. Neurology. 2013;81:1564–1565. doi: 10.1212/WNL.0b013e3182a9f57b. [DOI] [PubMed] [Google Scholar]
- Vos P.G. Swets test Services; 1988. Bourdon-Vos test handleiding. [Google Scholar]
- Wang X., Jiao D., Zhang X., Lin X. Altered degree centrality in childhood absence epilepsy: A resting-state fMRI study. J. Neurol. Sci. 2017;373:274–279. doi: 10.1016/j.jns.2016.12.054. [DOI] [PubMed] [Google Scholar]
- Watts D.J., Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30,918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.