Abstract
Adenylosuccinate lyase deficiency is a rare neurometabolic recessive disorder of purine metabolism characterized by a wide range of clinical manifestations.
We present a very mild phenotype of two siblings characterized by mild isolated cognitive disability, in absence of brain anomalies, seizures, EEG anomalies and without progression of disease. The two patients had unsuccessfully been investigated until clinical exome was performed. In both siblings, compound heterozygosity for two inherited missense variants in ADSL gene, c.76A>T (p.Met26Leu) and c.1187G>A (p.Arg396His), were detected. Analysis of the catabolic pathway of autophagy on EBV-transformed B lymphoblastoid cell derived from the male patient excluded the presence of any autophagy alterations at the basal level.
Further studies are necessary to understand the pathogenesis of the disease and to elucidate the potential role of autophagy in the development of ADSL deficiency.
Keywords: Adenylosuccinate lyase deficiency, Purine metabolism, Exome sequencing, Autophagy
Abbreviations: ADSL, Adenylosuccinate lyase deficiency; AICAr, Aminoimidazole carboxamide ribotide; SAICAR, succinyl-aminoimidazole carboxamide ribotide; AMP, Adenosine monophosphate; S- Ado, Succinyladenosine; S-AMP, Adenylosuccinate; MRI, magnetic resonance imaging
1. Introduction
Adenylosuccinate lyase deficiency (#MIM 103050) (ADSL) is a rare autosomal recessive disease first described by Jaeken and van den Berghe in 1984 [1] in three children with severe psychomotor delay and autism spectrum disorder. It is a defect of purine metabolism caused by biallelic mutations in ADSL gene leading to the accumulation of succinylnucleosides [2]. Adenylosuccinate lyase is involved in two pathways of purine nucleotide metabolism catalyzing the conversion of succinyl-aminoimidazole carboxamide ribotide (SAICAR) into aminoimidazole carboxamide ribotide (AICAR) and the formation of adenosine monophosphate (AMP) from adenylosuccinate (S-AMP) [3]. Adenylosuccinate lyase deficiency results in marked elevation of the succinylpurines succinyladenosine and SAICA-riboside in various body fluids, particularly in cerebrospinal fluid and urine [4].
ADSL deficiency clinical expression range from fatal to mild forms and include a wide spectrum of signs and symptoms [3]. Although a wide variability of clinical expression is described, different clinical phenotypes have emerged over the years based on the onset and severity of symptoms.
Three distinct types of ADSL deficiency have been described on a continuum spectrum of physical and clinical features.
Descriptive classification systems subdivided patient phenotypes in fatal neonatal form, severe type I and mild type II form. Fatal neonatal form is characterized by encephalopathy, intractable seizures and respiratory failure and leads to early death. The type I form include severe neurodevelopmental delay, early onset of seizures, autistic features and microcephaly. Type II form instead is characterized by later onset, slight to moderate psychomotor delay, seizure and transient contact disturbances [[5], [6], [7]].
There are no fixed parameters or defined score to classify patient into a specific class; furthermore, the mechanisms leading to a more severe phenotype are not yet fully understood.
The biochemical marker that seems to correlate with the severity of the disease is S-Ado/SAICAr ratio in body fluids. The lower the ratio, the more severe the clinical symptoms of the patients (neonatal fatal form S-Ado/SAICAr ratio in CSF<1, type I ratio ~ 1, type II ≥ 2) [8].
Of note, the wide range of essentially nonspecific manifestations and lack of awareness of the condition may prevent the correct diagnosis.
Here, we present a very mild phenotype of two siblings unsuccessfully investigated until clinical exome was performed. Furthermore, we investigated the catabolic pathway of autophagy on EBV-transformed B lymphoblastoid cell derived from the male patient, based on a recent report that described lipofuscin accumulation in glandular epithelium in a patient with ADSL deficiency most likely caused by a defect in autophagy [9].
2. Case report
Patient 1 and Patient 2 are two siblings, born from healthy unrelated German parents.
Patient 1 is a 19 year-old boy. Like his younger sister, he was born after an uneventful pregnancy; birth weight was 3,750 g (75° ct), length was 54 cm (75° ct) and head circumference was 36 cm (75° ct). APGAR score at 1 and 5 minute was 9 and 10 respectively. The neonatal period was normal with growth parameters in the normal range. Mild hypotonia, psychomotor and speech delay were noted before the age of two. He was sitting and walking unassisted before 18 and 30 months old, respectively. The patient has no seizures, autistic features or visual impairment nor dysmorphic facial features. Electroencephalography (EEG) and cerebral magnetic resonance imaging (MRI) were negative.
Patient 2 is a 14 years old girl. Her neonatal period was uneventful; birth weight was 3,600 g (50-75° ct), length was 49 cm (25-50° ct) and head circumference was 36 cm (75° ct). APGAR score at 1 and 5 minute was 10. At the age of 2, mild hypotonia psychomotor delay and very mild speech delay (less pronounced than her brother and current language skills are good) were noted. She was sitting and walking unassisted before 18 and 24 months old, respectively. When she was 10 years old, she presented absence-like episodes, and EEG was performed which did not show epileptic anomalies. No other signs and/or symptoms were noted.
A periodic neuropsychiatric evaluation was performed in both patients and documented mild developmental delay but specific tests have not been performed considering the very mild phenotype of the patients.
The patients have achieved almost all daily life personal autonomies (eat and dress independently, personal hygiene, they take public transportation to school for a distance of about five Kilometers, carry out the assigned homeworks), and showed improvement in language and motor skills.
Due to the evidence of overlapping clinical presentation, clinical exome was performed. In both siblings, two compound heterozygous missense variants in the ADSL gene were detected: c.76A>T (p.Met26Leu) and c.1187G>A (p.Arg396His), which had been inherited from the unaffected parents. Based on the ACMG criteria [10], both variants were classified as likely pathogenic, allowing the diagnosis of a mild form of Adenylosuccinate lyase deficiency. The R396H variant had been reported on ClinVar as disease-causing (VCV000204796.3) and has been reported in two studies in a compound heterozygous state with other missense variants in two ADSL deficiency patients [11,12]. Functional studies have shown that the R396H variant seems to affect a conserved active site residue and to have low residual enzyme activity and increased thermal instability [12,13]. Similarly, the M26L variant had previously been documented as an hypomorphic pathogenic variant [14].
Urine samples of patient 1 and 2 showed the presence of S-Ado (22.3 and 19.9 millimoles/mole creatinine respectively, normal range 0-5 millimoles/mole creatinine), SAICAr (6.2 and 5.4 millimoles/mole creatinine respectively, normal range 0-0.8 millimoles/mole creatinine) and S- Ado/SAICAr ratio of 3.6 and 3.7 respectively.
Clinical features of the two patients define a very mild phenotype of ADSL deficiency not yet described (Table 1).
Table 1.
Differences between three different phenotypes. PMD, psychomotor disability, MRI, magnetic resonance imaging, S-Ado, succinyladenosine, SAICAr, succinyl-aminoimidazole carboxamide ribotide.
| Severe form | Moderate/mild form | Very mild phenotype | |
|---|---|---|---|
| Seizure | Early onset, often drug refractory epilepsy | Late onset (between 2nd-4th year), often effective response | Absent |
| PMD | Severe, disease progression with developmental arrest and in some patients coma vigil | Moderate/Mild | Very mild, no disease progression, improvement in language and motor skills |
| Autistic features | Yes | Yes, often transient contact disturbance | No |
| MRI | Global supra- and infratentorial volume loss and delayed myelination | Milder nonspecific changes including slight cerebral atrophy | No alterations |
| S-Ado/SAICAr | ~ 1 | > 2 | > 3.5 |
3. Materials and methods
3.1. Generation of Epstein-Barr (EBV) transformed B cell lines
The study was conducted in accordance with the principles of Helsinki Declaration and informed consent was obtained.
EBV-transformed B lymphoblastoid cell lines were generated from peripheral blood mononuclear cells (PBMCs) of Patient 1 or of a healthy donor, as previously reported [15]. Briefly, 5×10 to the power of 6 of fresh or frozen PBMCs were infected and incubated with concentrated supernatant of B95-8 EBV producer cell line (American Type Culture Collection, Rockville, MD) in the presence of 1 μg/mL cyclosporin A (Sandoz, Vienna, Austria). All cell lines were cultured in complete medium consisting of RPMI 1640 (Thermo Scientific, Pittsburgh, PA, USA) supplemented with 10% foetal bovine serum (Thermo Scientific) and 2 mM Glutamax (Thermo Scientific).
3.2. Cell treatment
As lysosomal-inhibiting agents, we used 10 nM Concanamycin A (ConA, Sigma 27689), potent inhibitor of V-ATPase [16], for 2h.: by blocking this enzyme we cause alkalization of the lysosome and the consequent blocking of its activity and therefore the accumulation of autophagic substrates. It is used to study autophagic flow.
3.3. SDS-PAGE and western blotting
Following treatment cultures were rinsed with ice-cold PBS, and lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 1% Triton X-100, 150 mM NaCl, 0.25% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 5mM MgCl2) containing protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and phosphate inhibitors (20 mM NaF, 10 mM þ-glycerophosphate, 1 mM Na3VO4) via incubation in ice for 30 min. A clear supernatant was obtained by centrifugation of lysates at 15000 rpm for 15 min. Protein concentration was determined using the DC protein assay kits (BioRad # 5000111); and samples subjected to SDS-polyacrylamide gel electrophoresis followed by Western blotting described previously [17]. Following processing, PVDF membrane (Merck Millipore) were subjected to enhanced chemiluminescent substrates (Millipore WBKL50500) and exposed to Amersham Imager 600 (GE Healthcare Life Sciences); bands were quantified using Fiji version of ImageJ (NIH) software. Target proteins were normalized against ACTIN.
Antibodies for Western analyses: ADSL (sc-365623) from Santa Cruz Biotechnology; NDP52 (#60732) from Cell Signaling; p62/SQSTM1 (610832) from MBL; LC3B (NB100-2220) from Novus Biologics; ACTIN (A2066) from Sigma.
4. Results
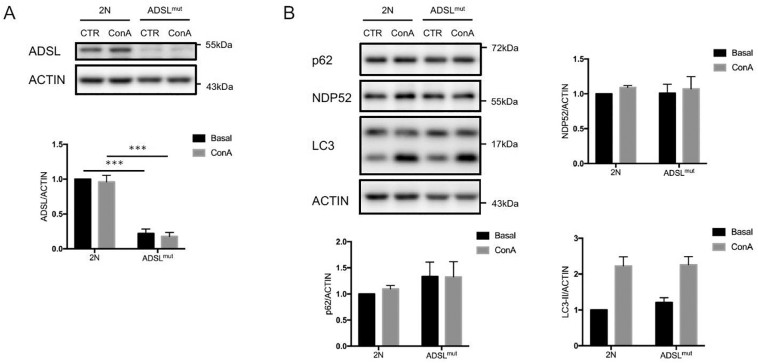
Studies conducted on generated EBV transformed B cell lines from Patient 1 (ADSLmut) showed a significant decrease of total ADSL protein levels compared to control cells (2N) (Fig. 1A). To exclude the possibility that this result was due to an elevated lysosomal degradation rate of ADSL, we repeated the analyses in the presence of Concanamycin A (ConA), a potent inhibitor of lysosomal acidification [16]. Under these conditions, we did not observe any ADSL accumulation or difference between ADSLmut and the control. Recently, Mastrangelo and collaborators described an abnormal accumulation of lipofuscin in granular epithelium of ADSL patients, reflecting the presence of defect in lysosomal clearance mechanisms [9]. We therefore investigated the autophagy flux in the presence or absence of ConA. However, we did not observe differences in the accumulation of LC3-II or of autophagic cargo receptors p62 and NDP52 (Fig. 1B), between control cells and EBV transformed B cells derived from Patients 1, excluding any autophagic alterations at the basal levels.
Fig. 1.
A. Epstein-Barr (EBV) transformed B cell lines from Patient 1 (ADSLmut) and Healthy donor (2N) were treated with ConA (10 nM) for 2h to block lysosome function. Whole-cell extracts from ADSLmut and 2N cells were analyzed by Western blot for ADSL and ACTIN. Quantification of ADSL normalized with ACTIN (n=3). B. ADSLmut and 2N cells, treated with vehicle or ConA (for 2h), were assessed for LC3 and for autophagic cargo receptors p62 and NDP52. All quantifications were normalized with ACTIN (n=3). All values are shown as the mean ± SEM. Statistical analysis was performed using two-way ANOVA with Tukey's multiple comparisons test. (***p<0.001). Immunoblots reported are from one representative experiment.
5. Discussion
Neurological manifestations in children, ranging from mild to severe, are common features in a variety of inherited metabolic/genetic diseases. The standard clinical practice includes the recognition of specific phenotype in addition to metabolic screening and genetic tests. However, many patients with this kind of disorder, often characterized by overlapping symptoms, are not given a specific diagnosis.
Adenylosuccinate lyase deficiency shows a wide spectrum of phenotype from slowly to rapidly progressing forms, so the majority of patients undergo a diagnostic odyssey where a series of single gene tests are used to search for a diagnosis.
There have been reports on the clinical utility of exome sequencing for the detection of rare variants in patients with a phenotype suspected to be due to a Mendelian genetic disorder [18]. For patients with nonspecific manifestations (e.g., intellectual disability without other recognizable distinguishing features) genome and exome sequencing can be clinically indicated. Furthermore, ADSL gene should be included in gene panels for isolated intellectual disability or psychomotor development delay. The identification of variants may identify the mode of inheritance and thus risks to the parents of recurrence in a subsequent child and risk to their relatives.
Here, clinical exome analysis allowed the diagnosis of a very mild form of ADSL deficiency characterized by isolated psychomotor delay without other manifestations. Furthermore, this peculiar clinical picture could define a new ADSL phenotype that has not yet been described and categorized.
Our report provides further evidence to recommend the clinical utility of exome sequencing in diagnosing patients with rare inherited metabolic disease (particularly recessive without a clear family history) and mild phenotype or nonspecific/unusual disease presentation that elude clinical diagnosis.
No clear genotype-phenotype correlation about identified mutations has been observed but it might be supposed the presence of genetic modifiers that may mitigate the final phenotype.
Our analysis also excludes the presence of any autophagy alterations in EBV-transformed B lymphoblastoid cells derived from this very mild form of ADSL deficiency, at least at basal levels. However, we cannot exclude the possibility that autophagy may be affected in patients with the most severe form of the disease.
Currently, there is no effective treatment for ADSL deficiency and only a few reports are available regarding the various therapeutic strategies to prevent intractable epilepsy. Of these, only two showed some beneficial effects (D-ribose and uridine administration) [19,20]; however, results have not been confirmed in further studies [21,22].
6. Conclusions
Exome sequencing allows revealing new phenotypes of known genotypes. Our two patients highlighted a new ADSL phenotype characterized by mild isolated cognitive disability, absence of brain anomalies, of seizures or EEG anomalies.
We suggest that even patients affected only by mild cognitive disability should be investigated for ADSL deficiency.
Further studies are necessary to understand the pathogenesis of the disease, in order to develop a more precise genotype-phenotype correlation and to elucidate the potential role of autophagy in the development of ADSL deficiency.
Financial disclosures
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The present paper does not contain other data apart from the ones that were already inserted (Data availability not applicable).
Editorial policies and ethical considerations
All the reported investigations and a specific permission for the publication of the results was obtained through a written informed parental consent.
Acknowledgements
We would acknowledge the patients, families and “Kindness for Kids” Foundation for their cooperation and support.
Footnotes
There are no prior publications or submissions with any overlapping information, including studies and patients. The work has not been and will not be submitted to any other journal while it is under consideration by Molecular Genetics and metabolism Reports. No author has any conflict of interest. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Marina Macchiaiolo wrote the first draft of the manuscript. Each author listed on the manuscript has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript. Paola Sabrina Buonuomo and Gerarda Mastrogiorgio were involved in clinical management of the patient. Matteo Bordi, Beatrice Testa, Gerrit Weber, Emanuele Bellacchio and Marco Tartaglia were involved in laboratory analysis and interpretation of data. Francesco Cecconi and Andrea Bartuli had the supervision of the work and follow patients during the time.
References
- 1.Jaeken J., Van den Berghe G. An infantile autistic syndrome characterised by the presence of succinylpurines in body fluids. Lancet. 1984;10(2(8411)):1058–1061. [PubMed] [Google Scholar]
- 2.Kmoch S., Hartmannová H., Stibnrková B. Human adenylosuccinate lyase (ADSL), cloning and characterization of full-length cDNA and its isoform, gene structure and molecular basis for ADSL deficiency in six patients. Hum. Mol. Genet. 2000;12(9(10)):1501–1513. doi: 10.1093/hmg/9.10.1501. [DOI] [PubMed] [Google Scholar]
- 3.Jurecka A., Zikanova M., Kmoch S. Adenylosuccinate lyase deficiency. J. Inherit. Metab. Dis. 2015;38(2):231–242. doi: 10.1007/s10545-014-9755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krijt J., Kmoch S., Hartmannová H. Identification and determination of succinyladenosine in human cerebrospinal fluid. J. Chromatogr. B Biomed. Sci. Appl. 1999;16(726(1-2)):53–58. doi: 10.1016/s0378-4347(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 5.Jurecka A., Jurkiewicz E., Tylki-Szymanska A. Magnetic resonance imaging of the brain in adenylosuccinate lyase deficiency: a report of seven cases and a review of the literature. Eur. J. Pediatr. 2012;171(1):131–138. doi: 10.1007/s00431-011-1503-9. [DOI] [PubMed] [Google Scholar]
- 6.Jurecka A., Opoka-Winiarska V., Rokicki D. Neurologic presentation, diagnostics, and therapeutic insights in a severe case of adenylosuccinate lyase deficiency. J. Child Neurol. 2012;27(5):645–649. doi: 10.1177/0883073811424465. [DOI] [PubMed] [Google Scholar]
- 7.Macchiaiolo M., Barresi S., Cecconi F. A mild form of adenylosuccinate lyase deficiency in absence of typical brain MRI features diagnosed by whole exome sequencing. Ital. J. Pediatr. 2017 Aug 2;43(1):65. doi: 10.1186/s13052-017-0383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Bergh F., Vincent M.F., Jaeken J. Residual adenylosuccinase activities in fibroblasts of adenylosuccinase-deficient children: parallel deficiency with adenylosuccinate and succinyl-AICAR in profoundly retarded patients and non-parallel deficiency in a mildly retarded girl. J. Inherit. Metab. Dis. 1993;16(2):415–424. doi: 10.1007/BF00710291. [DOI] [PubMed] [Google Scholar]
- 9.Mastrangelo M., Alfonsi C., Screpanti I. Broadening phenotype of adenylosuccinate lyase deficiency: A novel clinical pattern resembling neuronal ceroid lipofuscinosis. Mol. Genet. Metab. Rep. 2019 Aug 21;21:100502. doi: 10.1016/j.ymgmr.2019.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards S., Aziz N., Bale S., ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro M., Perez-Cerda C., Merinero B. Screening for adenylosuccinate lyase deficiency: clinical, biochemical and molecular findings in four patients. Neuropediatrics. 2002;33(4):186–189. doi: 10.1055/s-2002-34493. [DOI] [PubMed] [Google Scholar]
- 12.Zikanova M., Skopova V., Hnizda A. Biochemical and structural analysis of 14 mutant adsl enzyme complexes and correlation to phenotypic heterogeneity of adenylosuccinate lyase deficiency. Hum. Mutat. 2010 Apr;31(4):445–465. doi: 10.1002/humu.21212. [DOI] [PubMed] [Google Scholar]
- 13.De Zoysa Ariyananda L., Antonopoulos C., Currier J. In vitro hybridization and separation of hybrids of human adenylosuccinate lyase from wild-type and disease-associated mutant enzymes. Biochemistry. 2011 Mar 1;50(8):1336–1346. doi: 10.1021/bi101734q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Race V., Marie S., Vincent M.F. Clinical, biochemical and molecular genetic correlations in adenylosuccinate lyase deficiency. Hum. Mol. Genet. 2000 Sep 1;9(14):2159–2165. doi: 10.1093/hmg/9.14.2159. [DOI] [PubMed] [Google Scholar]
- 15.Caruana I., Weber G., Ballard B.C. K562-derived whole-cell vaccine enhances antitumor responses of CAR-redirected virus-specific cytotoxic T lymphocytes in vivo. Clin. Cancer Res. 2015 Jul 1;21(13):2952–2962. doi: 10.1158/1078-0432.CCR-14-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J.-H., McBrayer M.K., Wolfe D.M. Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 2015;12:1430–1444. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordi M., Darji S., Sato Y. mTOR hyperactivation in Down Syndrome underlies deficits in autophagy induction, autophagosome formation, and mitophagy. Cell Death Dis. 2019 Jul 22;10(8):563. doi: 10.1038/s41419-019-1752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biesecker L.G., Green R.C. Diagnostic clinical genome and exome sequencing. N. Engl. J. Med. 2014 Jun 19;370(25):2418–2425. doi: 10.1056/NEJMra1312543. [DOI] [PubMed] [Google Scholar]
- 19.Salerno C., Celli M., Finocchiaro R. Effect of D-ribose administration to a patient with inherited deficit of adenylosuccinase. Adv. Exp. Med. Biol. 1998;431:177–180. doi: 10.1007/978-1-4615-5381-6_34. [DOI] [PubMed] [Google Scholar]
- 20.Salerno C., Crifo C., Curatolo P. Effect of uridine administration to a patient with adenylosuccinate lyase deficiency. Adv. Exp. Med. Biol. 2000;486:75–77. doi: 10.1007/0-306-46843-3_14. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Duenas B., Sempere A., Campistol J. Novel features in the evolution of adenylosuccinate lyase deficiency. Eur. J. Paediatr. Neurol. 2012;16(4):343–348. doi: 10.1016/j.ejpn.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Jurecka A., Tylki-Szymanska A., Zikanova M. D−Ribose therapy in four Polish patients with adenylosuccinate lyase deficiency: absence of positive effect. J. Inherit. Metab. Dis. 2008 Dec;31(Suppl 2):S329–S332. doi: 10.1007/s10545-008-0904-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The present paper does not contain other data apart from the ones that were already inserted (Data availability not applicable).