Introduction
Mitochondrial disorders are rare diseases that are caused by mutations of either mitochondrial DNA or nuclear mitochondrial genes.1 The clinical manifestations of mitochondrial disorders can vary widely and there is typically a lack of genotype-phenotype correlation, which can make diagnosing the disease a challenge. Lactic acidosis can be the main manifestation in many patients with mitochondrial disorder, especially when combined with recurrent episodes of hypokalemia. Lactic acidosis may not always be present and may manifest itself only under physiological stress. Therefore, a high index of suspicion is required when evaluating such patients. Hypokalemia is another feature that can be seen in association with lactic acidosis due to intracellular shift of potassium, but this association is typically underrecognized.2 Here, we report a patient with long-standing history of recurrent episodes of hypokalemia and lactic acidosis who was diagnosed as having distal renal tubular acidosis (RTA) elsewhere, but was eventually found to have a mitochondrial gene mutation accounting for her clinical presentation.
Case Presentation
A 38-year-old woman with long-standing history of hypokalemia and metabolic acidosis was seen in the Nephrology Clinic for a second opinion regarding her electrolyte abnormalities. Her past medical history was notable for acid reflux, polycystic ovarian syndrome, hypertriglyceridemia, Hashimoto’s hypothyroidism, and anxiety. Her home medications included levothyroxine 200 μg daily, liothyronine 5 μg daily, sertraline 50 mg daily, omeprazole 40 mg daily, lubiprostone 24 mg daily, furosemide 20 mg daily as needed, metolazone 5 mg daily as needed, sodium bicarbonate 650 mg twice daily, and potassium chloride 20 mEq daily. She endorsed daily fatigue and muscle weakness and pain, especially with activity.
Her symptoms initially started at age 26 with off-and-on episodes of hypokalemia and metabolic acidosis. She had 2 documented episodes of hypokalemia and anion gap metabolic acidosis when she was 30 and 35 years of age before her current presentation without any identifiable etiology and without measurement of any lactate levels (Table 1). She was admitted to a hospital a year before her current presentation and was found to have profound anion gap metabolic acidosis with elevated lactate (13.9 mmol/l) and hypokalemia (2.3 mmol/l). Her elevated lactate was attributed to metformin that was started 2 months before her hospitalization for her polycystic ovarian syndrome.3 Potassium was supplemented and metformin was discontinued. She presented again 5 months later and was found to have hypokalemia (3.3 mmol/l) and metabolic acidosis (lactate of 4.0 mmol/l). Despite the presence of anion gap metabolic acidosis, she was mistakenly given a diagnosis of distal RTA and was started on potassium and sodium bicarbonate supplementation at that point.
Table 1.
Laboratory data
| 8 yr prior | 3 yr prior | 12 mo prior | 7 mo prior | Current visit | 2 d later (at dismissal) | Reference range | |
|---|---|---|---|---|---|---|---|
| Serum | |||||||
| Sodium, mmol/l | 140 | 140 | 136 | 140 | 136 | 143 | 135–145 |
| Potassium, mmol/l | 3.2 | 3.4 | 2.3 | 3.3 | 2.1 | 3.8 | 3.6–5.2 |
| Chloride, mmol/l | 108 | 103 | 98 | 105 | 80 | 106 | 98–107 |
| Bicarbonate, mmol/l | 13 | 21 | 11 | 19 | 38 | 24 | 22–29 |
| Creatinine, mg/dl | 0.67 | 0.7 | 0.67 | 0.7 | 0.78 | 0.72 | 0.59–1.04 |
| Anion gap | 19 | 16 | 20 | 16 | 18 | 13 | 7–15 |
| Magnesium, mg/dl | 0.7 | 1.7 | 2.5 | 2.2 | 1.7–2.3 | ||
| Albumin, g/dl | 4.3 | 3.4–5.4 | |||||
| Lactate, mmol/l | 13.9 | 4.0 | 5.1 | 1.9 | 0.5–2.2 | ||
| Arterial blood gas | |||||||
| pH | 7.55 | 7.43 | 7.35–7.45 | ||||
| pCO2, mm Hg | 39 | 32 | 32–45 | ||||
| pO2, mm Hg | 105 | 105 | 83–108 | ||||
| HCO3, mmol/l | 34 | 22 | 22–26 | ||||
| Urine | |||||||
| pH | 6.7 | 4.5–8.0 | |||||
| Sodium, mmol/l | <10 | ||||||
| Potassium, mmol/l | 10 | ||||||
| Chloride, mmol/l | 20 | ||||||
| Magnesium, mg/dl | 4.0 | ||||||
| Ammonium, mmol/l | 3–65 | ||||||
| Creatinine, mg/dl | 101 | ||||||
| 24-h potassium, mmol | 22.7 | 17–77 | |||||
| Endocrine | |||||||
| Cortisol, μg/dl | 12 (AM) 11 (PM) | 7–25 (AM) 2–14 (PM) | |||||
| TSH, mIU/l | 1.4 | 0.3–4.2 | |||||
| ACTH, pg/ml | 35 | 7.2–63 | |||||
| Creatinine kinase, U/l | 131 | 26–192 | |||||
| Aldosterone, ng/dl | 7.7 | <21 | |||||
| Renin activity, ng/ml | 17 | 2.9–24 |
ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone.
At the time of evaluation in the Nephrology Clinic, her physical examination was notable for short stature, blood pressure of 94/64 mm Hg and heart rate of 88 beats per minute. The rest of her physical examination was unremarkable. Laboratory workup was completed, which showed a serum potassium of 2.1 mmol/l (Table 1). Given the severe hypokalemia, she was admitted to the hospital. An electrocardiogram confirmed presence of prolonged QT (QTC interval 526 ms). Additional urine studies were obtained (Table 1). Given the low urinary potassium levels, we suspected that the hypokalemia was either due to gastrointestinal loss, previous use of diuretic, or shifting of potassium.
At the time of hospital admission, the patient had metabolic alkalosis in combination with anion gap metabolic acidosis (unlike her prior episodes when she primarily had a metabolic acidosis). The metabolic alkalosis in combination with low blood pressure, and low urinary sodium and chloride levels were most consistent with previous diuretic use. Patient confirmed that indeed she was taking diuretics (prescribed to her for lower extremity edema) on a regular basis before her current visit but that she had stopped all use a day before her current evaluation. She had started diuretics after her last emergency department visit (7 months before the current evaluation). She denied any diarrhea despite daily use of lubiprostone 24 mg. A potential shift of potassium related to her lactic acidosis also was considered as a potential contributor to her hypokalemia.2 Following admission to the hospital, she received intravenous normal saline and her potassium was aggressively supplemented, and she was subsequently dismissed.
A week later, she was seen again in the Nephrology Clinic. At that point, her metabolic acidosis and hypokalemia had resolved. Her prior episodes of metabolic acidosis were most consistent with an anion gap metabolic acidosis secondary to elevated L lactate levels. In the absence of evidence of any shock or systemic hypoperfusion, a type B lactic acidosis was suspected.4 In the absence of any obvious liver disease, malignancy, or diabetes, a mitochondrial disorder was considered. In addition, her prior episode of profound lactic acidosis when using metformin in the setting of a normal renal function and standard dose of metformin further raised suspicion for an underlying predisposing factor such as a mitochondrial disorder.
Given this suspicion, urinary organic acid, serum free carnitine (FC), acylcarnitine (AC), and AC/FC ratio were obtained and she was referred to the genetic clinic for further evaluation. Urine organic acid profile was normal. Her total carnitine and FC were slightly low at 33 nmol/l (reference 34–78 nmol/l) and 20 nmol/l (reference 25–54 nmol/l), respectively, but she had a normal AC/FC of 0.7 (reference 0.1–0.8).
During her genetic clinic visit, she was noted to have bilateral hearing loss (on finger rub test), weakness of −1/−2 in the bilateral biceps muscles, hip flexor muscle, and intrinsic hand muscles, in addition to short stature. Her family history was significant for short stature (grandmothers on both sides), hearing loss (mother and maternal aunt), vision problems (mother), and Hashimoto’s hypothyroidism (sister). Quantitative urine analysis of amino acids was normal, as was brain magnetic resonance imaging. Muscle biopsy (left vastus lateralis) showed nonspecific mild myopathy and type 2B fiber atrophy. Mitochondrial full genome analysis was performed, which revealed a large-scale mitochondrial DNA (mtDNA) deletion of approximately 8.1 kb, spanning mitochondrial genome 5791 to 13,917. This deletion was expected to affect partial complex I, and whole complex V and IV, including the following genes: MT-TC, MT-TY, MT-CO1, MT-TS1, MT-TD, MT-CO2, MT-TK, MT-ATP8, MT-ATP6, MT-CO3, MT-TG, MT-ND3, MT-TR, MT-ND4, MT-TH, MT-TS2, MT-TL2, and MT-ND5. The approximate heteroplasmy level of this deletion was <10%.
Discussion
Mitochondrial disorder is caused by impaired mitochondrial energy production secondary to mutations of either mtDNA or nuclear mitochondrial genes.1,5 Point mutations and large-fragment mtDNA deletions represent the 2 most common causes of primary mitochondrial disorders. Currently, there is no uniform set of tests that is recommended to evaluate a patient who is suspected to have mitochondrial disorder.1,5 Lactic acid typically accumulates in patients with mitochondrial disorder due to increased production of pyruvate, which in turn is due to increased glycolysis in the setting of reduced adenosine triphosphate (ATP) production related to abnormality in the electron transport chain.1,6 Elevated lactate levels are, however, neither specific nor sensitive for diagnosing mitochondrial disorders. This is because lactate levels can be elevated in other conditions, such as tissue ischemia.5 Similarly, some patients with mitochondrial disease may have elevated lactic acid levels only when under physiologic stress, as was the case in our patient. Therefore, high index of suspicion is required when evaluating patients who may have a mitochondrial disorder.
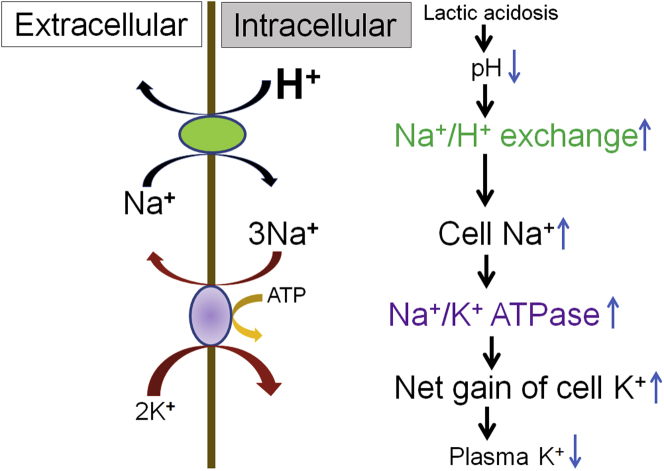
Notable in this patient’s presentation were her repeated episodes of hypokalemia in combination with her episodes of lactic acidosis. At the time of her current presentation, even though she had a profound metabolic alkalosis (pH of 7.55), she also had a superimposed anion gap metabolic acidosis (lactate of 5.1 mmol/l and anion gap of 18). This case highlights the importance of calculating the anion gap in all patients with acid-base disorder. When evaluating patients with unexplained hypokalemia, urinary potassium levels can be helpful in evaluating the cause. A low urinary potassium level suggests nonrenal potassium wasting, as could be seen with gastrointestinal losses (e.g., diarrhea), potassium shifting, or when renal potassium wasting has stopped (e.g., after discontinuing diuretic therapy). RTA is a major cause of urinary potassium loss that is typically seen in combination with nongap metabolic acidosis.6 In our patient, given the concomitant hypokalemia and metabolic acidosis, a diagnosis of distal RTA had been previously made. However, the predominant feature of anion gap metabolic acidosis (as opposed to nongap acidosis) and the low urinary potassium-to-creatinine ratio (10 mEq/g) at the time of her hypokalemia (2.1 mmol/l) argued against that diagnosis. In addition, a urinary acidification test was completed and was normal, further arguing against a diagnosis of distal RTA. Even though her hypokalemia was partly related to her off-and-on use of diuretics and likely accounted for the severity of her hypokalemia (2.1 mmol/l) at the time of her current presentation, an intracellular shift of potassium was also likely a contributing factor. Lactic acidosis results in a drop in intracellular pH. The low intracellular pH in turn activates the Na+/H+ pump in the cell, which pushes hydrogen out in an exchange for sodium. The influx of sodium intracellularly then activates the Na+/K+-ATPase pump that results in intracellular shift of potassium that leads to hypokalemia (Figure 1).2 This intracellular shift of potassium will be most noticeable when there is no underlying renal failure. This is in contrast to the outward shift of potassium that is seen in association with metabolic acidosis secondary to inorganic acids.
Figure 1.
Schematic of effects of lactic acidosis on plasma K+. Elevation of lactate, an organic acid, causes lactic acidosis, which results in reduction of intracellular pH. The low pH in turn activates the Na+/H+ exchange in the cell, which pushes H+ out in exchange for Na+. The influx of Na+ intracellularly then activates the Na+/K+-ATPase, which results in intracellular shift of K+, leading to hypokalemia. Adapted from Figure 4 in Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22:1981–1989.2 Copyright © 2011 by the American Society of Nephrology.
When a mitochondrial disease is suspected, other useful screening tests besides an elevated lactate would include urinary organic acid, serum FC, AC, and AC/FC ratio. Urine organic acid test can be helpful to evaluate the intermediates of the Krebs cycle, such as methylmalonate, and dicarboxylic acid, which can be elevated in patients with mitochondrial disorder. Carnitine is necessary for carrying long-chain fatty acids across the mitochondrial membrane and low carnitine levels and elevated AC/FC are suggestive of abnormal free fatty oxidation.1,5 None of these tests, however, are sensitive, and ultimately if the index of suspicion is high, genetic testing needs to be completed.
Mitochondrial disorder may have a syndromic or nonsyndromic manifestation.1,5 Our patient appeared to have a nonsyndromic manifestation at first glance, as lactic acidosis was the predominant feature in her clinical presentation. However, she also suffered from muscle pain on a daily basis, which was significantly exacerbated by exercise. Her muscle biopsy did reveal myopathy, which in combination with large-scale mtDNA loss was consistent with mitochondrial myopathy. Her short stature, hearing loss, and hypothyroidism also were likely related to her mitochondrial gene deletion. The association between her clinical features and mitochondrial gene deletion, however, was not obvious. In mitochondrial disorders, the variability of the phenotype is related to the degree of heteroplasmy, which is the proportion of deleted mtDNA within mitochondria.7 Our patient had a low heteroplasmy (<10%) and therefore the clinical impact of her gene deletion was not readily apparent.
Presently, there is no curative treatment for mitochondrial disorder. In addition to advising our patient to discontinue her diuretics, proton pump inhibitor, and laxative, she was also started on bicarbonate supplementation. The most commonly used dietary supplement ingredients for mitochondrial disease include vitamin E, coenzyme Q10, riboflavin, and carnitine.8 Therefore, she also was started on carnitine 3 g per day in 2 divided doses, coenzyme Q10 600 mg per day in 2 divided doses, and riboflavin 100 mg per day. The ketogenic diet has been considered as an alternative therapy for patients with mitochondrial disorders, particularly with complex I deficiency. Given her mitochondrial myopathy, however, we did not recommend it due to the potential deleterious effects of ketogenic diet on muscle cells. The patient was also advised to avoid medications that could adversely affect the mitochondrial function, including metformin, valproic acid, propofol, neuromuscular blocking agents, and statins, based on recommendations from the Mitochondrial Medicine Society.9
Conclusion
In conclusion, a mitochondrial disorder should be suspected in patients with unexplained lactic acidosis and hypokalemia, and a detailed history and physical examination is important, as it may yield other diagnostic clues, as was the case in our patient (Table 2).
Table 2.
Teaching points
| A mitochondrial disorder should be suspected in patients with unexplained lactic acidosis. |
| In a patient with unexplained lactic acidosis, it is important to be aware of mitochondrial disorder and to obtain a careful clinical history and physical examination of possible underlying mitochondrial disorder. Certain drugs, such as metformin, can worsen the lactic acidosis. An underlying predisposition should be suspected if lactic acidosis develops in the setting of normal kidney function and on a standard dose of metformin. |
| Hypokalemia associated with lactic acidosis. |
| Unlike inorganic acids that are associated with hyperkalemia, lactic acidosis results in an inward shift of potassium and hypokalemia. This is due to increased activity of Na+-K+-ATPase pump related to the low intracellular pH that develops from lactic acidosis. |
Disclosure
All the authors declared no competing interests.
References
- 1.Koenig M.K. Presentation and diagnosis of mitochondrial disorders in children. Pediatr Neurol. 2008;38:305–313. doi: 10.1016/j.pediatrneurol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson P.S., Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22:1981–1989. doi: 10.1681/ASN.2011040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalau J.D., Kajbaf F., Protti A. Metformin-associated lactic acidosis (MALA): moving towards a new paradigm. Diabetes Obes Metab. 2017;19:1502–1512. doi: 10.1111/dom.12974. [DOI] [PubMed] [Google Scholar]
- 4.Kraut J.A., Madias N.E. Lactic acidosis. N Engl J Med. 2014;371:2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 5.Alston C.L., Rocha M.C., Lax N.Z. The genetics and pathology of mitochondrial disease. J Pathol. 2017;241:236–250. doi: 10.1002/path.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallés P.G., Batlle D. Hypokalemic distal renal tubular acidosis. Adv Chronic Kidney Dis. 2018;25:303–320. doi: 10.1053/j.ackd.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Stefano G.B., Bjenning C., Wang F. Mitochondrial heteroplasmy. Adv Exp Med Biol. 2017;982:577–594. doi: 10.1007/978-3-319-55330-6_30. [DOI] [PubMed] [Google Scholar]
- 8.Hirano M., Emmanuele V., Quinzii C.M. Emerging therapies for mitochondrial diseases. Essays Biochem. 2018;62:467–481. doi: 10.1042/EBC20170114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh S., Goldstein A., Karaa A. Patient care standards for primary mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med. 2017;19(12) doi: 10.1038/gim.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]