Abstract
Introduction
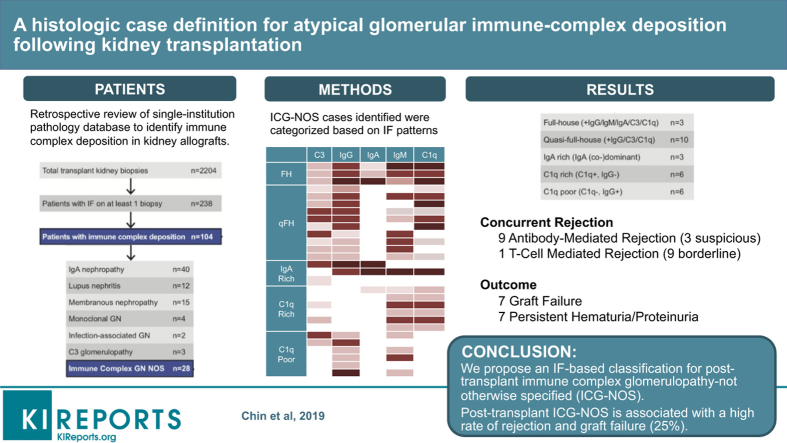
Immune-complex deposition in the transplanted kidney can present as well-phenotyped recurrent or de novo glomerular disease. However, a subset, herein termed immune-complex glomerulopathy not otherwise specified (ICG-NOS), defies classification. We quantified, categorized, and characterized cases of transplant ICG-NOS occurring at a single US academic medical center.
Methods
We retrospectively reviewed our single-institution pathology database (July 2007–July 2018) to identify and categorize all cases of immune-complex deposition in kidney allografts (based on immunofluorescence microscopy). We extracted clinicopathologic and outcome data for ICG-NOS (i.e., immune complex deposition not conforming to any well-characterized glomerular disease entity).
Results
Of 104 patients with significant immune deposits, 28 (27%) were classified as ICG-NOS. We created 5 mutually exclusive ICG-NOS categories: Full-house, Quasi-full-house, IgA-rich, C1q-rich, and C1q-poor. Overall, 16 (57%) patients met criteria for definite or possible allograft rejection, including 9 (32%) with antibody-mediated rejection (ABMR), 3 (11%) suspicious for ABMR, 1 (4%) with T-cell–mediated rejection (TCMR), and 9 (32%) with borderline TCMR. After a median follow-up of 2.3 (range, 0.1–14.0) years after biopsy, 7 (25%) allografts had failed and an additional 8 (29%) had persistent renal dysfunction (hematuria, 14%; proteinuria, 21%; and estimated glomerular filtration rate <60 ml/min per 1.73 m2, 11%).
Conclusion
In contrast to prior studies, our findings suggest that ICG-NOS is not necessarily a benign glomerular process and that there may be an association between ICG-NOS and alloimmunity. Our immunofluorescence-based classification provides a framework for future studies aiming to further elucidate ICG-NOS pathogenesis and prognosis.
Keywords: classification, glomerulonephritis, immune complex, pathology, transplant
Graphical abstract
Immune-complex deposition in the transplanted kidney can occur in the setting of a recurrent or de novo glomerular process, most commonly a well-phenotyped systemic or kidney-limited autoimmune disorder (e.g., lupus nephritis, membranous nephropathy, IgA nephropathy). However, cases failing to conform to recognized disease patterns, herein termed ICG-NOS, have also been described. In particular, prior literature describes case series of C1q-dominant nephropathy1,2 and IgM-dominant nephropathy3 in the kidney allograft while commenting on their uniqueness compared with other disease patterns.
However, existing studies lack a systematic approach to case identification and definition and thus report heterogeneous phenotypes and outcomes. Another nonarchetypical phenotype is that of nonlupus full-house nephropathy, defined as full-house immunofluorescence (IF) staining (i.e., positive staining for IgA, IgG, IgM, C3, and C1q) in the absence of other clinical or immunologic features of systemic lupus erythematosus. Nonlupus full-house nephropathy has been associated with poor clinical outcomes when affecting the native kidney,4,5 although outcomes in the transplanted kidney have not been described. C1q-dominant cases include those with variable amounts of IgG, IgM, and C3 (along with C1q) immune deposition, and have generally been associated with benign clinical outcomes when occurring in the transplanted kidney.1,2 In contrast, IgM-dominant immune deposition in the transplanted kidney has been associated with concurrent transplant rejection, albeit without significant differences in graft survival compared with matched transplanted kidney patients.3,6 Although these studies have been important in drawing attention to the phenomenon of ICG-NOS in the kidney allograft, they have tended to focus on specific features or subtypes of ICG-NOS rather than to examine the entire spectrum of disease, resulting in nonuniform and at times conflicting case definitions, nomenclatures, and conclusions. Accordingly, the incidence, spectrum, pathophysiology, clinical characteristics, and outcomes of post-transplant ICG-NOS remain enigmatic.
We designed and conducted the following study to systematically examine kidney allograft biopsies with evidence of immune-complex deposition by IF microscopy referred to a single academic institution. We aimed to (i) characterize the pathologic spectrum of ICG-NOS; (ii) propose a system for categorizing cases of ICG-NOS, using objective and externally applicable histologic case definitions; and (iii) describe clinical characteristics and outcomes in ICG-NOS, including comparisons across subgroups and to the existing literature.
Methods
The Stanford University School of Medicine Pathology Department provides nephropathology services to the Stanford Healthcare Kidney Transplant Program (adult) and the Lucile-Packard Children’s Hospital Kidney Transplant Program (pediatric). Kidney allograft biopsies are generally performed for a clinical indication in adults, whereas both protocol and indication biopsies are performed in children. Biopsy specimens are processed using standard protocols for light (LM), IF, and electron microscopy (EM).7,8 At our center, IF studies are performed on transplant biopsies only at the interpreting pathologist’s discretion.
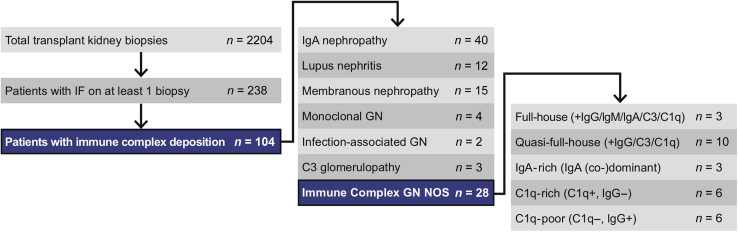
With the approval of the Stanford University Institutional Review Board (#37478), 1 renal pathologist (MLT) retrospectively searched for kidney transplant biopsies with an IF panel performed, and reviewed kidney allograft biopsy reports between July 2007 and July 2018 to assemble our cohort. We included all patients who had at least 1 transplant kidney biopsy referred to our department during the study period and for which IF was performed. Thereafter, we excluded cases with negative immune staining, a maximum of trace intensity staining for all immune reactants, with less than 2+ C3 and/or IgM staining as the only positive IF finding, or with C3 and/or IgM occurring only in a segmental sclerosis pattern as the only positive IF finding. The full biopsy reports for cases of interest were then reviewed by 2 renal pathologists (MLT and VC) as well as by 2 nephrologists with expertise in glomerular disease (MMOS) and transplant nephrology (XSC). Cases were categorized using standard histopathologic criteria in to well-phenotyped glomerular diseases (Supplementary Table S1). Conflicting opinions were handled by discussion and consensus. Cases failing to conform to a well-phenotyped histopathologic disease entity were designated as ICG-NOS and formed the focus of this study, all of which ultimately had at least 1+ IgG, IgA, and/or C1q (Figure 1).
Figure 1.
Case selection algorithm. Also see Table 1. GN, glomerulonephritis; IF, immunofluorescence; NOS, not otherwise specified.
To arrive at an IF-based categorization systemic for ICG-NOS, we considered the following:
-
(i)
IF patterns identified in our cohort, using a heat map to visualize common clusters, and
-
(ii)
ICG-NOS categories reported in the prior literature, with particular attention to reports of C1q- and IgM-rich deposits.1, 2, 3,6,7
For each patient with ICG-NOS, the index allograft biopsy slides and EM images were reviewed in-depth by a renal pathologist (MLT). All LM, IF, and EM features were characterized and quantified according to standard approaches. For LM, we recorded the number of glomeruli sampled, % global sclerosis, % segmental sclerosis, % crescents, % interstitial fibrosis and tubular atrophy, presence/absence of peritubular capillaritis, presence/absence of C4d staining, presence/absence of cellular rejection, and additional glomerular findings per Banff, including glomerular proliferation, leukocyte exudation, and basement membrane duplication.8, 9, 10 We defined mesangial changes (hypercellularity) as per the 2003 ISN/Renal Pathology Society classification for lupus nephritis (with mesangial hypercellularity or proliferation defined as 3 or more mesangial nuclei per mesangial zone), based on institutional practice. Other options included the Banff definition: “expansion of the matrix in the mesangial interspace to exceed the width of 2 mesangial cells in the average in at least 2 glomerular lobules,”9(p.1807) or the updated lupus classification defining hypercellularity as 4 or more mesangial nuclei, with a perceived lower sensitivity in detecting subtle mesangial changes.11 For IF, we recorded presence, pattern, and intensity of C3, IgG, IgA, IgM, C1q, kappa, and lambda staining. Finally, EM images were evaluated for the presence and location of electron-dense deposits, glomerular basement membrane changes, and podocyte effacement. For patients with more than 1 kidney biopsy occurring before or after the index case, biopsy reports also were reviewed.
Patient electronic medical records for all cases of ICG-NOS were then comprehensively reviewed by 2 investigators (KKC and XSC) to extract data for predefined clinical variables, including demographics (age, sex, race, ethnicity), type of transplant (living or deceased donor), time since transplant, cause of end-stage renal disease and whether this was histologically confirmed, relevant laboratory findings (serum creatinine, quantified urine protein and blood donor-specific antibody, antinuclear antibody, and complement C3 and C4 testing), immunosuppressive therapies prescribed before and after the index biopsy, and infection history (concurrent active infection and/or history of viral activation since transplantation). Long-term patient outcomes (including serum creatinine, urine protein and blood, allograft survival, and patient survival at last follow-up) also were recorded.
For statistical analyses, we compared LM, IF, EM, and clinical findings across ICG-NOS subgroups. Categorical variables were compared using Fisher’s exact test and continuous variables using Kruskal-Wallis test. A P value <0.05 was considered statistically significant.
Results
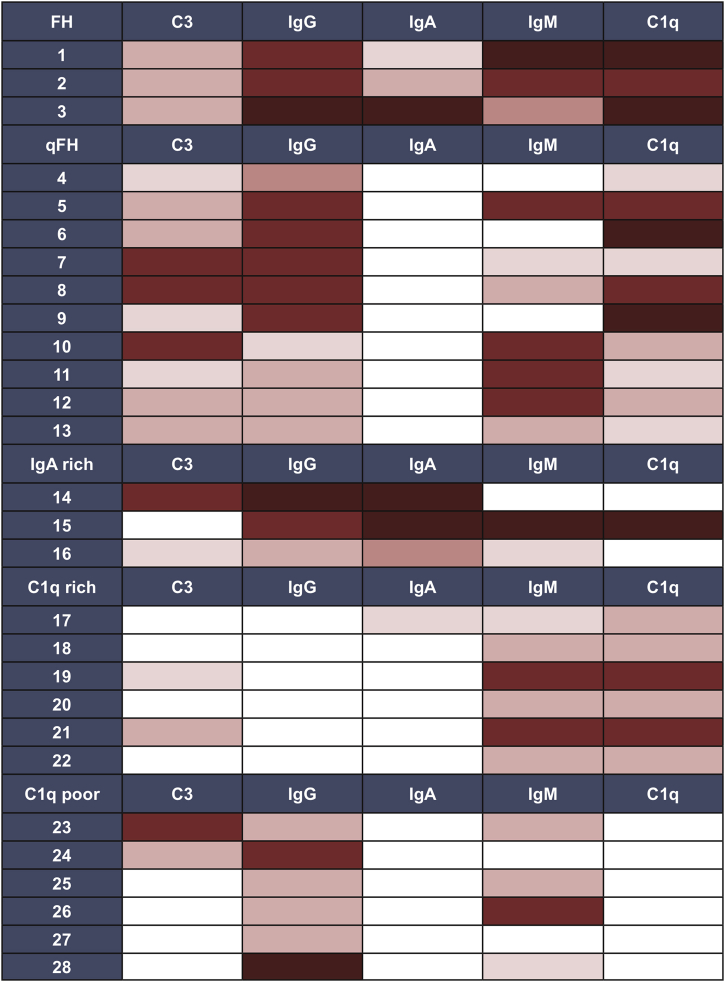
During the 11-year study period, 2204 renal transplant biopsies were processed and interpreted at our center. A total of 238 patients had IF performed on at least 1 biopsy. Of those 238 patients, 104 (44%) had immune-complex deposition in at least 1 kidney biopsy (the earliest-occurring biopsy during the study period was selected as the index biopsy). Of the 104 patients, 76 had immune-complex–mediated glomerular disease that could be classified into a well-established pathologic category (Supplementary Table S1). The focus of our study was the remaining ICG-NOS cohort (n = 28, Figure 1). Based on an in-depth review of IF patterns (Figure 2), interpreted in conjunction with IF patterns described in the existing literature, we created 5 intuitive subgroups based solely on IF findings: Full-House (+IgG/+IgM/+IgA/+C3/+C1q), Quasi-Full-House (+IgG/+C3/+C1q), IgA-rich (IgA-dominant/-codominant), C1q-rich (−IgG/+C1q), and C1q-poor (+IgG or +IgM/no C1q) (Table 1). The largest ICG-NOS subgroup was the 10-person quasi-full-house group, which demonstrated IgG, C3, and C1q positivity. All other groups had at least 3 patients and featured a variety of IF patterns (Figure 1 and Table 1). Although IF findings in the IgA-rich group could be compatible with a diagnosis of IgA nephropathy, we considered the 3 cases reviewed in this manuscript as not otherwise specified based on substantial IgG, C3, and/or C1q costaining that was more than expected for typical IgA nephropathy.
Figure 2.
Heatmap of immunofluorescence findings that informed our approach to categorizing patients into subgroups. White, no reactivity; pink to red to dark red indicates increasing intensity. FH full-house; qFH, quasi-full-house.
Table 1.
Case definition of immune-complex glomerulopathy not otherwise specified (ICG-NOS) subgroups
| ICG-NOS group | Definitiona | Number of patients |
|---|---|---|
| Full-house (ICG-FH) | +IgG/+IgM/+IgA/+C3/+C1q | 3 |
| Quasi-full-house (ICG-qFH) | +IgG/+C3/+C1qb | 10 |
| IgA-rich (ICG-IgA) | IgA (co-)-dominantc | 3 |
| C1q-rich (ICG-C1q+) | −IgG/+C1q | 6 |
| C1q-poor (ICG-C1q−) | +IgG/−C1q | 6 |
FH, full-house; qFH, quasi-full-house.
+ means at least trace staining for that Ig.
Can also be +IgM or +IgA, but not both.
Does not meet criteria for typical IgA nephropathy (Supplementary Table S1).
Table 2 describes the pathologic features of the 5 subgroups. By definition, IF and LM were performed in 100% of cases, and EM was performed in 21 (75%) cases. The most common glomerular pattern was mesangial hypercellularity or proliferation, present in 18 (64%) patients, whereas a sizable minority of 8 (29%) patients had normal LM findings. The IF staining pattern differed significantly across the subgroups, consistent with our case definitions. Five (18%) patients had positive C4d staining in peritubular capillaries, and 19 (70%) in the glomeruli. Four patterns of glomerular C4d staining were seen in glomeruli: (i) C4d matching glomerular deposits (n = 12), (ii) C4d matching glomerular deposits and additionally seen along capillary walls in a transplant glomerulopathy-like pattern (n = 5, three with capillary wall duplication by LM or EM, 2 with IF but no EM performed), (iii) glomerular endothelial staining and C4d staining of the peritubular capillaries (n = 2), and (iv) hyalinosis pattern in FSGS (n = 1). In the 21 (75%) patients who had EM performed, most immune-complex deposits were mesangial (14 patients, 67%), and a smaller percentage were mixed mesangial and subendothelial (5 patients, 24%).
Table 2.
Pathologic features, according to immune-complex glomerulopathy not otherwise specified (ICG-NOS) group
| Total (n = 28) | Full-house (+IgG/+IgM/+IgA/+C3/+C1q) (n = 3) |
Quasi-full-house (+IgG/+C3/+C1q) (n = 10) |
IgA-rich (IgA-codominant) (n = 3) |
C1q-rich (−IgG/+C1q) (n = 6) |
C1q-poor (+IgG/−C1q) (n = 6) |
P value | |
|---|---|---|---|---|---|---|---|
| Light microscopy | |||||||
| Number of glomeruli, median (range) | 16 (4–30) | 20 (10–26) | 17 (4–28) | 14 (6–21) | 16 (6–22) | 15 (12–30) | 0.34 |
| Glomerular injury pattern, n (%)a | |||||||
| Mesangial hyperplasia-proliferation | 18 (64) | 2 (67) | 5 (50) | 1 (33) | 5 (83) | 5 (83) | 0.40 |
| Capillary wall duplication | 7 (25) | 0 (0) | 1 (10) | 1 (33) | 3 (50) | 2 (33) | 0.34 |
| Focal and segmental glomerulosclerosis | 7 (25) | 1 (33) | 3 (30) | 1 (33) | 1 (17) | 1 (17) | 0.94 |
| Normal | 8 (29) | 1 (33) | 4 (40) | 2 (67) | 0 (0) | 1 (17) | 0.94 |
| % Global glomerulosclerosis, median (range) | 5.5 (0–58) | 0 (0–4) | 9 (0–50) | 7 (0–14) | 7 (0–50) | 4.5 (0–58) | 0.53 |
| % Interstitial fibrosis/tubular atrophy, median (range) | 10 (0–95) | 0 (0–0) | 13 (0–50) | 17.5 (5–30) | 20 (0–70) | 15 (0–95) | 0.50 |
| Histologic antibody-mediated rejection, n (%) | 0.74 | ||||||
| Glomerulitis + peritubular capillaritis | 12 (43) | 1 (33) | 3 (30) | 1 (33) | 4 (67) | 3 (50) | |
| Glomerulitis only | 3 (11) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 2 (33) | |
| Peritubular capillaritis only | 5 (18) | 1 (33) | 2 (20) | 1 (1) | 1 (17) | 0 (0) | |
| None | 8 (29) | 1 (33) | 4 (40) | 1 (33) | 1 (17) | 1 (17) | |
| Histologic T-cell–mediated rejection, n (%) | 0.07 | ||||||
| Banff 1A | 1 (4) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Borderline | 9 (32) | 0 (0) | 5 (50) | 1 (33) | 3 (50) | 0 (0) | |
| None | 18 (64) | 2 (67) | 5 (50) | 2 (67) | 3 (50) | 6 (100) | |
| No histologic rejection | 8 (29) | 1 (33) | 2 (20) | 1 (33) | 1 (17) | 1 (17) | 0.74 |
| Immunofluorescence microscopy | |||||||
| IgG, median (range) | 1.0 (0.0–4.0) | 2.0 (2.0–4.0) | 1.75 (0.5–2.0) | 2.0 (1.0–3.0) | 0.0 (0.0–0.0) | 1.0 (1.0–3.0) | 0.002 |
| IgA, median (range) | 0.0 (0.0–3.0) | 1.0 (0.5–3.0) | 0.0 (0.0–0.0) | 3.0 (1.5–3.0) | 0.0 (0.0–0.5) | 0.0 (0.0–0.0) | <0.001 |
| IgM, median (range) | 1.0 (0.0–3.0) | 2.5 (1.5–3.0) | 1.0 (0.0–2.0) | 0.5 (0.0–3.0) | 1.0 (0.5–2.0) | 0.8 (0.0–2.0) | 0.08 |
| C1q, median (range) | 1.0 (0.0–3.0) | 2.5 (1.0–3.0) | 1.0 (0.5–3.0) | 0 (0.0–3.0) | 1.0 (1.0–2.0) | 0.0 (0.0–0.0) | <0.001 |
| C3, median (range) | 0.75 (0.0–2.0) | 1.0 (1.0–1.0) | 1.0 (0.5–2.0) | 0.5 (0.0–2.0) | 0.0 (0.0–1.0) | 0.0 (0.0–2.0) | 0.06 |
| C4d, peritubular capillary, n (%) | 0.69 | ||||||
| positive | 5 (18) | 1 (33) | 0 (0) | 1 (33) | 2 (33) | 1 (17) | |
| Negative | 22 (79) | 2 (67) | 9 (90) | 2 (67) | 4 (67) | 5 (83) | |
| Not done | 1 (4) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | |
| C4d, glomerular, n (%) | 0.25 | ||||||
| Mesangial | 7 (25) | 0 (0) | 5 (50) | 1 (10) | 0 (0) | 1 (17) | |
| Capillary wall | 12 (43) | 1 (33) | 2 (20) | 2 (20) | 5 (83) | 2 (33) | |
| Negative | 7 (25) | 2 (67) | 2 (20) | 0 (0) | 1 (17) | 2 (33) | |
| Not done | 1 (4) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | |
| Electron microscopy | |||||||
| Electron microscopy performed, n (%) | 21 (75) | 3 (100) | 8 (80) | 1 (33) | 4 (67) | 5 (83) | 0.36 |
| Deposit location, n (%) | 0.34 | ||||||
| Mesangial | 14 (67) | 2 (67) | 6 (75) | 0 (0) | 2 (50) | 3 (60) | |
| Mesangial and subendothelial | 5 (24) | 1 (33) | 2 (25) | 1 (100) | 1 (25) | 0 (0) | |
| None | 3 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 2 (40) | |
| Feature of transplant glomerulopathy (glomerular basement membrane duplication or mesangiolysis) | 6 (29) | 1 (33) | 2 (25) | 0 (0) | 2 (50) | 1 (20) | 0.75 |
| Percent podocyte effacement, median (range) | 10 (0–100) | 5 (5–10) | 10 (0–80) | 30 (30–30) | 10 (0–10) | 10 (0–100) | 0.73 |
Some biopsies demonstrated more than 1 glomerular injury pattern.
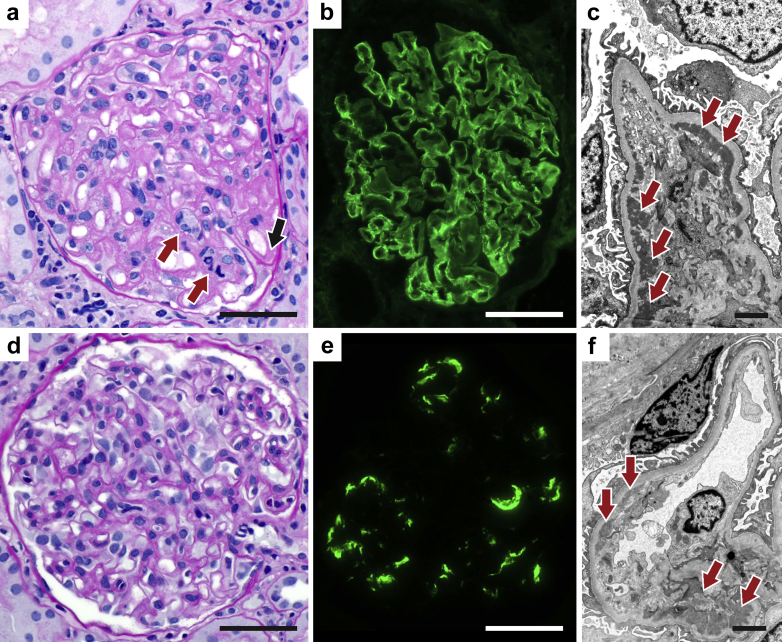
We specifically examined the cases for capillary wall duplication, a feature of transplant glomerulopathy. Capillary wall duplication by LM or EM was seen in a total of 11 cases (39%) and was especially common in the IgA-rich (2 of 3, 66%) and C1q-rich (3 of 6, 50%) groups, although differences across subgroups were not statistically significant (Figure 3, Supplementary Figures S1–S5).
Figure 3.
Biopsy findings. (a–c) Patient 3, full-house category. (a) Light microscopy (periodic acid-Schiff [PAS] stain) showing capillary wall double contours with deposit (black arrow) and endocapillary hypercellularity (red arrows). (b) Immunofluorescence microscopy (IgG immune staining) showing diffuse granular capillary wall deposit. (c) Electron microscopy showing abundant subendothelial and mesangial deposits (arrows). (d–f) Patient 9, quasi-full-house category. (d) Light microscopy (PAS stain) showing glomerulitis and slight mesangial hypercellularity. (e) Immunofluorescence microscopy (C1q immune staining) showing segmental mesangial and capillary wall staining. (f) Electron microscopy showing subendothelial and mesangial electron-dense deposits (arrows). Bars = (a,b,d,e) 50 μm and (c,f) 2 μm.
Twenty (71%) patients had histologic features of ABMR (peritubular or glomerular capillaritis). Considering clinical features also, 9 of these 20 patients (45%) met 2017 Banff criteria for ABMR: 1 (33%) in full-house, 1 (10%) in quasi-full-house, 1 (33%) in IgA-rich, 4 (67%) in C1q-rich, and 2 (33%) in C1q-poor. Of those 9, 5 met criteria by having positive C4d staining of their peritubular capillaries and positive donor-specific antibodies. The other 4 met criteria by having at least moderate microvascular inflammation with positive donor-specific antibodies. Three other patients (11%) met only 2 of 3 Banff criteria for ABMR and were labeled suspicious for ABMR. Only 1 patient had clear evidence of TCMR. Nine other patients (32%) had histologic features borderline for TCMR by Banff criteria. When stratified by indication for biopsy, 5 of 12 (42%) protocol biopsies were at least borderline for TCMR or suspicious for ABMR, including 2 cases of ABMR, whereas 11 of 16 (69%) indication biopsies were at least borderline for TCMR or suspicious for ABMR, including 1 case of Banff 1a TCMR with ABMR, and 7 cases of ABMR only. The difference in rejection rate in protocol versus indication biopsies is not statistically significant (P = 0.15).
Table 3 displays the clinical characteristics. The median patient age was 18 (range, 1–71) years, 50% were adults, and 61% were male. The most common race/ethnicities were Caucasian (29%) and Hispanic (29%). Cause of end-stage renal disease was most commonly unknown/unbiopsied, which included 4 cases of presumed diabetic and/or hypertensive nephropathy (36%), followed by nonimmune (32%), including polycystic kidney disease, methylmalonic acidemia, and reflux nephropathy. There were 2 (7%) cases of systemic autoimmune disease, namely systemic lupus erythematosus and eosinophilic granulomatosis with polyangiitis: in both cases, the nature of the glomerular deposits post-transplantation (IgG and IgM only in the lupus case, and C3, IgG, IgA [dominant], IgM in the polyangiitis case) were inconsistent with the cause of native kidney disease. Renal-limited glomerular diseases, including focal segmental glomerulosclerosis and IgA nephropathy, accounted for 7 (25%) causes of end-stage renal disease. At the time of biopsy, 6 (21%) patients had a recent or active infection, including bacteremia or a respiratory, gastrointestinal, or urinary tract infection. Nine (32%) patients had a history of viral activation post-transplantation, including positive serum titers for polyoma BK virus, Epstein-Barr virus, or cytomegalovirus, although none were known to be viremic at time of biopsy.
Table 3.
Clinical features, according to immune-complex glomerulopathy not otherwise specified (ICG-NOS) subgroup
| Total (n = 28) | Full-house (+IgG/+IgM/+IgA/+C3/+C1q) (n = 3) |
Quasi-full-house (+IgG/+C3/+C1q) (n = 10) |
IgA-rich (IgA-codominant) (n = 3) |
C1q rich (−IgG/+C1q) (n = 6) |
C1q poor (+IgG/−C1q) (n = 6) |
P value | |
|---|---|---|---|---|---|---|---|
| Age at transplantation, median (range) | 17.5 (1–71) | 18 (1–68) | 17 (2–71) | 24 (17–31) | 19 (15–54) | 14 (8–26) | 0.53 |
| Adult at transplantation, n (%) | 14 (50) | 2 (67) | 5 (50) | 2 (67) | 3 (50) | 2 (33) | 0.86 |
| Male, n (%) | 17 (61) | 3 (100) | 7 (70) | 2 (67) | 3 (50) | 2 (33) | 0.34 |
| Race/ethnicity, n (%) | 0.48 | ||||||
| Caucasian | 8 (29) | 0 (0) | 2 (20) | 1 (3) | 2 (33) | 3 (50) | |
| Hispanic | 8 (29) | 2 (67) | 2 (20) | 0 (0) | 3 (50) | 1 (17) | |
| African American | 2 (7) | 0 (0) | 1 (10) | 0 (0) | 1 (17) | 0 (0) | |
| Asian/Pacific Islander | 6 (21) | 1 (33) | 2 (20) | 2 (67) | 0 (0) | 1 (17) | |
| Other | 4 (14) | 0 (0) | 3 (30) | 0 (0) | 0 (0) | 1 (17) | |
| Cause of ESRD, n (%) | 0.47 | ||||||
| Nonimmune ESRDa | 9 (32) | 1 (33) | 3 (30) | 0 (0) | 1 (17) | 4 (67) | |
| Systemic autoimmune disease | 2 (7) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 1 (17) | |
| Glomerulonephritisb | 7 (25) | 1 (33) | 2 (20) | 1 (33) | 2 (33) | 1 (17) | |
| Unknown/unbiopsied | 10 (36) | 1 (33) | 5 (50) | 1 (33) | 3 (50) | 0 (0) | |
| Time from transplant to biopsy, years, median (range) | 2.1 (0.2–11.1) | 4.3 (1.6–5.2) | 2.0 (0.5–4.3) | 3.5 (0.9–9.0) | 2.1 (0.2–5.9) | 1.1 (0.4–11.1) | 0.75 |
| Biopsy indication, n (%) | 0.47 | ||||||
| Increased sCr | 7 (25) | 1 (33) | 2 (20) | 1 (33) | 2 (33) | 1 (17) | |
| Proteinuria | 3 (11) | 1 (33) | 2 (20) | 0 (0) | 0 (0) | 1 (17) | |
| Increased sCr and proteinuria | 4 (14) | 0 (0) | 2 (20) | 0 (0) | 1 (17) | 0 (0) | |
| Positive DSA | 1 (4) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | |
| Rejection follow-up | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | |
| Protocol | 12 (43) | 1 (33) | 4 (40) | 1 (33) | 2 (33) | 4 (67) | |
| Laboratory features at time of biopsy | |||||||
| sCr (mg/dl), median (range) | 1.2 (0.4–7.0) | 1.1 (0.7–1.5) | 1.1 (0.4–2.0) | 1.2 (1.0–2.8) | 1.2 (0.9–3.5) | 0.9 (0.7–7.0) | 0.54 |
| Hematuria, n (%) | 8/22 (36) | 1/3 (33) | 1/7 (14) | 2/3 (67) | 1/4 (25) | 3/5 (60) | 0.39 |
| Proteinuria, n (%) | 11/22 (59) | 1/3 (33) | 3/7 (43) | 1/3 (33) | 1/4 (25) | 3/5 (60) | 0.77 |
| Positive DSA, n (%) | 12/19 (63) | 1/1 (100) | 3/7 (43) | 1/2 (50) | 4/4 (100) | 3/5 (60) | 0.34 |
| ANA+, n (%) | 3/12 (25) | 1/1 (100) | 1/5 (20) | 0/2 (0) | 0/3 (0) | 1/1 (100) | 0.53 |
| Low C3, n (%) | 3/12 (25) | 1/1 (100) | 2/8 (25) | 0/2 (0) | 0/1 (0) | 0/0 (n/a) | 0.04 |
| Low C4, n (%) | 2/12 (17) | 1/1 (100) | 1/8 (12.5) | 0/2 (0) | 0/1 (0) | 0/0 (n/a) | 0.11 |
| Known infection at time of Bx, n (%) | 6 (21) | 1/3 (33) | 2/10 (20) | 0/3 (0) | 0/6 (0) | 3/6 (50) | 0.23 |
| History of viral activation | 9 (32) | 2/3 (67) | 3/10 (30) | 0/3 (0) | 2/6 (33) | 2/6 (33) | 0.54 |
| Antibody-mediated rejection | 0.10 | ||||||
| Confirmed | 9 (32) | 1 (33) | 1 (10) | 1 (33) | 4 (67) | 2 (33) | |
| Suspicious | 3 (11) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 2 (33) | |
| T-cell–mediated rejection | 0.07 | ||||||
| Confirmed | 1 (4) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Borderline | 9 (32) | 0 (0) | 5 (50) | 1 (33) | 3 (50) | 0 (0) | |
| No rejection | 12 (43) | 2 (67) | 5 (50) | 1 (33) | 2 (33) | 2 (33) | 0.83 |
| Treatment, n (%) | |||||||
| Steroid pulse | 9 (32) | 1 (33) | 4 (40) | 2 (67) | 2 (33) | 0 (0) | 0.31 |
| I.v. IG | 6 (21) | 1 (33) | 1 (10) | 1 (33) | 3 (50) | 0 (0) | 0.21 |
| Increase immunosuppression | 8 (29) | 0 (0) | 1 (10) | 1 (33) | 5 (83) | 1 (17) | 0.02 |
| No change | 14 (50) | 2 (67) | 5 (50) | 1 (33) | 1 (17) | 5 (83) | 0.20 |
| Yr of follow-up post-Bx, median (range) | 2.3 (0.1–14.0) | 1.9 (0.3–3.9) | 2.5 (0.6–14.0) | 1.1 (1.1–3.4) | 3.6 (0.6–7.9) | 2.5 (0.1–3.3) | 0.47 |
| Graft failure, n (%) | 7 (25) | 0 (0) | 2 (20) | 2 (67) | 2 (33) | 2 (33) | 0.79 |
| Yr to graft failure post-Bx, median (range)c | 3.0 (0.1–14.0) | n/a | 8.8 (3.6–14.0) | 1.1 | 2.3 (0.6–3.9) | 1.5 (0.1–3.0) | 0.42 |
| Laboratory features at time of last follow-upd | |||||||
| sCr at last follow-up (mg/dl), median (range) | 1.0 (0.5–5.2) | 1.0 (0.5–1.5) | 0.85 (0.5–2.4) | 3.3 (1.4–5.2) | 1.8 (0.9–3.0) | 0.8 (0.7–1.1) | 0.69 |
| Hematuria, n (%) | 4/16 (25) | 0/2 (0) | 1/6 (17) | 1/2 (50) | 1/2 (50) | 1/4 (25) | 0.70 |
| Proteinuria, n (%) | 6/17 (35) | 0/2 (0) | 2/7 (28) | 2/2 (100) | 1/2 (50) | 1/4 (25) | 0.26 |
| Follow-up biopsy with IF | 8/28 (29) | 1/3 (33) | 5/10 (50) | 0/3 (0) | 0/6 (0) | 2/6 (33) | 0.51 |
| Follow-up immune deposition | 0.11 | ||||||
| Persistent | 2 (25) | 1 (100) | 0 (0) | 1 (50) | |||
| Improved | 6 (75) | 0 (0) | 5 (100) | 1 (50) | |||
| Death, n (%) | 1 (4) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.07 |
ANA, antinuclear antibody; Bx, biopsy; DSA, donor-specific antibody; ESRD, end-stage renal disease; IF, immunofluorescence; sCr, serum creatinine.
For laboratory values at time of biopsy, the denominators (as shown in the first column) are frequently smaller than 28, as testing was not performed in all patients.
Includes presumed diabetic nephropathy, presumed hypertensive nephropathy, polycystic kidney disease, methylmalonic acidemia, reflux.
Of the 7 patients who had glomerulonephritis (GN) as a cause of ESRD, 5 had posttransplant GN that was phenotypically distinct from the original disease (focal and segmental glomerulosclerosis = 4, Churg-Strauss = 1). Two had potentially related disease: 1 patient with IgA nephropathy developed IgA-rich ICG-NOS; 1 patient with crescentic glomerulonephritis developed C1q+ ICG-NOS, but no evidence of crescents.
For patients who reached graft failure.
Excludes patents with graft failure.
The index biopsy was obtained at a median time of 2.1 years after transplantation (range, 0.2–11.1) and was most often done as a protocol biopsy (43%) or for the indication of increased serum creatinine (25%). Laboratory features at time of biopsy were notable for positive donor-specific antibody (12 of 19, 63%), proteinuria (11 of 22, 59%), and/or hematuria (8 of 22, 36%). In response to the biopsy findings, transplant nephrologists selected a variety of treatment choices. For 14 patients (50%), including 5 (36%) with concomitant definite or possible rejection, no changes were made. For the remaining 14 patients, including 11 (79%) with concomitant definite or possible rejection, i.v. IG, steroid pulses, and/or other immunosuppressive agents were prescribed.
Last follow-up occurred at a median of 2.3 years (range, 0.1–14.0 years) after the index biopsy. Overall, 7 patients (25%) experienced graft failure after a median of 3.0 years (range, 0.1–14.0 years) following the index biopsy, and 1 patient died (of stroke) with a functioning graft. Of the patients with functioning grafts (21 patients, 75%), 8 (38%) had evidence of ABMR at the time of biopsy and 1 (5%) had findings suspicious for ABMR, whereas 1 (5%) had TCMR and 9 (43%) had borderline TCMR. Eight (38%) had no evidence of rejection. At last follow-up of these 21 patients, 4 of 16 (25%) had persistent hematuria, 6 of 17 (35%) had proteinuria, and 3 (14%) had an estimated glomerular filtration rate less than 60 ml/min per 1.73 m2, with a median serum creatinine of 1.0 (range, 0.5–5.2) mg/dl. Overall, 8 of the 28 patients had subsequent biopsies that included IF: immune-complex deposition decreased in intensity in 6 patients (75%) and was stable in 2 (25%). Of the 6 patients with decreased immune-complex deposition, 3 had received an adjustment to their immunosuppression after the index biopsy, whereas maintenance immunosuppression was unchanged for the other 3. Two of the 6 patients had evidence of possible rejection. For these 8 patients with IF follow-up, creatinine increased at last follow-up in 3 patients, 2 of whom had graft failure. One graft failure occurred in a patient without rejection and with decreasing immune deposition over time, whereas the other graft failure occurred in a patient with rejection and persistent deposits.
Discussion
Glomerular disease is an important cause of kidney allograft loss. In a large study of long-term transplant outcomes in 1317 kidney transplant recipients, glomerular disease was responsible for 37% of the 330 graft failures.12 Although the most common forms of glomerular disease were recurrent disease and transplant glomerulopathy believed to be due to chronic ABMR, 10 cases (or 3% of all allograft failures) were attributed to de novo glomerular disease. Defining and better understanding of these less well understood glomerular disease entities was our primary objective.
To this end, we characterized our center’s experience with post-transplant ICG-NOS. Among 238 patients with IF performed in an allograft biopsy, we identified 28 patients with post-transplant ICG that did not conform to a well-phenotyped glomerular disease entity, which we termed ICG-NOS. Based on IF findings within this cohort, interpreted in the context of the existing literature pertaining to ICG-NOS, we developed a system for categorizing cases and described histologic and clinical characteristics within and across these categories. Unlike prior studies (Table 41, 2, 3,6,7,13) that often focused on a single IF pattern, we attempted to create a more systematic and comprehensive framework that addresses the full spectrum of ICG-NOS.
Table 4.
Summary of previous studies characterizing transplant immune-complex glomerulopathy (ICG) that cannot be readily classified under a known entity
| Author, journal, yr | Sample size (% all screened biopsies) | Case definition | Other IF features | Comparison to our case definitions | Key conclusions |
|---|---|---|---|---|---|
| Giannico et al.,6Human Pathology 2015 | 28 (3.5%) | Mesangial glomerulopathy without known history of ICG, and excluding IgA-dominant | 23 of 28 IgM dominant. 5 of 28 IgG dominant. 17 of 18 had concomitant C3/C1q. | All our categories except IgA-rich. Encompasses FH | High prevalence of concurrent rejection, both T-cell mediated (36% vs. 7% in control) and ABMR (25% vs. 4%). Rate of progression to graft failure (5 of 28) was not different from that in control (7 of 28), median follow-up 24 months. Authors hypothesize that ICG may not be clinically significant in terms of graft survival. |
| Lloyd et al.,7Human Pathology 2018 | 12 (1.7%) | Immune complexes not attributable to cause of native kidney failure | Typically codominant IgG and IgM; C1q present in 50% | All our categories except IgA-rich. Similar case-finding process | High prevalence of concurrent rejection (75%), including ABMR with DSAs (67%). High rate of progression with graft failure (7 of 12), median follow-up 36 mo. Authors hypothesize that the underlying mechanism for de novo ICG may be alloimmunity. |
| Said et al.,1Modern Pathology 2010 | 24 (0.1%) | C1q 2+ or higher, excluding native MPGN or lupus | Most also had C3, IgG, and IgM | All our categories except IgA-rich and C1q-poor | Lower prevalence of concurrent rejection compared with other studies (5 of 24); 50% had infection in the preceding 6 mo, but no evidence of infection-related GN on histology. Benign course in the absence of a second glomerular lesion |
| Kanai et al.,2BMC Nephrology 2018 | 5 (1.2%) | C1q 2+ or higher, no to mild proteinuria/hematuria | All cases had IgG, IgM, and C3 | qFH | Long-term mesangial C1q deposits accompanied by IgG, IgM, and C3 may be clinically silent. |
| Gough et al.,3Archives of Pathology and Laboratory Medicine 2005 | 9 (7.6%) | IgM immune complexes not otherwise attributable | +IgM with and without C3 | C1q-poor | Earlier onset of ICG (within 1 yr) compared to other studies. Viral infection in 2 of 9, no control group |
| Grau et al.,13Transplantation 2016 | 8 | Convenience sample of 51 transplant glomerulopathy cases. Included cases with immune complexes not otherwise attributable | Typically IgM-positive with variable C1q and C3 | Cases with CWD | In patients with known transplant glomerulopathy, ICG is present in 16% of cases and represents alloimmune response against non-MHC epitopes or mesangial targets. |
ABMR, antibody-mediated rejection; CWD, capillary wall duplication; DSA, donor-specific antibody; FH, full-house; GN, glomerulonephritis; IF, immunofluorescence; MPGN, membranoproliferative glomerulonephritis; qFH, quasi-full-house.
Of studies in the literature (Table 4), Lloyd et al.7 shares the most similar systematic case-finding process with our study. Lloyd et al.7 identified 12 patients with ICG-NOS and discovered a high prevalence of alloimmunity in those patients, with ABMR in 67%, TCMR in 33%, and positive donor-specific antibody in 63%. Based on this high prevalence of rejection, they hypothesized that alloimmunity might be the underlying mechanism for some cases of nonmembranous de novo immune-complex deposition. Grau et al.13 also reflected on an alloimmunity hypothesis and proposed that formation of allo-antibodies to non-HLA antigens and other glomerular constituents might be a possible mechanism for immune deposits in an animal model of transplant glomerulopathy. In our larger cohort, we also see a high prevalence of alloimmunity (at least 45% ABMR, albeit a lower rate of TCMR). Of note, transplant glomerulopathy is classically pauci-immune, although largely attributed to ABMR (anti-MHC antibodies). Our series, together with those of Lloyd et al.,7 Grau et al.,13 and others, raises the possibility of accompanying glomerular immune-complex deposition in a subset of transplant glomerulopathy cases. Nevertheless, the overlapping of histologic features between glomerulopathy attributable to ABMR versus those attributable to other autoimmune or infection-related forms of glomerular disease (i.e., leukocyte exudates-capillaritis, glomerular basement membrane duplication, and some patterns of C4d deposition), requires caution in diagnostic terminology and pathophysiologic inferences.14 An additional qualifier is the selection bias implicit in our retrospective study: even if ICG-NOS was clinically silent, detection would be more likely when rejection was concurrent. For instance, we note that the rate of concurrent rejection was higher when the biopsy was performed for a clinical indication, suggesting that rejection and not the ICG-NOS might have provided the trigger for performing the biopsy. The direction and nature of any hypothesized association between alloimmunity and ICG-NOS requires additional investigation in a larger study with less biased inclusion criteria. Our IF-based classification process provides a possible framework for approaching this topic.
At the same time, we propose that ICG-NOS is not necessarily clinically silent or an epiphenomenon of rejection, as 12 (43%) patients in our cohort lacked any evidence of concurrent rejection, of whom 5 had a clinically indicated biopsy for investigation of hematuria, proteinuria, or elevated creatinine that was likely attributable to the ICG-NOS.15,16
We also explored infection as a potential cause of ICG-NOS. In our cohort, 6 patients (21%) had known infection at the time of biopsy, and 9 patients (32%) had a history of viral activation. It is not possible to infer causality from these data, given the high background rate of infection in transplant recipients. We did not find any obvious culprit pathogenic organisms. Two patients in our cohort underwent simultaneous liver-kidney transplant for metabolic disease. Clinical studies in the literature have reported increased autoimmune serologic markers after liver transplantation, although the kidney biopsy literature is overshadowed by many patients with hepatitis B or C.17,18 Future studies are needed to fully investigate the putative link between infection, autoimmunity, alloimmunity, and ICG-NOS.
Considering graft outcomes for ICG-NOS, previous studies have differed in their conclusions. Table 4 summarizes these prior studies. Whereas Said et al.,1 Kanai et al.,2 and Giannico et al.6 concluded that ICG-NOS did not influence graft failure rates and are mostly clinically silent, Lloyd et al.7 had noted a high rate of progression to graft failure within a median follow-up of only 36 months. Said et al.1 and Kanai et al.2 had cohorts that were most similar to our ICG-C1q+ group, whereas Giannico et al.6 and Lloyd et al.7 had cohorts most similar to our ICG–quasi-full-house group. In our cohort, 7 (25%) patients reached graft failure with a relatively short median post-biopsy time of 3 years and only 3 of those patients had evidence of rejection at the time of biopsy. The inclusion of indication biopsies in our study biases us away from detection of subclinical disease. ICG-NOS may be clinically benign, as previously thought, or may portend a poor prognosis in some patients, even if the pathogenesis remains obscure.
The numbers in our ICG-NOS subgroups are too small to identify statistically significant between-group differences. Prior studies excluded cases with IgA-dominant or -codominant: we included 3 cases with an unusual constellation of deposits. It is therefore especially difficult to make inferences about this patient group: indeed, whether these cases are variants or misclassifications of IgA nephropathy or a unique entity requires further study.
We used the ISN/Renal Pathology Society classification for lupus nephritis of mesangial hypercellularity in looking at potential histologic correlates of glomerular deposit, rather than the updated classification or the Banff classification as outlined in the Methods section. Applying the alternative definitions, requiring 2 or 4 rather than 3 cells per mesangial area, would result in fewer or more cases with normal glomeruli in each category. However, given that our classification is predominantly IF-based, it would not alter our conclusion.
Our study has a number of limitations. A fundamental challenge in any retrospective study is case selection bias: a large proportion of biopsies in our study (57%) were done for clinical indications, resulting in an oversampling of cases with concurrent rejection or clinical abnormality, as previously discussed. Further, our center does not perform IF and EM on all allograft biopsies, but only in cases of hematuria or proteinuria, or if the glomeruli are morphologically abnormal and tissue is available; we therefore cannot comment on prevalence or incidence. Furthermore, EM samples were available for only 75% of cases. Another limitation is the incompleteness of clinical data, although this did not differ across subgroup categories. Although one of the largest studies to date to explore ICG-NOS post transplantation, our small sample size precluded meaningful between-group comparisons. Nevertheless, the trends we observe may enable hypothesis generation and our approach to categorizing cases will inform the design of future studies. Finally, findings from this single-center, retrospective study are not necessarily generalizable to other centers or eras, and should be validated in a larger, multicenter, and ideally international, patient cohort.
In summary, we present a series of 28 patients with ICG-NOS in the kidney allograft that were identified through a comprehensive review of all biopsies performed at a single academic center over 11 years. With the consensus of team members with expertise in glomerular disease, transplant nephrology, and kidney pathology, we constructed a framework for categorizing biopsies with ICG-NOS based on IF pattern. Although our findings require confirmation in a larger multicenter patient cohort, we note that ICG-NOS was associated with concurrent rejection, particularly ABMR, lending support to an alloimmunity hypothesis. In addition, although ICG-NOS can present in some patients with relatively benign clinical findings (including some cases picked up incidentally on a protocol biopsy), at other times it can be associated with renal impairment and graft failure, even in the absence of concomitant evidence of rejection. We welcome increased attention to, and reporting of, this phenomenon in other patient cohorts, so that the spectrum of pathophysiologies, clinicopathologic features and correlates, treatment responses, and long-term outcomes, of post-transplant ICG-NOS can be better understood.
Disclosure
All the authors declared no competing interests.
Footnotes
Table S1. Immune-complex glomerular disease: diagnostic patterns.
Figure S1. Patient 3, full-house category, additional images (see also Figure 3).
Figure S2. Patient 8, quasi-full-house category.
Figure S3. Patient 15, IgA-rich category.
Figure S4. Patient 19, C1q-rich category.
Figure S5. Patient 24, C1q-poor category.
Supplementary Material
References
- 1.Said S.M., Cornell L.D., Valeri A.M. C1q deposition in the renal allograft: a report of 24 cases. Mod Pathol. 2010;23:1080–1088. doi: 10.1038/modpathol.2010.92. [DOI] [PubMed] [Google Scholar]
- 2.Kanai T., Akioka Y., Miura K. Predominant but silent C1q deposits in mesangium on transplanted kidneys - long-term observational study. BMC Nephrol. 2018;19:82. doi: 10.1186/s12882-018-0874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gough J., Yilmaz A., Yilmaz S., Benediktsson H. Recurrent and de novo glomerular immune-complex deposits in renal transplant biopsies. Arch Pathol Lab Med. 2005;129:231–233. doi: 10.5858/2005-129-231-RADNGI. [DOI] [PubMed] [Google Scholar]
- 4.Wen Y.-K., Chen M.-L. Clinicopathological study of originally non-lupus “full-house” nephropathy. Ren Fail. 2010;32:1025–1030. doi: 10.3109/0886022X.2010.510614. [DOI] [PubMed] [Google Scholar]
- 5.Rijnink E.C., Teng Y.K.O., Kraaij T. Idiopathic non-lupus full-house nephropathy is associated with poor renal outcome. Nephrol Dial Transplant. 2017;32:654–662. doi: 10.1093/ndt/gfx020. [DOI] [PubMed] [Google Scholar]
- 6.Giannico G.A., Arnold S., Langone A. Non–immunoglobulin A mesangial immune complex glomerulonephritis in kidney transplants. Hum Pathol. 2015;46:1521–1528. doi: 10.1016/j.humpath.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd I.E., Ahmed F., Revelo M.P., Khalighi M.A. De novo immune complex deposition in kidney allografts: a series of 32 patients. Hum Pathol. 2018;71:109–116. doi: 10.1016/j.humpath.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Loupy A., Haas M., Solez K. The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17:28–41. doi: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roufosse C., Simmonds N., Clahsen-van Groningen M. 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weening J.J., D’Agati V.D., Schwartz M.M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 11.Bajema I.M., Wilhelmus S., Alpers C.E. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 12.El-Zoghby Z.M., Stegall M.D., Lager D.J. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527–535. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 13.Grau V., Zeuschner P., Immenschuh S. Immune complex-type deposits in the Fischer-344 to Lewis rat model of renal transplantation and a subset of human transplant glomerulopathy. Transplantation. 2016;100:1004. doi: 10.1097/TP.0000000000001068. [DOI] [PubMed] [Google Scholar]
- 14.Sethi S., Nasr S.H., De Vriese A.S., Fervenza F.C. C4d as a diagnostic tool in proliferative GN. J Am Soc Nephrol. 2015;26:2852–2859. doi: 10.1681/ASN.2014040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leal R., Pinto H., Galvão A. Nephrotic range proteinuria in renal transplantation: clinical and histologic correlates in a 10-year retrospective study. Transplant Proc. 2017;49:792–794. doi: 10.1016/j.transproceed.2017.01.066. [DOI] [PubMed] [Google Scholar]
- 16.Roberti I., Vyas S. Immune-mediated nephropathies in kidney transplants: recurrent or de novo diseases. Pediatr Transplant. 2016;20:946–951. doi: 10.1111/petr.12789. [DOI] [PubMed] [Google Scholar]
- 17.Foschi A., Zavaglia C.A., Fanti D. Autoimmunity after liver transplantation: a frequent event but a rare clinical problem. Clin Transplant. 2015;29:161–166. doi: 10.1111/ctr.12498. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Li B., Wang W., Lv J. Risk factors for new-onset chronic kidney disease in patients who have received a liver transplant. Exp Ther Med. 2018;15:3589–3595. doi: 10.3892/etm.2018.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.