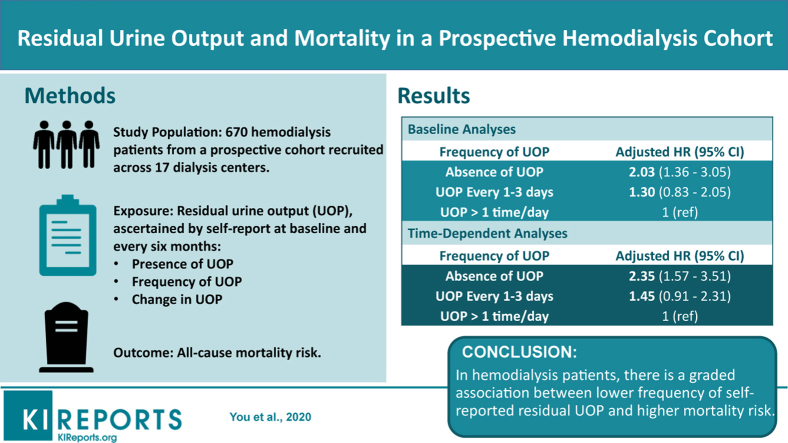
Abstract
Introduction
Although residual urine output (UOP) is associated with better survival and quality of life in dialysis patients, frequent measurement by 24-hour urine collection is burdensome. We thus sought to examine the association of patients’ self-reported residual UOP, as an alternative proxy of measured residual UOP, with mortality risk in a prospective hemodialysis cohort study.
Methods
Among 670 hemodialysis patients from the prospective multicenter Malnutrition, Diet, and Racial Disparities in Kidney Disease study, we examined associations of residual UOP, ascertained by patient self-report, with all-cause mortality. Patients underwent protocolized surveys assessing presence and frequency of UOP (absent, every 1–3 days, >1 time per day) every 6 months from 2011 to 2015. We examined associations of baseline and time-varying UOP with mortality using Cox regression.
Results
In analyses of baseline UOP, absence of UOP was associated with higher mortality in expanded case-mix adjusted Cox models (ref: presence of UOP): hazard ratio (HR), 1.78 (95% confidence interval [CI], 1.16–2.72). In analyses examining baseline frequency of UOP, point estimates suggested a graded association between lower frequency of UOP and higher mortality, although estimates for UOP every 1 to 3 days did not reach statistical significance (reference: UOP >1 time per day): HR, 1.29 (95% CI, 0.82–2.05) and HR, 1.97 (95% CI, 1.24–3.12) for UOP every 1 to 3 days and absence of UOP, respectively. Similar findings were observed in analyses of time-varying UOP.
Conclusion
In hemodialysis patients, there is a graded association between lower frequency of self-reported UOP and higher mortality. Further studies are needed to determine the clinical impact of more frequent assessment of residual UOP using self-reported methods.
Key words: end-stage renal disease, hemodialysis, mortality, residual kidney function, urine output
Graphical abstract
In the United States, approximately 120,000 patients with incident end-stage renal disease (ESRD) transition to dialysis each year, among whom 27% and 12% of patients commence therapy with an estimated glomerular filtration rate (eGFR) of 10 to <15 ml/min per 1.73 m2 and >15 ml/min per 1.73 m2, respectively.1 Hence, in contemporary practice, a large proportion of patients with incident ESRD are transitioning to hemodialysis with substantial residual kidney function and urine output (UOP). Although hemodialysis is thought to lead to more accelerated residual kidney function decline compared with peritoneal dialysis,2 some data suggest that hemodialysis patients experience greater preservation of kidney function than previously estimated (i.e., 70% and 14%–20% of patients with residual kidney function 1 and 3 to 5 years, respectively, after transitioning to dialysis3).
Among hemodialysis and peritoneal dialysis patients, a growing body of evidence has shown a graded association between preservation of residual UOP and kidney function with greater survival, presumably due to better volume control, solute clearance, and uremic toxin removal given its continuous nature.4, 5, 6, 7, 8 In current US clinical practice, residual UOP and kidney function are not routinely monitored in the maintenance hemodialysis population, as (i) frequent urine collection and measurement may be burdensome on patients, as well as (ii) being time-consuming, cost-incurring, and impractical for dialysis staff and providers. In addition, broader implementation of direct measurement of residual UOP and kidney function in hemodialysis patients may also be hindered by (iii) uncertainty regarding the optimal metric in this population.9,10
Thus, to better inform the field, we designed a prospective, multicenter study of well-characterized hemodialysis patients in which we conducted protocolized assessment of self-reported residual UOP every 6 months. In this study, we examined patterns in the presence and frequency of self-reported UOP at study entry and changes over time, as well as the associations of baseline and repeated (longitudinal) measures UOP with mortality in hemodialysis patients across Southern California.
Methods
Source Cohort
The source population was composed of incident and prevalent hemodialysis patients from the prospective Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease (MADRAD) study11, 12, 13, 14, 15 who were recruited from 17 outpatient dialysis units across Southern California over the period of October 2011 through November 2015. In this substudy of the MADRAD cohort, patients were included provided they were 18 years or older at the time of study entry (i.e., date of first survey of UOP assessment), had a diagnosis of ESRD, received thrice-weekly in-center hemodialysis for at least 4 consecutive weeks, completed at least 1 or more protocolized surveys of self-reported UOP, and signed a local institutional review board approved consent form. Patients were excluded if they were actively receiving peritoneal dialysis, had dialysis-dependent acute kidney injury, had a life expectancy of less than 6 months (e.g., stage IV cancer), or were unable to provide consent without a proxy (e.g., dementia). The study was approved by the institutional review committee of the University of California Irvine Medical Center.
Exposure Ascertainment
The exposure of interest was self-reported UOP ascertained from protocolized surveys administered every 6 months (i.e., semesters) to patients during their hemodialysis treatments. The self-administered survey included 2 questions regarding the presence and frequency of UOP. In primary analyses, we first examined the association of presence versus absence of UOP with all-cause mortality risk. In secondary analyses, we then examined the relationship between frequency of UOP, categorized as (i) absence of UOP, (ii) UOP every 1–3 days, and (iii) UOP >1 time per day, with all-cause mortality risk. In a subcohort of patients who underwent 2 or more surveys, we examined the association between change in UOP, categorized as (i) persistent presence of UOP, (ii) gain in UOP, (iii) loss in UOP, and (iv) persistent absence of UOP, with all-cause mortality risk in sensitivity analyses.
Outcome Ascertainment
The primary outcome of interest was all-cause mortality risk. At-risk time began the day following the first survey of UOP measurement, and patients were censored for kidney transplantation, transfer to a nonaffiliated outpatient dialysis unit or peritoneal dialysis, or at the end of the study period (December 7, 2015). Using a formal adjudication process, each semester information regarding mortality, censoring events, and associated dates from the preceding 6 months was collected from event forms completed by the MADRAD research coordinators and reviewed by 2 MADRAD study nephrologists (CMR and KK-Z), supplemented by medical records from the dialysis centers.
Statistical Analyses
Baseline characteristics across exposure groups were compared using χ2, analysis of variance, and Kruskal-Wallis tests according to data type. We first examined the relationship between relevant clinical characteristics with likelihood of (i) presence of UOP (ref: absence of UOP) and (ii) high frequency of UOP, defined as UOP >1 time per day (ref: UOP <1 time per day) at study entry using logistic regression.
To determine the long-term and short-term associations of presence of residual UOP with health outcomes, we examined both baseline and time-varying UOP with all-cause mortality risk using fixed and time-varying Cox proportional hazard models, respectively.16 Logistic regression and Cox regression models were analyzed using 3 incremental levels of covariate adjustment:
-
(i)
unadjusted model: included presence of UOP as the primary exposure of interest;
-
(ii)
case-mix analyses: adjusted for covariates in the unadjusted model, as well as age, sex, race, ethnicity, and diabetes; and
-
(iii)
expanded case-mix analyses: adjusted for covariates in the case-mix model, as well as dialysis vintage.
We a priori defined the expanded case-mix adjusted model as our preferred model, which forced into the model core sociodemographic measures and other confounders of the association between UOP and mortality. Given that dialysis adequacy may vary among patients with presence versus absence of UOP as a result of differential prescription parameters, we also conducted sensitivity analyses in which we incrementally adjusted for single pool Kt/V in (i) expanded case-mix+adequacy analyses. To also account for the impact of vascular access type on dialysis adequacy, as well as differential practice patterns across dialysis centers, we also conducted sensitivity analyses that incrementally adjusted for these covariates in (ii) expanded case-mix+vascular access analyses and (iii) expanded case-mix+center analyses.
Effect of modification of UOP-mortality associations on the basis of age, sex, race, ethnicity, dialysis vintage (i.e., dichotomized as <1 vs. ≥1 year as a proxy of incident vs. prevalent ESRD status, respectively, as well as <2 vs. ≥2 years), diabetes, serum creatinine (i.e., proxy of muscle mass), serum albumin, normalized protein catabolic rate, and body mass index strata were explored through the addition of 2-way interaction terms with presence of UOP separately using Wald testing. There were no missing data for age, sex, race, ethnicity, and diabetes, and the remaining covariates had <1% missing values at baseline except for serum creatinine, serum albumin, normalized protein catabolic rate, single pool Kt/V, and body mass index (13.0%, 11.8%, 11.2%, 12.8%, and 11.0% of patients with missing covariates, respectively). Multiple imputation was used to address missing covariate data. The proportional hazards assumption was confirmed graphically. Analogous approaches were used to examine the relationship between frequency of UOP (separately) and change in UOP with all-cause mortality risk. Analyses and figures were carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC), Stata version 13.1 (Stata Corporation, College Station, TX), and SigmaPlot version 12.5 (Systat Software, San Jose, CA).
Results
Study Population
Among 670 patients who met eligibility criteria (Supplementary Figure S1), the mean ± SD age of the cohort was 54.6 ± 14.5 years, among whom 44% were women, 34% were black, 48% were Hispanic, and 54% had diabetes. The mean ± SD dialysis vintage of the cohort was 50 ± 48 months. At study entry, 25.4% of patients reported absence of UOP, whereas 74.6% reported presence of UOP. In terms of frequency of UOP, 19.9%, 51.5%, and 3.3% of patients reported having UOP every 1 to 3 days, UOP >1 time per day, and presence of UOP at a nonspecified frequency.
Patients’ baseline characteristics stratified by absence versus presence of UOP are shown in Table 1. Compared with patients with absence of UOP, those with presence of UOP tended to be older; had a shorter dialysis vintage; had lower serum alkaline phosphatase, creatinine, potassium, and single pool Kt/V levels; had higher eGFR levels; and were less likely to have hyperkalemia (defined as serum potassium >5.0 mEq/l). When comparing crude rates of hyperkalemia across exposure groups, we also observed that hyperkalemia rates were higher in those with absence versus presence of urine output (3.34 vs. 3.05 events per 100 person-months, respectively). On stratifying patients according to frequency of UOP, compared with patients with absence of UOP, those with a high frequency of UOP (i.e., UOP >1 time per day) also tended to be older; were less likely to be white or black and were more likely to be Asian; had a shorter dialysis vintage; had lower serum creatinine, potassium, and single pool Kt/V levels; had higher eGFR levels; and were less likely to have hyperkalemia (Supplementary Table S1).
Table 1.
Baseline characteristics according to absence versus presence of self-reported urine output (UOP)
| Absence of UOP | Presence of UOP | Pa | |
|---|---|---|---|
| % (n) of patients | 25.4 (170) | 74.6 (500) | n/a |
| Age (yr), mean ± SD | 51.6 ± 14.6 | 55.6 ± 14.3 | 0.004 |
| Female, % (n) | 42.9 (73) | 44.2 (221) | 0.78 |
| Race, % (n) | 0.43 | ||
| White | 57.1 (97) | 55.0 (275) | |
| Black | 36.5 (62) | 32.8 (164) | |
| Asian | 5.3 (9) | 9.4 (47) | |
| Pacific Islander | 1.2 (2) | 2.2 (11) | |
| Alaskan/American Indian | 0.0 (0) | 0.2 (1) | |
| Other race | 0.0 (0) | 0.4 (2) | |
| Hispanic ethnicity, % (n) | 52.9 (90) | 46.8 (234) | 0.17 |
| Vintage (mo), mean ± SD | 87.0 ± 59.3 | 37.2 ± 36.3 | <0.001 |
| Vintage, % (n) | <0.001 | ||
| <1 yr | 2.4 (4) | 27.2 (135) | |
| 1 to <2 yr | 12.4 (21) | 20.9 (104) | |
| ≥2 yr | 85.2 (144) | 51.9 (258) | |
| Diabetes, % (n) | 48.8 (83) | 55.8 (279) | 0.11 |
| Vascular access, % (n) | 0.14 | ||
| AVF/AVG | 77.1 (131) | 70.6 (353) | |
| Catheter | 8.8 (15) | 14.6 (73) | |
| Unknown | 14.1 (24) | 14.8 (74) | |
| Laboratory tests, median (IQR) | |||
| Serum albumin (g/dl) | 4.1 (3.9, 4.3) | 4.0 (3.8, 4.2) | 0.15 |
| Alkaline phosphatase (IU/l) | 91 (74, 130) | 85 (65, 116) | 0.03 |
| Calcium (mg/dl) | 9.2 (8.7, 9.6) | 9.1 (8.7, 9.5) | 0.67 |
| Serum creatinine (mg/dl) | 10.6 (8.5, 12.7) | 9.3 (7.2, 11.4) | <0.001 |
| Ferritin (ng/ml) | 625 (405, 820) | 638 (395, 872) | 0.64 |
| Hemoglobin (g/dl) | 10.7 (10.1, 11.4) | 10.7 (10.1, 11.2) | 0.30 |
| Iron saturation (%) | 28 (22, 36) | 28 (22, 35) | 0.44 |
| nPCR (g/kg per day) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 0.18 |
| Phosphorus (mg/dl) | 5.1 (3.9, 6.1) | 4.9 (4.1, 5.9) | >0.99 |
| Potassium (mEq/l) | 5.1 (4.7, 5.4) | 4.8 (4.4, 5.2) | <0.001 |
| PTH (pg/ml) | 365 (226, 579) | 348 (218, 491) | 0.30 |
| Single pool Kt/V | 1.7 (1.5, 2.0) | 1.6 (1.4, 1.8) | <0.001 |
| Laboratory tests, % (n) | |||
| Potassium (mEq/l) | <0.001 | ||
| <3.5 | 0 (0) | 0 (0) | |
| 3.5–5.0 | 49.0 (77) | 65.6 (286) | |
| >5.0–5.5 | 29.9 (47) | 22.2 (97) | |
| >5.5 | 21.0 (33) | 12.2 (53) | |
| Body anthropometry, median (IQR) | |||
| BMI (kg/m2) | 26.6 (23.0, 30.2) | 26.5 (23.4, 31.7) | 0.78 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; nPCR, normalized protein catabolic rate; PTH, parathyroid hormone.
P values calculated by analysis of variance, χ2, or Kruskal-Wallis tests.
Clinical Characteristics Associated With Self-Reported UOP
In case-mix adjusted logistic regression analyses, clinical characteristics associated with higher likelihood of presence of UOP included older age, which persisted with further adjustment for expanded case-mix covariates (ref: absence of UOP) (Table 2). In contrast, being of black race, Hispanic ethnicity, and longer dialysis vintage, as well as having higher serum alkaline phosphatase, creatinine, potassium, and single pool Kt/V levels were associated with lower likelihood of presence of UOP; however, on adjustment for expanded case-mix covariates, only associations of serum creatinine, potassium, and single pool Kt/V levels with presence of UOP remained significant.
Table 2.
Clinical characteristics associated with self-reported presence of urine output (UOP) (Left; ref: absence of UOP) and high frequency of UOP (i.e., >1 time/day) (right; ref: <1 time/day)
| Presence of UOP |
High frequency of UOP |
|||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Case-mixa OR (95% CI) |
Expanded case-mixb OR (95% CI) |
Unadjusted OR (95% CI) |
Case-mixa OR (95% CI) |
Expanded case-mixb OR (95% CI) |
|
| Age (Δ10 yr) | 1.21 (1.07, 1.37) | 1.18 (1.03, 1.35) | 1.28 (1.10, 1.49) | 1.03 (0.92, 1.14) | 0.97 (0.87, 1.09) | 0.99 (0.88, 1.12) |
| Female (vs. male) | 1.05 (0.74, 1.50) | 1.02 (0.71, 1.45) | 1.21 (0.81, 1.81) | 1.17 (0.87, 1.59) | 1.15 (0.84, 1.56) | 1.31 (0.93, 1.83) |
| Race (vs. white) | ||||||
| Black | 0.93 (0.64, 1.36) | 0.62 (0.32, 1.19) | 0.85 (0.41, 1.76) | 1.03 (0.74, 1.43) | 0.73 (0.43, 1.25) | 1.00 (0.56, 1.77) |
| Asian | 1.84 (0.87, 3.90) | 1.27 (0.50, 3.21) | 1.60 (0.58, 4.45) | 2.32 (1.27, 4.25) | 1.68 (0.80, 3.51) | 2.17 (0.97, 4.85) |
| Pacific Islander | 1.94 (0.42, 8.91) | 1.32 (0.26, 6.73) | 1.95 (0.30, 12.65) | 1.18 (0.39, 3.58) | 0.79 (0.24, 2.63) | 0.97 (0.27, 3.51) |
| Alaskan/American Indian | — | — | — | — | — | — |
| Other race | — | — | — | — | — | — |
| White (vs. nonwhite) | 0.92 (0.65, 1.31) | 0.73 (0.31, 1.74) | 0.58 (0.22, 1.52) | 0.85 (0.63, 1.16) | 0.81 (0.41, 1.58) | 0.68 (0.33, 1.40) |
| Black (vs. nonblack) | 0.85 (0.59, 1.22) | 0.54 (0.32, 0.92) | 0.66 (0.37, 1.18) | 0.94 (0.68, 1.29) | 0.64 (0.42, 0.98) | 0.79 (0.50, 1.25) |
| Hispanic (vs. non-Hispanic) | 0.78 (0.55, 1.11) | 0.54 (0.33, 0.90) | 0.63 (0.36, 1.10) | 0.75 (0.56, 1.02) | 0.55 (0.36, 0.83) | 0.61 (0.40, 0.95) |
| Vintage (vs. < 1 yr) | ||||||
| 1 to <2 yr | 0.15 (0.05, 0.44) | 0.13 (0.04, 0.38) | — | 0.47 (0.28, 0.80) | 0.44 (0.26, 0.75) | — |
| ≥2 yr | 0.05 (0.02, 0.15) | 0.05 (0.02, 0.13) | — | 0.23 (0.15, 0.36) | 0.23 (0.15, 0.35) | — |
| Diabetes (vs. nondiabetes) | 1.32 (0.93, 1.88) | 1.17 (0.80, 1.71) | 0.68 (0.43, 1.06) | 1.26 (0.93, 1.71) | 1.36 (0.97, 1.89) | 1.00 (0.70, 1.43) |
| Serum albumin (Δ0.55 g/dl) | 0.93 (0.71, 1.20) | 0.98 (0.75, 1.29) | 1.06 (0.78, 1.44) | 0.95 (0.75, 1.19) | 0.96 (0.75, 1.22) | 1.02 (0.78, 1.32) |
| Alkaline phosphatase (Δ25 IU/l) | 0.91 (0.84, 0.98) | 0.91 (0.84, 0.98) | 0.92 (0.84, 1.00) | 0.92 (0.85, 1.00) | 0.92 (0.85, 1.00) | 0.95 (0.87, 1.03) |
| Calcium (Δ1 mg/dl) | 1.04 (0.79, 1.36) | 1.03 (0.78, 1.36) | 1.28 (0.94, 1.75) | 1.07 (0.85, 1.37) | 1.08 (0.85, 1.38) | 1.29 (0.98, 1.70) |
| Serum creatinine (Δ0.5 mg/dl) | 0.93 (0.90, 0.96) | 0.92 (0.89, 0.96) | 0.93 (0.89, 0.97) | 0.96 (0.94, 0.99) | 0.95 (0.92, 0.99) | 0.97 (0.94, 1.01) |
| Ferritin (Δ50 ng/ml) | 1.01 (0.98, 1.04) | 1.01 (0.98, 1.04) | 1.03 (1.00, 1.07) | 1.01 (0.99, 1.04) | 1.01 (0.99, 1.04) | 1.04 (1.01, 1.07) |
| Hemoglobin (Δ1 g/dl) | 0.87 (0.73, 1.03) | 0.86 (0.73, 1.02) | 0.98 (0.80, 1.20) | 0.84 (0.72, 0.98) | 0.84 (0.72, 0.98) | 0.89 (0.75, 1.05) |
| Iron saturation (Δ20%) | 0.99 (0.73, 1.34) | 1.03 (0.75, 1.40) | 1.14 (0.80, 1.62) | 0.94 (0.71, 1.23) | 0.96 (0.73, 1.28) | 1.07 (0.79, 1.44) |
| nPCR (Δ0.4g/kg per day) | 0.84 (0.65, 1.09) | 0.83 (0.63, 1.09) | 0.81 (0.59, 1.12) | 0.85 (0.68, 1.08) | 0.82 (0.64, 1.05) | 0.83 (0.64, 1.08) |
| Phosphorus (Δ1 mg/dl) | 0.98 (0.87, 1.11) | 1.02 (0.90, 1.16) | 0.96 (0.84, 1.11) | 0.99 (0.89, 1.10) | 0.99 (0.88, 1.10) | 0.95 (0.84, 1.07) |
| Potassium (Δ1) | 0.56 (0.42, 0.75) | 0.57 (0.42, 0.77) | 0.59 (0.42, 0.83) | 0.70 (0.54, 0.91) | 0.68 (0.52, 0.90) | 0.76 (0.57, 1.01) |
| PTH (Δ25 pg/ml) | 0.99 (0.97, 1.00) | 0.99 (0.97, 1.00) | 0.99 (0.97, 1.00) | 1.00 (0.98, 1.01) | 1.00 (0.98, 1.01) | 1.00 (0.99, 1.02) |
| spKt/V (Δ0.4) | 0.68 (0.55, 0.85) | 0.65 (0.51, 0.82) | 0.72 (0.56, 0.94) | 0.76 (0.62, 0.92) | 0.71 (0.57, 0.88) | 0.80 (0.64, 0.99) |
| BMI (Δ5 kg/m2) | 1.01 (0.89, 1.15) | 1.01 (0.88, 1.15) | 1.01 (0.87, 1.18) | 1.08 (0.96, 1.21) | 1.07 (0.95, 1.21) | 1.09 (0.96, 1.24) |
Bold estimates show statistically significant associations.
BMI, body mass index; CI, confidence interval; nPCR, normalized protein catabolic rate; OR, odds ratio; PTH, parathyroid hormone.
Case-mix is adjusted for age, sex, race (black), ethnicity, and diabetes.
Expanded case-mix is adjusted for age, sex, race (black), ethnicity, diabetes, and vintage.
In secondary analyses, being of black race, Hispanic ethnicity, and longer dialysis vintage, as well as having higher serum alkaline phosphatase, creatinine, and hemoglobin levels were associated with lower likelihood of having high frequency of UOP (i.e., UOP >1 time per day) in case-mix logistic regression analyses (ref: UOP <1 time per day) (Table 2). However, on adjustment for expanded case-mix covariates, only Hispanic ethnicity, higher serum ferritin levels, and higher single pool Kt/V were associated with lower likelihood of high frequency of UOP.
Presence Versus Absence of Self-Reported UOP and Mortality
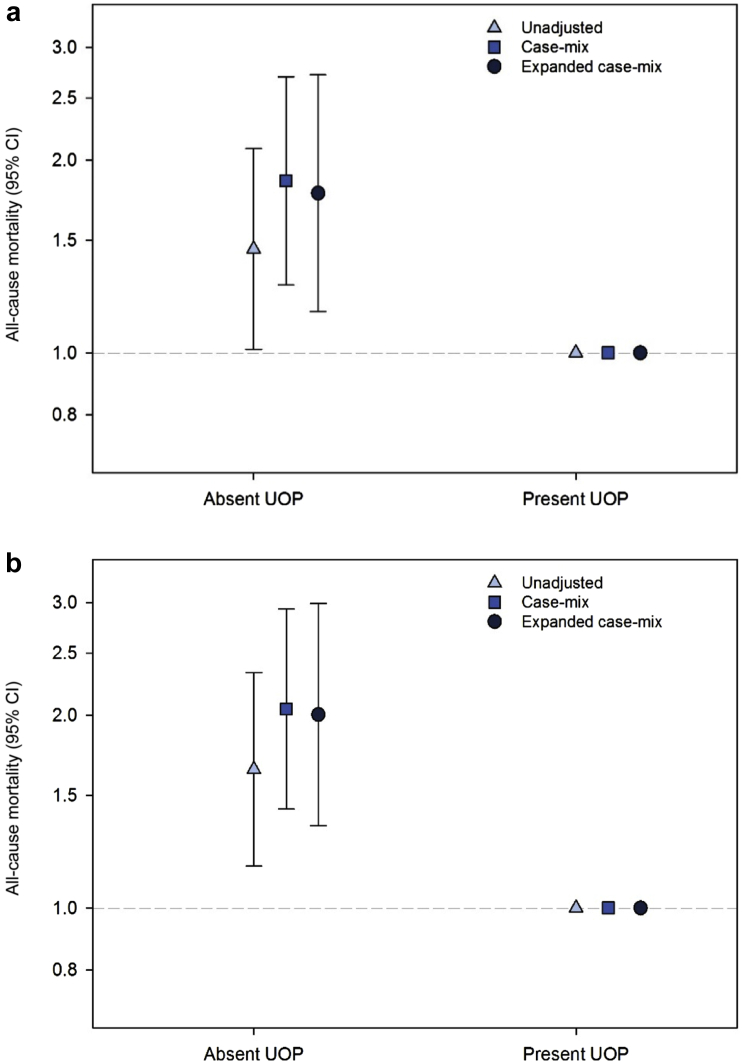
Patients contributed a total of 1566.2 patient-years of follow-up, during which time 135 all-cause death events occurred. The median (interquartile range) follow-up time was 2.40 (1.33, 3.51) years. In analyses of baseline UOP, absence of UOP was associated with higher mortality risk in unadjusted, case-mix, and expanded case-mix Cox models (ref: presence of UOP): HR, 1.45 (95% CI, 1.01–2.08), HR, 1.86 (95% CI, 1.28–2.70), and HR, 1.78 (95% CI, 1.16–2.72), respectively (Figure 1a and Supplementary Table S2). In analyses of time-varying UOP, even stronger associations between absence of UOP and higher mortality risk were observed in unadjusted, case-mix, and expanded case-mix Cox models (ref: presence of UOP): HR, 1.65 (95% CI, 1.16–2.33), HR, 2.05 (95% CI, 1.43–2.93), and HR, 2.01 (95% CI, 1.35–2.99), respectively (Figure 1b and Supplementary Table S2). These findings were robust in sensitivity analyses incrementally adjusted for dialysis adequacy, vascular access type, and dialysis center (Supplementary Table S2).
Figure 1.
Association between baseline (a) and time-varying (b) presence versus absence of self-reported urine output (UOP) with all-cause mortality risk. CI, confidence interval.
Frequency of Self-Reported UOP and Mortality
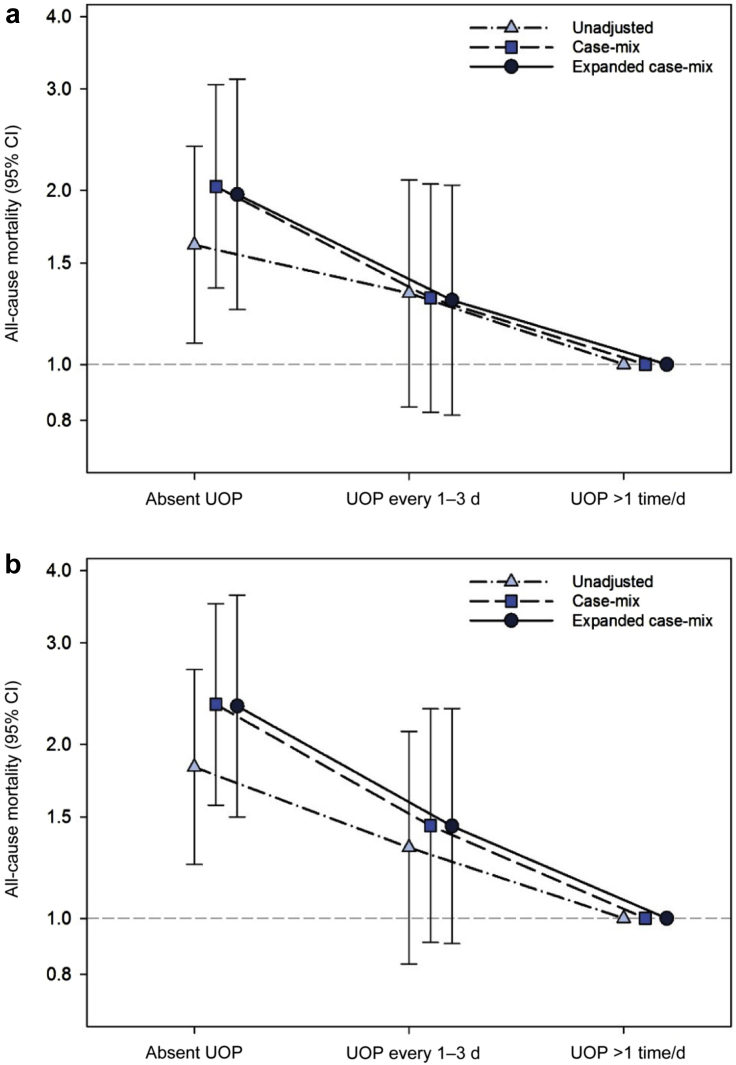
In secondary analyses examining frequency of UOP, point estimates of incrementally lower levels of self-reported UOP were linked with increasingly higher death risk. In analyses of baseline UOP, compared with having high frequency of UOP (i.e., UOP >1 time per day), having UOP every 1 to 3 days trended toward higher risk of death, and having absence of UOP was significantly associated with higher mortality risk in case-mix analyses: HR, 1.30 (95% CI, 0.83–2.05) and HR, 2.03 (95% CI 1.36–3.05), respectively (Figure 2a and Supplementary Table S3). These patterns of association persisted in analyses adjusted for expanded case-mix covariates, as well as those incrementally adjusted for dialysis adequacy, vascular access type, and dialysis center (Supplementary Table S3).
Figure 2.
Association between baseline (a) and time-varying (b) frequency of self-reported urine output (UOP) with all-cause mortality risk. CI, confidence interval.
Similarly, in analyses of time-varying UOP, compared with having high frequency of UOP, having UOP every 1 to 3 days trended toward higher mortality risk, whereas having absence of UOP was significantly associated with higher death risk in case-mix analyses: HR, 1.45 (95% CI, 0.91–2.31) and HR, 2.35 (95% CI, 1.57–3.51), respectively (Figure 2b and Supplementary Table S3). These patterns of association also persisted in analyses adjusted for expanded case-mix covariates, as well as those incrementally adjusted for dialysis adequacy, vascular access type, and dialysis center (Supplementary Table S3).
Self-Reported Urine Output and Mortality Across Clinically Relevant Subgroups
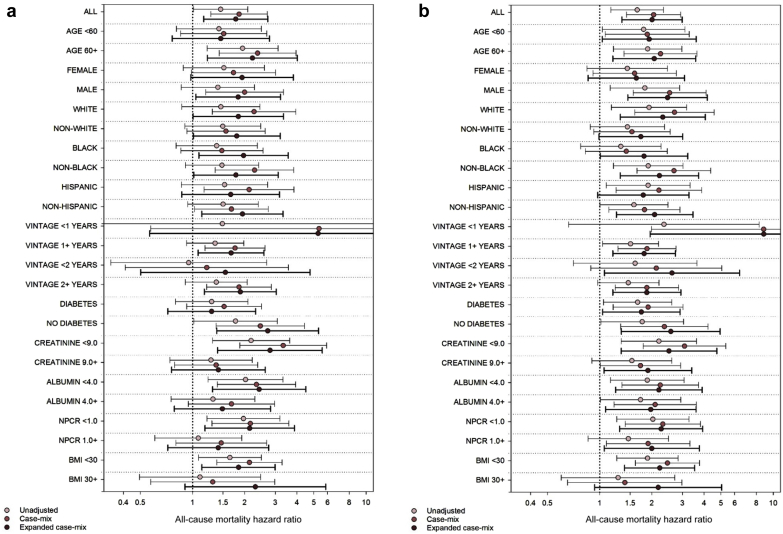
In expanded case-mix analyses of the association of baseline UOP with mortality risk stratified across clinically relevant subgroups, the nominal HR for presence of UOP was >1 across all groups (ref: absence of UOP) (Figure 3a and Supplementary Table S4). Nominal associations were statistically significant in the following subgroups: ≥60 years of age, males, whites, nonwhites, blacks, nonblacks, non-Hispanics, vintage ≥1 year, vintage ≥2 years, absence of diabetes, serum creatinine <9 mg/dl, serum albumin <4 g/dl, normalized protein catabolic rate <1 g/kg per day, and body mass index <30 kg/m2. Although the P values for interaction tests for all subgroups did not achieve statistical significance, there was a trend toward a significant interaction between UOP and serum creatinine (P-interaction = 0.06), such that absence of UOP was more potently associated with mortality among those with serum creatinine levels <9 versus ≥9 mg/dl, respectively.
Figure 3.
Association of baseline (a) and time-varying (b) presence versus absence of urine output (UOP) with all-cause mortality risk across clinically relevant subgroups. BMI, body mass index; NPCR, normalized protein catabolic rate.
In expanded case-mix analyses of the association of time-varying UOP with mortality risk stratified across clinically relevant subgroups, the nominal HR for presence of UOP was >1 across all groups (ref: absence of UOP) (Figure 3b and Supplementary Table S5). Nominal associations were statistically significant in the following subgroups: <60 years of age, ≥60 years of age, males, whites, blacks, nonblacks, non-Hispanic individuals, vintage <1 year, vintage ≥1 year, vintage <2 years, vintage ≥2 years, presence of diabetes, absence of diabetes, serum albumin <4 g/dl, serum albumin ≥ 4g/dl, normalized protein catabolic rate <1 g/kg per day, normalized protein catabolic rate ≥1 g/kg per day, and body mass index <30 kg/m2. However, the P values for interaction tests for all subgroups did not achieve statistical significance. A similar pattern of findings was observed following adjustment for expanded case-mix covariates.
Change in Self-Reported UOP and Mortality Risk
In a subset of 431 patients who underwent 2 or more surveys assessing UOP, we examined the relationship between change in self-reported UOP with mortality risk. Among these patients, 67.5%, 3.5%, 5.1%, and 23.9% reported persistent presence of UOP, gain in UOP, loss of UOP, and persistent absence of UOP over time (Supplementary Figure S2 and Supplementary Table S6). In case-mix analyses, compared with patients who reported persistent presence of UOP, those with persistent absence of UOP had significantly higher death risk: HR, 1.72 (95% CI, 1.04–2.84) (Supplementary Figure S3 and Supplementary Table S7). Although associations of gain in UOP and loss of UOP did not achieve statistical significance, point estimates suggested lower and higher death risk, respectively: HR, 0.92 (95% CI, 0.22–3.87) and HR, 1.41 (95% CI, 0.56–3.57), respectively. However, estimates were not statistically significant in analyses adjusted for expanded case-mix covariates, nor those incrementally adjusted for dialysis adequacy, vascular access type, and dialysis center (Supplementary Table S7).
Discussion
In this prospective, multicenter study of 670 hemodialysis patients who underwent protocolized assessment of self-reported UOP every 6 months, we found that three-quarters of the cohort reported presence of residual UOP. We observed that absence of UOP at study entry was associated with a 78% higher mortality risk using multivariable models that accounted for differences in case-mix covariates across exposure groups. These associations were even stronger in time-varying analyses that accounted for changes in UOP over time (i.e., 2-fold higher death risk).
A large body of evidence has demonstrated the benefits of residual UOP and/or kidney function on survival in both hemodialysis and peritoneal dialysis patients.17, 18, 19, 20, 21, 22, 23, 24, 25 For example, preservation of residual UOP may (i) attenuate interdialytic weight gains, resulting in lower ultrafiltration rates, intradialytic hypotension, and myocardial stunning, as well as (ii) better fluid balance,3, 4, 5, 6, 7, 8 subsequently reducing risk of left ventricular hypertrophy as a substrate for malignant ventricular arrhythmias.26 Native kidney function also provides greater clearance of middle and large molecular weight solutes versus hemodialysis.27, 28, 29 In addition, preservation of residual UOP and residual kidney function has been associated with reduced inflammation, better nutritional parameters, and greater health-related quality of life.22,30 Consequently, several studies in maintenance hemodialysis patients have shown that higher levels of residual urine volume are associated with lower mortality risk. First, in a prospective study of 734 incident hemodialysis patients who underwent urine volume assessment by questionnaire at study entry and 1-year follow-up by Shafi et al.,22 those who reported having baseline UOP of >250 ml/d as well as preserved UOP at 1 year had lower mortality risk. Then, in a retrospective cohort of 6358 incident hemodialysis patients from a large US dialysis organization who underwent 24-hour urine volume measurement at baseline and 1-year follow-up by Obi et al.,20 incrementally lower urine volume at 1 year (categorized as ≥1200 ml/d, 600 ml/d to <1200 ml/d, 300 ml/d to <600 ml/d, and <300 ml/d) as well as faster annual decline in urine volume was associated with higher risk of death. In another retrospective analysis of 1946 hemodialysis and peritoneal dialysis patients from Korea who underwent 24-hour urine volume measurement at a single point in time, each 0.1 l of urine volume was associated with incrementally greater survival.19 Most recently, among 37,474 patients from the international Dialysis Outcomes and Practice Patterns (DOPPS) study, those who reported baseline urine volume >200 ml/d had lower mortality risk; in a subcohort of 8388 patients from DOPPS Phase V who underwent more granular assessment of baseline urine volume (categorized as ≥1000 ml/d, 500 ml/d–999 ml/d, 200 ml/d–499 ml/d, and ≤200 ml/d), there was a graded association between lower urine volume and higher death risk.18
To our knowledge, ours is the first study to examine the longitudinal relationship between self-reported presence and frequency UOP ascertained by protocolized assessment every 6 months with mortality risk in hemodialysis patients. We found that approximately 20% and more than 50% of patients reported having frequent urination every 1 to 3 days or more than once per day, respectively; notably, the vast majority of these patients had a dialysis vintage of exceeding 1 year (i.e., 82% and 70%, respectively). The prevalence of preserved UOP is higher than previously reported estimates of preserved residual kidney function from less contemporary cohorts (i.e., ∼70%, 60%, and 30% after 1, 2, and 5 years of hemodialysis, respectively3), which may reflect the upward trend in eGFR at which patients with incident ESRD have been transitioning to hemodialysis over time.1 Furthermore, in analyses examining baseline and time-varying frequency of UOP, point estimates suggested a graded association between lower UOP frequency and higher mortality risk, although estimates for UOP every 1 to 3 days did not reach statistical significance. In a subcohort of 431 patients who underwent 2 or more surveys assessing UOP, we observed that approximately 68% of patients reported persistent presence of UOP who had lower mortality risk than those who experienced loss of UOP and persistent absence of UOP (∼5% and 24% of the cohort, respectively) over time.
Another notable finding of our study was the differential relationship between UOP with survival across subgroups of dialysis vintage and serum creatinine. In terms of the former, we examined patients dichotomized into subgroups of dialysis vintage of <1 versus ≥1 year as a proxy of incident versus prevalent ESRD status, respectively, In both baseline and time-varying analyses, point estimates demonstrated a trend toward absence of UOP being more potently associated with mortality among those with shorter versus longer dialysis vintage, suggesting more detrimental impact of loss of UOP among incident versus prevalent dialysis patients, respectively. In terms of the latter, we also observed a trend toward stronger associations between absence of UOP with higher mortality risk among patients with lower versus higher serum creatinine levels. In analyses of both baseline and time-varying UOP, point estimates for mortality were stronger in those with lower serum creatinine, and there was a trend toward a significant interaction in analyses of baseline UOP (P-interaction = 0.06). Given that serum creatinine is more representative of a marker of muscle mass in patients with ESRD (particularly with absence of UOP), these data suggest that associations between absence of UOP and higher mortality risk are heightened in those with worse body composition parameters.
Our findings underscore the prognostic importance of frequent and routine assessment of self-reported UOP in the hemodialysis population. Although the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines recommend routine quarterly measurement of residual kidney function in patients receiving peritoneal dialysis and twice-weekly hemodialysis,31 there is lack of guidance with respect to the thrice-weekly hemodialysis patients, many of whom may have substantial residual UOP as observed in our cohort. However, given that hemodialysis patients may be exposed to conditions and procedures that may possibly reduce residual UOP (e.g., intradialytic hypotension, contrast-enhanced imaging studies, nephrotoxic inflammatory mediators ensuing from dialysis tubing and impurities)4,32, 33, 34, 35 with potential adverse effects on volume and metabolic status,4 more frequent assessment is particularly warranted in this population. Moreover, consistent and recurring assessment of self-reported UOP may enhance dialysis patients’ awareness of the importance of residual kidney function, with favorable impact on their health behaviors (i.e., dietary fluid restriction) and self-engagement in their care. Although further research is needed to identify novel approaches and interventions that estimate and preserve residual UOP,4,9 more frequent and routine UOP assessment by patient-self-report (i.e., during weekly chairside hemodialysis rounds) can be conveniently incorporated in the clinical setting as a practical adjunct to direct quantitative assessment of residual UOP and kidney function by 24-hour urine collection.
Our study has a number of strengths, including its prospective examination of a well-defined hemodialysis cohort, with detailed collection of longitudinal data; protocolized assessment of self-reported UOP at repeated (i.e., 6-month) intervals among all patients who participated in the study (i.e., no selection bias due to restriction of the cohort to those who completed 24-hour urine collections); rigorous outcome adjudication procedures; and comprehensive adjustment for confounders of the residual UOP-mortality association with robust findings across multiple secondary and sensitivity analyses. However, several limitations of our study bear mention. First, we acknowledge that (i) self-reported UOP is a blunt metric of residual UOP, and that (ii) residual UOP does not equate with residual kidney function, and thus self-reported UOP should be used as an adjunct as opposed to a replacement for direct residual kidney function measurement by urine collection and other metrics. Second, we did not have data on use of certain medications (i.e., diuretics), dialysis treatment characteristics (i.e., interdialytic weight gain, ultrafiltration rate, intradialytic hypotension), and eGFR at the time of dialysis initiation, and therefore cannot exclude the possibility of residual confounding of UOP-mortality associations on this basis. However, given the possibility that these covariates may also function as mediators of UOP and mortality risk, non-inclusion in the multivariable models may be more ideal. Third, our prospective cohort was of moderate size, which may have had limited power to detect significant interactions across subgroups, which requires a substantially larger sample size. Fourth, we examined a mixed cohort of incident and prevalent hemodialysis patients, who may be affected by different types of biases with respect to survival and generalizability (i.e., patients with incident ESRD may have reduced generalizability, and patients with prevalent ESRD may be affected by survivor bias). Fifth, we lacked data on cause-specific mortality and hospitalization events. Finally, it is possible that these findings from a racially/ethnically diverse prospective cohort across Southern California may not be generalizable to geographic regions with distinct sociodemographic distributions and practice patterns. Indeed, the parent MADRAD cohort was composed of a younger study population compared with that of the broader U.S. dialysis population (i.e., racial/ethnic minorities have an earlier mean age of transition to ESRD).
In conclusion, in a prospective multicenter cohort of maintenance hemodialysis patients who underwent serial protocolized assessment of self-reported UOP every 6 months, we found that presence of residual UOP as well as increasing frequency of UOP was associated with greater survival. We also observed that persistent presence of UOP over time was associated with lower death risk. Our study suggests that frequent and routine assessment of self-reported UOP has prognostic utility in the hemodialysis population, and can be conveniently implemented at the chairside. At this time, further studies are needed to define management strategies that optimize and preserve UOP in the hemodialysis population.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors are supported by research grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, including K23-DK102903 (CMR), K24-DK091419 (KK-Z), R03–114642 (CMR), U01-KD102163 (KK-Z), and R44-DK116383 (KK-Z); and philanthropic grants from Mr. Louis Chang (KK-Z) and Dr. Joseph Lee (KK-Z, CMR). The sponsors did not have any role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Portions of these data have been presented in an abstract at the 2016 American Society of Nephrology Kidney Week Meeting from November 15–20, 2016 in Chicago, IL.
Footnotes
Table S1. Baseline characteristics according to frequency of self-reported urine output (UOP).
Table S2. Association between self-reported presence versus absence of urine output (UOP) and all-cause mortality risk.
Table S3. Association between self-reported frequency of urine output (UOP) and all-cause mortality risk.
Table S4. Associations of self-reported baseline absence of urine output (UOP) with all-cause mortality risk across clinically relevant subgroups (ref: presence of UOP).
Table S5. Associations of self-reported time-varying absence of urine output (UOP) with all-cause mortality risk across clinically relevant subgroups (ref: presence of UOP).
Table S6. Baseline characteristics according to self-reported change in urine output (UOP) over time.
Table S7. Association between change in self-reported urine output (UOP) and all-cause mortality risk.
Figure S1. Study algorithm for overall cohort creation.
Figure S2. Study algorithm for subcohort of patients eligible for self-reported change in urine output (UOP) analyses (i.e., patients who underwent 2 or more surveys assessing UOP over time).
Figure S3. Association between change in self-reported urine output (UOP) and all-cause mortality risk.
STROBE Statement.
Supplementary Material
References
- 1.U.S. Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2017. USRDS 2017 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. [Google Scholar]
- 2.Jansen M.A., Hart A.A., Korevaar J.C. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 3.Vilar E., Wellsted D., Chandna S.M. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009;24:2502–2510. doi: 10.1093/ndt/gfp071. [DOI] [PubMed] [Google Scholar]
- 4.Mathew A.T., Fishbane S., Obi Y. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int. 2016;90:262–271. doi: 10.1016/j.kint.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obi Y., Eriguchi R., Ou S.M. What is known and unknown about twice-weekly hemodialysis. Blood Purif. 2015;40:298–305. doi: 10.1159/000441577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee C.M., Ghahremani-Ghajar M., Obi Y. Incremental and infrequent hemodialysis: a new paradigm for both dialysis initiation and conservative management. Panminerva Med. 2017;59:188–196. doi: 10.23736/S0031-0808.17.03299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee C.M., Obi Y., Mathew A.T. Precision medicine in the transition to dialysis and personalized renal replacement therapy. Semin Nephrol. 2018;38:325–335. doi: 10.1016/j.semnephrol.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Rhee C.M., Unruh M., Chen J. Infrequent dialysis: a new paradigm for hemodialysis initiation. Semin Dial. 2013;26:720–727. doi: 10.1111/sdi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafi T., Levey A.S. Measurement and estimation of residual kidney function in patients on dialysis. Adv Chronic Kidney Dis. 2018;25:93–104. doi: 10.1053/j.ackd.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth-Manikowski S.M., Shafi T. Hemodialysis prescription for incident patients: twice seems nice, but is it incremental? Am J Kidney Dis. 2016;68:180–183. doi: 10.1053/j.ajkd.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrey A., You A.S., Kovesdy C.P. Dialysate potassium and mortality in a prospective hemodialysis cohort. Am J Nephrol. 2018;47(6):415–423. doi: 10.1159/000489961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee C.M., Chen Y., You A.S. Thyroid status, quality of life, and mental health in patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12:1274–1283. doi: 10.2215/CJN.13211216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee C.M., Nguyen D.V., Moradi H. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66:313–321. doi: 10.1053/j.ajkd.2015.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee C.M., You A.S., Nguyen D.V. Thyroid status and mortality in a prospective hemodialysis cohort. J Clin Endocrinol Metab. 2017;102:1568–1577. doi: 10.1210/jc.2016-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You A.S., Kalantar-Zadeh K., Lerner L. Association of growth differentiation factor 15 with mortality in a prospective hemodialysis cohort. Cardiorenal Med. 2017;7:158–168. doi: 10.1159/000455907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker F.W., de Mutsert R., van Dijk P.C. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int. 2008;74:994–997. doi: 10.1038/ki.2008.328. [DOI] [PubMed] [Google Scholar]
- 17.Bargman J.M., Thorpe K.E., Churchill D.N. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 18.Hecking M., McCullough K.P., Port F.K. Self-reported urine volume in hemodialysis patients: predictors and mortality outcomes in the international Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2019;74:425–428. doi: 10.1053/j.ajkd.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Lee M.J., Park J.T., Park K.S. Prognostic value of residual urine volume, GFR by 24-hour urine collection, and eGFR in patients receiving dialysis. Clin J Am Soc Nephrol. 2017;12:426–434. doi: 10.2215/CJN.05520516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obi Y., Rhee C.M., Mathew A.T. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol. 2016;27:3758–3768. doi: 10.1681/ASN.2015101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paniagua R., Amato D., Vonesh E. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 22.Shafi T., Jaar B.G., Plantinga L.C. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56:348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shemin D., Bostom A.G., Laliberty P. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 24.Termorshuizen F., Dekker F.W., van Manen J.G. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 25.van der Wal W.M., Noordzij M., Dekker F.W. Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant. 2011;26:2978–2983. doi: 10.1093/ndt/gfq856. [DOI] [PubMed] [Google Scholar]
- 26.Rhee C.M., Chou J.A., Kalantar-Zadeh K. Dialysis prescription and sudden death. Semin Nephrol. 2018;38:570–581. doi: 10.1016/j.semnephrol.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babb A.L., Ahmad S., Bergstrom J. The middle molecule hypothesis in perspective. Am J Kidney Dis. 1981;1:46–50. doi: 10.1016/s0272-6386(81)80011-x. [DOI] [PubMed] [Google Scholar]
- 28.Bargman J.M., Golper T.A. The importance of residual renal function for patients on dialysis. Nephrol Dial Transplant. 2005;20:671–673. doi: 10.1093/ndt/gfh723. [DOI] [PubMed] [Google Scholar]
- 29.Vilar E., Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24:487–494. doi: 10.1111/j.1525-139X.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang A.Y. Preserving residual kidney function in hemodialysis patients-back in the spotlight. J Am Soc Nephrol. 2016;27:3504–3507. doi: 10.1681/ASN.2016060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutlay S., Atli T., Koseogullari O. Thyroid disorders in hemodialysis patients in an iodine-deficient community. Artif Organs. 2005;29:329–332. doi: 10.1111/j.1525-1594.2005.29055.x. [DOI] [PubMed] [Google Scholar]
- 32.Dittrich E., Puttinger H., Schillinger M. Effect of radio contrast media on residual renal function in peritoneal dialysis patients--a prospective study. Nephrol Dial Transplant. 2006;21:1334–1339. doi: 10.1093/ndt/gfi023. [DOI] [PubMed] [Google Scholar]
- 33.Janousek R., Krajina A., Peregrin J.H. Effect of intravascular iodinated contrast media on natural course of end-stage renal disease progression in hemodialysis patients: a prospective study. Cardiovasc Intervent Radiol. 2010;33:61–66. doi: 10.1007/s00270-009-9715-3. [DOI] [PubMed] [Google Scholar]
- 34.Perl J., Bargman J.M. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068–1081. doi: 10.1053/j.ajkd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Weisbord S.D., Bernardini J., Mor M.K. The effect of coronary angiography on residual renal function in patients on peritoneal dialysis. Clin Cardiol. 2006;29:494–497. doi: 10.1002/clc.4960291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.