Cooperation of dehydroascorbate reductases and glutathione sets a threshold for ascorbate accumulation under high-light stress in Arabidopsis.
Abstract
Plants require a high concentration of ascorbate as a redox buffer for survival under stress conditions, such as high light. Dehydroascorbate reductases (DHARs) are enzymes that catalyze the reduction of DHA to ascorbate using reduced glutathione (GSH) as an electron donor, allowing rapid ascorbate recycling. However, a recent study using an Arabidopsis (Arabidopsis thaliana) triple mutant lacking all three DHAR genes (herein called ∆dhar) did not find evidence for their role in ascorbate recycling under oxidative stress. To further study the function of DHARs, we generated ∆dhar Arabidopsis plants as well as a quadruple mutant line combining ∆dhar with an additional vtc2 mutation that causes ascorbate deficiency. Measurements of ascorbate in these mutants under low- or high-light conditions indicated that DHARs have a nonnegligible impact on full ascorbate accumulation under high light, but that they are dispensable when ascorbate concentrations are low to moderate. Because GSH itself can reduce DHA nonenzymatically, we used the pad2 mutant that contains ∼30% of the wild-type GSH level. The pad2 mutant accumulated ascorbate at a wild-type level under high light; however, when the pad2 mutation was combined with ∆dhar, there was near-complete inhibition of high-light–dependent ascorbate accumulation. The lack of ascorbate accumulation was consistent with a marked increase in the ascorbate degradation product threonate. These findings indicate that ascorbate recycling capacity is limited in ∆dhar pad2 plants, and that both DHAR activity and GSH content set a threshold for high-light–induced ascorbate accumulation.
Plants accumulate ascorbate (ASC, also known as vitamin C) at very high levels in their tissues, especially in illuminated leaves (Smirnoff, 2018). The leaf ascorbate pool size is further enhanced under stress conditions, such as high irradiance (Dowdle et al., 2007). This antioxidant efficiently reacts with and detoxifies a number of reactive oxygen species (ROS), such as superoxide radical, singlet oxygen, and hydroxyl radical, in a nonenzymatic manner (Smirnoff, 2018). Although a chemical reaction between ASC and hydrogen peroxide (H2O2), another form of ROS, is very rare, plants have ascorbate peroxidases that can rapidly scavenge H2O2 using ASC as an electron donor (Asada, 1999; Maruta et al., 2016; Smirnoff and Arnaud, 2019). In addition, ASC serves as an electron donor for the recycling of tocopherol, a major fat-soluble antioxidant, from its oxidized form (Smirnoff, 2018). Thus, ASC as a soluble antioxidant plays a central role in cellular redox regulation by controlling ROS levels in plants. Furthermore, ASC is involved in a variety of biological processes, including iron uptake, hormone biosynthesis, anthocyanin accumulation, and the xanthophyll cycle (Müller-Moule et al., 2002; Grillet et al., 2014; Smirnoff, 2018), the latter of which dissipates excess excitation solar energy as heat (Müller-Moule et al., 2002).
In plants, ASC is synthesized from hexose through the d-Man/l-Gal pathway (Wheeler et al., 1998, 2015), in which GDP-l-Gal phosphorylases, encoded by the vitamin C-defective 2 (VTC2) and VTC5 genes, catalyze the rate-limiting step (Laing et al., 2007; Bulley et al., 2012; Yoshimura et al., 2014). The one-electron oxidation of ASC, for example, through the ascorbate peroxidase reaction, results in the formation of unstable monodehydroascorbate (MDHA) radicals, which can be recycled back to ASC through the activity of NAD(P)H-dependent MDHA reductases (MDARs; Hossain and Asada, 1985; Gallie, 2013). In illuminated chloroplasts, ferredoxin—the final electron acceptor in the photosynthetic electron transport chain—can also reduce MDHA (Asada, 1999). The MDHA radicals that escape from these reactions are spontaneously disproportionated into ASC and dehydroascorbate (DHA), a two-electron oxidized form. Reduced glutathione (GSH), another major soluble antioxidant, can reduce DHA into ASC in a nonenzymatic manner, but this reaction depends on the deprotonation of GSH to its thiolate form (GS−). Because the pKa of the GSH thiol group is high (∼9.0), the probability of GSH deprotonation is very low at a neutral pH, e.g. in the cytosol. The DHA reductases (DHARs) that catalyze the GSH-dependent DHA reduction allows plants to rapidly recycle ASC from DHA (Foyer and Halliwell, 1977; Gallie, 2013).
In higher plants, multiple isoforms of DHAR and MDAR are distributed in different subcellular compartments, including the cytosol, peroxisomes, chloroplasts, and/or mitochondria (Gallie, 2013). Arabidopsis has three functional genes that encode DHAR (DHAR1, DHAR2, and DHAR3). Two further DHAR-like sequences exist (At5g36270 and At1g19950), but these are likely pseudogenes (Dixon and Edwards, 2010). DHAR2 and DHAR3 are localized in the cytosol and chloroplast stroma, respectively (Noshi et al., 2016; Rahantaniaina et al., 2017). By contrast, the subcellular localization of DHAR1 is still obscure; Reumann et al. (2009) reported DHAR1 as a peroxisomal protein through proteomic and bio-imaging assays, whereas other studies using DHAR1 fused to a fluorescent protein showed that this enzyme was cytosolic (Grefen et al., 2010; Rahantaniaina et al., 2017). There are five genes encoding MDAR in Arabidopsis. MDAR1 is a dual-targeting protein that localizes to both the cytosol and peroxisomal matrix, whereas MDAR2 and MDAR3 are cytosolic (Lisenbee et al., 2005). MDAR4 is an enzyme attached to the peroxisomal membrane (Lisenbee et al., 2005), whereas MDAR5, also called MDAR6 or MDAR5/6, is localized to both chloroplasts and mitochondria (Obara et al., 2002).
The physiological importance of DHARs has been suggested by analysis of a transgenic tobacco (Nicotiana tabacum) line with reduced expression of cytosolic DHAR as well as an overexpression line. These studies showed the crucial roles of the cytosolic enzyme on the ascorbate pool size and redox state, stomata opening, photosynthesis, and plant growth (Chen et al., 2003; Chen and Gallie, 2004, 2006, 2008). In line with these findings, the Arabidopsis (Arabidopsis thaliana) dhar1 and dhar3 single mutants showed sensitivity to high irradiance under in vitro growth conditions, which was consistent with a slight decrease in their ascorbate contents compared to the wild type (Noshi et al., 2016, 2017). Other studies using DHAR-overexpression plants suggested that DHARs are crucial for keeping the ascorbate redox state and pool size very high (Gallie, 2013). In sharp contrast to these reports, Rahantaniaina et al. (2017) have recently generated an Arabidopsis triple-knockout mutant that lacked all three DHARs and had negligible DHAR activity, and found that the ascorbate pool size and redox states, as well as growth and development in this triple mutant, were indistinguishable from those in the wild type. More surprisingly, the triple knockout showed no effect on ascorbate profiles in a catalase-deficient mutant (cat2) background, in which a large amount of H2O2 was produced through photorespiratory glycolate oxidation (Kerchev et al., 2016), resulting in severe oxidative stress. Alternatively, DHARs were found to be required for the accumulation of oxidized glutathione (GSSG) and for the cell death triggered by the cat2-induced oxidative stress (Rahantaniaina et al., 2017). Based on these findings, it has been suggested that DHARs act as GSH dehydrogenases, rather than DHARs, to modulate H2O2-dependent redox signaling by controlling the glutathione redox state in the cat2 background (Rahantaniaina et al., 2017). Yet, the physiological significance of DHARs as ascorbate recycling enzymes remains largely unclear.
The aim of this study was to clarify if and to what extent DHARs contribute to the regulation of ascorbate pool size and redox state through its recycling in Arabidopsis. For this purpose, we herein focused on high light (HL) stress and generated a triple DHAR-knockout mutant that was further combined with either the vtc2-4 mutation (which causes ascorbate deficiency) or the phytoalexin-deficient 2-1 (pad2-1) mutation (which causes glutathione deficiency). Using these mutants, we investigated the impacts of DHARs on ascorbate profiles under HL stress in wild-type, ascorbate-deficient, and glutathione-deficient backgrounds. Our findings clearly indicate that the role of DHARs as ascorbate recycling enzymes is dependent on ascorbate pool size, and that cooperation of DHARs and GSH is required for ascorbate accumulation under HL stress in Arabidopsis.
RESULTS
Generation and Characterization of dhar1 dhar2 dhar3 Triple Mutants
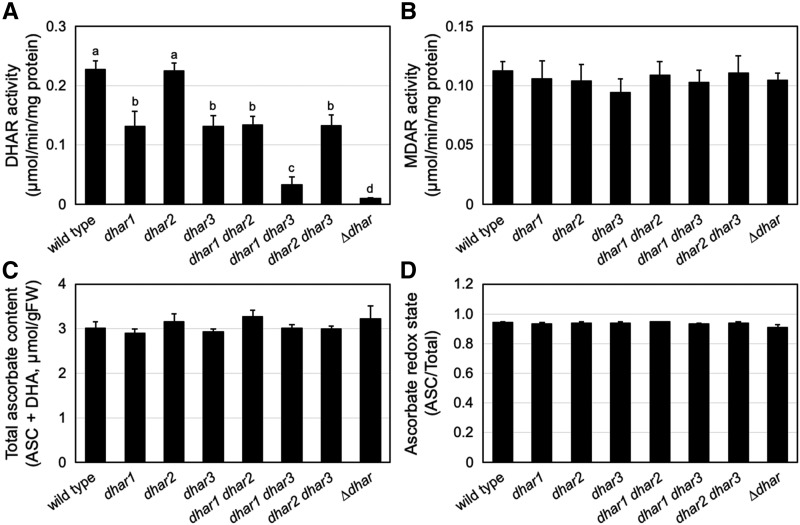
To investigate the contribution of DHARs to ascorbate recycling and pool size regulation in Arabidopsis, we tried to produce a triple mutant lacking all three DHARs, as reported by Rahantaniaina et al. (2017). For this purpose, dhar1 (SALK_029966), dhar2 (SALK_026089), and dhar3 (SAIL_435_A09), all of which have been used in recent studies (Noshi et al., 2016, 2017), were crossed with each other, resulting in the generation of double mutants dhar1 dhar2, dhar1 dhar3, and dhar2 dhar3. Among these double mutants, dhar1 dhar3 and dhar2 dhar3 were further crossed with each other to generate the triple-knockout mutant dhar1 dhar2 dhar3 (herein called ∆dhar), which was isolated from the F2 population by using PCR-based genotyping. In the previous work (Rahantaniaina et al., 2017), SALK_005238 was used to source the dhar1 mutation. Each gene knockout was confirmed by reverse transcription PCR (Supplemental Fig. S1). The measurement of DHAR activity revealed that DHAR1 and DHAR3 are the major isoforms, and that the triple ∆dhar mutant has negligible DHAR activity (Fig. 1A). DHAR loss-of-function did not affect MDAR activity (Fig. 1B). We did not observe any difference in the total ascorbate content (ASC + DHA) and ascorbate redox state (ASC/total) between the wild-type and mutant plants under low light (LL, 40–60 µmol photons m−2 s−1) growth conditions (Fig. 1, C and D). These findings are entirely consistent with recent reports (Noshi et al., 2016, 2017; Rahantaniaina et al., 2017).
Figure 1.
Characterization of the single, double, and triple mutants of DHARs. The wild-type and dhar mutant plants were grown under LL conditions for three weeks. The DHAR activity (A), MDAR activity (B), total ascorbate content (C), and ascorbate redox state (D) were measured. Data are presented as means ± se of more than three biological replicates. Different lowercase letters indicate significant difference (P < 0.05, Student’s t test).
As described above, the subcellular localization of DHAR1 remains unclear (Reumann et al., 2009; Grefen et al., 2010; Rahantaniaina et al., 2017). We also studied the subcellular location of DHAR1 using the GFP-fusion proteins. Because it is unclear whether the peroxisomal targeting signal, if present, exists at the N or C terminus of DHAR1, both DHAR1-GFP and GFP-DHAR1 fusion proteins were expressed in Arabidopsis leaves. In both cases, GFP fluorescence was detected only in the cytosol (Supplemental Fig. S2), which is consistent with the data from Grefen et al. (2010) and Rahantaniaina et al. (2017). Similar results were obtained when DHAR2-GFP, GFP-DHAR2, and GFP alone were expressed. Although further investigation will be required to clarify the potential peroxisomal localization of DHAR1, we herein consider DHAR1 as a cytosolic enzyme.
Contribution of DHARs to Ascorbate Recycling in HL Stress
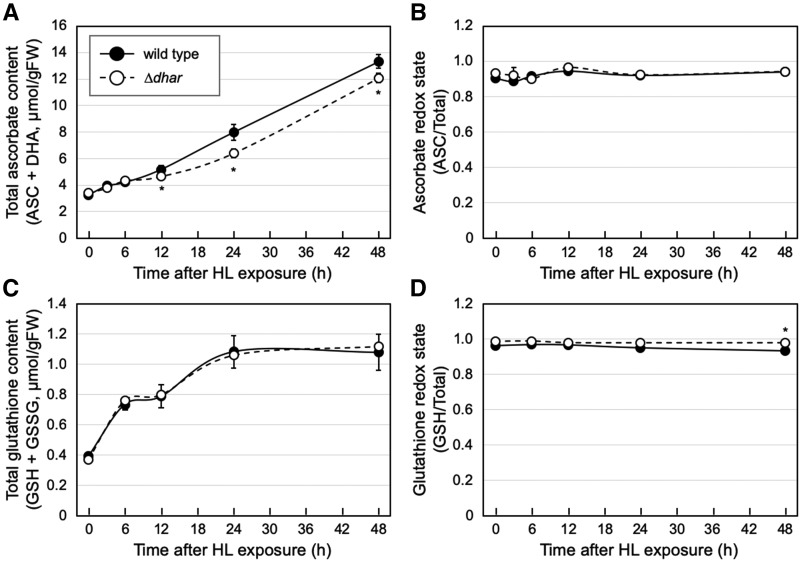
To facilitate the cellular use and biosynthesis of ascorbate, the 3-week–old plants grown in LL conditions were exposed to HL stress (1,500 µmol photons m−2 s−1). In this assay, we focused on ∆dhar and investigated the time-course effects of HL exposure on the ascorbate content and redox state of the wild-type and triple-mutant plants. In both genotypes, the total ascorbate content increased in response to HL exposure in a time-dependent manner. Although the total ascorbate content was not different between genotypes in the first 6 h, it was slightly, but significantly, lower in ∆dhar than in the wild-type genotype in the later periods (Fig. 2A). A maximum difference of ∼20% was observed after 24-h HL exposure. Nevertheless, at any period, no difference in the ascorbate redox state was observed between the wild-type and ∆dhar genotypes (Fig. 2B), implying that the turnover of DHA was very fast in Arabidopsis. These findings suggest that DHARs contribute, at least to a nonnegligible extent, to the full accumulation of ASC through its recycling in HL conditions. However, their contribution seems to be slight in HL conditions. This idea was supported by the result showing that no visible stress-sensitive phenotype was observed in ∆dhar (Supplemental Fig. S3A). Moreover, no significant difference was observed in the maximum quantum yield of PSII (Fv/Fm), a marker of photooxidative stress, between wild type and ∆dhar (Supplemental Fig. S3B).
Figure 2.
Ascorbate and glutathione profiles in ∆dhar under HL stress. The wild-type and ∆dhar plants were grown under LL conditions for 3 weeks and then exposed to HL stress for 48 h. The total ascorbate content (A), ascorbate redox state (B), total glutathione content (C), and glutathione redox state (D) were measured. Data are presented as means ± se of more than three biological replicates. Significant differences (Student’s t test): *P < 0.05 versus the value of the wild-type plant.
Furthermore, we measured the content of glutathione, because its reduced form (GSH) acts as an electron donor for the DHAR reaction. In both wild-type and ∆dhar plants, HL stress conditions enhanced the total glutathione content (reduced GSH + oxidized GSSG), although no clear difference was observed between their content values (Fig. 2C). In our experimental conditions, the glutathione redox state was hardly affected by HL stress (Fig. 2D). Nevertheless, both before and after HL stress, the glutathione redox state in the wild type was slightly but significantly lower (more oxidized) than that in ∆dhar. For example, after 48-h HL exposure, the redox state in wild-type plants was ∼0.93, whereas that in ∆dhar plants was 0.98 (Fig. 2D). These data resemble a recent finding that DHARs are crucial for GSH oxidation in the cat2 background (Rahantaniaina et al., 2017). However, it is difficult to deeply discuss the impact of DHARs on GSH oxidation because the change in the glutathione redox state was very small in this study.
Impacts of DHARs in the Ascorbate-Deficient vtc2-4 Background
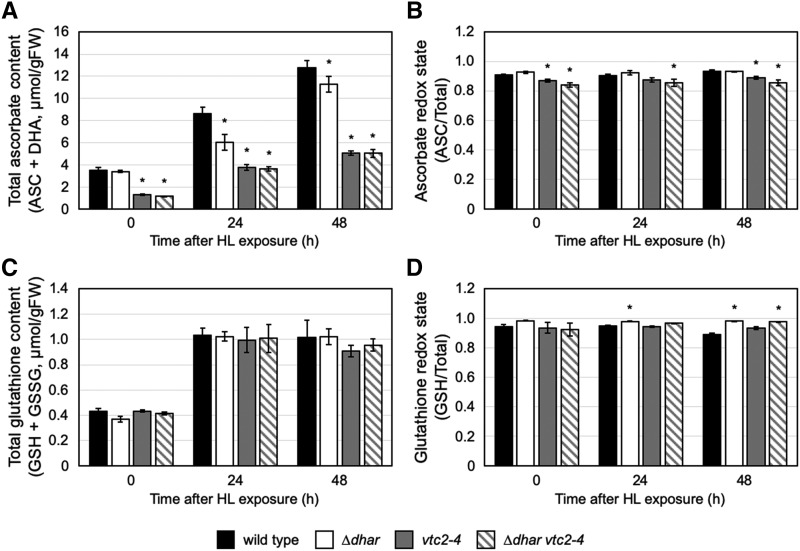
The difference in the total ascorbate levels between wild-type and ∆dhar plants was clear and statistically significant only in the later periods of HL stress when the ascorbate concentration was very high (Fig. 2). This led us to hypothesize that the DHAR reaction might be important only for such high accumulation of this compound. To further clarify the relationship between DHAR function and the cellular ascorbate concentration, we crossed ∆dhar with vtc2-4 (Lim et al., 2016), an ascorbate-deficient mutant, to generate the quadruple mutant ∆dhar vtc2-4 (Supplemental Fig. S4). vtc2-4 is a transfer DNA (T-DNA) insertion line disrupted in the VTC2 gene that encodes a major isoform of the GDP-l-Gal phosphorylase enzyme in the ascorbate biosynthesis pathway (Dowdle et al., 2007).
The quadruple mutant, as well as the vtc2-4 mutant, contained ∼30% of the wild-type ascorbate level under LL conditions (Fig. 3A). DHAR activity in the vtc2-4 mutant tended to be lower than that in the wild type. The ∆dhar vtc2-4 mutant, like ∆dhar, displayed negligible DHAR activity (Supplemental Fig. S4). An obvious photobleaching phenotype was observed in old leaves of vtc2-4 plants after 24-h HL exposure, but this phenotype was not exacerbated by the additional lack of DHARs (Supplemental Fig. S5). Indeed, no difference in the Fv/Fm value was observed between the vtc2-4 and ∆dhar vtc2-4 mutants (Supplemental Fig. S5). Interestingly, the total ascorbate level in the single vtc2-4 mutant increased in response to HL stress, although it was significantly lower in the mutant than in the wild type, both before and after HL stress. After 48-h HL exposure, the ascorbate content in the vtc2-4 mutant was ∼5.0 µmol g−1 fresh weight (FW), which was higher than the initial value of the ascorbate content in the wild-type plant (∼3.5 µmol g−1 FW, at 0 h; Fig. 3A). This might be due to the activation of another isoform of GDP-l-Gal phosphorylase encoded by the VTC5 gene. Importantly, such an increase in the ascorbate content was also observed in the quadruple mutant ∆dhar vtc2-4, and there was no difference in the ascorbate content between the vtc2-4 and ∆dhar vtc2-4 mutants (Fig. 3A). Thus, the observed difference in ascorbate content between wild type and ∆dhar plants in HL conditions (Figs. 2A and 3A) was not observed between vtc2-4 and ∆dhar vtc2-4 (Fig. 3A). Although the ascorbate redox states were not different between vtc2-4 and ∆dhar vtc2-4 plants, they were slightly lower than those in wild-type and ∆dhar plants (Fig. 3B). Our data suggest that DHARs play an important role in ascorbate recycling only when the ascorbate concentration is very high (e.g. in HL-exposed wild-type plants). Other pathway(s) would be able to almost completely substitute for DHARs when the ascorbate level is low to moderate, even in HL conditions (e.g. in HL-exposed vtc2-4 plants). A low ascorbate concentration, the lack of DHARs, or both did not largely affect the glutathione profiles in HL conditions (Fig. 3, C and D). One exception was that the glutathione redox state in the ∆dhar and ∆dhar vtc2-4 genotypes was slightly higher than that in wild type after 12- and/or 24-h exposure to HL stress.
Figure 3.
Ascorbate and glutathione profiles in the vtc2-4 and ∆dhar vtc2-4 mutants under HL stress. The wild-type, ∆dhar, vtc2-4, and ∆dhar vtc2-4 plants were grown under LL conditions for 3 weeks and then exposed to HL for 48 h. The total ascorbate content (A), ascorbate redox state (B), total glutathione content (C), and glutathione redox state (D) were measured. Data are presented as means ± se of more than three biological replicates. Significant differences (Student’s t test): *P < 0.05 versus the value of the wild-type plant. No significant difference in the ascorbate content was observed between the vtc2-4 and ∆dhar vtc2-4 plants, either before or after HL.
Effects of DHARs in the Glutathione-Deficient pad2-1 Background
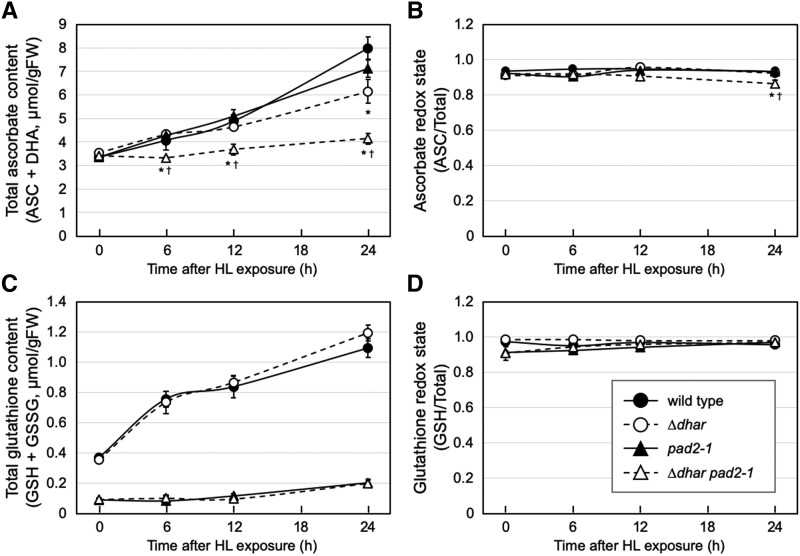
GSH serves as an electron donor for the DHAR reaction and also interacts with and reduces DHA to ASC in a nonenzymatic manner. The DHA reduction may occur in a nonenzymatic manner in vivo, as suggested by a computational estimation (Polle, 2001). We, therefore, investigated if the negligible DHAR activity was complemented through the nonenzymatic reduction of DHA by GSH. For this purpose, a glutathione-deficient mutant, pad2-1 (Parisy et al., 2007), was combined with ∆dhar to produce a ∆dhar pad2-1 quadruple mutant (Supplemental Fig. S4). We confirmed that the single pad2-1 mutant as well as the quadruple mutant contained ∼30% of the wild-type glutathione level under LL conditions and low glutathione concentration in HL conditions (Fig. 4C).
Figure 4.
Ascorbate and glutathione profiles of the pad2-1 and ∆dhar pad2-1 plants under HL stress. The wild-type, ∆dhar, pad2-1, and ∆dhar pad2-1 plants were grown under LL conditions for 3 weeks and then exposed to HL for 24 h. The total ascorbate content (A), ascorbate redox state (B), total glutathione content (C), and glutathione redox state (D) were measured. Data are presented as means ± se of at least three biological replicates. Significant differences (Student’s t test): *P < 0.05 and †P < 0.05 versus the values of the wild-type and ∆dhar plants, respectively.
The pad2-1 single mutation had a negligible impact on the ascorbate pool size and redox state under both LL and HL conditions (Fig. 4, A and B). Thus, only 30% of wild-type glutathione was found to be enough for maintaining high levels of ASC in HL, which is consistent with previous reports using glutathione-deficient mutants (e.g. Han et al., 2013). Furthermore, the ∆dhar pad2-1 quadruple mutant still contained ascorbate at the wild-type level under LL conditions. However, the HL-induced increase in ascorbate content observed in ∆dhar was almost completely inhibited in ∆dhar pad2-1 (Fig. 4A). Although the quadruple mutant ∆dhar pad2-1 still maintained a high ascorbate redox state in HL conditions, there was a slight decrease in the redox state after 24-h exposure to HL compared to the wild-type plant (Fig. 4B). Furthermore, the quadruple mutant showed high sensitivity to HL stress compared to its parental lines as well as the wild-type plant (Fig. 5A). Consistent with this, the decrease in Fv/Fm caused by HL exposure was more pronounced in the ∆dhar pad2-1 mutant (Fig. 5B). These findings suggest that GSH may compensate for the lack of DHARs under HL.
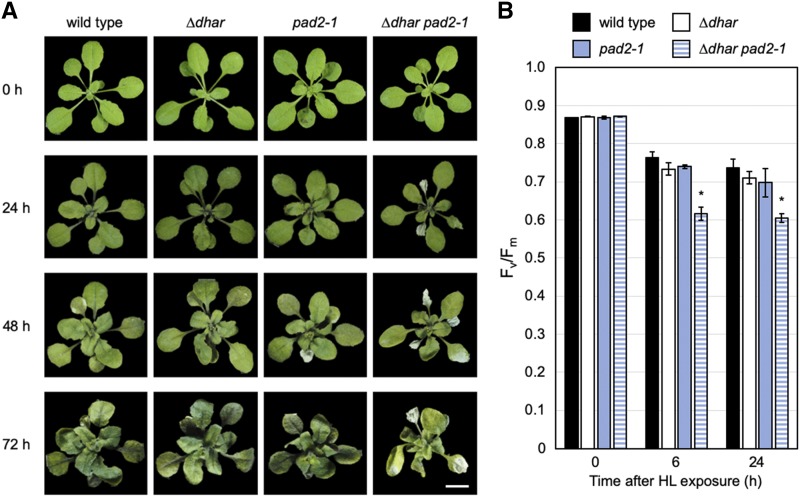
Figure 5.
Sensitivity of the pad2-1, ∆dhar, and ∆dhar pad2-1 plants to HL stress. The wild-type, ∆dhar, pad2-1, and ∆dhar pad2-1 plants were grown under LL conditions for 3 weeks and then exposed to HL for 72 h. A, Plants were photographed at the indicated times, and the rosettes were digitally extracted for comparison. Similar results were repeatedly obtained in three independent experiments. The representative results are shown. Scale bar = 1 cm. B, The Fv/Fm values before and after HL. Data are presented as means ± se of more than four biological replicates. Significant differences (Student’s t test): *P < 0.05 versus the value of the wild-type plant.
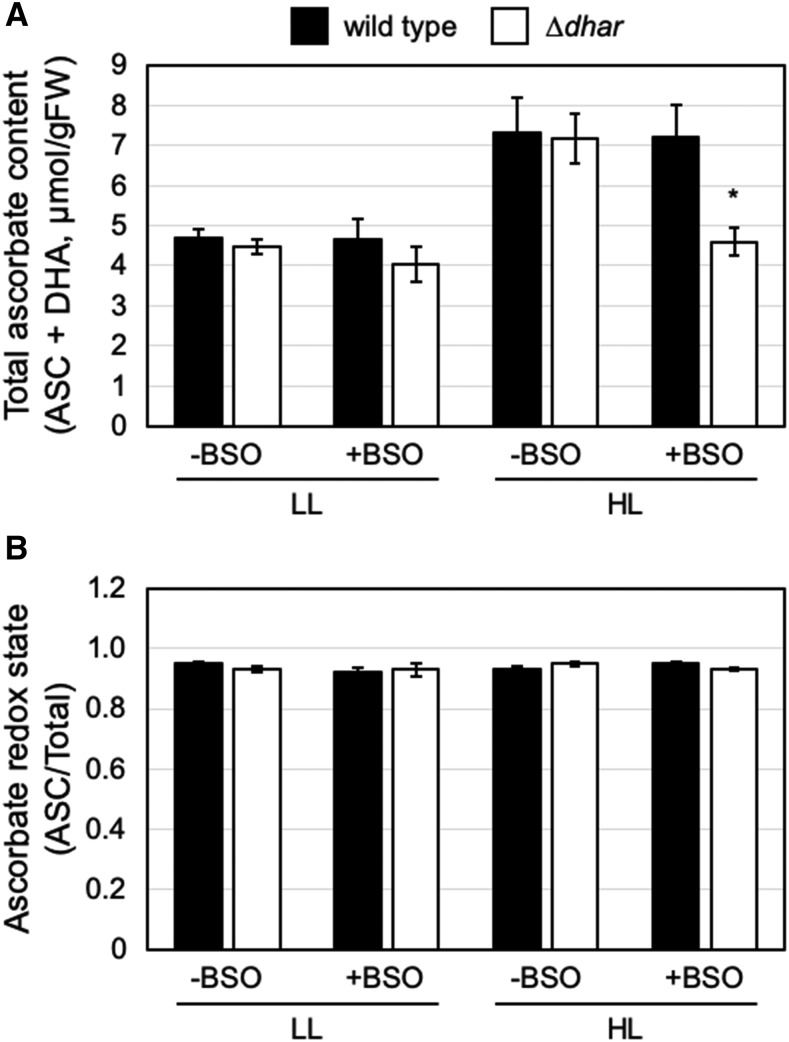
To investigate this in more detail, we used an inhibitor of glutathione biosynthesis, buthionine sulfoximine (BSO), to mimic the pad2-1 mutation. A treatment with 1 mm of BSO for 12 h decreased the glutathione content in Arabidopsis leaves to ∼27% of the mock control (Supplemental Fig. S6), creating intracellular conditions comparable to the effect of the pad2-1 mutation (Fig. 4C). After BSO treatment, wild-type and ∆dhar leaves were exposed to LL and HL conditions (40–60 and 1,500 µmol photons m−2 s−1, respectively) for 12 h. In wild-type leaves, the ascorbate content was higher in HL than in LL conditions and was not affected by the BSO treatment (Fig. 6). By contrast, ascorbate accumulation in HL conditions was strongly suppressed in ∆dhar leaves treated with BSO (Fig. 6). Together with the data from the ∆dhar pad2-1 mutant, the pharmacological data clearly supports our idea that GSH provides a functional substitute for DHAR activity in the accumulation of ascorbate in HL conditions.
Figure 6.
Impact of the lack of DHARs on ascorbate profiles under HL stress with BSO treatment. Leaves were excised from the 3-week–old wild-type and ∆dhar plants grown under LL conditions and treated with 1 mm of BSO solution or water (control) for 12 h in LL. Subsequently, the leaves were exposed to LL or HL for 12 h. The total ascorbate content (A) and the ascorbate redox state (B) were measured. Data are presented as means ± se of more than three biological replicates. Significant differences (Student’s t test): *P < 0.05 versus the value of the wild-type plant.
Levels of Ascorbate Degradation Products in ∆dhar pad2-1 Exposed to HL
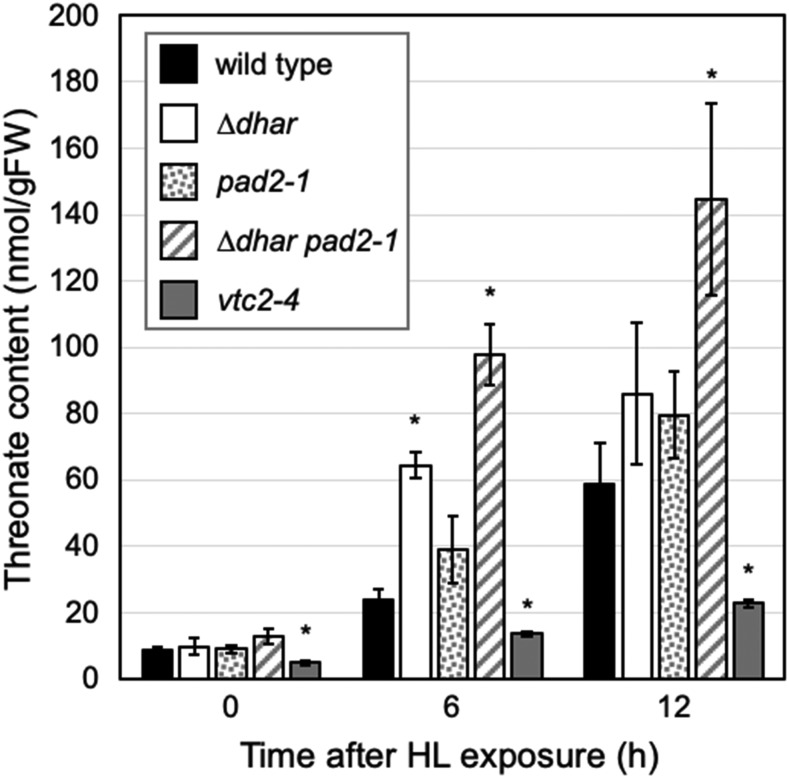
DHA is unstable and easily degraded into oxalate and l-threonate in plants (Green and Fry, 2005; Smirnoff, 2018). To clarify whether the ascorbate recycling capacity is limited in ∆dhar pad2-1, we attempted to measure the oxalate and threonate levels in wild-type and mutant leaves before and after HL exposure. The vtc2-4 mutant was also added to this assay to investigate whether ascorbate could be the source of these compounds. Oxalate was not detected in wild-type plants or in any of the mutant plants, suggesting a very fast turnover of this compound in Arabidopsis leaves (data not shown). However, threonate was detectable in both wild-type and mutant plants (Fig. 7). Under LL conditions, the threonate content in wild-type leaves was ∼8.7 nmol g−1 FW, which then increased upon HL exposure in a time-dependent manner. After 12-h HL exposure, the level of threonate in wild-type leaves was ∼58.9 nmol g−1 FW. Both before and after HL exposure, threonate levels in vtc2-4 leaves were significantly lower than those in wild-type leaves (Fig. 7), suggesting that this compound is produced largely through ascorbate degradation in Arabidopsis.
Figure 7.
Threonate content in the vtc2-4, pad2-1, ∆dhar, and ∆dhar pad2-1 plants under HL stress. Plants were grown under LL conditions for 3 weeks and then exposed to HL for 12 h. The threonate content in leaves was measured. Data are presented as means ± se of more than three biological replicates. Significant differences (Student’s t test): *P < 0.05 versus the value of the wild-type plant.
The accumulation of threonate under HL was also observed in the ∆dhar, pad2-1, and ∆dhar pad2-1 mutants (Fig. 7). Threonate levels in pad2-1 and ∆dhar tended to be higher than that in wild-type leaves. In fact, the threonate level in ∆dhar leaves was significantly higher than that in wild-type leaves after 6-h HL exposure. Compared to the pad2-1 and ∆dhar mutants as well as wild-type plants, the increase in threonate level was much more pronounced in the quadruple mutant ∆dhar pad2-1. Specifically, after 6- and 12-h HL exposure, the threonate levels in ∆dhar pad2-1 were ∼97.8 and 144.8 nmol g−1 FW, respectively, which were significantly higher than that in wild-type plants as well as in the parental lines (Fig. 7). Thus, the absence of ascorbate accumulation in ∆dhar pad2-1 is consistent with an increase in the level of the ascorbate degradation product threonate, indicating the low ascorbate recycling capacity of the ∆dhar pad2-1.
DISCUSSION
More than two decades ago, Morell et al. (1997) found that DHA (at 50 µm) caused the oxidative inactivation of a number of thiol enzymes that are regulated by the thioredoxin system. Moreover, thioredoxins and trypsin inhibitors were implied to have DHAR activity. Based on these findings, they proposed that the accumulation of significant amounts of DHA in plant cells is impossible, and that plants neither possess nor require a specific DHAR enzyme (Morell et al., 1997). This report raised a dispute (Foyer and Mullineaux, 1998; Morell et al., 1998), and subsequent genetic studies using DHAR overexpression, knockdown, and/or knockout lines indicated the physiological importance of DHARs in ascorbate recycling in plants, especially in tobacco (Chen et al., 2003; Chen and Gallie, 2004, 2006, 2008; Gallie, 2013; Noshi et al., 2016, 2017), after which the dispute was largely ignored. However, a recent study using ∆dhar did not identify a role for DHARs in maintaining the ascorbate pool size and redox state, even under oxidative stress caused by the cat2 mutation (Rahantaniaina et al., 2017), again calling the role of DHARs into question. To further address this long-standing debate, we herein investigated the impacts of DHARs on ascorbate profiles in wild-type, vtc2-4, and pad2-1 backgrounds under LL and HL conditions, which has improved our understanding of these processes.
Although the triple knockout mutant ∆dhar had no effect on ascorbate pool size and redox state under LL conditions (Fig. 1), HL-induced ascorbate accumulation observed in the wild type was partially inhibited in ∆dhar plants (Fig. 2). Although slight, the extent of inhibition (∼20%) was statistically significant and consistent with a small increase in the level of threonate (Fig. 7). A very low accumulation of threonate in the ascorbate-deficient vtc2-4 mutant suggests that ascorbate is a precursor of this compound in Arabidopsis (Fig. 7). Because ascorbate degradation starts from DHA in plants (except for some plant families, such as Vitaceae and Geraniaceae; see Smirnoff, 2018), these findings indicate that DHARs have a slight but nonnegligible contribution to the complete ascorbate accumulation capacity through ascorbate recycling under HL stress. This nonnegligible contribution of DHARs was, however, completely diminished in the vtc2-4 background (i.e. in quadruple mutant ∆dhar vtc2-4; Fig. 3). The distinct impacts of DHARs in the wild-type and vtc2-4 backgrounds suggest that DHARs are required only for very high accumulation of ascorbate and that they are dispensable when the concentration of ascorbate is low to moderate. When there is not a high concentration of ascorbate, other recycling systems are likely sufficient for ascorbate recycling in the absence of DHAR activity. Such a scenario provides an explanation for why DHARs had a negligible impact on ascorbate profiles under the oxidative stress caused by the cat2 mutation (Rahantaniaina et al., 2017). The ascorbate level in the cat2 mutant grown under long-day conditions (with a light intensity of 200-µmol photons m−2 s−1) was ∼3.6 to 3.7 µmol g−1 FW (Rahantaniaina et al., 2017), which was comparable to that in wild-type plants before HL exposure in this study (∼3.0–3.5 µmol g−1 FW; Figs. 1, 2, 3, and 4). However, this should be carefully studied in further experiments, because the cat2 mutant displays more severe oxidative stress symptoms, such as GSSG accumulation and subsequent cell death (Queval et al., 2007; Kerchev et al., 2016; Waszczak et al., 2016; Rahantaniaina et al., 2017), compared to that in wild-type plants exposed to HL (Fig. 2; Supplemental Fig. S3). Such severe oxidative stress would drastically stimulate the rate of ascorbate oxidation, providing a large amount of substrate for DHAR activity. The enhanced probability of the DHAR reaction was actually found to be associated with the accumulation of GSSG in the cat2 mutant (Rahantaniaina et al., 2017).
The contribution of DHARs in the wild-type background under HL conditions was, however, apparently slight, which is in line with our phenotypic data showing that growth and stress tolerance of ∆dhar plants was indistinguishable from that of wild-type plants. Thus, as discussed by Rahantaniaina et al. (2017), the data from previous and current studies using the Arabidopsis ∆dhar plants are largely inconsistent with those from the earlier tobacco studies, in which the knockdown of a cytosolic DHAR greatly affected the ascorbate pool size and redox state, growth, and biological processes (Chen et al., 2003; Chen and Gallie, 2004, 2006, 2008). The physiological importance of DHARs potentially varies depending on plant species; however, reverse genetic studies in other species are currently not available. A recent study in Chlamydomonas reinhardtii, a green alga that possesses only one DHAR gene encoding the chloroplastic enzyme, showed that DHAR expression knockdown drastically affects the ascorbate pool size and cell growth under HL stress (Lin et al., 2016). Alternatively, the discrepancy between Arabidopsis and tobacco plants might be caused by differences in their growth conditions; Arabidopsis in this study and in previous works (Rahantaniaina et al., 2017) were grown under controlled laboratory conditions, whereas transgenic tobacco plants were grown under natural light conditions in a glasshouse (Chen et al., 2003; Chen and Gallie, 2004, 2006, 2008). In natural environments, there is high fluctuation in light intensities, leading to severe oxidative inactivation of photosynthesis and subsequent ROS production (Suorsa et al., 2012; Takagi et al., 2016), conditions in which plants may require high ascorbate recycling capacities.
This study and previous studies using ∆dhar clearly indicate that a lack of DHARs can be largely compensated by other systems. Such compensation systems must include MDHA reduction by ferredoxins/MDARs and DHA reduction by GSH. We herein focused on and investigated the role of GSH using the pad2-1 and ∆dhar pad2-1 mutants. Although ascorbate accumulation in the pad2-1 single mutant tended to be lower than that in the wild type after 24 h HL exposure, no statistical difference was observed between the two ascorbate levels (Fig. 4). By contrast, the ascorbate accumulation in HL conditions was markedly affected in the quadruple mutant ∆dhar pad2-1, in which the HL-induced ascorbate accumulation was almost completely inhibited (Fig. 4). Similar results were obtained when GSH availability in ∆dhar was lowered by BSO treatment (Fig. 6). The absence of ascorbate accumulation in ∆dhar pad2-1 under HL was clearly consistent with a drastic increase in threonate levels (Fig. 7). These data strongly suggest that GSH itself can compensate for a lack of DHAR enzymes. The compensation by GSH would be provided by its nonenzymatic reduction of DHA because of the negligible DHAR activity in ∆dhar (Fig. 1; Rahantaniaina et al., 2017). Considering the high pKa value of the GSH thiol group, the probability of nonenzymatic DHA reduction is higher in more alkaline compartments, such as the mitochondrial matrix (pH 8.1), peroxisomes (8.4), and illuminated chloroplast stroma (>8.0), compared to the relatively neutral cytosolic compartment (7.2; the pH values were obtained from Shen et al., 2013). Thus, our genetic study suggests that the cooperation of the DHARs and GSH is crucial for ascorbate accumulation under HL. The ∆dhar pad2-1 quadruple mutant was highly sensitive to HL (Fig. 5). This phenotype could be explained by the absence of ascorbate accumulation, because ascorbate content in the quadruple mutant after 24-h HL exposure was almost comparable to that in vtc2-4, which was also sensitive to HL stress.
A recent kinetic model suggested that ascorbate recycling occurs mainly through MDAR activity, and the coupling between ascorbate and GSH is rare under nonstress conditions (an irradiance of 200-µmol photons m−2 s−1 in a 16-h photoperiod; Tuzet et al., 2019). This model agrees with our finding that ∆dhar pad2-1 accumulates ascorbate at the wild-type level under LL growth conditions (Fig. 4). The considerable inhibition of HL-induced ascorbate accumulation in ∆dhar pad2-1 indicates that the rate of ascorbate oxidation under stress conditions exceeds the capacity of MDAR activity, resulting in an enhanced probability of redox coupling between ascorbate and glutathione in both enzymatic and nonenzymatic manners. Nevertheless, the glutathione redox state remained very high in the ∆dhar pad2-1 mutant under HL stress, and no marked accumulation of GSSG was observed (Fig. 4). This might be explained by the low glutathione concentration in ∆dhar pad2-1, as GSSG accumulation in cat2 was found to be inhibited by the genetic mutations that cause glutathione deficiency (Han et al., 2013).
In this study, we could not observe the accumulation of DHA in significant amounts. In wild-type and mutant plants under HL, the ascorbate redox state was >0.84 in HL conditions. The lowest value was found in ∆dhar vtc2-4 before HL exposure (Fig. 3). Although the ascorbate recycling capacity was inhibited in ∆dhar pad2-1, this quadruple mutant retained the ascorbate redox state at 0.86 (after 24-h HL exposure; Fig. 4). This value was significantly lower than the wild-type value (0.93), although the difference was small. Similar results were obtained by Rahantaniaina et al. (2017), who found the ascorbate redox states to be >0.8 in all mutants (even in the cat2 background). These data might be in line with a previous assumption that the accumulation of significant amounts of DHA is impossible in plant cells (Morell et al., 1997). However, the low levels of DHA were not due to the limitation of DHA production through MDHA reduction, which was previously proposed by Morell et al. (1997), because ascorbate turnover was actually facilitated in ∆dhar pad2-1 in HL conditions. Thus, it is likely that DHA is unstable in Arabidopsis cells and its degradation is favored when ascorbate recycling capacity is limited. There may be an enzyme that catalyzes DHA degradation in plants, e.g. DHA lactonase. Further detailed discussion for this requires experimental evidence.
In conclusion, our genetic study indicates that the physiological significance of DHARs is dependent on ascorbate pool size, and that the cooperation of DHARs and GSH is required for ascorbate accumulation under HL stress in Arabidopsis. Based on our findings as discussed above, it is plausible that GSH itself provides a functional substitute for enzymatic DHA reduction in ascorbate recycling. However, at present, it remains to be clarified if the absence of ascorbate accumulation in HL-exposed ∆dhar pad2-1 is caused only by a low ascorbate recycling capacity. Because GSH plays pleiotropic roles in many biological processes (Noctor et al., 2011), it is possible that secondary effects of GSH deficiency occur in the absence of DHAR activity (e.g. inhibition of ascorbate biosynthesis). However, our findings strongly suggest that the physiological importance of DHARs as ascorbate recycling enzymes becomes more relevant when GSH availability is limited. It is very important to note that GSH deficiency can be caused by experimental manipulations, such as genetic mutations (e.g. pad2-1) or inhibitor treatments, and also by natural environmental conditions. For example, sulfur-deficient conditions result in a decrease in glutathione as well as other sulfur-containing compounds (Kandlbinder et al., 2004). Cadmium stress is also known to decrease GSH availability because GSH plays an indispensable role in detoxifying this metal (Howden et al., 1995). It will be interesting to investigate the impacts of DHARs on ascorbate pool size regulation in conditions under which there is a low GSH pool size.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 was used as the wild type. The T-DNA insertion lines dhar1 (SALK_029966), dhar2 (SALK_026089), dhar3 (SAIL_435_A09), and vtc2-4 (SAIL_769_H05) as well as pad2-1 were obtained from the Arabidopsis Biological Resource Center and were used for crossing. The point mutation (for pad2-1) and T-DNA insertions (for the other lines) were confirmed by DNA sequencing and genomic PCR, respectively.
Seeds were sown in soil (Jiffy-7; Jiffy Products) and stratified in darkness for 3–4 d at 4°C. Plants were then grown in a growth chamber (KCLP-1000CCFL-6-8L-CO2 high pressure system; NK) maintained at 22°C for 16 h of LL (40–60 µmol photons m−2 s−1) and at 20°C for 8 h of darkness. At 3 weeks, the plants were exposed to HL stress (∼1,500 µmol photons m−2 s−1) for up to 3 d without a period of darkness. For the HL stress assay, we used a white LED lighting unit (High-Luminous-Energy Panel unit; Ushio Lighting), which allowed HL exposure without heating (the temperature around HL-exposed leaves was ∼24°C to 25°C).
For the BSO assay, leaves from 3-week–old plants were excised and treated with 1 mm of BSO solution or water (control) for 12 h in LL. Subsequently, the leaves were exposed to LL or HL for 12 h. Because ascorbate biosynthesis is regulated by circadian rhythms, the HL and BSO assays were started after 4 h of illumination. The fully expanded leaves from at least three plants were used for enzyme assays and ascorbate and glutathione measurements as one biological replicate.
Semiquantitative Reverse Transcription PCR
Total RNA was extracted using RNAiso Plus (Takara) and treated with DNase to remove the genomic DNA. The first strand of complementary DNA (cDNA) was synthesized using reverse transcriptase (ReverTra Ace) with an oligo(dT) primer. The total RNA extraction and cDNA synthesis were performed according to the manufacturer’s instructions. Primers used are listed in Supplemental Table S1.
Enzyme Assays
Arabidopsis leaves (0.5 g) frozen in liquid nitrogen were ground and homogenized with 300 μL of potassium phosphate buffer (50 mm at pH 7.0) containing 1 mm of EDTA. After centrifugation (15,300g) for 20 min at 4°C, the supernatant was used for enzyme assays. For MDAR activity measurement, the extract (50 μL) was added to 950 μL of the reaction mixture containing 50 mm of potassium phosphate buffer at pH 7.0, 1 mm of ASC, and 0.2 mm of NADH. The reaction was started by the addition of 0.2 units of ascorbate oxidase. The decrease in A340 was monitored, and the activity was calculated using an absorbance coefficient of 6.2 mm−1 cm−1. For DHAR activity measurement, the extract (50 μL) was added to 950 μL of the reaction mixture containing 50 mm of potassium phosphate buffer at pH 7.0, 0.1 mm of DHA, and 2.5 mm of GSH. The increase in A265 was monitored, and the activity was calculated using an absorbance coefficient of 14 mm−1 cm−1.
Ascorbate and Glutathione Measurement
Ascorbate measurement was performed using an ultra-fast liquid chromatography system (Prominence UFLC; Shimadzu) equipped with a C-18 column (cat. no. LUNA C18-2, 150 × 4.6 nm; Shimadzu), according to Shiroma et al. (2019). Total ascorbate was measured after reducing DHA by incubating it with 10 mm of Tris (2-carboxyethyl) phosphine hydrochloride. DHA was calculated as the difference between the total and reduced ascorbate. The glutathione content was measured according to Noctor et al. (2016) without any modification.
Subcellular Localization of DHAR1 and DHAR2
The cDNAs encoding DHAR1 and DHAR2 were cloned into the donor vector, pDONR221, and then recloned into the destination vectors to express chimeric proteins fused to GFP under the control of the CaMV 35S promoter. We used the destination vectors pGWB505 and pGWB506 (Nakagawa et al., 2007), in which GFP was fused to the C- and N-termini of DHARs, respectively. Primer sequences are listed in Supplemental Table S1. To transiently express the fusion proteins or GFP alone, pGWB505/DHARs, pGWB506/DHARs, and pGWB506 (empty vector) were absorbed onto tungsten particles (1.0 μm in diameter) according to the manufacturer’s instructions (Tanaka). Rosette leaves were harvested from the 4-week–old Arabidopsis wild-type plants grown in soil under LL growth conditions and then placed onto a 2% (w/v) agar plate. The leaves were bombarded with 4 μL of DNA-coated tungsten particles (1 μg of DNA) placed onto the plastic holder using a GIE-III IDERA particle gun (Tanaka) at a helium pressure of 4 kgf cm−2 under a vacuum of 600 mm Hg. Subsequently, the agar plate was filled with water to prevent desiccation. After being incubated overnight at 22°C in the dark, the leaves were viewed with a TCS SP5 confocal laser scanning microscope (Leica Microsystems) using an HCX PL APO CS 63.0 9 1.20 WATER UV objective lens (Leica Microsystems). The fluorescence of GFP and chlorophyll was detected at 500 to 530 and 680 to 700 nm, respectively.
Chlorophyll Fluorescence Measurements
Chlorophyll fluorescence in Arabidopsis leaves was measured at 22°C with a Closed FluorCam 800MF (Photon Systems Instruments). The Fv/Fm ratio of Arabidopsis leaves was determined after dark adaptation for 30 min according to Yabuta et al. (2007).
Oxalate and Threonate Measurement
Oxalate and threonate extraction was performed according to Miyagi et al. (2010) with minor modification. Fully expanded leaves excised from at least three plants were frozen in liquid nitrogen and ground. Approximately 50 mg of the powdered sample was transferred to a frozen tube and homogenized in 150 μL of 100% (v/v) methanol. Thereafter, 150 μL of the internal standard solution consisting of 100 μm of 1,4-piperazine diethane sulfonic acid, 100 μm of MES, and 100 mm of HCl was added. The homogenate was centrifuged for 5 min at 15,300 × g (4°C). The supernatant was transferred to a 3-kD cutoff filter (Millipore) after centrifugation (15,300g, 30 min), and the filtrate was then analyzed by capillary electrophoresis triple‐stage quadrupole mass spectrometry as described in Miyagi et al. (2019).
Data Analyses
The statistical analyses of data were based on Student’s t tests. Calculations were performed on more than three independent biological replicates (see figure legends). In all experiments, except for the measurement of chlorophyll fluorescence, fully expanded leaves from more than three plants were pooled and used as one biological replicate.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DHAR1 (At1g19570), DHAR2 (At1g75270), DHAR3 (At5g16710), MDAR1 (At3g52880), MDAR2 (At5g03630), MDAR3 (At3g09940), MDAR4 (At3g27820), MDAR5 (At1g63940), VTC2 (At4g26850), and VTC5 (At5g55120).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Transcription of DHARs in single, double, and triple dhar mutants.
Supplemental Figure S2. Subcellular localization of DHAR1 and DHAR2.
Supplemental Figure S3. Sensitivity of ∆dhar to HL stress.
Supplemental Figure S4. Generation of the ∆dhar vtc2-4 and ∆dhar pad2-1 mutant plants.
Supplemental Figure S5. Sensitivity of vtc2-4 and ∆dhar vtc2-4 to HL stress.
Supplemental Figure S6. Inhibition of glutathione biosynthesis by the BSO treatment.
Supplemental Table S1. List of primers used.
Acknowledgments
The authors thank Tsuyoshi Nakagawa (Shimane University) for providing the Gateway vectors and Dr. Kohji Nishimura (Shimane University) for technical help with confocal microscopy.
Footnotes
This work was supported by the Japan Society for the Promotion of Science (KAKENHI grant no. 18K19179 to T.M. and T.O., and grant nos. 17H03807 and 19K22284 to T.I. and T.M.), and the Japan Science Society (Sasakawa Scientific Research Grant to Y.T.).
Articles can be viewed without a subscription.
References
- Asada K.(1999) The water–water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K, Andre C, Brewster D, Karunairetnam S, Allan AC, et al. (2012) Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol J 10: 390–397 [DOI] [PubMed] [Google Scholar]
- Chen Z, Gallie DR(2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16: 1143–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR(2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142: 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR(2008) Dehydroascorbate reductase affects non-photochemical quenching and photosynthetic performance. J Biol Chem 283: 21347–21361 [DOI] [PubMed] [Google Scholar]
- Chen Z, Young TE, Ling J, Chang SC, Gallie DR(2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100: 3525–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Edwards R(2010) Glutathione transferases. Arabidopsis Book 8: e0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N(2007) Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52: 673–689 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B(1977) Purification and properties of dehydroascorbate reductase from spinach leaves. Phytochemistry 16: 1347–1350 [Google Scholar]
- Foyer CH, Mullineaux PM(1998) The presence of dehydroascorbate and dehydroascorbate reductase in plant tissues. FEBS Lett 425: 528–529 [DOI] [PubMed] [Google Scholar]
- Gallie DR.(2013) The role of l-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot 64: 433–443 [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC(2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature 433: 83–87 [DOI] [PubMed] [Google Scholar]
- Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, Blatt MR(2010) A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J 64: 355–365 [DOI] [PubMed] [Google Scholar]
- Grillet L, Ouerdane L, Flis P, Hoang MT, Isaure MP, Lobinski R, Curie C, Mari S(2014) Ascorbate efflux as a new strategy for iron reduction and transport in plants. J Biol Chem 289: 2515–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G(2013) Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal 18: 2106–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Asada K(1985) Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J Biol Chem 260: 12920–12926 [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS(1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandlbinder A, Finkemeier I, Wormuth D, Hanitzsch M, Dietz KJ(2004) The antioxidant status of photosynthesizing leaves under nutrient deficiency: Redox regulation, gene expression and antioxidant activity in Arabidopsis thaliana. Physiol Plant 120: 63–73 [DOI] [PubMed] [Google Scholar]
- Kerchev P, Waszczak C, Lewandowska A, Willems P, Shapiguzov A, Li Z, Alseekh S, Mühlenbock P, Hoeberichts FA, Huang J, et al. (2016) Lack of GLYCOLATE OXIDASE1, but Not GLYCOLATE OXIDASE2, attenuates the photorespiratory phenotype of CATALASE2-Deficient Arabidopsis. Plant Physiol 171: 1704–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Wright MA, Cooney J, Bulley SM(2007) The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci USA 104: 9534–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B, Smirnoff N, Cobbett CS, Golz JF(2016) Ascorbate-deficient vtc2 mutants in Arabidopsis do not exhibit decreased growth. Front Plant Sci 7: 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ST, Chiou CW, Chu YL, Hsiao Y, Tseng YF, Chen YC, Chen HJ, Chang HY, Lee TM(2016) Enhanced ascorbate regeneration via dehydroascorbate reductase confers tolerance to photo-oxidative stress in Chlamydomonas reinhardtii. Plant Cell Physiol 57: 2104–2121 [DOI] [PubMed] [Google Scholar]
- Lisenbee CS, Lingard MJ, Trelease RN(2005) Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase. Plant J 43: 900–914 [DOI] [PubMed] [Google Scholar]
- Maruta T, Sawa Y, Shigeoka S, Ishikawa T(2016) Diversity and evolution of ascorbate peroxidase functions in chloroplasts: More than just a classical antioxidant enzyme? Plant Cell Physiol 57: 1377–1386 [DOI] [PubMed] [Google Scholar]
- Miyagi A, Noguchi K, Tokida T, Usui Y, Nakamura H, Sakai H, Hasegawa T, Kawai-Yamada M(2019) Oxalate contents in leaves of two rice cultivars grown at a free-air CO2 enrichment (FACE) site. Plant Prod Sci 22: 407–411 [Google Scholar]
- Miyagi A, Takahashi H, Takahara K, Hirabayashi T, Nishimura Y, Tezuka T, Kawai-Yamada M, Uchimiya H(2010) Principal component and hierarchical clustering analysis of metabolites in destructive weeds; polygonaceous plants. Metabolomics 6: 146–155 [Google Scholar]
- Morell S, Follmann H, de Tullio M, Häberlein I(1997) Dehydroascorbate and dehydroascorbate reductase are phantom indicators of oxidative stress in plants. FEBS Lett 414: 567–570 [DOI] [PubMed] [Google Scholar]
- Morell S, Follmann H, de Tullio M, Häberlein I(1998) Dehydroascorbate reduction: The phantom remaining. FEBS Lett 425: 530–531 [DOI] [PubMed] [Google Scholar]
- Müller-Moulé P, Conklin PL, Niyogi KK(2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128: 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, et al. (2007) Improved Gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 71: 2095–2100 [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH(2016) Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ 39: 1140–1160 [DOI] [PubMed] [Google Scholar]
- Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH(2011) Glutathione. Arabidopsis Book 9: e0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshi M, Hatanaka R, Tanabe N, Terai Y, Maruta T, Shigeoka S(2016) Redox regulation of ascorbate and glutathione by a chloroplastic dehydroascorbate reductase is required for high-light stress tolerance in Arabidopsis. Biosci Biotechnol Biochem 80: 870–877 [DOI] [PubMed] [Google Scholar]
- Noshi M, Yamada H, Hatanaka R, Tanabe N, Tamoi M, Shigeoka S(2017) Arabidopsis dehydroascorbate reductase 1 and 2 modulate redox states of ascorbate-glutathione cycle in the cytosol in response to photooxidative stress. Biosci Biotechnol Biochem 81: 523–533 [DOI] [PubMed] [Google Scholar]
- Obara K, Sumi K, Fukuda H(2002) The use of multiple transcription starts causes the dual targeting of Arabidopsis putative monodehydroascorbate reductase to both mitochondria and chloroplasts. Plant Cell Physiol 43: 697–705 [DOI] [PubMed] [Google Scholar]
- Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F(2007) Identification of PAD2 as a gamma-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J 49: 159–172 [DOI] [PubMed] [Google Scholar]
- Polle A.(2001) Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol 126: 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Miginiac-Maslow M, van Breusegem F, Noctor G(2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52: 640–657 [DOI] [PubMed] [Google Scholar]
- Rahantaniaina MS, Li S, Chatel-Innocenti G, Tuzet A, Issakidis-Bourguet E, Mhamdi A, Noctor G(2017) Cytosolic and chloroplastic DHARs cooperate in oxidative stress-driven activation of the salicylic acid pathway. Plant Physiol 174: 956–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S, Quan S, Aung K, Yang P, Manandhar-Shrestha K, Holbrook D, Linka N, Switzenberg R, Wilkerson CG, Weber AP, et al. (2009) In-depth proteome analysis of Arabidopsis leaf peroxisomes combined with in vivo subcellular targeting verification indicates novel metabolic and regulatory functions of peroxisomes. Plant Physiol 150: 125–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L(2013) Organelle pH in the Arabidopsis endomembrane system. Mol Plant 6: 1419–1437 [DOI] [PubMed] [Google Scholar]
- Shiroma S, Tanaka M, Sasaki T, Ogawa T, Yoshimura K, Sawa Y, Maruta T, Ishikawa T(2019) Chloroplast development activates the expression of ascorbate biosynthesis-associated genes in Arabidopsis roots. Plant Sci 284: 185–191 [DOI] [PubMed] [Google Scholar]
- Smirnoff N.(2018) Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic Biol Med 122: 116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Arnaud D(2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221: 1197–1214 [DOI] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24: 2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi D, Takumi S, Hashiguchi M, Sejima T, Miyake C(2016) Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol 171: 1626–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzet A, Rahantaniaina MS, Noctor G(2019) Analyzing the function of catalase and the ascorbate-glutathione pathway in H2O2 processing: Insights from an experimentally constrained kinetic model. Antioxid Redox Signal 30: 1238–1268 [DOI] [PubMed] [Google Scholar]
- Waszczak C, Kerchev PI, Mühlenbock P, Hoeberichts FA, Van Der Kelen K, Mhamdi A, Willems P, Denecker J, Kumpf RP, Noctor G, Messens J, et al. (2016) SHORT-ROOT deficiency alleviates the cell death phenotype of the Arabidopsis catalase2 mutant under photorespiration-promoting conditions. Plant Cell 28: 1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler G, Ishikawa T, Pornsaksit V, Smirnoff N(2015) Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. eLife 4: 6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N(1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393: 365–369 [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S(2007) Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot 58: 2661–2671 [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Nakane T, Kume S, Shiomi Y, Maruta T, Ishikawa T, Shigeoka S(2014) Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Biosci Biotechnol Biochem 78: 60–66 [DOI] [PubMed] [Google Scholar]