Introduction
Oxalate is a highly oxidized dicarboxylic acid ubiquitous to the human diet and produced as an end product of amino acid metabolism. Lacking endogenous oxalate-metabolizing enzymes, humans are vulnerable to oxalate-induced toxicity. By contrast, the human intestinal microbiota harbors various microbes that can degrade oxalate.1 Particularly Oxalobacter formigenes, a human commensal that uses oxalate as its sole energy source, has been associated with lower urinary oxalate in human and animal models.2,3 Dietary oxalate had been commonly implicated in calcium oxalate kidney stones, but has only recently been recognized as a cause of nephropathy.4 Although several case reports have now described renal failure from excessive dietary intake of oxalate,5 the role played by oxalate metabolism in the gut microbiome in this condition remains uncertain. However, recent data on the role of the microbiome, particularly O formigenes, in human oxalate metabolism suggest that a link between changes in O formigenes colonization and oxalate nephropathy is plausible. We present a case of oxalate nephropathy leading to acute kidney injury induced by excessive tea ingestion. Fecal analysis demonstrated marked defects in stool microbiome oxalate-degrading capacity, and molecular analyses confirmed the absence of O formigenes colonization.
To the best of our knowledge, this is one of the first reports demonstrating an absence of an important commensal oxalate degrader, O formigenes, in the intestinal microbiota in the setting of acute kidney injury induced by excessive oxalate ingestion. Our data suggest that gut microbiota may play a key role in preventing oxalate-induced kidney injury.
Case Presentation
A 52-year-old man with a history of hypertension, hyperlipidemia, anxiety, and alcohol abuse presented to the emergency room with 1 week of malaise, abdominal discomfort, nausea, fatigue, and hiccoughing. Three months before admission, he underwent a prostate biopsy and received 1 g intramuscular ceftriaxone and 1 g intramuscular gentamycin. His recent history was otherwise notable only for the recent cessation of daily alcohol intake.
His physical examination was unremarkable but his laboratory tests showed blood urea nitrogen 93 mg/dl, creatinine 10.9 mg/dl, sodium 127 mmol/l, bicarbonate 14 mmol/l, anion gap 16 mmol/l, phosphorus 8.6 mg/dl, and venous pH 7.24. His urinalysis and kidney ultrasound were unremarkable. Laboratory data were normal 6 months before admission (blood urea nitrogen 8 mg/dl, creatinine 0.7 mg/dl, urinalysis negative).
His creatinine continued to trend up to 11.25 mg/dl, and the patient remained oliguric despite the administration of normal saline. Hemodialysis was initiated on hospital day 3, and a kidney biopsy was performed 2 days later.
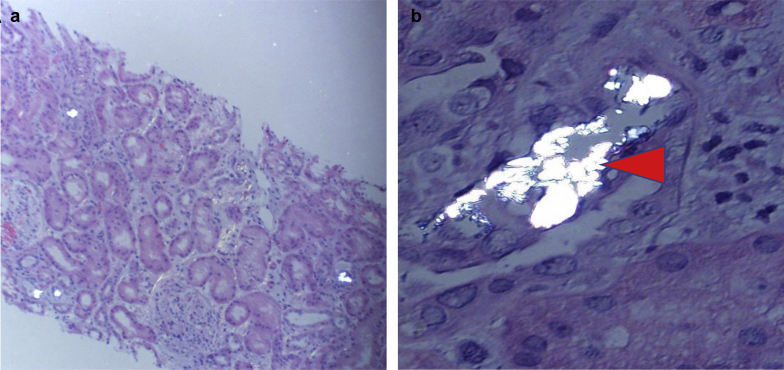
Kidney biopsy showed frequent intratubular calcium oxalate crystals associated with diffuse and moderate acute tubular injury on light microscopy. There was tubular dilatation, simplification, cytoplasmic vacuolization, and flattening in the tubular epithelium, and tubular epithelial cells showed focal brownish granules within the cytoplasm (Figure 1). Immunofluorescence microscopy was essentially negative. Electron microscopy showed 30% visceral epithelial cell foot process effacement with segmental microvillous transformation of the podocytes.
Figure 1.
Polarization at low and high power showing oxalate casts within the tubules (hematoxylin and eosin stain). (a) original magnification ×100; (b) original magnification ×400. Arrowhead demonstrates intratubular oxalate crystals.
On further questioning, the patient described drinking a large amount of black and iced tea (>1–2 gallons/day) since discontinuing alcohol intake approximately 4 months before admission. He denied any other ingestions.
Plasma and urine oxalate concentrations were checked after the third hemodialysis session and were elevated despite the presumed removal of oxalate during hemodialysis (Table 1).
Table 1.
Subject’s data
| 24-h urine oxalatea (mg) | Plasma oxalate (μmol/l)a | qPCR for O formigenes (sample 1) | qPCR for O formigenes (sample 2) | ODA (sample 1) | ODA (sample 2) |
|---|---|---|---|---|---|
| 51 (<44) | 18 (<1.9) | 0b | 0b | Negative | Negative |
ODA, oxalate degradation assay; O formigenes, Oxalobacter formigenes; qPCR, quantitative polymerase chain reaction.
Checked after 3 hemodialysis sessions.
Below limit of detection 1 × 103 copies/μl.
Urine output increased after 3 sessions of intermittent hemodialysis, and further dialysis was held. On discharge, his creatinine decreased to 3.6 mg/dl. Three months after discharge, his kidney function had recovered with a creatinine level of 1.2 mg/dl.
DNA Isolation
Fecal samples were collected from the subject during hospitalization on 2 consecutive days (days 8 and 9) and stored at −80 oC until DNA was extracted, using the MoBio 96-well extraction kit (Hilden, Germany), following the manufacturer’s instructions.
Oxalate Degradation Assay
Donor feces were inoculated into O formigenes–specific growth medium supplemented to 25 mmol/l with potassium oxalate as described previously.6 Samples were incubated at 37oC for 10 days. Degradation of oxalate by O formigenes was assessed by the addition of 100 mM CaCl2 to culture supernatant. In the presence of O formigenes, complete degradation of oxalate in the medium at 10 days is expected, and the addition of CaCl2 will not result in detectable calcium oxalate precipitation (oxalate degradation assay [ODA]–positive test). Conversely, in the absence of O formigenes, oxalate is not completely degraded, and the addition of CaCl2 leads to a white precipitate (ODA-negative test).
Quantitative Polymerase Chain Reaction
To quantitate the number of copies of Oxalyl-CoA decarboxylase, quantitative polymerase chain reaction was performed using the LightCycler 480 SYBR Green І Master Mix and run in the LightCycler 480 system (Roche, Indianapolis IN) as described.6 The O formigenes–specific oligonucleotide primers used were as follows: forward, 5ʹ-GTGTTGTCGGCATTCCTATC-3ʹ and reverse 5ʹ-TTGGGAAGCAGTTGGTGG -3ʹ. Samples were prepared under a laminar flow hood, with each reaction well containing 10 μl of the master mix, 1 μl of each 10 μM primer, 6 μl of water, and 2 μl of DNA template. Cycling conditions included an initial incubation at 95°C for 10 minutes, followed by 40 cycles at 95 °C for 23 seconds, annealing at 63 oC for 20 seconds, and extension at 70 oC for 40 seconds.
We first determined the presence of O formigenes colonization indirectly using a culture-based method through the ODA. ODA on separate 2 fecal samples showed no significant oxalate degradation at 10 days, whereas fecal samples from O formigenes–positive healthy subjects and a growing culture of O formigenes (positive control) had a positive ODA. We next confirmed absence of O formigenes colonization using quantitative polymerase chain reaction on fecal DNA. Two fecal samples tested in duplicate were negative (Table 1).
Discussion
Oxalate nephropathy secondary to high dietary oxalate, particularly from black tea,7 has been recently described, but why some subjects are susceptible to this event and, in particular, whether changes in gut microbiota are responsible, has, to our knowledge, never been evaluated. Multiple bacteria have the potential to degrade oxalate, including O formigenes, Lactobacillus sp., Bifidobacterium sp., Escherichia coli, and others. O formigenes is a gut commensal that requires oxalate for its survival. As a specialist oxalate degrader, it is thought to be the most important bacteria for oxalate degradation in the human intestines.1 Lack of O formigenes has been associated with higher urinary and plasma oxalate in stone formers,2 raising the possibility that lack of this bacterium would be associated with higher urinary oxalate in healthy adults, particularly when oxalate intake or absorption is high. Jiang et al.3 showed that in the absence of O formigenes, urinary oxalate increased in healthy adults fed a high-oxalate and low-calcium diet. In contrast, in O formigenes–positive subjects, the bacteria responded by increasing colonization density 10-fold, and urinary oxalate remained normal. In our case, the patient was drinking copious amounts of tea not containing a significant amount of calcium, thereby mimicking the oxalate load used in the experiments of Jiang et al.3 Oxalate content of tea has been estimated at 14 mg/cup (224 mg/gallon), and the patient described drinking more than 1 to 2 gallons per day for several months. In the absence of O formigenes, our patient’s ability to metabolize the high oxalate load was impaired, leading to high urinary oxalate exposure over an extended period with subsequent oxalate nephropathy (Table 2).
Table 2.
Teaching points
| Excessive dietary oxalate can induce acute kidney injury. |
| We hypothesize a role of the gut microbiota, particularly Oxalobacter formigenes, in protecting against oxalate nephropathy. |
| More studies are needed to confirm this hypothesis. |
Oxalate is a toxic terminal metabolite that can induce renal injury and inflammation, and calcium oxalate crystals increase the expression of specific genes that encode for transcriptional activators in kidney epithelial cell cultures.8 The mechanisms underlying kidney injury have been explored in several animal studies. Sterile inflammation from the intracellular nucleotide-binding domain, leucine-rich repeat-containing receptor, pyrin domain-containing-3 inflammasome activation has been described as a key mechanism underlying the toxicity.9 Highlighting the extent of the toxicity, a chronic kidney disease model of oxalate feeding appears to be a reproducible model of chronic kidney disease that recapitulates the clinical manifestations of chronic kidney disease in humans.4
Our patient recovered his normal kidney function at follow-up, possibly because of short-term exposure to oxalate. In contrast to a recent systematic review of published biopsy-confirmed cases of secondary oxalate nephropathy that described 108 patients with oxalate nephropathy, of whom none had a complete recovery, 42% had partial recovery, and 58% had no recovery and remained dialysis-dependent.5
Why this patient lacked O formigenes is uncertain. One possibility is that the recent use of antibiotics in our patient may have eradicated O formigenes, as it is sensitive to gentamycin and several other commonly used antibiotics. Alternatively, the patient might have been of the 50% to 70% of American adults lacking O formigenes colonization at baseline. Another less likely hypothesis is that the patient’s acute kidney injury induced loss of O formigenes and a decrease in the microbiome’s oxalate-degrading function.
Conclusion
We present a case of oxalate nephropathy secondary to high dietary oxalate in a patient not colonized with O formigenes. We propose a potential mechanism by which this bacterium can protect against cases of oxalate nephropathy secondary to dietary oxalate loads. More studies are needed to confirm this hypothesis and identify therapies to restore colonization for people at risk for acute kidney injury.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Supported in part by the Oxalosis and Hyperoxaluria Foundation–American Society of Nephrology Career Development Award and Rare Kidney Stone Consortium grant U54KD083908, a part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, the National Center for Advancing Translational Sciences (NCATS), and funded through a collaboration between the NCATS and National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Abratt V.R., Reid S.J. Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv Appl Microbiol. 2010;72:63–87. doi: 10.1016/S0065-2164(10)72003-7. [DOI] [PubMed] [Google Scholar]
- 2.Siener R., Bangen U., Sidhu H. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 2013;83:1144–1149. doi: 10.1038/ki.2013.104. [DOI] [PubMed] [Google Scholar]
- 3.Jiang J., Knight J., Easter L.H. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol. 2011;186:135–139. doi: 10.1016/j.juro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulay S.R., Eberhard J.N., Pfann V. Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. Am J Physiol Renal Physiol. 2016;310:F785–F795. doi: 10.1152/ajprenal.00488.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumlertgul N., Siribamrungwong M., Jaber B.L., Susantitaphong P. Secondary oxalate nephropathy: a systematic review. Kidney Int Rep. 2018;3:1363–1372. doi: 10.1016/j.ekir.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pebenito A.M., Liu M., Nazzal L., Blaser M.J. Development of a humanized murine model for the study of Oxalobacter formigenes intestinal colonization. J Infect Dis. 2019;220:1848–1858. doi: 10.1093/infdis/jiz370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syed F., Mena-Gutierrez A., Ghaffar U. A case of iced-tea nephropathy. N Engl J Med. 2015;372:1377–1378. doi: 10.1056/NEJMc1414481. [DOI] [PubMed] [Google Scholar]
- 8.Hammes M.S., Lieske J.C., Pawar S. Calcium oxalate monohydrate crystals stimulate gene expression in renal epithelial cells. Kidney Int. 1995;48:501–509. doi: 10.1038/ki.1995.320. [DOI] [PubMed] [Google Scholar]
- 9.Knauf F., Asplin J.R., Granja I. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013;84:895–901. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]