Abstract
STUDY QUESTION
How are rotating night shift schedules associated with age at menopause among a large, national cohort of shift working nurses?
SUMMARY ANSWER
Our findings suggest that working rotating night shifts with sufficient frequency may modestly accelerate reproductive senescence among women who may already be predisposed to earlier menopause.
WHAT IS KNOWN ALREADY
Younger age at menopause has been associated with increased risk of adverse health outcomes, particularly those linked to reproduction. Night work has been associated with reproductive dysfunction, including disruption of menstrual cycle patterns.
STUDY DESIGN, SIZE, DURATION
This cohort study was conducted among 80 840 women of the Nurses’ Health Study 2 (NHS2), with prospective follow-up from 1991 through 2013. Loss-to-follow-up of the NHS2 is estimated to be <10%.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We assessed the association between cumulative and current rotating night shift work and age at natural menopause over 22 years of follow-up (1991–2013). Cox proportional hazards models were used to estimate hazard ratios (HR) for menopause, adjusted for age, smoking status, body mass index, physical activity, alcohol consumption, reproductive factors and exogenous hormone use.
MAIN RESULTS AND THE ROLE OF CHANCE
Over follow-up, 27 456 women (34%) reached natural menopause. Women who worked 20 or more months of rotating night shifts in the prior 2-year had an increased risk of earlier menopause (multivariable-adjusted (MV)-HR = 1.09, 95% CI: 1.02–1.16) compared to women without rotating night shift work. This risk was stronger among women undergoing menopause or otherwise censored under age 45 years (MV-HR = 1.25, 95% CI: 1.08–1.46), than it was for those continuing in the study when >45 years old (MV-HR = 1.05, 95% CI: 0.99–1.13). Working 10 or more years of cumulative rotating night work was also associated with higher risk of menopause among women reaching menopause under age 45 (MV-HR10–19 years = 1.22, 95% CI: 1.03–1.44; MV-HR≥20 years = 1.73, 95% CI: 0.90–3.35), though not over the age of 45 years (MV-HR10–19 years = 1.04, 95% CI: 0.99–1.10; MV-HR≥20 years = 1.01, 95% CI: 0.89–1.15).
LIMITATIONS, REASONS FOR CAUTION
The degree to which observed effects of rotating night shifts on age at natural menopause are due to circadian disruption, rather than fatigue and stress associated with working more demanding schedules, is uncertain due to potential residual confounding by these factors.
WIDER IMPLICATIONS OF THE FINDINGS
This is the first study to assess the effects of night work on menopausal timing among a larger national cohort of shift working women. Women already prone to earlier menopause may further truncate their reproductive lifetime by working schedules comprising day as well as night shifts.
STUDY FUNDING/COMPETING INTEREST(s)
This study was supported by Center for Disease Control and Prevention/The National Institute for Occupational Safety and Health Grant 5R01OH009803 (PI: Schernhammer E), as well as UM1 CA176726 from the National Institute of Health. The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the article; and decision to submit the article for publication. The authors have no conflicts of interest.
Keywords: menopause, rotating night work, shift schedules, fertility, ovulation, circadian disruption
Introduction
Age at natural menopause has important implications on women’s health. Earlier menopause has been associated with increased risk of cardiovascular disease, osteoporosis, shorter life expectancy and even cognitive decline while women who go through menopause when they are older are at greater risk for breast, endometrial and ovarian cancers (Gold, 2011; Ruth et al., 2016). Considerable variation exists in age at natural menopause, the reasons for which are not fully understood. Menopause occurs when a minimum number of oocytes cease to be viable, the variation in timing of which is thought to be chiefly dependent on rates of follicle atresia (Gougeon, 1996; Gold, 2011). While a strong genetic component is undeniable (He et al., 2009; Stolk et al., 2012, 2009), familial studies estimating variation in age at menopause attributable to heritable factors (Snieder et al., 1998; de Bruin et al., 2001; van Asselt et al., 2004; Murabito et al., 2005) have been highly variable and suggest environmental exposures contribute substantially to age at menopause. Though there is debate in the epidemiologic literature, modifiable risk factors for age at menopause have been identified. The strongest of these include smoking-related exposures, lower parity and socioeconomic status, while evidence for other candidates such as diet, body fat composition or oral contraceptive use have been less conclusive (Gold, 2011; Ruth et al., 2016). To date, these factors are unlikely to explain a substantial portion of variation attributable to exogenous causes (Voorhuis et al., 2010).
A factor that may impact age at menopause is night work, previously linked to adverse reproductive outcomes including changes in menstrual cycle patterns (Chung et al., 2005; Su et al., 2008; Lawson et al., 2011) as well as higher risk of preterm delivery (Bonzini et al., 2007) and miscarriage (Fernandez et al., 2016), suggesting an effect of circadian disruption on ovulation and fertility. The increased risk of hormonally triggered cancers among long-term night shift workers, including endometrial (Viswanathan et al., 2007) and breast cancer (Schernhammer et al., 2001, 2006), lends further support to the notion that circadian perturbations may alter reproductive signaling.
Desynchronization from environmental circadian cues, as experienced by night workers, may impact menopausal timing through chronic dysregulation of gonadal function. Exposure to artificial light during dark hours suppresses and alters the timing of nocturnal pineal melatonin secretion (Brainard et al., 1997). Though causal relationships have not been substantiated, observations that endogenous melatonin decreases during puberty (Waldhauser et al., 1984; Attanasio et al., 1985), that higher levels are coincident with functional hypogonadism (Berga et al., 1988; Laughlin et al., 1991), or that circulating melatonin has been inversely correlated with estrogen levels in amenorrhoeic women (Okatani and Sagara, 1994) could suggest an overall inhibitory effect of melatonin on ovarian activity. Thus, a decrease in nocturnal melatonin may promote gonadal activity, potentially delaying menopause. Conversely, indications that melatonin protects follicle integrity (Cruz et al., 2014; Tamura et al., 2017), thereby imparting resistance to atresia, and evidence of the importance of synchronized endocrine signaling and clock gene expression across central and peripheral targets on mammalian ovarian function (Sellix, 2015; Simonneaux and Bahougne, 2015), suggest night work-associated circadian disruption may translate into earlier menopause. However, whether night shift work affects the age at onset of menopause has not been studied to date.
We investigated the association between rotating night shift work and age at natural menopause within the Nurses’ Health Study 2 (NHS2) cohort. Our findings will add insight on the impact of night work schedules on ovarian function and further elucidate the role of circadian regulation in human reproduction.
Materials and Methods
Study population
The NHS2 is an ongoing prospective cohort of 116 429 female registered nurses in the USA between ages 25 and 42 at its start in 1989 (Willett, 2013). Biennial questionnaires have been collected since baseline, capturing lifestyle, environmental and occupational exposures, as well as medication use and medical conditions; follow-up to date exceeds 90%. The Institutional Review Board of Brigham and Women’s Hospital (Boston, MA) approved this study, and informed consent was implied by the return of the initial questionnaire.
Rotating night shift work exposure
Rotating night shift work, to be distinguished from exclusive night work, signifies schedules that include working nights concurrently with either evenings or days. Hypothesized to be more disruptive to circadian-regulated processes than exclusive night work, it has been studied previously as a risk factor for adverse reproduction-linked outcomes in Nurses’ Health Study cohorts (Schernhammer et al., 2001, 2006). The 1989 NHS2 questionnaire queried number of prior years (1–2, 3–5, 6–9, 10–14, 15–19 or 20+) worked with the following item: ‘What is the total number of years during which you worked rotating night shifts (at least 3 nights/month in addition to days/evenings in that month)?’. Subsequent 1991, 1993, 1995, 1997, 2001, 2005, 2007 and 2011 questionnaires queried number of months (none, 1–4, 5–9, 10–14, 15–19 and 20 or more) in which at least 3 nights per month, in addition to days and evenings, had been worked in the past 2 years. The 2001 and 2005 questionnaires queried multiple consecutive 2-year intervals so that complete rotating night shift work exposure through 2005 was captured. As rotating shift work exposure was not captured in 2009, values were carried forward from the previous questionnaire for those who reported working current jobs involving rotating night shifts. Women who did not report working such jobs were assigned zero months of rotating night shifts for 2007 through 2009.
Cumulative months worked rotating night shifts prior to 1989 was estimated by taking the midpoint of each category and multiplying by 12. Women who reported working 20 or more years were assigned 240 months of cumulative rotating night shift work. Cumulative months worked over follow-up was estimated by adding the midpoints of each category across biennial questionnaires; for the open-ended’ 20 or more’ category, we conservatively assigned 20 months. In the event of missing data, rotating night shift work was carried forward for one questionnaire cycle.
Age at natural menopause
On each biennial NHS2 questionnaire, participants were asked whether their menstrual periods had ceased, at what age, and if this was due to natural menopause, chemotherapy or radiation, or surgery. When reported age at menopause was inconsistent across questionnaires, the first reported age was used.
Ascertainment of covariates
Potential confounders were selected a priori. Age, smoking (Mikkelsen et al., 2007; Pokoradi et al., 2011; Sarac et al., 2011), parity (Parazzini, 2007), body mass index (Kato et al., 1998), oral contraceptive use (Pokoradi et al., 2011), age at menarche (Parazzini, 2007), alcohol consumption (Torgerson et al., 1997; Brett and Cooper, 2003) and physical activity (Nagata et al., 2012) have previously been associated, to varying degrees, with timing of natural menopause. Breastfeeding has also been speculated to impact this process (Weinstein et al., 2003). Sleep duration was also considered as a potential confounder.
Statistical analyses
Women were excluded if they did not return the study baseline 1991 questionnaire or had missing or incomplete records for rotating night shift work exposure at, or prior to study baseline. Further exclusion criteria included natural or medically induced menopause, use of menopausal hormone therapy (HT), or cancer diagnosis (excluding non-melanoma skin cancer) on, or prior to the 1993 questionnaire.
Cox proportional hazards models were used to estimate the effect of rotating night shift work on timing of natural menopause. The time metameter was age in months, effectively controlling all models for age (i.e. all models were age-adjusted). Women entered the cohort at their age at the 1993 biennial questionnaire return date. A counting process data structure (Therneau, 2000) was used to handle time-dependent covariates and accommodate delayed entry, permitting women to enter the cohort at differing ages. The main exposure, rotating night shift work and covariate values, were updated at each biennial questionnaire.
In our main exposure, we defined rotating night shift work as recent number of months worked rotating night shifts, i.e. during the 2-year interval prior to the preceding questionnaire return to ensure temporality (i.e. shift work occurred prior to reported age at menopause). Secondarily, we updated ‘lifelong’ years of rotating night shifts worked to the end of the prior 2-year questionnaire cycle. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Women were censored at cancer diagnosis, death, hysterectomy, bilateral oophorectomy, report of periods ceasing due to chemotherapy or radiation, or initiation of HT use. Censoring at cancer diagnosis and HT use was due to potential effects of cancer or chemotherapy (Sklar, 2005) on menstruation, and exogenous hormone medications interfering with menopausal timing (Jernstrom et al., 2003) and inducing menstruation (Furness et al., 2009), respectively. Further, women were censored for failing to complete two consecutive questionnaires, and if missing rotating night shift data on two consecutive questionnaires.
Sensitivity analyses
Violations of the proportional hazards assumption were assessed visually (Hosmer, 1999). The relative hazard of menopause for both recent and lifelong rotating shift work exposure definitions varied with age (i.e. the risk of earlier menopause among rotating night shift workers relative to women who did not work these schedules was not constant as women grew older); we, therefore, divided the time metameter (i.e. age) at the average age in this cohort, i.e. 45 years, and present stratified results. Age- and multivariable-adjusted hazard ratios (HRs) were estimated for women younger and older than 45 years for both exposure definitions. We plotted two sets of multivariable-adjusted cumulative probability functions of remaining premenopausal comparing only women who did not work any months of rotating night shifts in the prior two years to those who worked 20 or more. The first set included women 30–45 years old, the second, those 45 years and older. Due to literature suggesting that diabetes may increase risk of earlier menopause, sensitivity analyses were conducted excluding women with Type I and Type II diabetes prior to baseline (Wellons et al., 2017). As smoking is one of the most reliable exogenous determinants of menopausal timing, we investigated the impact of substituting smoking status with pack-years.
Results
As of the 1991 questionnaire return, 80 840 women met eligibility criteria. Of these, 61% of women reported working rotating night shifts prior to 1989 and 38% reported working rotating night shifts between 1989 and 2011. Of women who reported working rotating night shifts prior to 1989, the mean (SD) cumulative duration was 70.5 (67.5) months. Of those who reported rotating night shifts only after the 1989 baseline, the mean cumulative duration worked over follow-up was 36.9 (46.6) months. Working rotating night schedules was less common with age. While 23% of women under 40 years reported working any rotating night shifts in the previous 2 years, only 8% over 50 years did so.
The distribution of covariates by duration of rotating night shift work between 1989 and 1991 questionnaire return dates is shown in Table I. Women who worked any rotating night shifts during this interval were more likely to be current smokers. Rotating night shift workers were also younger (mean difference of 1.3 years), more physically active (median increase of 0.8 metabolic equivalents/week) and experienced less sleep (i.e. <7 h/night). Finally, they were more often nulliparous, though when they had children, did so at a younger age.
Table I.
Age and age-standardized characteristics in 1991 according to months of rotating night shifts worked among 80 840 women in the Nurses’ Health Study 2.
| Rotating night shift work between 1989 and 1991 | ||||
|---|---|---|---|---|
| Characteristic | None | 1–9 months | 10–19 months | ≥20 months |
| N | 62 205 | 8768 | 4077 | 5790 |
| Age, years (mean, SD) | 36.5 (4.5) | 35.2 (4.7) | 34.9 (4.7) | 35.5 (4.7) |
| Alcohol consumption, g/d (median, quartiles) | 0.9 (0–3.5) | 0.9 (0–3.5) | 0.9 (0–3.5) | 0.9 (0–2.9) |
| BMI, kg/m2 (mean, SD) | 24.2 (5.0) | 25.2 (5.6) | 25.4 (5.7) | 25.6 (5.9) |
| Physical Activity, METs‡/wk (median, IQR) | 12.5 (5.0–26.5) | 12.7 (5.0–27.3) | 14.1 (5.7–30.2) | 13.8 (5.4–30.2) |
| Smoking Status (%) | ||||
| never smoker | 67 | 64 | 64 | 64 |
| past smoker | 22 | 21 | 23 | 20 |
| current smoker | 11 | 15 | 14 | 15 |
| Oral contraceptive use (%) | ||||
| Never used | 17 | 16 | 15 | 17 |
| 1–47 mos | 41 | 42 | 43 | 42 |
| 48–95 mos | 25 | 24 | 25 | 24 |
| ≥96 mos | 17 | 17 | 17 | 17 |
| Parity (%) | ||||
| Nulliparous | 26 | 32 | 28 | 28 |
| 1 child | 19 | 17 | 17 | 16 |
| 2 children | 35 | 31 | 32 | 34 |
| 3+ children | 20 | 20 | 23 | 22 |
| Age at first birth (%)† | ||||
| Nulliparous | 26 | 32 | 28 | 28 |
| ≤20 y | 6 | 9 | 10 | 10 |
| 21–25 y | 28 | 28 | 29 | 30 |
| 26–30 y | 31 | 24 | 26 | 26 |
| >30 y | 10 | 7 | 7 | 6 |
| Total time breast fed (%)† | ||||
| Never breast fed | 25 | 27 | 28 | 30 |
| Breast fed ≤ 1 y | 34 | 35 | 32 | 32 |
| Breast fed > 1 y | 41 | 37 | 40 | 38 |
| Sleep, h/night (%) | ||||
| <7 | 27 | 34 | 34 | 39 |
| 7–8 | 68 | 62 | 62 | 56 |
| ≥9 | 5 | 4 | 5 | 5 |
| Age at menarche (%) | ||||
| <12 y | 24 | 25 | 25 | 25 |
| 12–13 y | 59 | 58 | 57 | 56 |
| ≥14 y | 18 | 18 | 18 | 19 |
Values are means (SD), median (quartiles) or percentages of non-missing values. Category percentages may not sum to 100 due to rounding.
†Parous women only.
‡Metabolic equivalent tasks.
Of the 80 840 eligible women at baseline 49 748 were right-censored prior to end of follow-up, 33 450 of which was due to rotating shift work exposure data missing for two consecutive cycles (n = 19 742) and HT use (n = 13 708). Of those not censored, 27 456 (57%) women had natural menopause; 2524 of which occurred among those under 45 years. Mean age of natural menopause for these women was 50 (SD ± 4.0 years). There were 3636 women remaining eligible for natural menopause at the end of study follow-up (i.e. the 2013 questionnaire return date).
The association between recent rotating night shift work and onset of natural menopause is presented in Table II. Age and multivariable-adjusted models indicate a moderately increased risk of earlier menopause for women with ≥10 months of rotating night shift work in the previous 2-year interval. The point estimate was largest for women who had worked 10–19 months of rotating night shifts (multivariable-adjusted (MV) HR: 1.11, 95% CI: 1.01–1.21), and was similar to that for ≥20 months (MV HR: 1.09, 95% CI: 1.02–1.16). This highest exposure level was more strongly associated with earlier menopause for women younger than 45 years (MV HR: 1.25; 95% CI: 1.08–1.46).
Table II.
Adjusted hazard ratios (HRs) and 95% CIs of natural menopause by months of rotating night shifts worked during the prior 2 years among 80 840 women of the Nurses’ Health Study 2, with prospective follow-up from 1991 through 2013.
| Months of rotating night shift work in past 2 years | Mean age at menopause (years, SD) | Number of Events | Age-adjusted HR (95% CI) | Multivariable-adjusted HR* (95% CI) | Multivariable-adjusted HR*a (95% CI) | Multivariable-adjusted HR*b (95% CI) |
|---|---|---|---|---|---|---|
| Overall cohort | ||||||
| None | 49.9 (3.8) | 24 731 | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) |
| 1–9 | 48.5 (4.4) | 1187 | 1.09 (1.03–1.16) | 1.06 (1.00–1.12) | 1.05 (0.99–1.12) | 1.06 (1.00–1.12) |
| 10–19 | 48.1 (5.0) | 483 | 1.12 (1.03–1.23) | 1.11 (1.01–1.21) | 1.10 (1.00–1.20) | 1.10 (1.01–1.21) |
| ≥20 | 48.5 (4.5) | 1055 | 1.10 (1.04–1.17) | 1.09 (1.02–1.16) | 1.08 (1.02–1.15) | 1.09 (1.02–1.16) |
| Subcohort censored at younger than 45 years | ||||||
| None | 41.4 (2.8) | 2066 | 1.0 (ref.) | 1.0 (ref.) | NA*c | NA*d |
| 1–9 | 40.5 (3.6) | 182 | 1.04 (0.90–1.21) | 0.96 (0.83–1.12) | ||
| 10–19 | 39.9 (3.9) | 90 | 1.23 (1.00–1.52) | 1.13 (0.91–1.39) | ||
| ≥20 | 40.9 (2.9) | 186 | 1.37 (1.18–1.59) | 1.25 (1.08–1.46) | ||
| Subcohort censored at 45 years or older | ||||||
| None | 50.7 (2.9) | 22 665 | 1.0 (ref.) | 1.0 (ref.) | NA*c | NA*d |
| 1–9 | 50.0 (2.7) | 1005 | 1.10 (1.03–1.17) | 1.07 (1.00–1.14) | ||
| 10–19 | 49.9 (2.9) | 393 | 1.10 (1.00–1.22) | 1.09 (0.99–1.21) | ||
| ≥20 | 50.1 (2.9) | 869 | 1.06 (0.99–1.13) | 1.05 (0.99–1.13) | ||
*Hazard ratios adjusted for age, smoking status (never, past or current smoker), age at first birth and parity combined (nulliparous; age at first birth <24, 1–2 children; age at first birth 24–29, 1–2 children; age at first birth >29, 1–2 children; age at first birth <23, >2 children; age at first birth 24–29, >3 children; age at first birth >29, >2 children), body mass index (<18.5, 18.5 to 20, >20–22.5, >22.5–25, >25–30 and >30 kg/m2), duration of oral contraceptive use (0, 1–23, 24–47, 48–71, 72–95, 96–119 and >120 months), duration breast fed (never, ≤1 y, >1 y), alcohol consumption (0, >0–1, >1–4, >4–8, >8–12, >12 g/wk), physical activity (≤3, >3–9, >9–19, >19–27, >27–42, >42 metabolic equivalent tasks/wk), age at menarche (≤9, 10, 11, 12, 13, 14, 15, ≥16 y) and sleep in 24 h (≤4, 5, 6, 7, 8, ≥9 h).
aAdditional adjustment for years worked rotating shifts prior to 1989.
bAdditional adjustment for cumulative rotating shift work over follow-up.
cAdditional adjustment for year worked rotating shifts prior to 1989 not shown for age-stratified analyses.
dAdditional adjustment for cumulative rotating shift work over follow-up not shown for age- stratified analyses.
Table III shows the association between lifelong rotating night shift exposure and natural menopause. Working 11–20 years conveyed a slightly higher risk of earlier menopause compared to never having worked rotating night shifts, though the corresponding multivariable-adjusted effect estimate had a lower bound of one (HR: 1.06; 95% CI: 1.00–1.11). The point estimate was similar for the smaller number of women who worked >20 years of rotating night shifts (HR: 1.08; 95% CI: 0.95–1.22). Younger women (i.e. those <45 years) working more than 10 years of rotating shifts had an increased risk of earlier menopause. Working 11–20, and >20, years of rotating shifts was associated with a 22% (MV HR: 1.22; 95% CI: 1.03–1.44) and 73% (MV HR: 1.73; 95% CI: 0.90–3.35) increased risk, respectively, though only nine women in the highest exposure category under the age of 45 had the outcome.
Table III.
Adjusted hazard ratios (HRs) and 95% CIs of natural menopause by lifetime cumulative history of rotating night shift work among 80 840 women of the Nurses’ Health Study 2, with prospective follow-up from 1991 through 2013.
| Years of cumulative rotating night shift work | Mean age at menopause (years, SD) | Number of events | Age-adjusted HR (95% CI) | Multivariable-adjusted HR* (95% CI) |
|---|---|---|---|---|
| Overall cohort | ||||
| None | 49.8 (3.9) | 8292 | 1.0 (ref.) | 1.0 (ref.) |
| 0–5 | 49.7 (4.0) | 13 452 | 1.02 (0.99–1.05) | 1.02 (0.99–1.04) |
| 5–10 | 49.7 (4.0) | 3429 | 1.05 (1.01–1.09) | 1.03 (0.99–1.07) |
| 10–15 | 49.8 (3.8) | 1454 | 1.09 (1.03–1.16) | 1.06 (1.00–1.12) |
| 15– 20 | 49.9 (3.8) | 580 | 1.09 (1.00–1.19) | 1.04 (0.96–1.14) |
| >20 | 50.8 (3.1) | 249 | 1.08 (0.95–1.22) | 1.02 (0.90–1.16) |
| Subcohort censored at younger than 45 years | ||||
| None | 41.3 (3.0) | 727 | 1.0 (ref.) | 1.0 (ref.) |
| 0–5 | 41.2 (3.0) | 1274 | 1.08 (0.99–1.18) | 1.07 (0.98–1.18) |
| 5–10 | 41.3 (3.0) | 333 | 1.13 (1.00–1.29) | 1.10 (0.96–1.25) |
| 10–15 | 41.4 (2.5) | 127 | 1.24 (1.02–1.49) | 1.13 (0.93–1.36) |
| 15–20 | 41.8 (2.0) | 54 | 1.72 (1.30–2.26) | 1.51 (1.14–2.00) |
| >20 | 43.0 (1.0) | 9 | 2.10 (1.09–4.05) | 1.73 (0.90–3.35) |
| Subcohort censored at 45 years or older | ||||
| None | 50.6 (2.9) | 7565 | 1.0 (ref.) | 1.0 (ref.) |
| 0– 5 | 50.6 (2.9) | 12 178 | 1.01 (0.98–1.04) | 1.01 (0.98–1.04) |
| 5–10 | 50.6 (2.8) | 3096 | 1.02 (1.00–1.08) | 1.02 (0.98–1.06) |
| 10–15 | 50.6 (2.8) | 1327 | 1.08 (1.02–1.12) | 1.06 (1.00–1.12) |
| 15–20 | 50.7 (2.9) | 526 | 1.05 (0.96–1.26) | 1.01 (0.93–1.11) |
| >20 | 51.1 (2.7) | 240 | 1.06 (0.93–1.38) | 1.01 (0.89–1.15) |
*Hazard ratios adjusted for age, smoking status (never, past or current smoker), age at first birth and parity combined (nulliparous; age at first birth <24, 1–2 children; age at first birth 24–29, 1–2 children; age at first birth >29, 1–2 children; age at first birth <23, >2 children; age at first birth 24–29, >3 children; age at first birth >29, >2 children), body mass index (<18.5, 18.5–20, >20–22.5, >22.5–25, >25–30 and >30 kg/m2), duration of oral contraceptive use (0, 1–23, 24–47, 48–71, 72–95, 96–119 and >120 months), duration breast fed (never, ≤1 y, >1 y), alcohol consumption (0, >0–1, >1–4, >4–8, >8–12, >12 g/wk), physical activity (≤3, >3–9, >9–19, >19–27, >27 to 42, >42 metabolic equivalent tasks/wk), age at menarche (≤9, 10, 11, 12, 13, 14, 15, ≥16 y) and sleep in 24 h (≤4, 5, 6, 7, 8, ≥9 h).
Excluding women who had diabetes prior to baseline and adjusting multivariable models using pack-years instead of smoking status did not materially affect results.
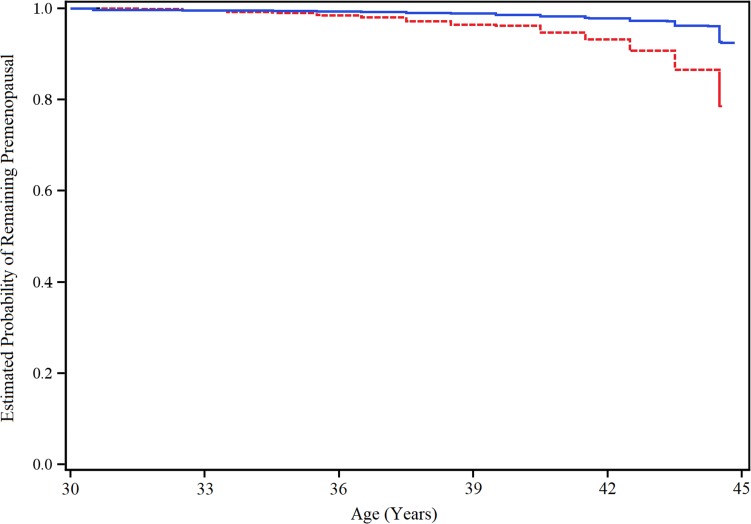
Multivariable-adjusted plotted cumulative probabilities of remaining premenopausal demonstrate that women 45 years and younger who worked ≥20 months during the prior 2-year interval were more likely to experience menopause earlier relative to those who worked none (Fig. 1).
Figure 1.
Multivariable-adjusted cumulative probability of remaining premenopausal until 45 years of age across women who never worked rotating night shifts (solid blue line), and those who worked 20 or more months of rotating night shifts (hashed red line), during the prior 2 years. Adjusted for age, smoking status (never, past or current smoker), age at first birth and parity combined (nulliparous; age at first birth <24, 1–2 children; age at first birth 24–29, 1–2 children; age at first birth >29, 1–2 children; age at first birth <23, >2 children; age at first birth 24–29, >3 children; age at first birth >29, >2 children), body mass index (<18.5, 18.5–20, >20–22.5, >22.5–25, >25–30 and >30 kg/m2), duration of oral contraceptive use (0, 1–23, 24–47, 48–71, 72–95, 96–119 and >120 months), duration breast fed (never, ≤1 y, >1 y), alcohol consumption (0, >0–1, >1–4, >4–8, >8–12, >12 g/wk), physical activity (≤3, >3–9,> 9–19, >19–27, >27–42, >42 metabolic equivalent tasks/wk), age at menarche (≤9, 10, 11, 12, 13, 14, 15, ≥16 y) and sleep in 24 h (≤4, 5, 6, 7, 8, ≥9 h).
Discussion
This work denotes the first prospective investigation of the association of night work with menopausal timing. Due to the complexity of central reproductive regulation, competing mechanisms support that women working more rotating night shifts over their reproductive lifetime could experience either delayed or advanced menopausal onset. Our findings suggest that women who work rotating night shifts are at increased risk of earlier menopause. Overall, a modest effect was observed for those who worked 10 or more months during the previous 2 years. Risk of earlier menopause was more pronounced for women under 45 years, for night work measured as both recent and lifelong exposures. This suggests that risk of early ovarian failure may be exacerbated by working rotating night shifts with sufficient frequency.
The increased risk of earlier menopause among rotating night shift workers does not support a net stimulatory effect of circadian disruption on ovulation. The hypothesis that circadian disruption may delay menopause via suppression of nocturnal melatonin secretion is given credence by indications of seasonal variation in human fertility. Higher conception rates have been observed during the summer in more seasonally photoperiod-diverse environments (Rojansky et al., 1992) or by latitude (Batschelet et al., 1973) corresponding to seasonal variation in nocturnal melatonin (Kauppila et al., 1987). Mechanistic evidence for melatonin as a neuroendocrine regulator of fertility is found in seasonally breeding animal models that, while not fully elucidated, have been long acknowledged (Revel et al., 2009; Dardente, 2012). It remains unclear whether human reproductive function is materially subject to melatonin-mediated photoperiodic regulation analogous to those of seasonal breeders (Bronson, 2004).
Our findings suggest an adverse impact of circadian disruption on ovulation. Whether this is mediated by nocturnal melatonin suppression is uncertain. It is becoming recognized that coordinated timing of clock gene expression across central and peripheral sites is crucial for maintaining physiological function. Out of phase expression caused by changing environmental cues, the strongest of which is the diurnal light cycle, can jeopardize these processes. The importance of coordinated timing across the hypothalamic pituitary ovarian axis (HPOA) in ovulation is exemplified in mice by observation of clock gene regulation of both the preovulatory luteinizing hormone surge in gonadotrophs (Chu et al., 2013) and time-dependent receptor mediated sensitivity to this hormone by theca cells (Mereness et al., 2016), which trigger their differentiation into luteinized cells of the corpus luteum (Young and McNeilly, 2010). Shorter or more irregular menstrual cycles in night shift workers (Chung et al., 2005; Su et al., 2008; Lawson et al., 2011; Wang et al., 2016) underscore the relevance of clock-regulated processes in human ovulation and the potential dysfunction imparted by out of phase circadian stimuli.
The observation that women predisposed to early menopause may experience accelerated ovarian failure due to working sufficiently frequent rotating night shifts is novel. The major determinant of menopausal timing is the remaining number of viable candidate follicles from birth (Gougeon, 1996). Until half way through the reproductive lifetime, atresia is the most common fate of these follicles (McGee and Hsueh, 2000); however, we have no reason to suspect women with a lower baseline follicle count, or greater predisposition to atresia, would self-select rotating night work. The elevated risk of earlier menopause for women under 45 years who worked 10 or more months of recent rotating night shifts during the previous 2 years suggests that the most influential exposure window begins once the menopausal cascade is underway.
It has been suggested that melatonin may act as an anti-apoptotic agent within the follicle through receptor mediated signaling or directly as an antioxidant (Tamura et al., 2012). The typically higher levels of melatonin in follicular fluid may be diminished in women who work frequent rotating night shifts due to compromised nocturnal pineal production, exacerbating follicle depletion with sufficient night work exposure.
Psychological stress has been associated with higher levels of oxidative stress and markers of declining ovarian reserve in premenopausal women (Epel et al., 2004; Bleil et al., 2012), potentially contributing to earlier menopause. More generally, an effect of stress on central reproductive signaling is found in animal models demonstrating altered pituitary gonadotropin secretion in response to cortisol infusion (Pierce et al., 2009). Glucocorticoids, the levels of which become elevated in response to stress, are thought to interact with the HPOA at multiple sites, with potential adverse outcomes for fertility and ovulation (Whirledge and Cidlowski, 2010). It may be that working rotating nights can be chronically stress-inducing, making it difficult to separate independent effects of circadian disruption and the stress response on menopausal onset when using shift work as a surrogate exposure. Support for elevated stress in night workers can be found in studies that have observed alterations in cortisol profiles (Weibel et al., 1996) or truncations of the cortisol quiescent period in circadian phase-advanced volunteers (Caufriez et al., 2002; Griefahn et al., 2006).
Cassou et al. (2007) observed that both currently working ‘high-strain jobs’ and working prior jobs involving ‘difficult schedules’ were associated with earlier menopause. Given evidence that nurses are commonly called upon to work long shifts, it is plausible that a substantial proportion of participants in the NHS2 may have met these criteria, though these were not clearly identifiable given the available data. The observation that women may work fewer rotating night shifts with increasing age may be explained in part by changes in chronotype, which tend to shift more towards morning type with age (Fischer et al., 2017): women with this chronotype tend to find intermittent night shifts more strenuous (van de Ven et al., 2016). As such, in addition to choosing more socially normative schedules afforded by occupational seniority, some women may avoid rotating night work as they approached menopause due to reduced biological tolerance. Whether the higher risk of early menopause among those working rotating night shift schedules observed in this study was exacerbated by chronotype-related tolerance is uncertain. While the NHS 2 did capture morning-evening preference on the 2009 questionnaire, 29% of the baseline cohort did not complete this item and data collection done at this time may have no longer reflected chronotype at time of rotating shift work exposure effect for the oldest subgroup. Further, stratifying the sample by the 2009 morning vs evening preference did not materially change findings (data not shown).
First-reported age at menopause in the NHS1 has been validated as being reliable, at least insofar as remaining fairly consistent on the subsequent biennial questionnaire for most women (Colditz et al., 1987) Repeated questionnaire assessments of menopausal status also served to decrease misclassification. The potential for increased recall error with more temporally distant reporting (den Tonkelaar, 1997) further supports our use of first-reported age to define the timing of natural menopause. Despite indications that self-reported menopausal age in the NHS2 is of relatively high validity, some misclassification may have arisen due to the defining criteria. Unlike the World Health Organization definition of menopause which requires periods to have ceased for 12 months (WHO Scientific Group on Research on the Menopause in the 1990s, 1994), the NHS2 definition is limited to querying whether a woman’s periods had ceased.
In addition to the novelty of this research, a key strength is the study power afforded by the NHS2, the largest cohort of female shift-workers of its kind. Despite this, power was limited for analyses restricted to women right-censored by 45 years as <10% of eligible women reported natural menopause by this age. A limitation is that rotating night shift schedule duration comprised the only routinely collected data on night work in the NHS2. The potential for misclassification due to not having more detailed shift schedule information has been discussed in previous work using NHS2 data to study rotating night work effects on reproduction-linked outcomes (Schernhammer et al., 2006; Lawson et al., 2011). Nevertheless, the NHS2 remains one of the only sizable cohorts in existence that collects longitudinal data on night work and potential confounding factors. The quality of the self-reported data has been previously validated for many of the items captured on the ongoing biennial questionnaires, such as self-reported menopausal status (Colditz et al., 1987).
Conclusion
Our findings suggest that premenopausal women who exceed a threshold of rotating night shift work may be at risk of moderately accelerated menopausal onset, an effect that is most pronounced among younger women potentially at elevated risk for early ovarian failure. So far, we can only speculate the degree to which disruptive circadian stimuli, through cascade effects on central and peripheral HPOA targets, including nocturnal melatonin suppression, may play a role. Further study of the interplay between circadian signaling and biological mediators of stress among shift workers, in addition to validation of this finding among studies using more detailed shift schedule data is recommended. A suggested starting point is that future occupational cohorts capture measures of ‘shift system’ and ‘shift intensity’ to supplement cumulative night work measures exemplified by the main exposures examined in this study, as recommended by the International Agency for Research on Cancer in their investigation of the carcinogenic potential of night work (Stevens et al., 2011).
Acknowledgements
We thank the thousands of participants in the Nurses’ Health Study 2.
Authors' roles
D.S., S.S. and E.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: E.S., J.A.K., D.S. Acquisition, analysis or interpretation of data: All authors. Drafting of the article: D.S. Critical revision of the article for important intellectual content: all authors. Statistical analysis: D.S., B.R., J.R. Obtaining funding: E.S. Administrative, technical or material support: D.S. Study supervision: E.S.
Funding
Center for Disease Control and Prevention/The National Institute for Occupational Safety and Health (Grant 5R01OH009803) (PI: Schernhammer E), as well as (UM1 CA176726) from the National Institute of Health. The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the article for publication.
Conflict of interest
None.
References
- Attanasio A, Borrelli P, Gupta D. Circadian rhythms in serum melatonin from infancy to adolescence. J Clin Endocrinol Metab 1985;61:388–390. [DOI] [PubMed] [Google Scholar]
- Batschelet E, Hillman D, Smolensky M, Halberg F. Angular-linear correlation coefficient for rhythmometry and circannually changing human birth rates at different geographic latitudes. Int J Chronobiol 1973;1:183–202. [PubMed] [Google Scholar]
- Berga SL, Mortola JF, Yen SS. Amplification of nocturnal melatonin secretion in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 1988;66:242–244. [DOI] [PubMed] [Google Scholar]
- Bleil ME, Adler NE, Pasch LA, Sternfeld B, Gregorich SE, Rosen MP, Cedars MI. Depressive symptomatology, psychological stress, and ovarian reserve: a role for psychological factors in ovarian aging? Menopause 2012;19:1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: a systematic review. Occup Environ Med 2007;64:228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms 1997;12:537–546. [DOI] [PubMed] [Google Scholar]
- Brett KM, Cooper GS. Associations with menopause and menopausal transition in a nationally representative US sample. Maturitas 2003;45:89–97. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Are humans seasonally photoperiodic? J Biol Rhythms 2004;19:180–192. [DOI] [PubMed] [Google Scholar]
- Cassou B, Mandereau L, Aegerter P, Touranchet A, Derriennic F. Work-related factors associated with age at natural menopause in a generation of French gainfully employed women. Am J Epidemiol 2007;166:429–438. [DOI] [PubMed] [Google Scholar]
- Caufriez A, Moreno-Reyes R, Leproult R, Vertongen F, Van Cauter E, Copinschi G. Immediate effects of an 8-h advance shift of the rest-activity cycle on 24-h profiles of cortisol. Am J Physiol Endocrinol Metab 2002;282:E1147–E1153. [DOI] [PubMed] [Google Scholar]
- Chu A, Zhu L, Blum ID, Mai O, Leliavski A, Fahrenkrug J, Oster H, Boehm U, Storch KF. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology 2013;154:2924–2935. [DOI] [PubMed] [Google Scholar]
- Chung FF, Yao CC, Wan GH. The associations between menstrual function and life style/working conditions among nurses in Taiwan. J Occup Health 2005;47:149–156. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319–325. [DOI] [PubMed] [Google Scholar]
- Cruz MH, Leal CL, Cruz JF, Tan DX, Reiter RJ. Essential actions of melatonin in protecting the ovary from oxidative damage. Theriogenology 2014;82:925–932. [DOI] [PubMed] [Google Scholar]
- Dardente H. Melatonin-dependent timing of seasonal reproduction by the pars tuberalis: pivotal roles for long daylengths and thyroid hormones. J Neuroendocrinol 2012;24:249–266. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Bovenhuis H, van Noord PA, Pearson PL, van Arendonk JA, te Velde ER, Kuurman WW, Dorland M. The role of genetic factors in age at natural menopause. Hum Rep 2001;16:2014–2018. [DOI] [PubMed] [Google Scholar]
- den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 1997;27:117–123. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Pro Natl Acad Sci USA 2004;101:17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez RC, Marino JL, Varcoe TJ, Davis S, Moran LJ, Rumbold AR, Brown HM, Whitrow MJ, Davies MJ, Moore VM. Fixed or rotating night shift work undertaken by women: implications for fertility and miscarriage. Semin Reprod Med 2016;34:74–82. [DOI] [PubMed] [Google Scholar]
- Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US—influence of age and sex. PLoS One 2017;12:e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness S, Roberts H, Marjoribanks J, Lethaby A, Hickey M, Farquhar C. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst Rev 2009;15:CD000402. [DOI] [PubMed] [Google Scholar]
- Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996;17:121–155. [DOI] [PubMed] [Google Scholar]
- Griefahn B, Kuenemund C, Robens S. Shifts of the hormonal rhythms of melatonin and cortisol after a 4 h bright-light pulse in different diurnal types. Chronobiol Int 2006;23:659–673. [DOI] [PubMed] [Google Scholar]
- He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet 2009;41:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Survival Analysis. INC., New York: John Wiley & Sons, 1999. [Google Scholar]
- Jernstrom H, Bendahl PO, Lidfeldt J, Nerbrand C, Agardh CD, Samsioe G. A prospective study of different types of hormone replacement therapy use and the risk of subsequent breast cancer: the women’s health in the Lund area (WHILA) study (Sweden). Cancer Causes Control 2003;14:673–680. [DOI] [PubMed] [Google Scholar]
- Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol 1998;51:1271–1276. [DOI] [PubMed] [Google Scholar]
- Kauppila A, Kivela A, Pakarinen A, Vakkuri O. Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. J Clin Endocrinol Metab 1987;65:823–828. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Loucks AB, Yen SS. Marked augmentation of nocturnal melatonin secretion in amenorrheic athletes, but not in cycling athletes: unaltered by opioidergic or dopaminergic blockade. J Clin Endocrinol Metab 1991;73:1321–1326. [DOI] [PubMed] [Google Scholar]
- Lawson CC, Whelan EA, Lividoti Hibert EN, Spiegelman D, Schernhammer ES, Rich-Edwards JW. Rotating shift work and menstrual cycle characteristics. Epidemiology 2011;22:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000;21:200–214. [DOI] [PubMed] [Google Scholar]
- Mereness AL, Murphy ZC, Forrestel AC, Butler S, Ko C, Richards JS, Sellix MT. Conditional deletion of Bmal1 in ovarian Theca cells disrupts ovulation in female mice. Endocrinology 2016;157:913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TF, Graff-Iversen S, Sundby J, Bjertness E. Early menopause, association with tobacco smoking, coffee consumption and other lifestyle factors: a cross-sectional study. BMC Public Health 2007;7:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murabito JM, Yang Q, Fox C, Wilson PW, Cupples LA. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab 2005;90:3427–3430. [DOI] [PubMed] [Google Scholar]
- Nagata C, Wada K, Nakamura K, Tamai Y, Tsuji M, Shimizu H. Associations of physical activity and diet with the onset of menopause in Japanese women. Menopause 2012;19:75–81. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Sagara Y. Amplification of nocturnal melatonin secretion in women with functional secondary amenorrhoea: relation to endogenous oestrogen concentration. Clin Endocrinol (Oxf) 1994;41:763–770. [DOI] [PubMed] [Google Scholar]
- Parazzini F. Determinants of age at menopause in women attending menopause clinics in Italy. Maturitas 2007;56:280–287. [DOI] [PubMed] [Google Scholar]
- Pierce BN, Stackpole CA, Breen KM, Clarke IJ, Karsch FJ, Rivalland ET, Turner AI, Caddy DJ, Wagenmaker ER, Oakley AE et al. Estradiol enables cortisol to act directly upon the pituitary to suppress pituitary responsiveness to GnRH in sheep. Neuroendocrinology 2009;89:86–97. [DOI] [PubMed] [Google Scholar]
- Pokoradi AJ, Iversen L, Hannaford PC. Factors associated with age of onset and type of menopause in a cohort of UK women. Am J Obstet Gynecol 2011;205:34.e31–13. [DOI] [PubMed] [Google Scholar]
- Revel FG, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Melatonin controls seasonal breeding by a network of hypothalamic targets. Neuroendocrinology 2009;90:1–14. [DOI] [PubMed] [Google Scholar]
- Rojansky N, Brzezinski A, Schenker JG. Seasonality in human reproduction: an update. Hum Rep 1992;7:735–745. [DOI] [PubMed] [Google Scholar]
- Ruth KS, Perry JR, Henley WE, Melzer D, Weedon MN, Murray A. Events in early life are associated with female reproductive ageing: a UK Biobank Study. Sci Rep 2016;6:24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarac F, Oztekin K, Celebi G. Early menopause association with employment, smoking, divorced marital status and low leptin levels. Gynecol Endocrinol 2011;27:273–278. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology 2006;17:108–111. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 2001;93:1563–1568. [DOI] [PubMed] [Google Scholar]
- Sellix MT. Circadian clock function in the mammalian ovary. J Biol Rhythms 2015;30:7–19. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Bahougne T. A multi-oscillatory circadian system times female reproduction. Front Endocrinol (Lausanne) 2015;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst Monogr 2005;34:25–27. [DOI] [PubMed] [Google Scholar]
- Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab 1998;83:1875–1880. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castano-Vinyals G, Davis S, Frings-Dresen MH, Fritschi L et al. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med 2011;68:154–162. [DOI] [PubMed] [Google Scholar]
- Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet 2012;44:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk L, Zhai G, van Meurs JB, Verbiest MM, Visser JA, Estrada K, Rivadeneira F, Williams FM, Cherkas L, Deloukas P et al. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat Genet 2009;41:645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SB, Lu CW, Kao YY, Guo HR. Effects of 12-hour rotating shifts on menstrual cycles of photoelectronic workers in Taiwan. Chronobiol Int 2008;25:237–248. [DOI] [PubMed] [Google Scholar]
- Tamura H, Kawamoto M, Sato S, Tamura I, Maekawa R, Taketani T, Aasada H, Takaki E, Nakai A, Reiter RJ et al. Long-term melatonin treatment delays ovarian aging. J Pineal Res 2017;62. [DOI] [PubMed] [Google Scholar]
- Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y et al. The role of melatonin as an antioxidant in the follicle. J Ovarian Res 2012;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM.. Modeling Survival Data: Extending The Cox Model. New York: Springer-Verlag, 2000. [Google Scholar]
- Torgerson DJ, Thomas RE, Campbell MK, Reid DM. Alcohol consumption and age of maternal menopause are associated with menopause onset. Maturitas 1997;26:21–25. [DOI] [PubMed] [Google Scholar]
- van Asselt KM, Kok HS, Pearson PL, Dubas JS, Peeters PH, Te Velde ER, van Noord PA. Heritability of menopausal age in mothers and daughters. Fertil Steril 2004;82:1348–1351. [DOI] [PubMed] [Google Scholar]
- van de Ven HA, van der Klink JJ, Vetter C, Roenneberg T, Gordijn M, Koolhaas W, de Looze MP, Brouwer S, Bultmann U. Sleep and need for recovery in shift workers: do chronotype and age matter? Ergonomics 2016;59:310–324. [DOI] [PubMed] [Google Scholar]
- Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res 2007;67:10618–10622. [DOI] [PubMed] [Google Scholar]
- Voorhuis M, Onland-Moret NC, van der Schouw YT, Fauser BC, Broekmans FJ. Human studies on genetics of the age at natural menopause: a systematic review. Hum Reprod Update 2010;16:364–377. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Weiszenbacher G, Frisch H, Zeitlhuber U, Waldhauser M, Wurtman RJ. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet 1984;1:362–365. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gu F, Deng M, Guo L, Lu C, Zhou C, Chen S, Xu Y. Rotating shift work and menstrual characteristics in a cohort of Chinese nurses. BMC women’s health 2016;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel L, Spiegel K, Follenius M, Ehrhart J, Brandenberger G. Internal dissociation of the circadian markers of the cortisol rhythm in night workers. Am J Physiol 1996;270:E608–E613. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Gorrindo T, Riley A, Mormino J, Niedfeldt J, Singer B, Rodriguez G, Simon J, Pincus S. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol 2003;158:782–791. [DOI] [PubMed] [Google Scholar]
- Wellons MF, Matthews JJ, Kim C. Ovarian aging in women with diabetes: an overview. Maturitas 2017;96:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol 2010;35:109–125. [PMC free article] [PubMed] [Google Scholar]
- WHO Scientific Group on Research on the Menopause in the 1990s (1994: Geneva, Switzerland) & World Health Organization (1996). Research on the Menopause in the 1990s: Report of a WHO Scientific Group Geneva: World Health Organization. http://www.who.int/iris/handle/10665/41841
- Willett WC. The Nurses’ Health Study. 2013. Boston, Description of Nurses’ Health Study Cohorts.
- Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction 2010;140:489–504. [DOI] [PubMed] [Google Scholar]