Abstract
A major paradigm in cancer immunotherapy is to use checkpoint inhibitors to break regulatory mechanisms that usually guard the host against autoimmune diseases. CTLA-4-targeting immunotherapy was the first example that help to establish this paradigm. However, the clinically tested anti-CTLA-4 antibodies exhibit suboptimal efficacy but high toxicity. Recent studies have demonstrated that immunotherapy-related adverse events (irAE) and the cancer immunotherapeutic effect (CITE) represent distinct and therapeutically separable activities of anti-CTLA-4 antibodies. The former is attributable to inactivation of CTLA-4 checkpoint, while the latter is due to selective depletion of regulatory T cells (Treg) in tumor microenvironment. Here we argue that for safer and more effective CTLA-4-targeting immune therapy, one should preserve rather than inhibit the CTLA-4 checkpoint while enhancing the efficacy and selectivity of Treg-depletion in tumor microenvironment.
Keywords: CTLA-4, cancer Immunotherapy, immunotherapy-related adverse events, checkpoint blockade immunotherapy, regulatory T cells
Immune tolerance checkpoints and cancer immunotherapy
The immune system defends the host against infections and malignancies. These mighty tasks are carried out by leukocytes that recognize pathogens and cancer cells through either pattern-recognition receptors (innate immunity) or clonally distributed antigen-receptors including B-cell surface immunoglobulin and T cell receptors (adaptive immunity). Ligand recognition by these receptors triggers complicated cascades of cellular and molecular interactions that not only numerically increases the number of leukocytes (expansion), but also qualitatively changed the properties of the cells (activation and differentiation). Given the brutal force of immune response, multiple layers of regulatory mechanisms are necessary to ensure the immune response is normally controlled to avoid self-destruction.
The immune response is by no means foolproof: cancer occurs because the immune response does not eliminate all malignant cells, while autoimmune disease develops when the immune responses were misdirected against host cellular components even including DNA and caused tissue injuries. Cancer immunotherapy aims to activate the immune system to destroy cancer. One way to achieve this goal is to release negative regulatory mechanisms of immune response to boost cancer immunity. This approach, collectively developed by many laboratories, was called checkpoint blockade by Dr. James Allison and colleagues [1]. Programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) are the two checkpoints that has been successfully targeted in clinics [2]. A recent essay has questioned whether it is appropriate to call anti-PD-1 and anti-PD-1 ligand 1 (PD-L1) therapeutics as checkpoint inhibitors, as the authors believe these drugs correct what has gone awry in cancer rather than breaking a physiologically important immune tolerance mechanism [3]. In this perspective, we will discuss the challenges of CTLA-4 checkpoint blockade in cancer immunotherapy and propose a new paradigm for safer and more effective CTLA-4 targeting in cancer immunotherapy.
CTLA-4: a Treg-intrinsic immune checkpoint against fatal autoimmune diseases
Linsley and colleagues initially reported in 1991 that CTLA-4 is the high affinity receptor for B7/BB1, a cell surface protein now called CD80 [4]. It was subsequently revealed that B7-2 (CD86) protein was another CTLA-4 ligand [5] [6, 7]. CTLA-4 function was initially inferred by effect of anti-CTLA-4 antibodies. The ability to anti-CTLA-4 antibodies to stimulate T cell activation was also first reported by Linsley et al. who suggested CTLA-4 as an co-activator for T cells [4]. Subsequently, Bluestone and Allison groups [8, 9] showed that activation of T cells can be achieved with antigen-binding fragments (Fab, see Glossary) of anti-CTLA-4 antibodies. Since monovalent Fab was considered incapable of inducing receptor signaling , the Fab-enhanced T-cell activation was interpreted in the context that the antibody have blocked a negative regulator for T cell activation. Therefore, the data constituted suggestive evidence that CTLA-4 is a negative regulator for T cell activation [8, 9].
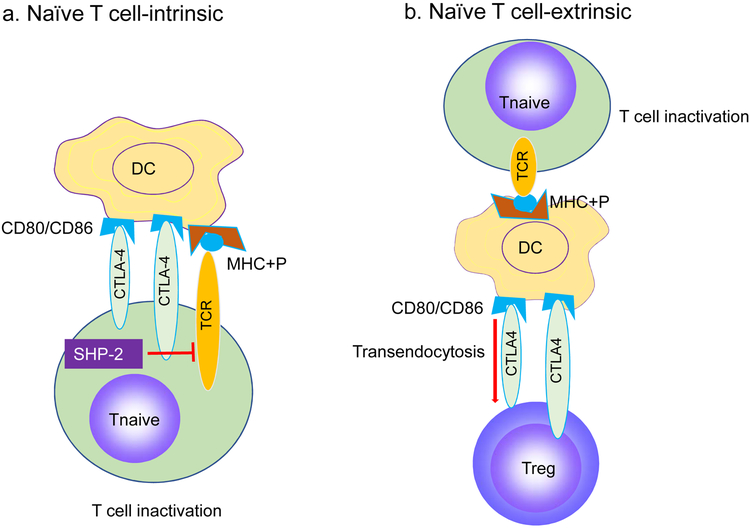
Around the same time, Mak and Sharpe groups independently reported that germline deletion of Ctla4 cause severe autoimmune diseases with early lethality [10, 11]. More recent data demonstrate that mutation of either CTLA4 [12, 13] or the lipopolysaccharide-responsive and beige-like anchor (LRBA) gene [13, 14] (which is required for CTLA-4 recycling) genes caused autoimmune diseases in humans. These data established CTLA-4 as a crucial checkpoint against autoimmune diseases. These data were interpreted by many as evidence for a cell-intrinsic negative regulatory function of CTLA-4. As a molecular mechanism, two groups reported that engagement of CTLA-4 triggers association of a tyrosine phosphatase SHP-2 (Src homology region 2 domain-containing phosphatase-2) [15, 16]. This was presumed to down-regulation of signal transduction by the T-cell receptor (TCR) on naïve T cells after TCR binding by its ligand, the complex between the major histocompatibility complex (MHC) and antigenic peptides (MHC+P) present on dendritic cells (DC) (Figure 1a). The work suggested SHP-2 as critical transducer for negative signaling from CTLA-4 [15, 16] (Figure 1a). However, T cell-intrinsic inhibitory effect of CTLA-4 has not been resolved historically [17-20]. In particular, since T-lineage-specific deletion of Shp2 inhibit T cell activation, genetic data did not support SHP-2 as negative regulator for T cell function [21]. These considerations prompted us to question whether CTLA-4 is a negative signaling molecule for T cell activation and whether cancer immunotherapy can be achieved by inhibiting physiological function of CTLA-4 [22, 23].
Figure 1. New Alternative ways to explore physiological function of CTLA-4.
a. Traditional view: CTLA-4 is a naïve T cell (Tn)-intrinsic inhibitor of T cell activation. Engagement of CTLA-4 on naïve T cells by CD80/CD86 on dendritic cells (DC)? triggers association of SHP-2, which cause down-regulation of signal transduction by the T-cell receptor (TCR). b. Proposed view: CTLA-4 works primarily as an essential positive regulator of regulatory T cells (Treg), perhaps functions by transendocytosis of CD80/CD86 on DC. As a result, naïve T cells lack costimulatory signal derived from interaction between CD28 on naïve T cells and CD80/CD86 on DC but receive TCR ligand, the complex of major histocompatibility complex antigen and peptide (MHC+P), a condition that causes T cell inactivation.
A major challenge to the notion of CTLA-4 as cell-intrinsic negative regulator for T cell activation come from the elegant chimera analysis from Bachmann and colleagues [24] who reported that in chimera consisting of Ctla4+/+ and Ctla4−/− bone marrow, the inherent pathogenicity of Ctla4−/− immune system are completely abrogated by Ctla4+/+ hematopoietic cells. These data demonstrate that CTLA-4 works in trans to protect host against autoimmune diseases. The lack of cell intrinsic negative regulatory effect from CTLA-4 signaling was further demonstrated by the data that Ctla4−/− T cells had no advantage over Ctla4+/+ T cells in clonal expansion following lymphocytic choriomeningitis virus (LCMV), Leishmania major and mouse mammary tumor virus infection [25]. Since T cells expand more than 10,000 fold during first week of LCMV infection [26], releasing T cells from cell-intrinsic negative regulatory activity would have substantially increased the clonal size of the Ctla4−/− T cells. The chimera mice studies refuted the cell-intrinsic inhibitory effect during T cell activation and established a concept that CTLA-4 mediates immune regulation of cells that are genetically devoid of the Ctla4 gene, i.e. CTLA-4 works in trans.
The in trans mode of immunoregulation by CTLA-4 provided a useful clue to the cellular basis for CTLA-4 function, as the cells capable of suppress autoimmune diseases in trans is called regulatory T cells (Treg), which have emerged as an essential guard against lethal autoimmune diseases as genetic inactivation of the gene encoding the forkhead box P3 protein (FOXP3), the master regulator of Treg [27, 28] leads to lethal autoimmune diseases in mouse [29] and humans [30, 31]. Therefore, it is intriguing to speculate whether the trans-suppressive function of CTLA-4 may be due to its function on Treg.
Bluestone’s laboratory was the first to report that CTLA-4 is almost exclusively expressed in Tregs under homeostatic condition [32], which in retrospect is predictable as Ctla4 is a target gene of Foxp3, the master regulator of Treg [33]. By showing that Treg-specific deletion of Ctla4 recapitulated the phenotype of mice with germline deletion of Ctla4 and that Ctla4 deficiency abrogated the function of Treg [34], Sakaguchi’s laboratory provided the definitive data which establishes CTLA-4 as the immune checkpoint that prevents autoimmune diseases by conferring function to Tregs.
Despite extensive literature on the molecular interactions responsible for CTLA-4-mediated immune regulation, there is no consensus on how CTLA-4 contributed to Treg cell function. Samson group showed thatCTLA-4 on Treg down-regulated CD80/CD86 on DCs by transendocytosis [35] (Figure 1b). Since CD80/CD86 is critical for activation of T cells after the TCR was stimulated by MHC and peptide complex, such down-regulation would abrogate the DC-induced T cell activation [35]. This is an attractive mechanism of CTLA-4-mediated immune suppression as it explains CTLA-4’s recycling [36], Treg-intrinsic requirement for Ctla4 for mouse survival and the above mentioned data from chimera studies by Bachmann. The fact that mutations in LRBA, the gene which is critical for CTLA-4 recycling, caused autoimmune diseases in human [13, 14] lends further support to the concept.
Challenges in targeting CTLA-4 in cancer immunotherapy
The Allison laboratory reported in 1996 that antibody targeting CTLA-4 induced rejection of established tumors in the mice [37]. These observations inspired Medarex Inc (later acquired by Bristol-Myers Squibb, Inc) to generate anti-human CTLA-4 antibody for treatment of melanoma. After a decade-long clinical testing, the anti-CTLA-4 antibody Ipilimumab was shown to improve survival of melanoma patients in a large phase III clinical trial [38]. This antibody has taken a historical significance as it is the first T-cell targeting antibody that activates host immunity against cancer. Another longstanding effort to develop a different anti-CTLA-4 antibody, the Tremelimumab by Pfizer, Inc and AstraZeneca Inc, however, has not reached statistical endpoint in phase III trials [39]. Therefore, clinical performance of CTLA-4 targeting is mainly based on a single antibody. In contrast, PD-1/PD-L1 (originally called B7H1) targeting (hereby called anti-PD therapy) has been validated by a large cohort of clinical products in multiple cancers.
Compared with anti-PD therapy, CTLA-4 targeting faces two related challenges: suboptimal efficacy and increased toxicity. In a direct head-to-head comparison between Nivolumab (anti-PD-1) and Ipilimumab in adjuvant therapy of advanced melanoma, Weber et al. [40] reported that, in a large clinical trial involving 906 patients with resected stage III and IV melanoma, patients receiving monotherapy with Nivolumab showed 70.5% recurrence-free survival (RFS) over 1 year period, while those receiving Ipilimumab showed have 60.5% RFS (P<0.001). Remarkably, severe (grades 3-4) immunotherapy-related adverse events (irAE) were reported among 14.4% Nivolumab-treated patients and 46.2% Ipilimumab-treated patients. As a result of severe toxicity, treatment was discontinued among 9.7% of Nivolumab-treated patients and 42.6% of Ipilimumb-treated patients. In a large phase III clinical trial in patients with advanced melanoma, Ipilimumab-treated arm had a median survival of 19.9 months, Nivolumab-treated arm had a median survival of 36.9 months, while the Ipilimumab and Nivolumab combination arm had a median survival of more than 60 months [41]. The rates of severe irAE for Ipilimumab, Nivolumab, and the Ipilimumab+Nivolumab arms were 28%, 23% and 59% respectively [41]. As a result, while anti-PD therapy has been approved in rapidly expanding indications such as melanoma, lung cancer, liver cancer, kidney cancer, lymphoma, as well as cancer with high microsatellite instability, anti-CTLA-4 monotherapy largely limits to melanoma [3].
Despite these challenges, there is a continuing interest in targeting CTLA-4 for cancer immunotherapy as Ipilimumab has shown to improve therapeutic response to anti-PD-1 antibody, as evidenced by improved overall survival, objective response rate and progression-free survival [42-48]. Moreover, cancer patients receiving Ipilimumab exhibit very long survival if they survived more than three years [49]. However, severe irAE occurred at exceptionally high levels, reaching 73-90% among patients receiving Ipilimumab and Nivolumab combination in neoadjuvant therapy setting [50, 51]. Since irAE is basically an autoimmune disease-like state, the increased incidence should have been predicted by the current paradigm of disrupting a critical checkpoint that ensures immune tolerance to self-antigens.
CTLA-4 checkpoint blockade is neither necessary nor sufficient for cancer immunotherapy
The observations of anti-CTLA-4 vs anti-PD in humans differ sharply from those in preclinical models where anti-PD reagents are typically less effective than anti-CTLA-4 antibodies in cancer immunotherapy [52-55]. While it is possible that preclinical models did not reflect human diseases, it is of interest to ask whether the low efficacy and high toxicity of CTLA-4 in humans is due to either inherent limit of the CTLA-4 target or because of a fundamental knowledge gap on how to target CTLA-4. Recent studies have offered exciting new insights on the fundamental cause of therapeutic efficacy vs irAEs.
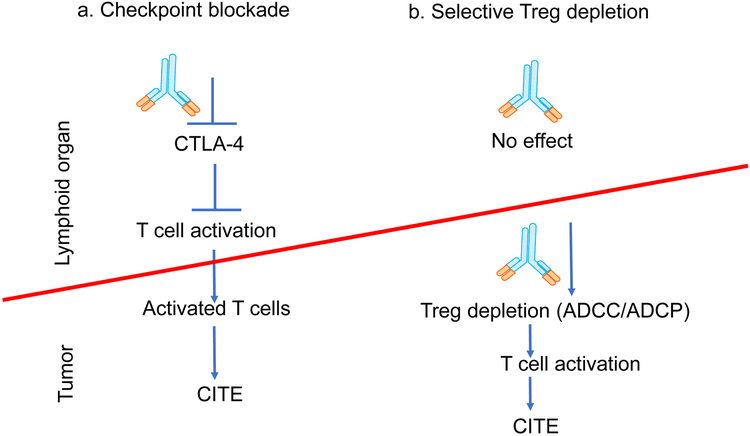
Cancer immunotherapeutic effect (CITE) of anti-CTLA-4 antibodies has been interpreted on basis of checkpoint blockade hypothesis [1] which stipulates that the anti-CTLA-4 antibodies block the naïve T cell-intrinsic CTLA-4 checkpoint by inhibiting CTLA-4 interaction with CD80 and CD86 (Figure 2a). This allows activation of tumor-reactive T cells which then migrate into tumors to confer therapeutic effect (Figure 2a). However, it has not been tested whether blocking CTLA-4 interaction with its physiological ligands, CD80 and CD86 is either necessary or sufficient for cancer therapeutic effect of anti-CTLA-4 antibodies. We have recently provided several lines of evidence that demonstrate CTLA-4 checkpoint blockade is neither necessary nor sufficient for cancer therapeutic effect [55]:
Figure 2. Recent insights on how anti-CTLA-4 antibodies promote cancer immunity.
a. Traditional view: checkpoint blockade hypothesis stipulates that the anti-CTLA-4 antibodies block the cell-intrinsic checkpoint of T cell activation in naïve T cells, allowing activation of tumor-reactive T cells which then migrate into tumors to confer cancer immunotherapeutic effect (CITE). b. Recent view: anti-CTLA-4 antibodies deplete Treg in tumor, leading to activation and increased effector function of tumor-specific T cells in the tumors and increased CITE. Here, lymphoid organs refer to secondary lymphoid organs where immune responses are initiated, including spleen and lymph nodes.
First, while clinically effective Ipilimumab blocks CD80/CD86-CTLA-4 interaction if CD80/CD86 are provided in soluble phase, it is largely ineffective in blocking this interaction when CD80/CD86 and CTLA-4 are expressed on cell surface. In particular, Ipilimumab barely block CTLA-4-mediated transendocytosis either in mice that express human CTLA-4 or in vitro when the receptor and ligands are expressed on separate cells.
Second, in heterozygous mice that co-dominantly expressed mouse and human CTLA-4 molecules (CTLA4h/m), antibodies that bind to human but not mouse CTLA-4 molecules are incapable of blocking the physiological function of CTLA-4 but effectively induced tumor rejection.
Third, two mutant antibodies that have lost the ability to block the CD80/CD86-CTLA-4 interaction are fully capable of causing tumor rejection in mice expressing human CTLA-4 protein.
Fourth, an antibody against host FcγR, which should not affect the ability of anti-CTLA-4 to block CTLA-4 binding to its ligands, abrogated CITE.
These data demonstrate that blocking CTLA-4 interaction with CD80 and CD86 is neither necessary nor sufficient for CITE. Therefore, they call for reappraisal of the CTLA-4 checkpoint blockade hypothesis for cancer immunotherapy and brings attention to an alternative mechanism by which anti-CTLA-4 antibodies can induce tumor rejection [23].
The alternative mechanism arises from understanding the role of interaction of Fc fragments of anti-CTLA-4 antibodies with specific host cellular receptors for IgG Fc (FcγR. where antibody-coated cells are eliminated by cells expressing FcγR by processes known as antibody-dependent cell-mediated cytotoxicity (ADCC) and/or antibody-dependent cell-mediated phagocytosis (ADCP) (Figure 2b). Several laboratories have reported that interaction between CTLA-4 antibodies and the activating Fc receptors on host cells are critical for selective depletion of Treg in the tumor, leading to tumor rejection. Thus, Selby et al. showed that the therapeutic effect of anti-mouse CTLA-4 antibodies is determined by their ability to engage Fc receptors for ADCC/ADCP [53], while our group showed that an antibody that reacted with FcγRII and FcγRIII, abrogated immunotherapeutic effect of an anti-human CTLA-4 antibody, Ipilimumab [55]. The work highlighted the essential role of FcγRII and FcγRIII in tumor rejection. These findings are further supported by genetic data that shows that targeted mutations of activating FcγR abrogates immunotherapeutic effect of anti-mouse CTLA-4 antibodies [56, 57]. More recently, Vargas et al. [58] demonstrated that ADCC activity of chimeric anti-mouse CTLA-4 antibodies against Treg is essential for tumor rejection in mice with human FcγR system. In mouse models, the depletion is selective for tumor-infiltrating Treg because they express much higher levels of CTLA-4 [53] [55]. While not critically validated, it is also possible that the tumor microenvironment consists of more effector cells capable of mediating ADCC/ADCP (Figure 2b).
In a mouse model that fully recapitulates irAE of Ipilimumab in human, depletion of Treg was observed in neither lymphoid nor other target organs of the inflammatory cells induced by immunotherapy [54]. Clinical data lend further support to this notion. Thus, Treg depletion in melanoma samples has been shown to associate with clinical outcome in melanoma patients receiving Ipilimumab [59, 60]. Although Sharma et al have observed an increase in absolute Treg number in cancer samples from Ipilimumab-treated patients when compared to those from untreated, a careful examination of the data presented showed that the apparent increase was due overwhelming increase of CD4 T cells, and that there is a substantial reduction of % Treg among CD4 T cells in Ipilimumab treated melanoma patients [61]. In agreement the potential contribution of Treg depletion, Sharma et al suggested that Ipilimumab activity may be further increased by enhancing ADCC [61]. Indeed, a new version of Ipilimumab with afucosylated Fc has been developed to improve ADCC activity. This drug is being tested in clinic (Clinical Trial Numberi: NCT03369223). However, since the CTLA-4 binding properties are not changed, this approach should do little to reduce irAE associated with checkpoint inactivation. In contrast, significantly higher toxicity has been observed in non-human primates receiving the new version than those that received the old version of Ipilimumabii, perhaps due to enhanced ADCC function of new version.
Preserving CTLA-4 checkpoint for safe and more effective immunotherapy
Given the essential role for CTLA-4 checkpoint in preventing autoimmune diseases, the prevailing concept to induce tumor rejection by this checkpoint must be considered only as the last resort. When human CTLA4 gene knock in mice were used to evaluate multiple anti-CTLA-4 antibodies for therapeutic efficacy and autoimmunity, it was found that the anti-CTLA-4 antibody that caused the most efficient tumor rejection actually induced the least anti-DNA antibodies [62]. These data suggest that autoimmunity is not a necessary price for cancer immunity conferred by anti-CTLA-4 antibodies.
Furthermore, by administrating anti-CTLA-4 antibodies in 10-day old mice, we were able to fully recapitulate pathological findings of irAE in cancer patients receiving either Ipilimumab or combination of anti-CTLA4 and anti-PD therapies [54]. Using this model, we again demonstrated that it is possible to identify antibodies with minimal adverse effect but optimal immunotherapeutic activity for cancer [54].
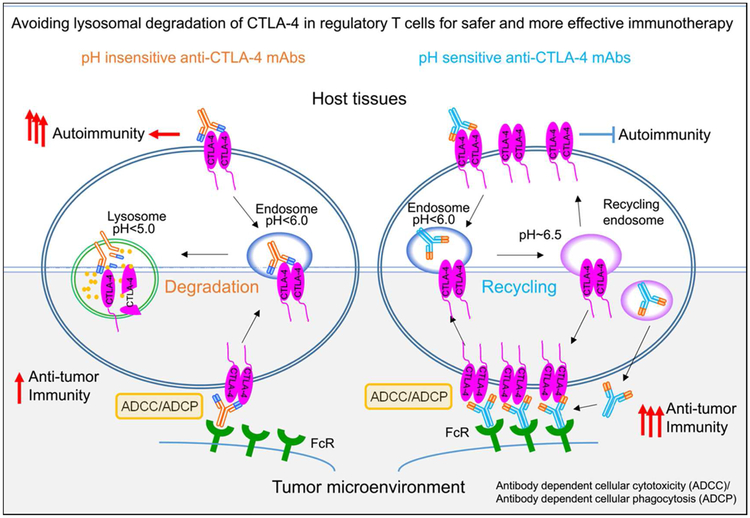
An important issue is how anti-CTLA-4 antibodies can cause autoimmune diseases. Our recent studies have demonstrated that the toxicity of anti-CTLA-4 antibodies is independent of its ability to block CTLA-4:CD80/CD86 interaction [54]. To understand how anti-CTLA-4 antibodies cause irAE, we evaluated the impact of irAE-prone and non-prone antibodies for their impact on CTLA-4 trafficking in the cells [63]. Our data demonstrated that irAE-prone anti-CTLA-4 antibodies such as Ipilimumab prevent CTLA-4 recycling and direct CTLA-4 into lysosomal degradation. In contrast, those anti-CTLA-4 antibodies that cause no irAE preserve CTLA-4 recycling and cause no lysosomal degradation. Pharmacological inhibition of CTLA-4 recycling confers irAE activity to a non-irAE-inducing antibody, confirming the importance of preserving CTLA-4 recycling in avoiding irAE.
To understand how irAE-prone and non-prone antibodies differentially affect intracellular CTLA-4 trafficking, we considered the possibilities that these two class of antibodies may differ in their pH-sensitivity in CTLA-4 binding. As illustrated in Figure 3, since early endosomes undergo acidification as they mature into late endosome and lysosomes, pH-sensitive anti-CTLA-4 antibodies would dissociate from CTLA-4 and thus allow it to recycle to cell surface. In contrast, the pH-insensitive antibodies would continue to bind to CTLA-4 and prevent it from recycling to cell surface to exert their physiological function.
Figure 3. A converging mechanism for safer and more effective cancer immunotherapy.
The properties of clinically used antibodies are shown on the left: the pH-insensitive antibodies cause down-regulation of CTLA-4 through lysosomal degradation. In host tissues, CTLA-4 down-regulation causes autoimmune diseases; while in the tumor CTLA-4 down-regulation reduces ADCC activity and thus anti-cancer efficacy. In contract, as depicted on the right, pH-sensitive antibodies do not cause CTLA-4 degradation as it allows recycling of both antibodies and CTLA-4 molecules. Preserving the CTLA-4 checkpoint on host tissues prevents autoimmune diseases, while preserving CTLA-4 in tumor microenvironment and increasing bioavailability of anti-CTLA-4 antibodies ensures high ADCC ligand density, and thus better Treg depletion and anti-tumor activity.
We have reported two lines of evidence in support of this notion [63]:
First, we found that while irAE-prone antibodies are pH-insensitive as they bound CTLA-4 at comparable efficacy between pH 4.5-7.0 and directed CTLA-4 into lysosomal degradation. In contrast, the non-irAE prone antibodies are pH-sensitive as they dissociated progressively from CTLA-4 at pH below 6.0 and allowed CTLA-4 recycling to cell surface by the LRBA-dependent mechanism.
Second, increasing the pH sensitivity of by antibody engineering prevented antibody-triggered lysosomal CTLA-4 down-regulation and dramatically attenuates irAE.
Surprisingly, in addition to preserving CTLA-4 immune checkpoint function, pH-sensitivity also preserve CTLA-4 as target for ADCC and ADCP [63]. This, together with recycling of anti-CTLA-4 antibodies that allowed increased antibody bioavailability, made pH-sensitive anti-CTLA-4 antibodies more effective in intra-tumor regulatory T-cell depletion and rejection of large established tumors [63]. Our data establish a new paradigm for cancer research that allows for abrogating irAE while increasing CITE of anti-CTLA-4 antibodies, as diagramed in Figure 3.
Conclusions and Future Perspectives
Accumulating data reviewed herein have established that CTLA-4 is a critical immune checkpoint against autoimmune diseases and that blocking this checkpoint is neither necessary nor sufficient for cancer immunotherapy. It is therefore inescapable that one could improve safety of CTLA-4 antibodies without losing clinical efficacy for cancer immunotherapy by preserving rather than inhibiting CTLA-4 checkpoint function. What is unexpected is that preserving CTLA-4 checkpoint also allows more effective depletion of Treg in cancer microenvironment and thus improve therapeutic efficacy. The new concept, if proven through future clinical studies, would mark a paradigm shift in cancer immunotherapy.
Moreover, while systemic depletion of Treg by anti-CTLA-4 antibodies have been ruled out [59, 60], the impact of the antibodies on organs underlying immunotherapy-related event has not been carefully scrutinized in humans (see Outstanding Questions). A systemic approach would allow us to fully discern how the clinically used anti-CTLA-4 antibodies inactivated CTLA-4 checkpoint and to engineer or select antibodies that preserves rather than inhibit the CTLA-4 checkpoint.
Outstanding Questions.
Is preserving cell surface CTLA-4 function sufficient to avoid anti-CTLA-4 antibody-induced adverse events?
What are the mechanism for selective depletion of Treg in tumor microenvironment?
Would clinical studies confirm the benefit of the antibodies that preserve CTLA-4 checkpoint in cancer patients?
Can the concept of preserving immune checkpoint for safer and more effective cancer therapy applicable to anti-PD cancer immunotherapy?
Finally, compared with anti-PD therapeutics, anti-CTLA-4 antibodies are dosed at much lower doses and durations due to their high toxicity which has prevents the anti-CTLA-4 antibodies to reach their full therapeutic potential. With improved safety without losing efficacy, more patients may tolerate higher doses, it might be possible to test the potential of anti-CTLA-4 as a monotherapy beyond melanoma.
Highlights.
Cancer therapeutic effect (CITE) of anti-CTLA-4 antibodies is due to selective depletion of tumor-infiltrating regulatory T cells (Treg)
Immunotherapy-related adverse events (irAE) attributable to CTLA-4 inactivation
pH-insensitive antibodies direct CTLA-4 to lysosomal degradation
pH-sensitive anti-CTLA-4 antibodies minimize irAE by preserving CTLA-4 recycling
Preserving CTLA-4 recycling enhances selective depletion of tumor infiltrating Treg
Preserving the CTLA-4 checkpoint allows safer and more effective immunotherapy
ACKNOWLEDGEMENT
We thank our colleagues, Drs. Xuexiang Du, Yan Zhang, Mingyue Liu, Fei Tang and Martin Devenport for their contribution to studies underlying the ideas discussed herein. This work is supported by National Institute of Health (AI64350, CA227671) and Melanoma Research Alliance Team Research Award (559400) and a grant from OncoImmune, Inc.
GLOSSARY
- Adjuvant therapy:
cancer treatments for patients administrated after surgery.
- Clonal expansion:
the process that leads to increased frequency of T or B cells with a unique antigen receptor.
- Dendritic cells (DC):
the major cell types that present antigen to activate T cells
- Fab:
a fragment of immunoglobulin that mediate binding to antigen.
- Fc:
a crystalizable fragment of immunoglobulin.
- Neoadjuvant therapy:
cancer treatments for patients administrated before surgery.
- Transendocytosis:
cellular receptor-mediated uptake and internalization of ligand on another cell.
- Tumor microenvironment:
The normal cells, molecules, and blood vessels that surround and feed a tumor cell.
Footnotes
DISCLAIMER STATEMENT: The authors are among the co-founders and have equity interest in OncoImmune, Inc.
RESOURCES
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Korman AJ et al. (2006) Checkpoint blockade in cancer immunotherapy. Advances in Immunology 90, 297–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei SC et al. (2018) Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 8 (9), 1069–1086. [DOI] [PubMed] [Google Scholar]
- 3.Sanmamed MF and Chen L (2019) A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 176 (3), 677. [DOI] [PubMed] [Google Scholar]
- 4.Linsley PS et al. (1991) CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 174 (3), 561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y et al. (1993) A major costimulatory molecule on antigen-presenting cells, CTLA4 ligand A, is distinct from B7. J Exp Med 178 (5), 1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman GJ et al. (1993) Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice [see comments]. Science 262 (5135), 907–9. [DOI] [PubMed] [Google Scholar]
- 7.Hathcock KS et al. (1993) Identification of an alternative CTLA-4 ligand costimulatory for T cell activation [see comments]. Science 262 (5135), 905–7. [DOI] [PubMed] [Google Scholar]
- 8.Walunas TL et al. (1994) CTLA-4 can function as a negative regulator of T cell activation. Immunity 1 (5), 405–13. [DOI] [PubMed] [Google Scholar]
- 9.Krummel MF and Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation [see comments]. J Exp Med 182 (2), 459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tivol EA et al. (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3 (5), 541–7. [DOI] [PubMed] [Google Scholar]
- 11.Waterhouse P et al. (1995) Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4 [see comments]. Science 270 (5238), 985–8. [DOI] [PubMed] [Google Scholar]
- 12.Kuehn HS et al. (2014) Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 345 (6204), 1623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou TZ et al. (2017) Identifying functional defects in patients with immune dysregulation due to LRBA and CTLA-4 mutations. Blood 129 (11), 1458–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo B et al. (2015) AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 349 (6246), 436–40. [DOI] [PubMed] [Google Scholar]
- 15.Lee KM et al. (1998) Molecular basis of T cell inactivation by CTLA-4 [In Process Citation]. Science 282 (5397), 2263–6. [DOI] [PubMed] [Google Scholar]
- 16.Marengere LE et al. (1996) Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4 [published errata appear in Science 1996 Dec 6;274(5293)1597 and 1997 Apr 4;276(5309):21]. Science 272 (5265), 1170–3. [DOI] [PubMed] [Google Scholar]
- 17.Bai XF et al. (2002) B7-CTLA4 interaction promotes cognate destruction of tumor cells by cytotoxic T lymphocytes in vivo. Blood 99 (8), 2880–9. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y et al. (1997) CTLA-4-B7 interaction is sufficient to costimulate T cell clonal expansion. J Exp Med 185 (7), 1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng P et al. (1998) B7-CTLA4 interaction enhances both production of antitumor cytotoxic T lymphocytes and resistance to tumor challenge. Proc Natl Acad Sci U S A 95 (11), 6284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallarino F et al. (1998) B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med 188 (1), 205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TV et al. (2006) Conditional deletion of Shp2 tyrosine phosphatase in thymocytes suppresses both pre-TCR and TCR signals. J Immunol 177 (9), 5990–6. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y (1997) Is CTLA-4 a negative regulator for T-cell activation? Immunol Today 18 (12), 569–72. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y and Zheng P (2018) How Does an Anti-CTLA-4 Antibody Promote Cancer Immunity? Trends Immunol 39 (12), 953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmann MF et al. (1999) Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol 163 (3), 1128–31. [PubMed] [Google Scholar]
- 25.Bachmann MF et al. (2001) Normal pathogen-specific immune responses mounted by CTLA-4-deficient T cells: a paradigm reconsidered. Eur J Immunol 31 (2), 450–8. [DOI] [PubMed] [Google Scholar]
- 26.Butz EA and Bevan MJ (1998) Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8 (2), 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori S et al. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299 (5609), 1057–61. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA and Rudensky AY. (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 4, 330–334. [DOI] [PubMed] [Google Scholar]
- 29.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 27 (1), 68–73. [DOI] [PubMed] [Google Scholar]
- 30.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 27 (1), 20–1. [DOI] [PubMed] [Google Scholar]
- 31.Wildin RS et al. (2002) Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet 39 (8), 537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salomon B et al. (2000) B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12 (4), 431–40. [DOI] [PubMed] [Google Scholar]
- 33.Chen C et al. (2006) Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J Biol Chem 281 (48), 36828–34. [DOI] [PubMed] [Google Scholar]
- 34.Wing K et al. (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322 (5899), 271–5. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi OS et al. (2011) Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332 (6029), 600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linsley PS et al. (1996) Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 4 (6), 535–43. [DOI] [PubMed] [Google Scholar]
- 37.Leach DR et al. (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271 (5256), 1734–6. [DOI] [PubMed] [Google Scholar]
- 38.Hodi FS et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363 (8), 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribas A et al. (2013) Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. Journal of Clinical Oncology 31 (5), 616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber J et al. (2017) Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 377 (19), 1824–1835. [DOI] [PubMed] [Google Scholar]
- 41.Larkin J et al. (2019) Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 381 (16), 1535–1546. [DOI] [PubMed] [Google Scholar]
- 42.Tawbi HA et al. (2018) Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med 379 (8), 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellmann MD et al. (2018) Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 378 (22), 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motzer RJ et al. (2018) Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 378 (14), 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolchok JD et al. (2017) Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 377 (14), 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larkin J et al. (2015) Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373 (1), 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Postow MA et al. (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372 (21), 2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolchok JD et al. (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369 (2), 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schadendorf D et al. (2015) Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 33 (17), 1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blank CU et al. (2018) Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 24 (11), 1655–1661. [DOI] [PubMed] [Google Scholar]
- 51.Amaria RN et al. (2018) Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 24 (11), 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selby MJ et al. (2016) Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, In Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PLoS One 11 (9), e0161779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selby MJ et al. (2013) Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 1 (1), 32–42. [DOI] [PubMed] [Google Scholar]
- 54.Du X et al. (2018) Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res 28 (4), 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du X et al. (2018) A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res 28 (4), 416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simpson TR et al. (2013) Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 210 (9), 1695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bulliard Y et al. (2013) Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. Journal of Experimental Medicine 210 (9), 1685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arce Vargas F et al. (2018) Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 33 (4), 649–663 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarhini AA et al. (2014) Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 9 (2), e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romano E et al. (2015) Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A 112 (19), 6140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma A et al. (2019) Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin Cancer Res 25 (4), 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lute KD et al. (2005) Human CTLA4 knock-in mice unravel the quantitative link between tumor immunity and autoimmunity induced by anti-CTLA-4 antibodies. Blood 106 (9), 3127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y et al. (2019) Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Research 29 (8), 609–627. [DOI] [PMC free article] [PubMed] [Google Scholar]