Abstract
Vaginal cytology is the most common method of monitoring the estrous cycle in rats; however, this test requires specific technical training and can be subject to interpretation. Vaginal impedance offers a quicker and less technically challenging alternative and has been used successfully to identify estrus in normally cycling breeder rats. We hypothesize that vaginal impedance can also be used to stage the estrous cycle in rats that have been given luteinizing hormone releasing hormone (LHRH) for timed mating. Vaginal impedance measurements and vaginal cytology were performed in LHRH-primed female rats (n = 36) at the expected peak of proestrus and paired with proven stud males. Breeding success was determined by gross necropsy to detect embryo implantation sites in the female rats. We found that the predictive rates of vaginal cytology and impedance measurement for proestrus were similar; however, both methods resulted in high proportions of false positive and false negative determinations (28% and 31%, respectively). We further hypothesized that females respond to LHRH at variable rates, resulting in variable times of peak proestrus. To test this, vaginal impedance measurements were performed multiple times throughout the expected day of proestrus in LHRH-primed female rats (n = 36). Females were either paired with a male 24 h after reaching the proestrus threshold (n = 18) or paired according to our standard protocol at 1300 h on the day after the expected proestrus (n = 18). Sequential measurements reduced false positive and negative rates (14% and 8%, respectively). Pregnancy rates did not differ based on the time of pairing during expected estrus. Overall, we determined vaginal impedance can be more successful than vaginal cytology at identifying proestrus in the rat, but only if multiple measurements are taken.
Abbreviations: LHRH, luteinizing hormone releasing hormone; SD, Sprague–Dawley
Quick, efficient, and accurate staging of estrous has important implications for the use of breeding technologies, the development of animal models, and on controlling sex-related effects in biomedical research. Hormonal fluctuations during the estrous cycle are commonly thought to add a confounding variable to research studies, even though evidence supports the contrary.20 Due to concerns about the impact of fluctuating hormones in female animals, many investigators use only male animals, which potentially leads to male sex bias in some disciplines.1 In May of 2014, the NIH announced a multifaceted plan to improve the sex balance in research,7 potentially increasing the need to stage the estrous cycle in female animals used for biomedical purposes. In addition, modern transgenic technology has revived the rat as a popular and valuable biomedical model. Despite this, the use of rats is constrained by limits of reproductive technology, specifically low embryo survival rates that require the euthanasia of large numbers of females for development of transgenic animals.6,13,16 Staging the estrous cycle is therefore vitally important for estrous synchronization and reducing the number of animals required for the production of transgenic rats.
Vaginal cytology is considered the ‘gold standard’ method for staging the estrous cycle and has been used for nearly a century in the rat.18 However, vaginal cytology is time-consuming and requires technical expertise outside the usual skill set of many laboratory or husbandry technicians. Vaginal cytology uses the natural cyclicity in the appearance of cells shed into the vaginal lumen to identify the stage of the estrous cycle. Performing this assessment requires both technical skill to collect the specimens and histologic skill to evaluate the cellular pattern.18
Vaginal impedance is a valuable alternative to cytology that has been successfully used in normally cycling female rats to evaluate the stage of the estrous cycle.2,22 Impedance measurements reflect the naturally occurring changes in electrical resistance of the vaginal mucosa during the different stages of the cycle.3 A single individual can perform measurements in seconds without the need for subjective interpretation of the results. A value ≥ 3 kiloohms (kΩ) indicates proestrus while the other 3 stages of the cycle have values lower than 3 kΩ.17
While vaginal impedance is commonly used to assess normally cycling females, data are not available to demonstrate whether impedance measurements can be used in females given luteinizing hormone releasing hormone (LHRH) agonist, a common procedure for synchronization of estrous.4 The goal of the current study was to evaluate the accuracy of vaginal impedance measurements compared with vaginal cytology in determining whether a female is in proestrus after being given 40 µg LHRH agonist 72 h previously. The use of vaginal impedance instead of cytology has the potential to reduce technician training time, the time needed to perform estrous cycle staging, and numbers of animals needed for breeding colonies or experiments by allowing experimental variation due to the estrous cycle to be better monitored and controlled.
Materials and Methods
Ethics Statement.
This study was approved by the University of Missouri Institutional Animal Care and Use Committee and conformed to all guidelines put forward by the Guide.14 At the beginning of the study, animals were confirmed to be free of common rat infectious agents using a quarterly sentinel monitoring program through IDEXX BioAnalytics (IDEXX Bioresearch SPF Rat Profiles, Quarterly and Annual; https://tinyurl.com/y2z2cvf5). Trained animal care staff monitored animals daily for food and water consumption and overall health status, with no adverse conditions or health outcomes noted.
Animals.
Research-naïve female Rattus norvegicus SAS Sprague–Dawley rats (Crl:SD; n = 72; hereby referred to as SD; Charles River, Kingston, NY) were obtained between 10 to 12 wk of age. Rats were housed in groups of 4 animals per ventilated cage (Thoren Maxi-Miser Interchangeable System, Hazelton, PA) containing paper chip bedding (SSP, Watertown, TN) and Carefresh nesting material (Ferndale, WA). All animals were kept on a 14:10 light-dark cycle (lights on, 0600 h; lights off, 2000 h) and provided with ad libitum autoclaved feed (Lab Diet 5001, St Louis, MO) and sulfuric acid-treated water. Proven, adult, Sprague–Dawley (SD) males (that is, males that had previously produced offspring) were used for breeding and kept at the same vivarium conditions as females, except for being singly housed when not breeding. Rats were euthanized using carbon dioxide inhalation at a fill rate of 10% to 30% of the chamber volume per minute and secondary cervical dislocation. An outline of the methods used in the following studies can be found in Figure 1.
Figure 1.
Protocol for evaluating the effectiveness of using vaginal impedance to stage estrous in female rats given LHRH by (A) comparing vaginal impedance and vaginal cytology, and (B) evaluating impedance values throughout the expected day of proestrus. (A) Three groups of 12 female SD rats between 10-12 wk of age were injected with 0.2 mL luteinizing hormone releasing hormone (LHRH) at 0900 h on day 0. At 1300 h on day 3, vaginal impedance measurements and vaginal cytology samples were taken (in that order). Females were paired with proven males 24 h later (day 4). At 0700 h on day 5, females were inspected for vaginal plugs. Ten days postmating, females were euthanized and evaluated for pregnancy status. (B) Three groups of 12 female Crl:SD rats between 10-12 wk of age were injected with 0.2 mL (40 µg) LHRH at 0900 h on day 0. On day 3, the expected day of proestrus, vaginal impedance measurements were taken at 0900, 1100, 1300, 1500 and 1700 h, or until the female reached an impedance value ≥ 3kΩ. The next day (day 4), half of the females were paired with a proven male exactly 24 h after their positive proestrus impedance reading and half were paired at 1300 h per standard protocols.4 The next morning at 0700 h, females were inspected for vaginal plugs and were euthanized 10 d after mating to evaluate pregnancy status.
Luteinizing Hormone Releasing Hormone.
A 200 ug/mL stock of luteinizing hormone releasing hormone (LHRH) was made by diluting 5 mg of [des-Gly,10 D-Ala6]-LH-RH ethylamide acetate salt hydrate (L4513; Sigma-Aldrich, St. Louis, MO) in 25 mL of pharmaceutical grade, nonbacteriostatic, sterile water (Cat. no. 00409488710, Hospira, Lake Forest, IL). Aliquots (1 mL) were stored at -80 °C for up to 3 mo. Females were given 40 μg IP (0.2 mL) within 30 min of thawing on ice.
Vaginal Impedance.
Vaginal impedance was measured with the Rat Vaginal Impedance Checker (Model MK-11; Muromachi Kikai, Tokyo, Japan) immediately before vaginal cytology was performed. Measurements were performed by 2 individuals. One person held the rat in a 4-finger grasp while immobilizing the hindlimbs and the other handled the probe and measurement. A 4.5 mm diameter plastic probe containing 2 silver ring electrodes 3 mm apart (RP-45A Rat Probe; Muromachi Kikai, Tokyo, Japan) was inserted into the vagina and held stable while lightly pressing down on the caudoventral abdomen to ensure complete contact with the metal electrodes. The threshold for confirmation of proestrus was ≥ 3.0 kΩ. The probe was cleaned with 70% ethanol, purified water, and a sterile gauze between use in each rat.
Vaginal Cytology.
Vaginal cytology was performed by vaginal lavage using single-use, blunted, glass pipettes (Fisherbrand 5 2/4”, Fisher Scientific, Suwanee, GA) and sterile PBS. The tip of the pipette was gently inserted 5 to 10 mm into the vaginal orifice and approximately 0.2 mL of PBS was flushed into the vagina 2 to 3 times until cloudy. A single drop of sample was placed onto a glass slide in a thin layer and allowed to air dry. A new pipette was used for each rat. Each slide was stained with Diff-Quik (RAL Diagnostics, Martillac, France) and examined by light microscopy. The stage of the estrous cycle was determined by a trained veterinarian who was blind to the vaginal impedance measurements.
Vaginal Cytology compared with Vaginal Impedance.
Upon receipt, female SD rats (n = 36) were acclimated to vivarium conditions for 4 to 5 d before experimental manipulation. Beginning at 0900 h on day 0, rats were manually restrained for a single LHRH IP injection into the right caudoventral abdomen. Beginning at 1300 h on day 3, the expected day of proestrus, each rat was manually restrained for vaginal impedance measurement and cytology. At 1300 h on day 4, the expected day of estrus, females were paired for mating with a proven breeder Crl:SD male regardless of their stage of the estrous cycle. Females were checked for vaginal plugs the following morning (day 5) by carefully extending blunt-end forceps into the vaginal canal to expand the opening and visualize the white plug. Plug status was recorded and females were then cohoused in groups of 4 rats. Regardless of plug status, females remained in the study, and, ten days after mating, females were euthanized to confirm pregnancy by gross observation of embryo development within the uterine horns.
Sequential Vaginal Impedance.
Upon receipt, female SD rats (n = 36) were acclimated to vivarium conditions and injected with LHRH on day 0, as described above. Beginning at 0900 h on day 3, each female was manually restrained for vaginal impedance measurement. Measurements were taken for each female at 0900, 1100, 1300, 1500, and 1700 h, or until a female reached a value greater than or equal to 3.0 kΩ (the threshold signaling proestrus). Half of these females were paired with a male exactly 24 h after their proestrus threshold reading, and half were paired at 1300 h on day 4. Any females that did not exceed a reading of 3 kΩ were included in the group paired with a male at 1300 h on day 4. Females were checked for vaginal plugs the next morning (day 5) by carefully extending blunt-end forceps into the vaginal canal to expand the opening and visualize the white plug. Plug status was recorded and females were then cohoused in groups of 4. Regardless of plug status, ten days after mating, females were euthanized to confirm pregnancy by gross observation of embryo development within the uterine horns.
Data Collection and Statistical Analysis.
Vaginal cytology samples were categorized as either proestrus, estrus, or diestrus for each female (n = 36) (Figure 2). Stage of the estrous cycle was determined by visualization of the sample under light microscopy according to previously developed criteria.18 Briefly, proestrus (12 to 14 h) is characterized by a dominance of round, nucleated epithelial cells of uniform size. Estrus (25 to 27 h) is distinguished by the appearance of irregularly shaped, translucent, cornified epithelial cells lacking a nucleus. Metestrus (6 to 8 h) characterized by a predominance of leukocytes and occasional nucleated cells (approximately 10:1 ratio). A dominance of leukocytes with occasional round, nucleated epithelial cells characterizes diestrus (approximately 3:1 ratio; 55 to 57 h). Ten 200× fields per smear were analyzed and cellular frequencies were determined to distinguish the phases. Each cytology sample was given a designation and compared with the female's impedance measurement. Cytology and impedance were compared with pregnancy outcome for each female to get false positive and false negative rates for each method. Sequential vaginal impedance measurements were measured for each female (n = 36) and analyzed based on when that female was paired with a male (24 h after reaching an impedance reading ≥ 3 kΩ as compared with pairing at 1300 h) and whether the female was pregnant at necropsy. Categorical frequency data was analyzed using a log-linear analysis for a 3-way contingency table of cross-categorized frequency data (VassarStats 3-Way Contingency Table calculator at http://vassarstats.net/abc.html).
Figure 2.
Representative images of Diff-Quik (modified Giemsa)-stained vaginal smears from rats showing the relative cellular composition of 3 out of the 4 stages of the estrous cycle at 200× magnification. (A) Proestrus; cells tend to appear in clumps with an abundance (more than 80%) of basal and nucleated intermediate and superficial epithelial cells. (B) Estrus; More than 75% nonnucleated (cornified) superficial squamous cells. (C) Diestrus; more than 60% polymorphonuclear cells with occasional nucleated epithelial cells.
Results
Vaginal Cytology compared with Vaginal Impedance.
Of the 36 female rats used to compare vaginal cytology and vaginal impedance, 18 (50%) reached a vaginal impedance reading ≥ 3 kΩ at 1300 h on the expected day of proestrus. Eighteen (50%) females also had vaginal cytology readings that placed them in proestrus at 1300 h on the expected day of proestrus. These evaluations agreed with each other 78% of the time (14/18 rats). We confirmed vaginal plugs in 12 females – 7 (58%) of which were confirmed in proestrus by impedance measurement. A χ2 test of independence was performed to examine the relationship between impedance value and plug status. The relation between these variables was not significant, X2 (1, n = 36) = 2.9, p = 0.09. Of the 14 females that tested positive for proestrus by both cytology and impedance, only 5 (36%) were confirmed pregnant at the 10-d postmating necropsy evaluation (Table 1).
Table 1.
Comparison of vaginal impedance value, vaginal cytology reading, and pregnancy status at necropsy
| Vaginal cytology |
|||||||||
| Diestrus |
Proestrus |
Estrus |
|||||||
| P | NP | P | NP | P | NP | ||||
| Vaginal impedance | ≥ 3 kΩ | 3 | 1 | 5 | 9 | 0 | 0 | ||
| < 3 kΩ | 7 | 3 | 3 | 1 | 1 | 3 | |||
P, pregnant, NP, not pregnant
The 4 females that had a vaginal impedance reading ≥ 3kΩ but did not have a vaginal cytology positive for proestrus all had cytology confirming diestrus (Table 1). Of these females, 3 were confirmed pregnant at necropsy. Of the females that did not reach an impedance reading ≥ 3 kΩ, 10 were confirmed to be in diestrus through vaginal cytology, 4 confirmed in proestrus, and 4 confirmed in estrus (Table 1). Eleven of these females were confirmed pregnant at necropsy (7 from the diestrus group, 3 from the proestrus group, and one from the estrus group) (Table 1). Vaginal impedance measurement and vaginal cytology had the same false positive (28%) and false negative (31%) proportions. Likewise, sensitivity, specificity and accuracy were the same for both methods. Sensitivity (the proportion of females confirmed pregnant that were correctly identified as being in proestrus on the expected day – “true positive”) was 42%, with a 95% Confidence Interval (CI) of 20% to 66%] and specificity (the proportion of females confirmed not pregnant that were correctly identified as not being in proestrus on the expected day – “true negative”) was 41%, with a 95% CI of 18% to 69%] (Figure 3). Accuracy (the number of correct assessments out of all assessments, or the probability a female will be assigned the correct stage of the estrous cycle) was 42%.
Figure 3.
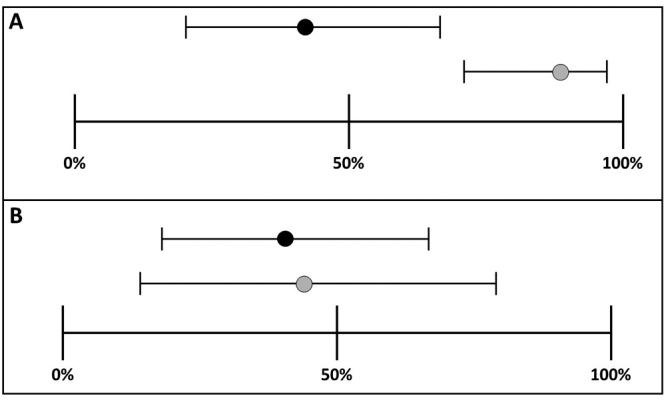
Ninety-five percent confidence intervals around estimates of sensitivity and specificity (A) Sensitivity for single (black circle) and sequential impedance readings (gray circle). 95% CI around estimate of sensitivity for sequential readings is narrower than for single readings. (B) Specificity for single reading (42%) and sequential readings (44%).
Sequential Vaginal Impedance.
Of the 36 females tested with sequential measurements, 29 (81%) reached a vaginal impedance reading ≥ 3kΩ on the expected day of proestrus (Figure 4). Of these proestrus “positive” females, 20 (67%) were confirmed to have a vaginal plug after mating overnight with a proven stud male. A χ2 test of independence found a significant relationship between impedance measurement and plug positive status [X2 (1, n = 36) = 3.9, P < 0.05, meaning that proestrus impedance value is strongly correlated with mating and plug positive status]. Twenty-four of the 29 sequentially tested females (83%) were confirmed pregnant at necropsy 10 d after mating (Table 2). No significant difference in pregnancy rates was found between females having undergone one impedance reading or multiple readings, X2 (1, n = 36) = 1.60, p = 0.21.
Figure 4.
Time at which females reached an impedance reading ≥ 3kΩ on day 3. Once females reached a reading of greater than or equal to 3kΩ, they were not measured again to reduce the chance of inducing pseudopregnancy.
Table 2.
Comparison of vaginal impedance value, pairing schedule, and pregnancy status at necropsy
| Pairing schedule |
||||||
| 24 h after impedance reading ≥ 3 kΩ |
1300 h (standard protocol) |
|||||
| P | NP | P | NP | |||
| Vaginal impedance | ≥ 3 kΩ | 14 | 4 | 10 | 1 | |
| < 3 kΩ | n/a | n/a | 3 | 4 | ||
P, pregnant, NP, not pregnant, n/a, not applicable
Seven females failed to reach an impedance measurement ≥ 3kΩ during the expected day of proestrus. Of these females, 3 were confirmed pregnant at necropsy. False positive and negative rates for sequential measurements were 14% and 8%, respectively. Sensitivity and specificity were recorded for sequential measurements. Sensitivity is the ability of a test (impedance) to correctly identify “true positive” subjects (females in proestrus). Specificity is the ability of a test (impedance) to correctly identify “true negative” subjects (females not in proestrus). Sensitivity and specificity of sequential vaginal impedance measurement were 89% [95% CI 71% to 98%] and 44% [95% CI 14% to 79%], respectively (Figure 3). Accuracy of sequential measurement was 78%. Log-linear analysis for a 2 × 2 × 2 contingency table of cross-categorized frequency data from sequential measurement data (impedance ≥ or < 3kΩ, pairing schedule, and pregnancy status) revealed that pregnancy rates differed significantly between females reaching the ≥ 3kΩ impedance value threshold for proestrus and those that did not (χ2 (1) = 4.3, P < 0.05). Females reaching an impedance value ≥ 3kΩ were significantly more likely to be confirmed pregnant at necropsy. However, time of pairing after sequential measurement did not affect pregnancy rates.
Log-linear analysis for a 2 × 2 × 2 contingency table of cross-categorized frequency data was used to compare the method of measurement (single or sequential), impedance value (≥ 3 kΩ or < 3 kΩ), and pregnancy status. Pregnancy rates differed significantly between females with an impedance value ≤ 3 kΩ (χ2 (1) = 3.9, P < 0.05) and females reaching an impedance value ≥ 3 kΩ regardless of method used. The females with the ≥ 3 kΩ impedance measurement were significantly more likely to be confirmed pregnant at necropsy. In addition, the method of measurement (single or sequential) had a significant effect on impedance values (χ2 (1) = 7.6, p < 0.01), with sequential measurement resulting in more females confirmed in proestrus by impedance value (≥ 3 kΩ) than with the single impedance reading.
Discussion
Overall, single vaginal impedance readings in female rats hormonally primed with LHRH resulted in equivalent false positive and negative rates as did vaginal cytology (28% and 31%, respectively) on the expected day of proestrus. This suggests that, for monitoring proestrus, vaginal impedance is as informative as vaginal cytology and supports its use as an alternative method. In addition, sequential vaginal impedance measurement resulted in much lower false positive and negative rates than did vaginal cytology (14% and 8% compared with 28% and 31%, respectively). Females reaching an impedance value greater than or equal to 3 kΩ were significantly more likely to be pregnant at necropsy than those not reaching this threshold. However, pairing schedule (pairing at 24 h after reaching impedance threshold as compared with pairing at 1300 h per our standard protocol) had no effect on pregnancy rates. In addition to the within-subjects design of the current study, future studies could include animals measured solely by impedance or cytology to ensure that using both means to measure the stage of the estrous cycle do not confound the results.
Vaginal impedance measurement is a quicker and more efficient way to monitor for proestrus than cytology. Multiple references for vaginal cytology in the rat are available; however, contemporary references tend to have relatively abbreviated information regarding the stages and lack comprehensive illustrations for training new technicians.11,12,18,19 In particular, limited information is provided on how to differentiate stages of the estrous cycle during transitional periods. Given how quickly rats cycle through the different stages of the estrous cycle, a single cytology sample may have characteristics of multiple stages. This could explain how some females in very early proestrus could be categorized as in diestrus by cytology (if lymphocytes remained in the sample) but proestrus by impedance.
To use cytology, an individual must have training in microscopy, knowledge of the variations that may be encountered on cytology, the ability to recognize artifactual changes, and the ability to interpret the overall characteristics of the sample beyond simple cell counts. To achieve appropriate proficiency in performing cytologic evaluation, trainees are advised to follow groups of animals for several cycles, checking cytology daily, to become familiar with their colony.8 Months of training may be necessary before a technician has the skills required to accurately stage the estrous cycle in animals. Furthermore, repeated cytologic sampling of animals during technician training has the potential to stimulate the neuroendocrine reflex, resulting in pseudopregnancy and/or diestrus cytology, creating abnormal estrous cyclicity and confounding training.4,27
In contrast to cytology, vaginal impedance relies on unambiguous numerical data to provide technicians with information within seconds regarding whether an animal is or is not in proestrus. If the probe leads are appropriately contacting the vaginal mucosa, the reading is accurate. The ease of performing impedance saves technician time and lowers costs by eliminating both the lengthy training and the time spent collecting, drying, staining, and reading each cytology sample.
The current study also supports the use of vaginal impedance over vaginal cytology to reduce animal numbers needed for breeding colonies and animal studies. By performing sequential vaginal impedance measurements, only 3 of the 36 females were categorized as not in proestrus by impedance measurement, but were confirmed pregnant at necropsy (false negative). This was a much lower false negative rate than seen for the single measurement and vaginal cytology group. In breeding colonies, false negatives are more problematic than false positives because mistakenly eliminating females extends the time required to acquire needed animal numbers for breeding colonies and research studies. Also, although 4-d estrous cycles are much more common in young female rats,9,18,19 some regularly cycling females with 5-d cycles may have been missed with the method we used for our study. In addition, multiple impedance measurements may result in mechanical stimulation of the cervix and cause higher pregnancy rates,13,27 but only if the measurements are performed incorrectly and the probe is inserted farther into the vaginal canal than is recommended.
Vaginal impedance does have limitations when considering overall estrous cycle monitoring. Impedance can only distinguish proestrus from the other 3 stages of estrous, because values less than 3 kΩ are nonspecific for estrus, metestrus, and diestrus. Therefore, investigators working in fields such as reproductive biology may require full estrous cycle monitoring, for which vaginal cytology is still the gold standard. Circulating hormone levels may not correspond to vaginal impedance or, depending on the age of the animal, to vaginal cytology.23,26 Previous studies correlating patterns of hormone fluctuation with vaginal cytology lack sufficient information regarding age and breeding status of females, making these results difficult to interpret.5,10.
We chose to use a single dose injection of LHRH as our method of estrous synchronization because it is a common and standard technique.4 A single-dose of LHRH provokes a preovulatory-like luteinizing hormone (LH) surge; however, studies have demonstrated variable success with single-dose LHRH inducing ovulation.15,21,24 This may explain why we saw low pregnancy success in our studies (approximately 53% and 75% for single and sequential monitoring respectively). Studies evaluating prolonged release of LHRH to achieve synchronization have had better results; this method may prove useful in the future, when coupled with impedance measurement.25
Overall, our findings suggest that vaginal impedance is equal to or better than vaginal cytology at detecting proestrus in females given LHRH. Laboratories with time constraints would be equally as successful using a single impedance measurement to detect proestrus as using cytology; however, laboratories with the means to perform multiple measurements over the expected day of proestrus will have better results than using vaginal cytology. Our data, along with vaginal impedance's ease of use, efficiency, and reduction in animal handling and stress, when compared with cytology, support the use of impedance measurement as an alternative and highly effective tool to determine proestrus in the rat.
Acknowledgments
This research was supported by the Office of the Director of the National Institutes of Health under award numbers P40 OD11062 (ECB) and T32 OD011126 (ECB, KLC). CC was supported by funding from The American Society of Laboratory Animal Practitioners (ASLAP) and IDEXX-Bioresearch as part of the University of Missouri Veterinary Research Scholars Program (VRSP). We thank Dr Yuksel Agca for training in vaginal cytology.
References
- 1.Beery AK, Zucker I. 2011. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35:565–572. 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartoš L. 1975. Oestral cycle phase determination by means of electrical impedance measurements of the vaginal mucosus membrane in the rat. Physiol Bohemoslov 24:427. [Google Scholar]
- 3.Bartoš L. 1977. Vaginal impedance measurement used for mating in the rat. Lab Anim 11:53–55. 10.1258/002367777780959148. [DOI] [PubMed] [Google Scholar]
- 4.Borjeson TM, Pang J, Fox JG, Garcia A. 2014. Administration of luteinizing hormone releasing hormone agonist for synchronization of estrus and generation of pseudopregnancy for embryo transfer in rats. J Am Assoc Lab Anim Sci 53:232–237. [PMC free article] [PubMed] [Google Scholar]
- 5.Butcher RL, Collins WE, Fugo NW. 1974. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 94:1704–1708. 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 6.Charreau B, Tesson L, Soulilou JP, Pourcel C, Anegon I. 1996. Transgenesis in rats: technical aspects and models. Transgenic Res 5:223–234. 10.1007/BF01972876. [DOI] [PubMed] [Google Scholar]
- 7.Clayton JA, Collins FS. 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509:282–283. 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cora MC, Kooistra L, Travlos G. 2015. Vaginal cytology of the laboratory rat and mouse: Review and criteria for staging of the estrous cycle using stained vaginal smears. Toxicol Pathol 43:776–793. 10.1177/0192623315570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman ME. 1988. The ovarian cycle of the rat, p 1893–1928. In: Knobil E, Neil J, editors. Physiology of reproduction. New York (NY): Raven Press. [Google Scholar]
- 10.Gay VL, Midgley AR, Niswender GD. 1970. Patterns of gonadotrophin secretion associated with ovulation. Fed Proc 29:1880–1887. [PubMed] [Google Scholar]
- 11.Goldman JM, Murr AS, Cooper RL. 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80:84–97. 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 12.Hubscher CH, Brooks DL, Johnson JR. 2005. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem 80:79–87. 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- 13.Iannaccone P, Galat V. 2014. Production of transgenic rats, p 251–273. In Pinkert CA, editor. Transgenic animal technology: a laboratory handbook. New York (NY): Elsevier. [Google Scholar]
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 15.Jin YK, Bae HS, Lee JY, Yum SY, Kim KM, Koo OJ, Jang G, Ailia MJ. 2018. The effect of gonadotropin-releasing hormone agonist on superovulation and estrous synchronization in female Sprague– Dawley rat. Reprod Fertil Dev 30:231–239. 10.1071/RDv30n1Ab197. [DOI] [Google Scholar]
- 16.Kawamata M, Ochiya T. 2011. Gene-manipulated embryonic stem cells for rat transgenesis. Cell Mol Life Sci 68:1911–1915. 10.1007/s00018-011-0669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koto M, Miwa M, Togashi M, Tsuji K, Okamoto M, Adachi J. 1987. [[A method for detecting the optimum day for mating during the 4-day estrous cycle in the rat; measuring the value of electrical impedance of the vagina]]. Jikken Dobutsu 36:195–198 [[[Article in Japanese]]]. [DOI] [PubMed] [Google Scholar]
- 18.Long JA, Evans HM. 1922. The oestrous cycle in the rat and its associated phenomena. Berkeley (CA): University of California Press. [Google Scholar]
- 19.Mandl AM. 1951. The phases of the oestrous cycle in the adult white rat. J Exp Biol 28:576–584. [Google Scholar]
- 20.Prendergast BJ, Onishi KG, Zucker I. 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40:1–5. 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Pursley JR, Mee MO, Wiltbank MC. 1995. Synchronization of dairy cows using PGF2α and GnRH. Theriogenology 44:915–923. 10.1016/0093-691X(95)00279-H. [DOI] [PubMed] [Google Scholar]
- 22.Ramos SD, Lee JM, Peuler JD. 2001. An inexpensive meter to measure differences in electrical resistance in the rat vagina during the ovarian cycle. J Appl Physiol(1985) 91:667–670. 10.1152/jappl.2001.91.2.667. [DOI] [PubMed] [Google Scholar]
- 23.Singletary SJ, Kirsch AJ, Watson J, Karim BO, Huso DL, Hurn PD, Murphy SJ. 2005. Lack of correlation of vaginal impedance measurements with hormone levels in the rat. Contemp Top Lab Anim Sci 44:37–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson KE, Stevenson JS, Lamb GC, Grieger DM, Löest CA. 1999. Follicular, hormonal, and pregnancy responses of early postpartum suckled beef cows to GnRH, norgestomet, and prostaglandin F2α. J Anim Sci 19:1823–1832. 10.2527/1999.7771823x. [DOI] [PubMed] [Google Scholar]
- 25.Vickery BH, McRae GI. 1980. Synchronization of oestrus in adult female rats by utilizing the paradoxical effects of an LH-RH agonist. J Reprod Fertil 60:399–402. 10.1530/jrf.0.0600399. [DOI] [PubMed] [Google Scholar]
- 26.Weixelbaumer KM, Drechsler S, Wehrenpfennig P, Khadem A, Bahrami S, Tichy A, Palme R, Osuchowski MF. 2014. Estrus cycle status defined by vaginal cytology does not correspond to fluctuations of circulating estrogens in female mice. Shock 41:145–153. 10.1097/SHK.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 27.Westwood FR. 2008. The female rat reproductive cycle: a practical histologic guide to staging. Toxicol Pathol 36:375–384. 10.1177/0192623308315665. [DOI] [PubMed] [Google Scholar]





