Abstract
Accurate assessment of coagulation in porcine studies is essential. We sought to establish normal values for porcine rotational thromboelastometry (ROTEM) according to the American Society for Veterinary Clinical Pathology guidelines and to assess the effects of various preanalytical parameters on those measurements. Healthy Yorkshire-cross pigs (n = 81; 46 males and 35 females) were anesthetized. By using a 18-gauge needle attached to a vacuum phlebotomy tube, blood was acquired from the cranial vena cava. Tubes were filled in the following order: evacuation clot tube, EDTA tube, heparin tube, and 2 citrate tubes. The citrate tubes were randomly assigned to 30 min with or without constant agitation on a rocker. The following parameters were reported according to the manufacturer's recommendations: clotting time, clot formation time, α, (tangent to the clot formation curve when the clot firmness is 20 mm), clot firmness after 10 and 20 min, maximal clot firmness, maximum lysis, and lysis indexes at 30 and 45 min. Reference intervals were reported as mean ± 2 SD (parametric distribution) or 2.5th and 97.5th percentile of the population's results (nonparametric distribution). The effects of sex, sampling order, and agitation on ROTEM results were analyzed through linear regression. Neither sex nor sample agitation influenced any of the ROTEM parameters. Combined reference intervals were established for each ROTEM parameter by pooling data from the nonagitated tubes for both male and female pigs. This study is the first to establish ROTEM reference intervals from a large number of male and female adult Yorkshire-cross pigs and to provide a detailed description of preanalytical sample processing.
Abbreviations: α, tangent to the clot formation curve when the clot firmness is 20 mm; A10, clot firmness after 10 minutes; A20, clot firmness after 20 minutes; CT, clotting time; CFT, clot formation time; EXTEM, Extrinsic assay panel; Li30, lysis index at time 30; INTEM, Intrinsic assay panel ; Li45, lysis index at time 45; LRL 90%, 90% confidence interval of the lower fence of the reference limit; MCF, maximum clot firmness; ML, maximum lysis; ROTEM, rotational thromboelastometry; URL 90%, 90% confidence interval of the upper fence of the reference limit
Despite differences between the porcine and human coagulation systems,15,19 pigs remain a species of choice for translational research,10 specifically in thrombosis and hemostasis.5,20,22 Rotational thromboelastometry (ROTEM; Instrumentation Laboratory, Bedford, MA) is a diagnostic tool that allows for simple and rapid benchtop evaluation of coagulation function. With rotational viscoelastometry, the elastic properties of a clot are measured from formation until lysis by immersing a pin into the blood sample. Whereas coagulation times provide key information regarding coagulation factor activity, rotational viscoelastometry is a user-friendly benchtop method that allows evaluation of the entire coagulation cascade (including platelet function and fibrinolysis), which is reflective of the cell-based coagulation model.2,7 Animal studies have shown the potential for ROTEM to assist in understanding, preventing, and treating various conditions, including acute traumatic coagulopathy,12,23,24 cerebral injury,13 cardiopulmonary bypass,9 and sepsis.31
Translational research bridges the gap between laboratory concepts and clinical practice. Biomedical research in animals allows for reproducible development and refinement of novel therapies. It is important to establish rotational thromboelastometry reference values in species used as models in coagulation research. Although many studies report the use of ROTEM in pigs, only one reported reference values.30 Furthermore, no studies report normal ROTEM values in a large cohort of Yorkshire-cross pigs, which are commonly used in our laboratory and in translational research in general. In addition, no available study satisfies the “National Institute of Health's expectation that scientists will account for the possible role of sex as a biological variable in vertebrate animal and human studies.”18 Finally, previous studies do not follow recent guidelines regarding the use of viscoelastometry7 or reference values determination4 in veterinary medicine, thus limiting the scientific impact and reproducibility of their results.
We sought to establish normal ROTEM Intrinsic and Extrinsic (INTEM and EXTEM) values for a large cohort of male and female Yorkshire-cross pigs by following the most recent veterinary viscoelastometry guidelines.4,7 Furthermore, it is common practice to place samples on a tube rocker for a 30-min preanalytical incubation period. Consequently, our secondary aim was to assess the effects of preanalytical sample rocking on ROTEM values.
Materials and Methods
This study was approved (protocol no. FDG20180031A) by the IACUC at David Grant USAF Medical Center (Travis Air Force Base, CA). After blood sampling, animals were used for other IACUC-approved experiments within our facility. Nonspecific pathogen-free animals were procured from the University of California-Davis (4%; Davis, CA), S and S Farms (81%; Ranona, CA), and Oak Hill Genetics (15%; El Nido, CA). Animals were acclimated for 10 d prior to the experiment. All animals were housed to provide visual, olfactory, and, when possible, tactile contact with conspecifics. All animal care and use were in compliance with the Guide for the Care and Use of Laboratory Animals in an AAALAC-accredited facility.14
The study population comprised 81 Yorkshire-cross pigs (Sus scrofa; 46 males and 35 females; age, 5 to 6 mo), which were apparently healthy, in light of absence of clinical signs of systemic illness (such as inappetence, diarrhea, fever, cough, or lameness) and with normal CBC (VetScan HM5, Abaxis, Union City, CA) and biochemistry (Vet Axcel, Alfa Wassermann, West Caldwell, NJ) profiles. Animals were food-fasted overnight before use but had free access to water. Pigs were anesthetized by using tiletamine–zolazepam (6.6 mg/kg IM; Fort Dodge Animal Health, Fort Dodge, IA). We used an 18-gauge needle attached to a blood collection system (Vacutainer, BD Monoject, Franklin Lake, NJ) to obtain the following blood samples from the right cranial vena cava: 1) 1 mL in a clot tube for fibrinogen quantification, 2) 2 sodium citrate tubes (2 mL each) for ROTEM analysis, 3) 2 mL in an EDTA tube for counting platelets. In addition, sodium citrate tubes contained 3.2% buffered sodium citrate at a strict 1:9 ratio of citrate to blood, yielding a final citrate concentration of 10.8 mM; 1 of the 2 tubes was selected randomly and placed on a rocker during the incubation, and the other was not. For samples 2 and 3, the blood was gently mixed with the anticoagulant in the tube. Fibrinogen concentration was measured on the nonagitated tube (VETSCAN VSpro, Abaxis). The methods used to collected samples 1 and 3 satisfied current recommendations regarding reporting of ROTEM results.7
ROTEM analysis was conducted in accordance with the most recent guidelines for the establishment of reference values4 and the use of rotational viscoelastometry in veterinary medicine.7 All samples used for ROTEM measurement were held at room temperature for 30 min prior to analysis, which was performed according to manufacturer's guidelines. ROTEM quality control was performed once daily prior to analysis. The following INTEM and EXTEM parameters were analyzed according to the manufacturer's recommendations: clotting time (CT), clot formation time (CFT), α angle, clot firmness after 10 min (A10), clot firmness after 20 min (A20), maximal clot firmness (MCF), maximum lysis (ML), lysis index at 30 min (Li30), and lysis index at 45 min (Li45).
Statistical analysis.
Methodology for establishing reference values for laboratory testing in veterinary species has been standardized by the American Society for Veterinary Clinical Pathology.4 Data were analyzed by using Reference Value Adviser version 2.1.6 This freeware assesses data distribution normality according to the Anderson–Darling test. Symmetry distribution is verified by robust methods. Outliers are detected by using the Dixon and Tukey tests. Accordingly, reference intervals are reported as either mean ± 2 SD or the 2.5th to 97.5th percentiles. In addition, the 90% CI of the lower and upper reference limits are reported for both distributions.4 The effects of sex, sampling order, and sample agitation on individual ROTEM parameters were assessed through logistic regression. Significance was set as a P value less than 0.01, to adjust for multiple comparisons. The effects of Hct, fibrinogen concentration, and platelet count were assessed by using linear regression.
Results
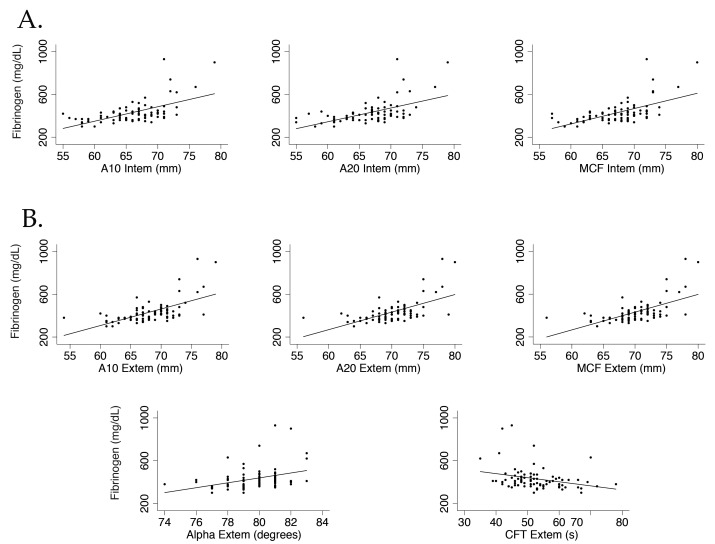
The median (2.5th to 97.5th percentiles) platelet count (× 109/L) was 235 (52 to 401), 233 (66 to 423), and 234 (68 to 407) in male pigs, female swine, and combined for both sexes, respectively. The median (2.5th to 97.5th percentiles) Hct (%) was 27.8 (22.5 to 30.1), 26.9 (21.7 to 31.5), and 27.0 (21.7 to 31.3) in males, females, and both sexes combined, respectively, and serum fibrinogen concentration (mg/dL) was 385 (300 to 529), 420 (340 to 570), and 400 (300 to 534). Preanalytical sample agitation and Hct had no effects on INTEM or EXTEM results (P > 0.01). Sampling order had no influence on INTEM parameters but significantly affected the EXTEM results for CFT (P = 0.005) and α (P = 0.006). Fibrinogen concentration influenced the INTEM results for A10 (r2 = 0.33, P < 0.001), A20 (r2 = 0.30, P < 0.001), and MCF (r2= 0.33, P < 0.001) and the EXTEM results for A10 (r2 = 0.38, P < 0.001), A20 (r2 = 0.38, P < 0.001), MCF (r2 = 0.38, P < 0.001), α (r2 = 0.12, P < 0.001), and CFT (r2 = 0.08, P = 0.003; Figure 1).
Figure 1.
Statistically significant relationships between serum fibrinogen concentration and various (A) INTEM and (B) EXTEM ROTEM parameters in swine.
Sex and platelet count did not influence any of the ROTEM results (P > 0.01). Because preanalytical sample agitation had no effects on INTEM or EXTEM results, combined reference intervals were established for each ROTEM parameter by merging data from the nonagitated tubes of male and female pigs (Tables 1 and 2).
Table 1.
INTEM reference intervals in swine
| CT (s) | CFT (s) | α (°) | A10 (mm) | A20 (mm) | MCF (mm) | ML (%) | Li30 (%) | Li45 (%) | |
| Male pigs | |||||||||
| Mean | 165 | 55 | 79 | 66 | 66 | 67 | 14 | 95 | 89 |
| 1 SD | 51 | 15 | 2 | 4 | 5 | 4 | 5 | 3 | 6 |
| CV (%) | 30.9 | 27.3 | 2.5 | 6.1 | 7.6 | 6.0 | 35.7 | 3.2 | 6.7 |
| Median | 167 | 52 | 80 | 66 | 67 | 68 | 14 | 96 | 90 |
| Range | 78–338 | 40–135 | 74–82 | 58–73 | 46–74 | 58–74 | 5–23 | 87–99 | 60–97 |
| RI | 78–326 | 40–126 | 74–82 | 58–73 | 48–74 | 58–74 | 5–23 | 87–99 | 64–97 |
| LRL 90% | 78–83 | 40–42 | 74–77 | 58–59 | 46–60 | 58–61 | 5–7 | 87–89 | 60–82 |
| URL 90% | 221–338 | 65–135 | 82–82 | 71–73 | 71–74 | 72–74 | 21–23 | 99–99 | 95–97 |
| Female pigs | |||||||||
| Mean | 168 | 51 | 80 | 67 | 68 | 68 | 14 | 96 | 90 |
| 1 SD | 66 | 7 | 3 | 5 | 5 | 4 | 4 | 3 | 4 |
| CV (%) | 39.3 | 13.7 | 3.8 | 7.5 | 7.4 | 5.9 | 28.6 | 3.1 | 4.4 |
| Median | 157 | 50 | 80 | 67 | 68 | 68 | 13 | 96 | 90 |
| Range | 70–359 | 40–71 | 68–83 | 54–77 | 56–78 | 56–78 | 8–23 | 86–99 | 80–95 |
| RI | 72–338 | 40–69 | 74–86 | 57–76 | 58–77 | 59–77 | 7–23 | 90–102 | 83–98 |
| LRL 90% | 62–88 | 38–42 | 71–77 | 54–60 | 56–61 | 56–62 | 7–8 | 88–92 | 81–85 |
| URL 90% | 278–399 | 63–77 | 83–87 | 74–78 | 74–79 | 75–79 | 20–25 | 100–104 | 96–99 |
| Both sexes combined | |||||||||
| Mean | 166 | 54 | 79 | 66 | 67 | 68 | 14 | 95 | 89 |
| 1 SD | 57 | 15 | 3 | 5 | 5 | 5 | 5 | 3 | 5 |
| CV (%) | 34.3 | 27.8 | 3.8 | 7.6 | 7.5 | 7.4 | 35.7 | 3.2 | 5.6 |
| Median | 162 | 52 | 80 | 67 | 68 | 68 | 14 | 96 | 90 |
| Range | 70–359 | 40–135 | 64–83 | 42–77 | 46–78 | 46–78 | 5–23 | 86–99 | 60–97 |
| RI | 78–337 | 40–123 | 68–83 | 54–76 | 55–77 | 56–77 | 6–23 | 87–99 | 80–96 |
| LRL 90% | 70–82 | 40–41 | 64–76 | 42–58 | 46–60 | 46–61 | 5–8 | 86–90 | 60–82 |
| URL 90% | 269–359 | 78–135 | 82–83 | 73–77 | 72–78 | 73–78 | 22–23 | 99–99 | 95–97 |
α, α angle; A10, clot firmness after 10 min; A20, clot firmness after 20 min; CFT, clot formation time; CT, clotting time; Li30, lysis index at 30 min; Li45, lysis index at 45 min; LRL 90%, 90% confidence interval of the lower fence of the reference limit; MCF, maximal clot firmness; ML, maximal lysis; RI, reference interval; URL 90%, 90% confidence interval of the upper fence of the reference limit.
Table 2.
EXTEM reference intervals in swine
| CT (s) | CFT (s) | α (°) | A10 (mm) | A20 (mm) | MCF (mm) | ML (%) | Li30 (%) | Li45 (%) | |
| Male pigs | |||||||||
| n | 46 | 46 | 45 | 45 | 45 | 45 | 46 | 46 | 46 |
| Mean | 59 | 55 | 80 | 68 | 69 | 70 | 9 | 98 | 94 |
| 1 SD | 8 | 13 | 2 | 4 | 4 | 4 | 3 | 2 | 3 |
| CV (%) | 13.6 | 23.6 | 2.5 | 5.9 | 5.8 | 5.7 | 33.3 | 2.0 | 3.2 |
| Median | 60 | 54 | 80 | 68 | 70 | 70 | 9 | 99 | 95 |
| Range | 39–76 | 40–127 | 76–82 | 60–74 | 62–75 | 62–75 | 3–16 | 94–100 | 88–98 |
| RI | 40–75 | 40–118 | 76–82 | 60–74 | 62–75 | 62–75 | 3–16 | 94–100 | 88–98 |
| LRL 90% | 39–47 | 40–46 | 76–76 | 60–62 | 62–64 | 62–63 | 3–4 | 94–96 | 88–90 |
| URL 90% | 70–76 | 69–127 | 81–82 | 73–74 | 74–75 | 74–75 | 14–16 | 100–100 | 98–98 |
| Female pigs | |||||||||
| n | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Mean | 59 | 54 | 80 | 69 | 70 | 71 | 8 | 99 | 95 |
| 1 SD | 8 | 9 | 2 | 4 | 4 | 4 | 3 | 1 | 2 |
| CV (%) | 13.6 | 16.7 | 2.5 | 5.8 | 5.7 | 5.6 | 37.5 | 1.0 | 2.1 |
| Median | 59 | 56 | 79 | 68 | 69 | 70 | 8 | 99 | 96 |
| Range | 39–71 | 35–74 | 76–83 | 61–79 | 64–80 | 64–80 | 4–16 | 95–100 | 90–98 |
| RI | 42–76 | 35–74 | 76–83 | 62–79 | 63–78 | 63–78 | 4–15 | 96–101 | 91–100 |
| LRL 90% | 38–46 | 31–40 | 75–77 | 60–63 | 61–78 | 62–65 | 3–5 | 95–97 | 89–92 |
| URL 90% | 72–80 | 69–78 | 82–84 | 75–82 | 76–80 | 75–80 | 13–17 | 100–102 | 99–100 |
| Both sexes combined | |||||||||
| n | 81 | 81 | 80 | 80 | 80 | 80 | 81 | 81 | 81 |
| Mean | 59 | 54 | 80 | 68 | 70 | 70 | 9 | 98 | 95 |
| 1 SD | 8 | 8 | 2 | 4 | 4 | 4 | 3 | 1 | 3 |
| CV (%) | 13.6 | 14.8 | 2.5 | 5.9 | 5.7 | 5.7 | 33.3 | 1.0 | 3.2 |
| Median | 59 | 54 | 80 | 68 | 69 | 70 | 8 | 99 | 95 |
| Range | 39–76 | 35–74 | 76–83 | 60–79 | 62–80 | 62–80 | 3–16 | 94–100 | 88–98 |
| RI | 39–71 | 40–73 | 76–82 | 61–78 | 63–79 | 63–79 | 4–16 | 95–100 | 90–98 |
| LRL 90% | 39–44 | 35–42 | 76–77 | 60–62 | 62–64 | 62–64 | 3–4 | 94–96 | 88–90 |
| URL 90% | 70–76 | 68–74 | 82–83 | 74–79 | 75–80 | 75–80 | 114–16 | 100–100 | 98–98 |
α, α angle; A10, clot firmness after 10 min; A20, clot firmness after 20 min; CFT, clot formation time; CT, clotting time; Li30, lysis index at 30 min; Li45, lysis index at 45 min; LRL 90%, 90% confidence interval of the lower fence of the reference limit; MCF, maximal clot firmness; ML, maximal lysis; RI, reference interval; URL 90%, 90% confidence interval of the upper fence of the reference limit. n < 81 pigs for some parameters due to the presence of outliers.
Discussion
Following the most recent veterinary guidelines, we established ROTEM (INTEM and EXTEM) reference intervals in a large cohort of male and female adult pigs.4,7 In addition, we demonstrated that neither sex nor platelet count significantly altered ROTEM results in our cohort, and preanalytical sample agitation and sampling order did not affect most INTEM and EXTEM results.
Swine are frequently used in translational medicine for several reasons. First, their cardiovascular and coagulation functions are close to those of humans. Second, large tissue samples can be acquired, which is a major advantage when compared with smaller mammals, such as rats and mice. In addition, due to their comparable size to humans, device development in pigs can be rapidly converted to clinical medicine in both pediatric and adult patients. Swine are thus a well-established model for coagulation and thrombosis research in various fields.5,20,22 Therefore, having establishing normal reference intervals for pigs is important to interpretation of relevant data.
Human and pigs do show some differences in coagulation and thrombosis. Several studies have described a more procoagulant profile in pigs when compared with humans. In one study, CT was more than twice higher in humans than in pigs,27 and in another, MCF was higher in pigs than in humans.27 Indeed, in our current study, pigs exhibited a faster rate of fibrin formation evidenced by higher EXTEM A10, A20, and MCF values when compared with humans (Table 3), although no direct comparison could be made due to the design of the study. In addition, pigs demonstrated stronger clots with better viscoelastic properties, indicated by shorter CFT, greater α angle, and higher MCF values; these alterations are associated with higher serum fibrinogen concentrations in pigs compared with humans. Elevation of serum fibrinogen concentration is a significant contributor to hypercoagulable profiles in humans21 and dogs,28 because it provides a direct substrate for the clot, improves platelet bridging, and increases blood viscosity.17 The reference intervals for the porcine lysis indices include values of 100% for Li30 and Li45. These results suggest, as previously mentioned for dogs,11 that viscoelastic evaluations seem poorly sensitive to detect hypofibrinolysis. Some authors have proposed adding tissue plasminogen activator to blood samples to increase the ability of the viscoelastic assays to identify alterations of fibrinolysis in humans16 and dogs.3
Table 3.
INTEM and EXTEM reference intervals in humans8
| CT (s) | CFT (s) | α (°) | A10 (mm) | A20 (mm) | MCF (mm) | ML (%) | Li30 (%) | Li45 (%) | |
| INTEM RI | 100–240 | 30–110 | 70–83 | 44–66 | 50–71 | 50–72 | < 15 | 94–100 | not done |
| EXTEM RI | 38–79 | 34–159 | 63–83 | 43–65 | 50–71 | 50–72 | < 15 | 94–100 | 85–100 |
α, α angle; A10, clot firmness after 10 min; A20, clot firmness after 20 min; CFT, clot formation time; CT, clotting time; Li30, lysis index at 30 min; Li45, lysis index at 45 min; MCF, maximal clot firmness; ML, maximal lysis; RI, reference interval.
Our results did not show significant differences in ROTEM parameters between male and female pigs. These results are similar to those of a previous study in dogs that was aimed at establishing reference intervals for thromboelastographic parameters.1 Some human studies suggest that women have a more hypercoagulable profile than men.26,29 Our study did not take into account the stage of the sexual cycle of the pigs, which could have influenced our results.
Sampling order had an effect on CFT and α in the EXTEM panel. This unexpected finding can be attributed to variation in platelet activation. The biologic significance of this observation remains unknown. Researchers should use consistent technique when sampling blood for ROTEM analysis.
Our study has several limitations. First, we evaluated a heterogeneous cohort of animals procured from 3 farms. Genetic variability may lead to different results for animals from other sources. Second, blood was sampled after induction of general anesthesia, which is common in porcine biomedical research. Although no available study describes the effects of tiletamine and zolazepam on porcine rotational viscoelastometry, anesthesia with ketamine, halothane, nitrous oxide, and oxygen has no effect on conventional coagulation parameters.25 Finally, we did not establish reference intervals for the other cartridges available for the ROTEM system (FIBTEM, HEPTEM, APTEM).
This study is the first to establish ROTEM normal values in a large number of male and female adult Yorkshire-cross pigs by following the most recent guidelines regarding the use of viscoelastometry7 and reference interval determination4 in veterinary medicine. Preanalytical agitation and animal sex had no effect on ROTEM results. Our findings provide valuable information for researchers using porcine models.
Acknowledgments
The views expressed in this material are those of the authors and do not reflect the official policy or position of the US Government, the Department of Defense, the Department of the Air Force, or the University of California–Davis. The animals involved in this study were procured, maintained, and used in accordance with the Laboratory Animal Welfare Act of 1966, as amended, and the Guide for the Care and Use of Laboratory Animals (National Research Council). The work reported herein was performed under United States Air Force Surgeon General-approved Clinical Investigation no. FDG20170015A. This study was funded by The United States Air Force, Headquarters, Office of the Surgeon General. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the David Grant USAF Medical Center, Clinical Investigation Facility, and administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and USAF-DGMC-CIF.
References
- 1.Bauer N, Eralp O, Moritz A. 2009. Reference intervals and method optimization for variables reflecting hypocoagulatory and hypercoagulatory states in dogs using the STA Compact automated analyzer. J Vet Diagn Invest 21:803–814. 10.1177/104063870902100606. [DOI] [PubMed] [Google Scholar]
- 2.Crochemore T, Piza FMT, Rodrigues RDR, Guerra JCC, Ferraz LJR, Corrêa TD. 2017. A new era of thromboelastometry. Einstein (Sao Paulo) 15:380–385. 10.1590/s1679-45082017md3130.[Article in English, Portuguese]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher DJ, Rozanski EA, Brainard BM, de Laforcade AM, Brooks MB. 2015. Assessment of the relationships among coagulopathy, hyperfibrinolysis, plasma lactate, and protein C in dogs with spontaneous hemoperitoneum. J Vet Emerg Crit Care (San Antonio) 26:41–51. 10.1111/vec.12346. [DOI] [PubMed] [Google Scholar]
- 4.Friedrichs KR, Harr KE, Freeman KP, Szladovits B, Walton RM, Barnhart KF, Blanco-Chavez J. 2012. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41:441–453. 10.1111/vcp.12006. [DOI] [PubMed] [Google Scholar]
- 5.Frith D, Cohen MJ, Brohi K. 2012. Animal models of trauma-induced coagulopathy. Thromb Res 129:551–556. 10.1016/j.thromres.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 6.Geffré A, Concordet D, Braun J-P, Trumel C. 2011. Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. 2011. Vet Clin Pathol 40:107–112. 10.1111/j.1939-165X.2011.00287.x. [DOI] [PubMed] [Google Scholar]
- 7.Goggs R, Brainard B, de Laforcade AM, Flatland B, Hanel R, McMichael M, Wiinberg B. 2014. Partnership on rotational viscoelastic test standardization (PROVETS): evidence-based guidelines on rotational viscoelastic assays in veterinary medicine. 2014. J Vet Emerg Crit Care (San Antonio) 24:1–22. 10.1111/vec.12144. [DOI] [PubMed] [Google Scholar]
- 8.Görlinger K, Dirkmann D, Hanke A. 2016. Rotational thromboelastometry (ROTEM®), p 267–298. Chapter 18. In: Gonzalez E, Moore HB, Moore EE, editors. Trauma induced coagulopathy. Switzerland: Springer; 10.1007/978-3-319-28308-1_18 [DOI] [Google Scholar]
- 9.Gronchi F, Perret A, Ferrari E, Marcucci CM, Fleche J, Crosset M, Schoettker P, Marcucci C. 2014. Validation of rotational thromboelastometry during cardiopulmonary bypass: A prospective, observational in-vivo study. Eur J Anaesthesiol 31:68–75. 10.1097/EJA.0b013e328363171a. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez K, Dicks N, Glanzner WG, Agellon LB, Bordignon V. 2015. Efficacy of the porcine species in biomedical research. Front Genet 6:1–9. 10.3389/fgene.2015.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanel RM, Chan DL, Conner B, Gauthier V, Holowaychuk M, Istvan S, Walker JM, Wood D, Goggs R, Wiinberg B. 2014. Systematic evaluation of evidence on veterinary viscoelastic testing part 4: Definitions and data reporting. J Vet Emerg Crit Care (San Antonio) 24:47–56. 10.1111/vec.12145. [DOI] [PubMed] [Google Scholar]
- 12.Hatch Q, Debarros M, Eckert M, Satterly S, Nelson D, Porta R, Lesperance R, Long W, Martin M. 2014. Acute coagulopathy in a porcine venous hemorrhage and ischemia reperfusion model. Am J Surg 207:637–641. 10.1016/j.amjsurg.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Hvas CL, Fenger-Eriksen C, Hoyer S, Sorensen B, Tonnesen E. 2013. Hypercoagulation following brain death cannot be reversed by the neutralization of systemic tissue factor. Thromb Res 132:300–306. 10.1016/j.thromres.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 15.Kessler U, Grau T, Gronchi F, Berger S, Brandt S, Bracht H, Marcucci C, Zachariou Z, Jakob SM. 2011. Comparison of porcine and human coagulation by thrombelastometry. 2011. Thromb Res 128:477–482. 10.1016/j.thromres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper GJ, Kleinegris MC, van Oerle R, Spronk HM, Lancé MD, Ten Cate H, Henskens YM. 2016. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb J 14:1 10.1186/s12959-016-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laharrague PF, Cambus JP, Fillola G, Corberand JX. 1993. Plasma fibrinogen and physiological aging. Aging (Milano) 5:445–449. [DOI] [PubMed] [Google Scholar]
- 18.Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Wetherington CL, Makris S, Arnold AP, Einstein G, Miller VM, Sandberg K, Maier S, Cornelison TL, Clayton JA. 2017. Considering sex as a biological variable in preclinical research. FASEB J 31:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno T, Tsukiya T, Takewa Y, Tatsumi E. 2018. Differences in clotting parameters between species for preclinical large animal studies of cardiovascular devices. J Artif Organs 21:138–141. 10.1007/s10047-017-1003-4. [DOI] [PubMed] [Google Scholar]
- 20.Münster AM, Olsen AK, Bladbjerg EM. 2002. Usefulness of human coagulation and fibrinolysis assays in domestic pigs. Comp Med 52:39–43. [PubMed] [Google Scholar]
- 21.Nielsen VG, Cohen BM, Cohen E. 2005. Effects of coagulation factor deficiency on plasma coagulation kinetics determined via thrombelastography: critical roles of fibrinogen and factors II, VII, X and XII. Acta Anaesthesiol Scand 49:222–231. 10.1111/j.1399-6576.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 22.Olsen AK, Kornerup Hansen A, Jespersen J, Marckmann P, Bladbjerg E-M. 1999. The pig as a model in blood coagulation and fibrinolysis research. Scand J Lab Anim Sci 26:214–224. [Google Scholar]
- 23.Ponschab M, Schochl H, Keibl C, Fischer H, Redl H, Schlimp CJ. 2015. Preferential effects of low volume versus high volume replacement with crystalloid fluid in a hemorrhagic shock model in pigs. BMC Anesthesiol 15:1–10. 10.1186/s12871-015-0114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prat NJ, Montgomery R, Cap AP, Dubick MA, Sarron JC, Destombe C, May P, Magnan P. 2015. Comprehensive evaluation of coagulation in swine subjected to isolated primary blast injury. Shock 43:598–603. 10.1097/SHK.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 25.Roussi J, Andre P, Samama M, Pignaud G, Bonneau M, Laporte A, Drouet L. 1996. Platelet functions and haemostasis parameters in pigs: absence of side effects of a procedure of general anaesthesia. Thromb Res 81:297–305. 10.1016/0049-3848(96)00001-1. [DOI] [PubMed] [Google Scholar]
- 26.Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, Huang H, Pinto R, Speers V, Reis M, Rizoli SB. 2009. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res 42:1210–1217. 10.1590/S0100-879X2009001200015. [DOI] [PubMed] [Google Scholar]
- 27.Siller-Matula JM, Plasenzotti R, Spiel A, Quehenberger P, Jilma B. 2008. Interspecies differences in coagulation profile. Thromb Haemost 100:397–404. 10.1160/TH08-02-0103. [DOI] [PubMed] [Google Scholar]
- 28.Smith SA, McMichael MA, Gilor S, Galligan AJ, Hoh CM. 2012. Correlation of hematocrit, platelet concentration, and plasma coagulation factors with results of thromboelastometry in canine whole blood samples. Am J Vet Res 73:789–798. 10.2460/ajvr.73.6.789. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian A, Albert V, Saxena R, Agrawal D, Pandey RM. 2014. Establishing a normal reference range for thromboelastography in North Indian healthy volunteers. Indian J Pathol Microbiol 57:43–50. 10.4103/0377-4929.130896. [DOI] [PubMed] [Google Scholar]
- 30.Velik-Salchner C, Schnürer C, Fries D, Müssigang PR, Moser PL, Streif W, Kolbitsch C, Lorenz IH. 2006. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thromb Res 117:597–602. 10.1016/j.thromres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Velik-Salchner C, Streif W, Innerhofer P, Maier S, Knotzer H, Pajk W, Klingler A, Mittermayr M, Haas T. 2009. Endotoxinemia-induced changes in coagulation as measured by rotation thrombelastometry technique and conventional laboratory tests: results of a pilot study on pigs. Blood Coagul Fibrinolysis 20:41–46. 10.1097/MBC.0b013e32831be9ad. [DOI] [PubMed] [Google Scholar]